Abstract
Objective
CDK9 controls the activation of primary inflammatory response genes. We determined whether CDK9 inhibition protects cartilage from the catabolic effects of pro-inflammatory cytokines.
Methods
Human chondrocytes were challenged with different pro-inflammatory stimuli (IL-1β, lipopolysaccharides, and TNFα), in the presence or absence of the CDK9 inhibitor Flavopiridol, or siRNA. The mRNA expression of inflammatory mediators, catabolic, and anabolic genes were determined by real-time PCR. Cartilage explants were incubated with IL-1β, with or without Flavopiridol, for 6 days. Cartilage matrix degradation was assessed by the release of glycosaminoglycan (GAG) and cleaved Type II collagen (Col2a) peptides.
Results
CDK9 inhibition by Flavopiridol, or knockdown by siRNA, effectively suppressed iNOS mRNA induction by all three pro-inflammatory stimuli. Results from NFkB-targets PCR array showed that Flavopiridol suppressed the induction of a broad range of inflammatory mediator genes (59 out of 67 tested) by IL-1β. CDK9 inhibition also suppressed induction of catabolic genes MMP 1, 3, 9, 13, and ADAMTS4, 5; but did not affect the basal expression of anabolic genes such as Col2a, aggrecan, and COMP, and housekeeping genes. Flavopiridol had no apparent short-term cytotoxicity as assessed by glucose-6-phosphate dehydrogenase activity. Finally, in IL-1β-treated cartilage explants, Flavopiridol reduced the release of matrix degradation products GAG and cleaved Col2a peptides, but did not affect long-term chondrocyte viability.
Conclusion
CDK9 activity is required for the primary inflammatory response in chondrocytes. Flavopiridol suppresses the induction of inflammatory mediators and catabolic genes to protect cartilage from the deleterious effects of pro-inflammatory cytokines, without impacting cell viability and functions.
Keywords: CDK9, inflammatory cytokines, primary response genes, Flavopiridol, chondrocytes, cartilage
Introduction
Osteoarthritis (OA) affects more than half of the people over age 65 in the United States. OA is a degenerative disease of the articular joints characterized by slow but progressive lost of cartilage. The main protein component of articular cartilage is a fibrillar network of type II collagen (Col2a), which provides tensile strength to the cartilage. The compressive stiffness of the cartilage is provided by the proteoglycan components, through their attraction of water molecules. Although the etiology of OA remains incompletely understood, various inflammatory conditions that cause damage to the collagens and proteoglycans in cartilage are suspected of initiating OA.
Pro-inflammatory cytokines are induced by a variety of stress conditions in cartilage, including joint overloading and physical damage such as occurs in sports-related injuries. Pro-inflammatory cytokines, such as interleukin 1 beta (IL-1β) and tumor necrosis factor alpha (TNFα), elicit a cascade of events that activate inflammatory mediator genes and apoptosis in chondrocytes (reviewed by (1)). Pro-inflammatory cytokines can also induce the expression of proteinases that degrade cartilage matrix, including matrix metallopeptidases (MMPs), aggrecanases, and cathepsins (reviewed by (2)). Therefore, a stretagy to effectively suppress the inflammatory response in cartilage may prevent or delay the onset of osteoarthritis.
Acute tissue stress and inflammatory signaling activate primary response genes (PRG) that do not require de novo protein synthesis. Recent advances demonstrate that despite their initiation by diverse signaling pathways, the transcriptional activation of most, if not all, PRG is similarly controlled by a general transcription factor (3, 4), namely the cyclin-dependent kinase 9 (CDK9). It was believed for many years that the rate-limiting step in transcriptional activation of PRG is the recruitment of transcription factors and RNA Polymerase II (Pol II) complex to the promoters. However, recent studies show that in order for these PRG to be activated rapidly, in their basal and unstimulated states, the Pol II complex is already pre-assembled and producing short mRNA transcripts (3, 4). In the absence of inflammatory signals, Pol II remains paused ~40 base-pair downstream of the transcription start site. Upon inflammatory stimulus, CDK9 is recruited to the transcription complex by the Bromodomain-containing protein (Brd) 4 through its associated with acetylated histones (8, 9). Once recruited, CDK9 phosphorylates Pol II to induce a conformational change that allows Pol II to enter possessive elongation to efficiently transcribe full-length mRNAs (reviewed in (5)). Thus, CDK9 regulation represents a central mechanism of activating PRG transcription and has a broad impact on many aspects of biological functions.
Given that CDK9 controls a common mechanism of all PRG activation, it is an attractive target for anti-inflammatory therapy (reviewed in (6)). The objective of this study is to determine whether CDK9 inhibition effectively suppress the inflammatory response in chondrocytes and protect cartilage from the catabolic effects of pro-inflammatory cytokines in vitro.
Methods
Articular chondrocytes and cartilage explants
Human primary chondrocytes and cartilage explants were isolated from cartilage tissues obtained from total knee arthroplasty (15 donors, age 44–80 years, all with end stage OA) with IRB approval and cultured in DMEM with 10% FBS as described (7). The chondrocytes were used for experiments within 3–5 days without passaging to avoid dedifferentiation. A total of 5 cartilage explant donors were used for matrix degradation studies (Figure 5). All other experiments were performed with chondrocytes from at least 3 individual donors. Full thickness bovine cartilage explants were isolated from the stifle joints of young veal calves using 6-mm biopsy punches and maintained in DMEM with 10% FBS.
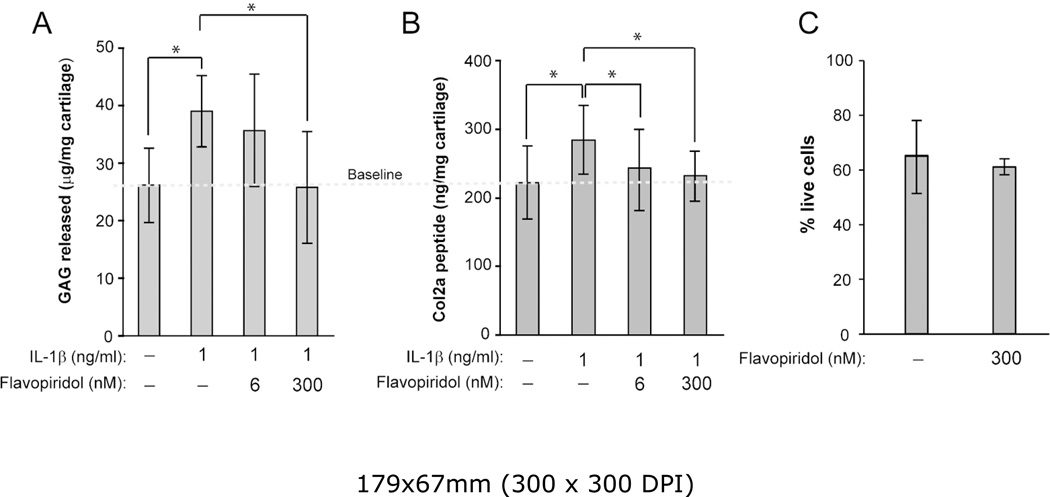
Figure 5. CDK9 inhibition protects cartilage from the catabolic effects of IL-1β.
(A) GAG breakdown in cartilage explants. Human arthritic cartilage explants (3mm cubes) were treated with 1 ng/ml IL-1s and the indicated concentrations of Flavopiridol for 6 days (media change at day 3). GAG released into the medium was measured by DMMB assays and normalized to the wet weight of the explants. Treatment with IL-1β alone caused cartilage degradation as indicated by increased GAG release. In the presence of 300nM Flavopiridol, GAG release returned to baseline. (B) Col2a degradation in cartilage explants. Human cartilage explants (n=5 donors) were treated with 1 ng/ml IL-1s and the indicated concentrations of Flavopiridol for 6 days (media change at day 3). Cleaved Col2a peptides released into the medium was measured by C2C ELSIA and normalized to the wet weight of the explants as described in the Methods. Treatment with IL-1β alone caused cartilage degradation as indicated by increased Col2a peptides. In the presence of Flavopiridol, Col2a peptides release returned to baseline. For all the above experiments, each data point was the mean +/− standard deviation from five different donors. (*p<0.05). (C) Long-term Flavopiridol treatment does not reduce chondrocyte viability. Bovine cartilage explants (6 mm full thickness disk) (n=3 different donors) were treated with 300 nM Flavopiridol for 6 days. The explants were sliced in half and stained with LIVE/DEAD stain as described in the Methods. The numbers of live and dead cells in three random fields were counted and the percentages of live cells were calculated.
Treatments of chondrocytes with inflammatory stimuli and small molecule inhibitors
Primary chondrocytes were seeded in 12-well plates at a density of 2×104 cells/cm2 and allowed to reach ~80% confluence (~4×104 cells/cm2). The cells were treated with 10 ng/ml lipopolysaccharide (LPS) (Sigma), 10 ng/ml IL-1β (R&D System), or 10 ng/ml TNFα (R&D System) for various time, with or without pharmacological inhibitors. The inhibitors used in this study included Flavopiridol (Sigma), JQ-1 (a kind gift from Dr. James Bradner, Harvard University (8)), BS-181 HCl (Selleckchem), and SNS-032 (Selleckchem). After the treatment, the cells were washed 3 times with phosphate buffered saline and harvested for gene and protein expression analysis.
Lentiviral siRNA constructs
The CDK9-targeting siRNA used in this study (AGGGACATGAAGGCTGCTAAT) was inserted into the AgeI and EcoRI sites of the lentiviral vector pLKO.1 (plasmid #8453, www.Addgene.com). A siRNA targeting GFP was used as control. Lentiviral particles were generated and tittered as described (9). Human chondrocytes were seeded at 1×104 cells/cm2 in 12-well plate. Lentiviral particles harboring CDK9- or GFP-targeting siRNA were then added at a multiplicity of infection of 10, in the presence of 1 Ng/ml polybrene (American Bioanalytical). The media was replaced after 16 hours and the cells were used for experiments after 5 days. Knockdown of CDK9 was confirmed by Western blotting.
Quantitative real-time (RT) PCR
For the determination of individual mRNA expression, cytokine/inhibitor-treated chondrocytes in 12-well plates were harvested by scraping and transferred to Eppendorf tubes, followed by cell lysis and reverse transcription to generate cDNA using the Cells-to-CT kit (Ambion) according to the manufacturer’s instructions. 2 ul of cDNA was used for quantitative RT-PCR (in a final volume of 10 ul) performed in triplicate in a 7900HT RT-PCR system with gene-specific Taqman probes (Applied Biosystem Inc.) according to the manufacturer’s conditions. Results were normalized to 18s rRNA and calculated as fold-change in mRNA expression relative to untreated control, using the 2−ΔΔ CT method. The probes used are as follow: iNOS, Hs01060345_m1; MMP1, Hs00899658_m1; MMP3, Hs00968305_m1; MMP9, Hs03234579_m1; MMP13, Hs0023992_m1; ADAMTS4, Hs00192708_m1; ADAMTS5, Hs00199841_m1; ACAN, Hs00202971_m1; COMP, Hs00154339_m1; COL2A, Hs01060345_m1; 18s rRNA, 4319413E. For PCR array analysis, IL-1β/Flavopiridol-treated chondrocytes grown in 10 cm plates were harvested and total RNA was isolated using the RNeasy Mini Kit (Qiagen). RNA quality and quantity were assessed with a Nanodrop 2000 spectrophotometer (Thermo Scientific) and ~500 ng of total RNA was reverse transcribed using a Superscript First-Strand kit (Invitrogen).
RT-PCR was performed in a 7900HT PCR system using the PCR Arrays for Human NFκB Signaling Targets (Qiagen, cat# 330231), according to the manufacturers’ protocol. PCR array data were analyzed by the accompanying online analysis software provided by Qiagen at www.qiagen.com.
Cytotoxicity/Viability Assays
For determining the short-term cytotoxic effects of Flavopiridol, chondrocytes were seeded in 96-well plates at 1, 5, or 10 × 103 cells per well and treated with 300 nM Flavopiridol for 5 hours. Cytotoxicity was assessed using the Vybrant Cytotoxicity Assay kit (Invitrogen, cat# V23111), according to the manufacturer’s protocol to measure soluble and total glucose 6-phosphate dehydrogenase (G6PD) activity with resazurin as substrates. Fluorescence was detected using a Synergy HT plate reader (Bio-TEK Instruments Inc.) with excitation and emission filters set at 528 and 635 nm, respectively.
For determining long-term effects of Flavopiridol on the viability of chondrocytes in cartilage, bovine cartilage explants (6–mm disk) were cultured in 6-well plates in DMEM and 10% FBS, in the presence or absence of 300 nM Flavopiridol for 6 days, with media changed every other day. The live and dead cells were stained using the LIVE/DEAD Viability/Cytotoxicity kit (Invitrogen, cat# L3224) according to the manufacturer’s protocol. The percentages of live and dead cells were determined by counting the cell numbers at three random fields of the cross-section images of explants captured using a fluorescence microscope. A total of 3 different donors were used.
Western blot analysis
Chondrocytes grown in 12-well plates were harvested and lysed with sample loading buffer (50 mM Tris-HCl, pH 6.8; 100 mM dithiothreitol; 4% 2-mercaptoethanol; 2% sodium dodecyl sulfate; 10% glycerol). Lysates were resolved by 4–12% SDS-polyacrylamide gels and transferred onto nitrocellulose membranes (BioRad). The membranes were blocked with 3% skim milk in TBST (25 mM Tris-HCl, pH 7.5; 125 mM NaCl; 0.1% Tween 20), followed by incubation with rabbit anti-CDK9 (0.6 ug/ml) (10) mouse anti-MMP13 (1:500 dilution) (Calbiochem, cat# IM78), or mouse anti-GAPDH (1:5000) (Ambion, cat# AM4300) at 4°C overnight. Blots were then probed with horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology), and reactive protein bands were visualized with Western Lightning Plus-ECL (Perkin Elmer) on radiographic films.
Assessment of cartilage degradation
Human cartilage explants (~3mm cubes) were treated with 1 ng/ml IL-1β for 6 days, in the presence or absence of 6 or 300 nM Flavopiridol (with media change on day 3). The amount of glycosaminoglycan (GAG) released into the media from day 3 to day 6 was determined by the colorimetric dimethylmethylene blue dye-assay, with chondroitin sulfate as standard (11). The release of Col2a degradation products into the media was determined by measuring the amount of cleaved Col2a peptides (12) with the C2C ELISA kit (IBEX Pharmaceuticals) according to the manufacturer’s protocol.
Statistical analysis
Values of all measurements were expressed as the mean + standard deviation. Changes in gene expression were analyzed by one-way ANOVA with SPSS 16.0 software. The fold-change in mRNA was used as variables to compare samples between different treatment groups. The least significant difference post-hoc analysis was conducted with a significance level of P<0.05.
Results
The role of CDK9 on the induction of the primary response gene iNOS
Although the rate-limiting step for transcriptional activation of primary inflammatory response genes in lymphocytes is controlled by CDK9 (3, 4), whether CDK9 plays a similar role in articular chondrocytes has not been investigated. Therefore, we take advantage of a widely used and well-characterized pharmacological CDK9 inhibitor, Flavopiridol, to determine the role of CDK9 in the activation of PRG in chondrocytes. To activate PRG, chondrocytes in culture were treated with IL-1β, in the presence or absence of 300 nM Flavopiridol (the effective plasma concentration determined in clinical trials (13)). The induction of the stress response gene iNOS (inducible nitric oxide synthase) (14) was determined at various time points. The results showed that iNOS mRNA was unchanged after 1 hour of IL-1β treatment, but was induced significantly after 3 and 5 hours (Figure 1A, open bars). However, co-treatment with Flavopiridol completely suppressed the induction of iNOS by IL-1β (Figure 1A, grey bars). These results indicate a time-dependent induction of iNOS by IL-1β that is sensitive to Flavopiridol treatment.
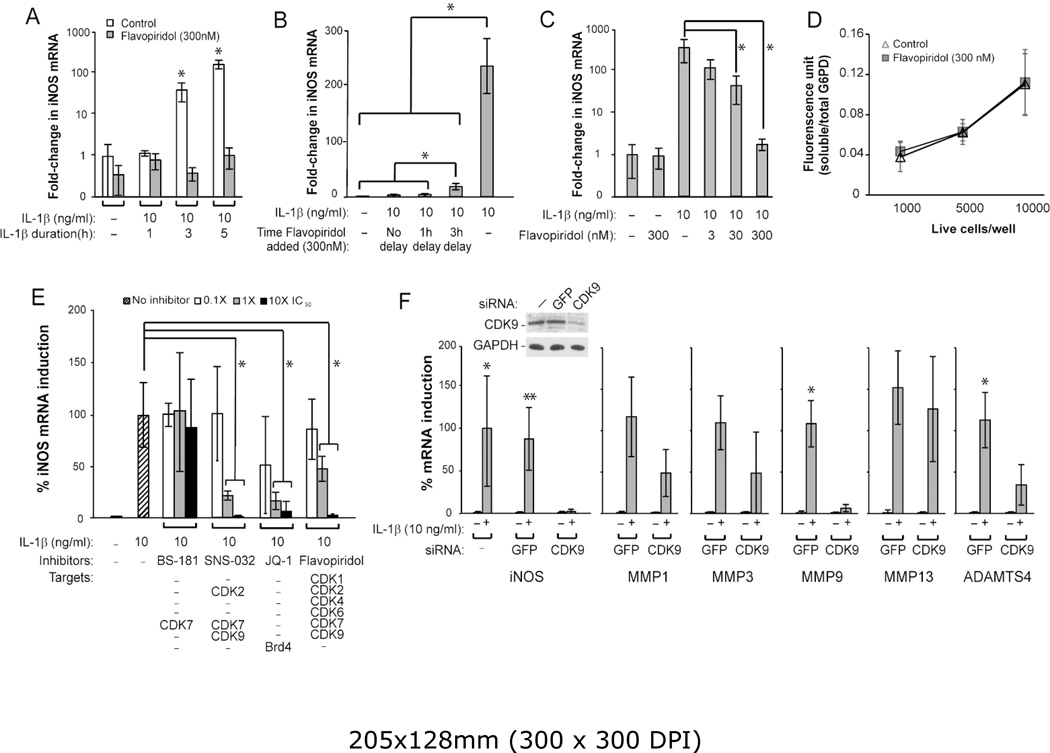
Figure 1. Characterization of the effects of CDK9 inhibition by Flavopiridol on iNOS induction.
(A) Kinetics of IL-1β-induced iNOS expression. Human chondrocytes were treated with 10 ng/ml IL-1β, with and without 300 nM Flavopiridol, for the indicated time. The fold-induction of iNOS mRNA relative to untreated control was determined by quantitative RT-PCR. (B) Time window of administration of Flavopiridol for iNOS suppression. Chondrocytes were treated with IL-1β for a total of 5 hours, with Flavopiridol added at different delayed time points to determine the window of opportunity for effective suppression of iNOS induction. (C) Dose-dependent suppression of iNOS induction by Flavopiridol. Chondrocytes were treated with IL-1β for 5 hours, with co-treatments of Flavopiridol at various concentrations to determine the dose-response for suppressing iNOS induction. (D) Cytotoxicity assays. Chondrocytes seeded in 96-wells at the indicated cell density were treated with 300 nM Flavopiridol for 5 hours, followed by measurement of soluble/total G6PD activity to determine the cytotoxic effects of Flavopiridol. (E) Suppression of iNOS induction by different inhibitors. Chondrocytes were treated with IL-1β for 5 hours in the presence of various small molecule inhibitors at the indicated concentrations. The induction of iNOS mRNA by IL-1β was determined and the maximum iNOS induction by IL-1β in the absence of inhibitor was set to 100%. The selected IC50 of various drugs based on their kinase inhibition are: BS-181, 21 nM for CDK7; SNS-032, 60 nM for CDK7 (used in this experiment) and 4 nM for CDK9; Flavopiridol, 30 nM for CDK9. JQ-1 is not a kinase inhibitor but prevents CDK9 recruitment to PRG promoters through suppressing the binding of Brd4 to acetylated histones at an IC50 of 300 nM. (F) siRNA-mediated depletion of CDK9 impairs iNOS, and catabolic gene induction. Chondrocytes were transduced with lentiviral particles harboring siRNA against CDK9, or GFP as control. After 5 days, cells were treated with IL-1β for 5 hours and harvested for Western and mRNA analysis. While IL-1β induction of iNOS was not significantly different between the control (*) and GFP siRNA (**) groups, iNOS induction was markedly suppressed by CDK9 knockdown. The IL-1β-induced mRNA expression of MMP1, 3, 9, 13 and ADAMTS4 in cells with GFP siRNA was similar to that of the control (not shown), but their induction were reduced by CDK9 siRNA. For all the above experiments, each data point was the mean +/− standard deviation from three different donors. (*, ** indicates p<0.05)
The above results demonstrated the effects of Flavopiridol administered concurrently with the inflammatory cytokines. We next tested whether a delay in the addition of Flavopiridol could still suppress iNOS induction by IL-1β. Chondrocytes were treated with IL-1β for a total of 5 hours, with either no delay, or a 1- or 3-hour delay in the addition of Flavopiridol. The data showed that when compared to no Flavopiridol treatment (~235-fold iNOS induction), the addition of Flavopiridol markedly repressed iNOS induction if Flavopiridol was administered at no delay (down to ~2.7-fold induction) and at a 1-hour delay (~4-fold) (Figure 1B). Although less effective, a 3-hour delay still allowed significant repression of iNOS induction (~18-fold) (Figure 1B). These results indicate that there is at least a 3-hour window of opportunity for administering Flavopiridol to suppress iNOS induction after the initial treatment of IL-1β. We next demonstrated that Flavopiridol suppressed iNOS induction by IL-1β in a dose-dependent manner, with the most effective dose at 300 nM (Figure 1C). Importantly, there was no apparent cytotoxicity of Flavopiridol at the highest dose, in terms of G6PD activity, on cultured chondrocytes within the 5 hours time frame tested (Figure 1D).
Besides CDK9, Flavopiridol has off-target effects that include other CDKs (listed in Figure 1E). While these CDKs do not affect PRG activation directly, we never the less used additional CDK inhibitors to rule out their involvement in suppressing iNOS induction. To this end, we tested the abilities to suppress iNOS induction by the following three small molecule inhibitors: BS-181 HCl (specific for CDK7) (15), SNS-032 (targeting CDK2, 7, and 9) (16), and JQ-1 (specific for Brd4, which is required for CDK9’s function (8)). The data showed that only SNS-032, JQ-1, and Flavopiridol, but not BS-181, suppressed iNOS induction in a dose-dependent manner (Figure 1E), thus effectively ruling out the involvement of CDK7 in IL-1β-induced iNOS transcription. It is important to note that unlike the other CDK inhibitors tested here, JQ-1 does not directly inhibit the kinase activity of CDK, but rather, it prevents the association of acetylated histones with Brd4 (8), which specifically recruits CDK9 to the promoters for activation of transcription of PRG (4, 5). Therefore, the above results strongly suggest that CDK9 is involved in iNOS induction. To definitively prove this, we used siRNA to specifically knockdown CDK9 expression in chondrocytes and determined the effects on iNOS induction. The results showed that in CDK9-depleted cells (confirmed by Western blot, Figure 1F inset), IL-1β failed to induce iNOS. Similar results were obtained when other catabolic genes such as MMP1, 3, 9, 13, and ADAMTS4 were examined (Figure 1F), thus demonstrating the requirement of CDK9 in catabolic gene induction. Taken together, our results clearly show the specific involvement of CDK9, but not other CDKs, in the induction of iNOS by IL-1β. Since Flavopiridol is the first small molecule CDK inhibitor in clinical trials with well-characterized pharmacokinetics, it has the highest potential for translation into clinical studies, and therefore, it is used exclusively in the remainder of this study.
CDK9 controls the activation of inflammatory response from diverse signals
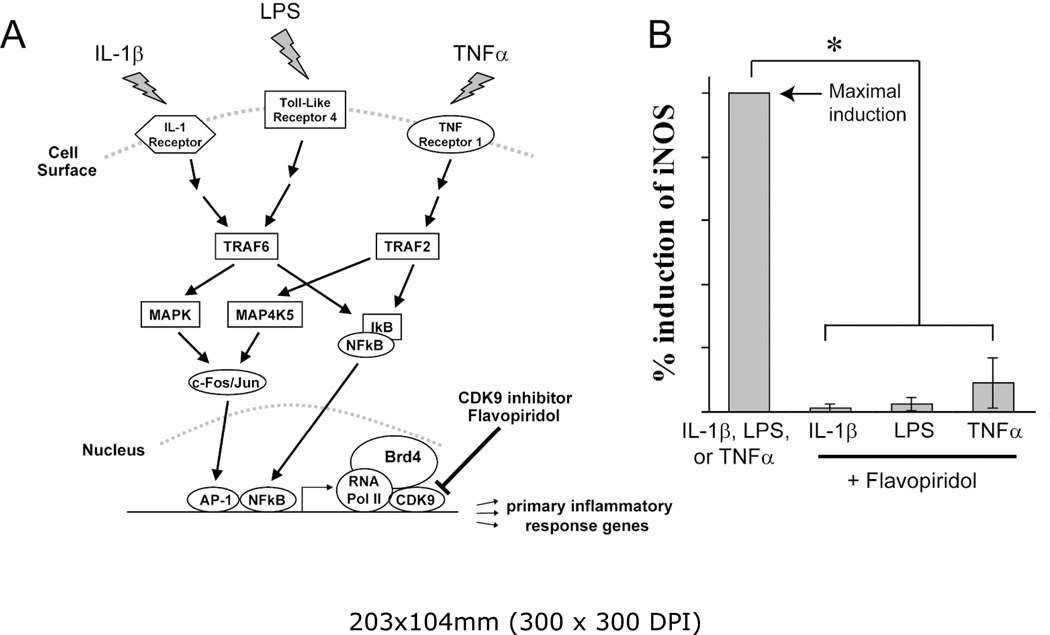
PRG are activated by diverse inflammatory signals. However, regardless of the sources, most inflammatory signals converge onto the rate-limiting step of transcriptional elongation of PRG, which is controlled by CDK9. In order to illustrate this, three different inflammatory signaling pathways were selected; namely, IL-1β, lipopolysaccharides (LPS), and Tumor Necrosis Factor alpha (TNFα). The cellular response to IL-1β, LPS, or TNFα is mediated by three distinct pathways – activation of the IL-1Receptor, Toll-Like Receptor 4, or TNF Receptor 1, respectively (Figure 2A). Chondrocytes were treated with three inflammatory stimuli independently, in the presence or absence of the CDK9 inhibitor Flavopiridol. The mRNA expression of iNOS, a common effector gene for all three pathways (14), was then determined. The results showed that Flavopiridol greatly suppressed the activation of iNOS expression by all three pathways (Figure 2B), demonstrating the effectiveness and broad range of Flavopiridol in preventing inflammatory response from diverse signals. Thus our data confirmed previous finding in other cellular systems (4, 5) and established CDK9 as a central regulatory point for primary inflammatory response in chondrocytes.
Figure 2. The CDK9 inhibitor Flavopiridol is effective against different inflammatory stimuli.
(A) Activation of inflammatory genes by diverse signals. Multiple pro-inflammatory stimuli, such as IL-1β, LPS, and TNFα activate their respective cell surface receptors. These signals are then transmitted through different intracellular mediators/pathways, which ultimately converge on CDK9-dependent transcription of inflammatory genes. Brd4 functions to recruit CDK9 to activated promoters. (B) Flavopiridol is effective against multiple inflammatory stimuli. Human chondrocytes (n=3 different donors) in monolayer culture were treated with different inflammatory stimuli (10ng/ml of either IL-1β, LPS, or TNFα) with or without 300 nM Flavopiridol for 5 hours. iNOS mRNA was quantified by real-time PCR as a measure of inflammatory response. The induction of iNOS by each stimulus alone was arbitrarily set to 100% (first bar) and compared to the respective value obtained in sample co-treated with each inflammatory stimulus and Flavopiridol. Results were the mean +/− standard deviation from three different donors (*p<0.05).
CDK9 inhibition prevents the activation of a broad spectrum of inflammatory response genes
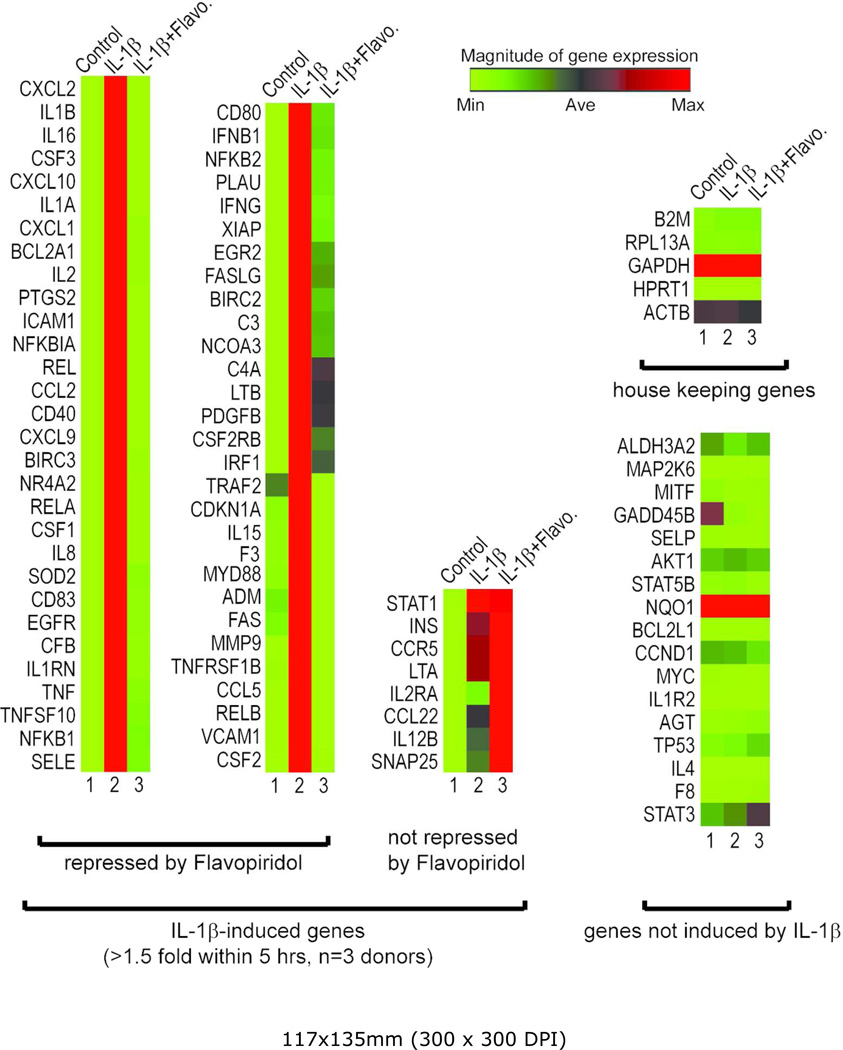
To further investigate the effects of CDK9 inhibition on the activation of other inflammatory mediators, the gene expression profiles of chondrocytes treated with IL-1β for 5 hours were determined by real-time PCR arrays. The PCR array contained 84 key genes responsive to NFκB signal transduction (Qiagen), which is central to the regulation of multiple cellular processes such as inflammatory, immunity, and stress responses. The gene expression profiles from three chondrocyte donors were averaged, and are presented as heat maps in which low and high relative expressions are represented by green and red colors, respectively (Figure 3). The array data in numerical format is included as supplementary data. The results showed that IL-1β strongly activated the majority of the 84 NFκB-target genes tested (Figure 3, compared lane 1& 2), while CDK9 inhibition by Flavopiridol almost completely abolished the effects of IL-1β (lane 3). On average, across three chondrocyte donors, CDK9 inhibition repressed IL-1β activity by a magnitude of greater than 86%, and suppressed 59 out of 67 NFκB-target genes that were activated at least 1.5-fold by IL-1β. Importantly, house-keeping genes, as well as genes not induced by IL-1β, were not affected by Flavopiridol. These data demonstrated that CDK9 can be targeted to effectively suppress only the activation of a cascade of downstream inflammatory response genes, without affecting the basal expression of non-responsive genes.
Figure 3. Flavopiridol effectively suppresses the induction of a broad range of inflammatory mediators.
Primary human chondrocytes (n=3 different donors) in monolayer culture were treated with 10 ng/ml IL-1β with or without 300 nM Flavopiridol for 5 hours. Gene expression was analyzed using real-time PCR Array for NFκB targets (Qiagen) and shown here as heat map (Green=minimum expression, Red=maximum expression). Of the 84 NFκB target genes tested, 67 were induced greater than 1.5-fold by IL-1β (compare lanes 1 & 2). Flavopiridol almost completely abolishes the effects of IL-1β in 59 of these 67 genes (lane 3). Importantly, housekeeping genes and non-inducible genes are unaffected by either IL-1β or Flavopiridol.
CDK9 inhibition prevents the activation of catabolic genes, but has no effects on basal expression of anabolic genes in chondrocytes
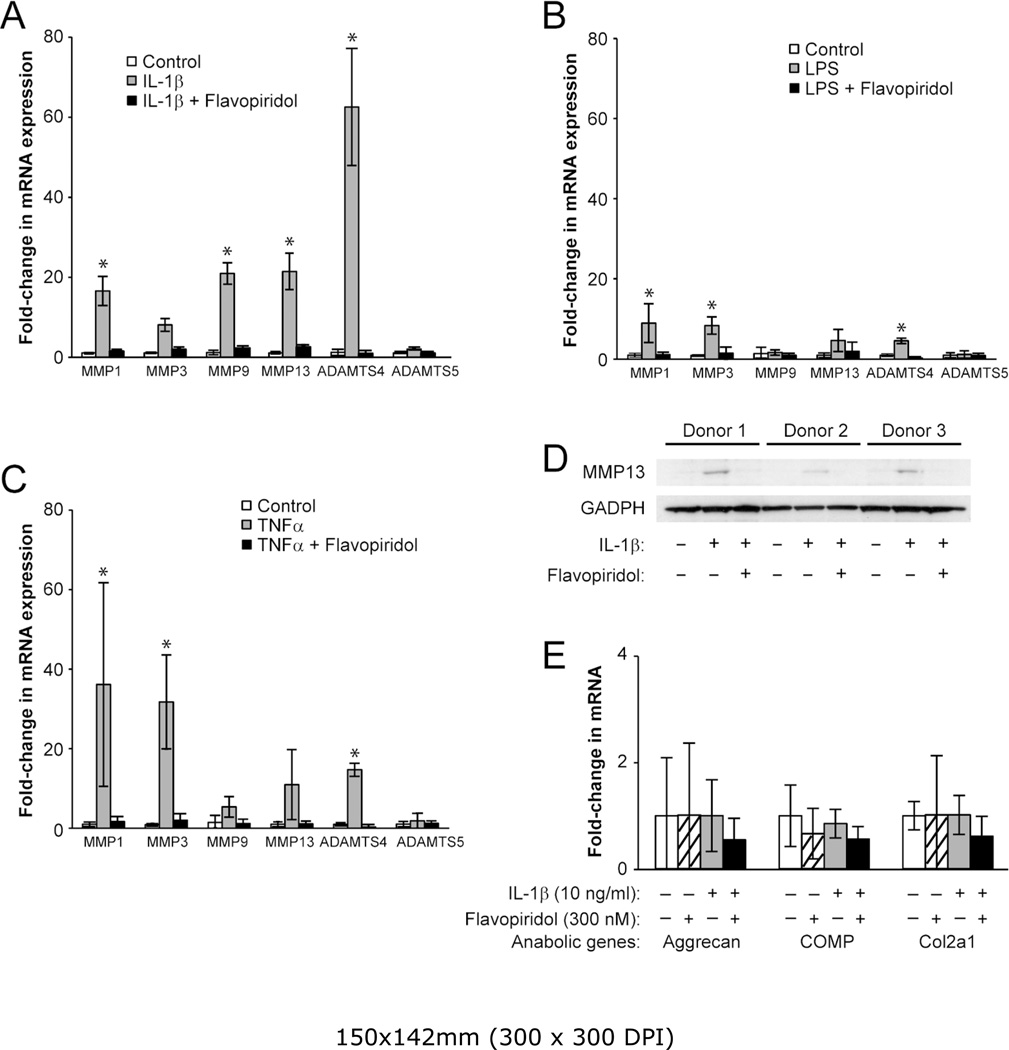
Besides activating the acute phase inflammatory genes, pro-inflammatory cytokines such as IL-1β and TNFα can also stimulate the expression of catabolic genes in chondrocytes (2, 17). These catabolic genes include the various matrix MMPs and ADAMTS (a disintegrin and metalloproteinase with a thrombospondin type 1 motif) that degrade the cartilage matrix. Given the role of CDK9 in activation of inflammatory genes, we next examined the effects of CDK9 inhibition on the induction of MMPs and ADAMTS in chondrocytes treated independently with IL-1β, LPS, and TNFα for 5 hours. The results showed that IL-1β-mediated upregulation of MMP1, 3, 9, and 13, as well as ADAMTS4 mRNAs, was markedly suppressed by co-treatment with Flavopiridol (Figure 4A). Similar trends were observed in LPS- or TNFα-treated samples (Figure 4B & C). These data indicated that CDK9 inhibition prevented the transcriptional activation of catabolic genes in chondrocytes. Next, we sought to confirm the upregulation of MMP13 mRNA at the protein level, which is implicated in collagen degradation and osteoarthritis (18). Chondrocytes were treated with IL-1β, with and without Flavopiridol, for 2 days. Cell-associated active MMP13 protein (~48 kDa) was then detected by Western blots. The data showed that MMP13 protein expression was elevated in all three donors treated with IL-1β, but remained at basal levels with IL-1β and Flavopiridol treatment (Figure 4D). Thus, the results on protein expression corroborate with the mRNA expression profiles of MMP13. On the other hand, the mRNA expression of selected anabolic genes (Aggrecan, Cartilage Oligomeric Matrix Protein (COMP), and Collagen 2a) in chondrocytes was not significantly affected by IL-1β or Flavopiridol within the same 5-hours time frame (Figure 4E). Taken together, the above results demonstrate that Flavopiridol selectively suppresses only the induction of catabolic genes by pro-inflammatory stimuli, while having negligible effects on the basal expression of anabolic genes.
Figure 4. CDK9 inhibition prevents induction of MMP and ADAMTS expression by various inflammatory stimuli.
(A–C) Primary chondrocytes (n=3 different donors) were treated with either 10 ng/ml of IL-1β, LPS, or TNFα, with or without 300 nM Flavopiridol for 5 hours, and the relative mRNA expression of the cartilage degrading enzymes MMP-1,-3,-9,-13, and ADAMTS4 and ADAMTS5 was determined by real-time PCR. (D) Flavopiridol suppresses MMP13 protein expression. Human chondrocytes (n=3 different donors) grown in 6-well plates were treated with 10 ng/ml IL-1β, with or without 300 nM Flavopiridol for 2 days. Cell-associated active MMP13 (~48kD) was detected by Western blot. (E) Flavopiridol does not affect basal expression of anabolic genes. Chondrocytes were treated with IL-1β and/or Flavopiridol for 5 hours and the mRNA expression of the cartilage matrix genes aggrecan, COMP, and Col2a were determined by RT-PCR. For all the above experiments, each data point was the mean +/− standard deviation from three different donors (*p<0.05).
CDK9 inhibition protects cartilage from the catabolic effects of IL-1β
Since CDK9 inhibition suppresses the activation of inflammatory genes and catabolic enzymes in chondrocytes, we next determined whether Flavopiridol can protect cartilage from the deleterious effects of pro-inflammatory cytokines. Human arthritic cartilage explants were isolated and cultured in media containing 1 ng/mL IL-1β, in the presence or absence of Flavopiridol for 6 days. Note that the concentration of IL-1β was reduced from the 10 ng/ml used in short-term monolayer culture, to a level similar to those detected in the synovial fluids of inflamed joints (19–21). Degradation of cartilage matrix was then assessed by measuring the release of GAG and Col2a cleavage peptides into the culturing media. As expected, IL-1β increased the amount of both GAG (Figure 5A) and Col2a peptides (Figure 5B) released into the media (compared first and second bars). However, the concentrations of both GAG and Col2a peptides were reduced by 6 nM Flavopiridol and returned to baseline levels by 300 nM Flavopiridol (Figure 5A & B). Thus, our data provides evidence that CDK9 inhibition prevents the catabolic destruction of cartilage by IL-1β. Importantly, the percentages of live/dead cells were similar in both untreated and Flavopiridol-treated bovine cartilage explants (Figure 5C). This result indicates that prolonged treatment of cartilage with Flavopiridol did not have an adverse effect on the viability of chondrocytes in cartilage explants. Taken together, our data indicate that CDK9 inhibition protects cartilage explants from the catabolic effects of IL-1β.
Discussion
The etiology of primary OA remains incompletely understood and the involvement of the inflammatory response is somewhat controversial. However, it is well established that damage to the collagen network originates around chondrocytes at the cartilage matrix surface (22). Since inflammatory response induces chondrocyte apoptosis and cartilage matrix breakdown (2), there are several anti-OA strategies that target either specific branches of the inflammatory signaling cascade (e.g. IL-1, IL-6, TNFα, and NFκB inhibitors) (17, 23, 24), or the downstream events such as apoptosis with caspase inhibitors (1). However, because inflammation can be induced by a variety of stimuli, the above individual approaches would have limited effectiveness in handling the diverse simultaneous challenges in a biological system, as well as limited abilities in efficiently suppressing a broad range of inflammatory mediator expression. A novel approach to addressing these limitations is to directly target CDK9, which activates transcription of primary inflammatory response genes. Using the pharmacological CDK9 inhibitor Flavopiridol, we have shown that cartilage can be protected from the harmful effects of pro-inflammatory cytokines.
Our results demonstrate for the first time in chondrocytes that Flavopiridol effectively suppresses the acute response to multiple inflammatory stimuli (Figure 2), and prevents the induction of a broad range of inflammatory mediators (Figure 3), as well as catabolic genes that contribute to the degradation of the cartilage matrix (Figure 4). In most cases, Flavopiridol almost completely abolishes the activation of inflammatory mediator expression. For example, from the PCR array data (Fig 3 and supplemental data), IL-1β induced expression of IL-6 by 492-fold, but only 4.2-fold in the presence of Flavopiridol, representing a 99.2% repression of IL-1s-dependent transcription. Importantly, our data demonstrate the selectivity of Flavopiridol-mediated inhibition, in which only the IL-1β-inducible genes are suppressed, but not the basal expression of non-inducible genes, housekeeping genes (Figure 3), and the anabolic genes (Col2a, COMP, and aggrecan) in chondrocytes (Figure 4E). The gene expression profiles are further supported by the experiments demonstrating Flavopiridol can effectively prevent cartilage degradation induced by IL-1β (Fig 5A & B). The reduction in matrix degradation products was not due to changes in cell viability in cartilage treated with Flavopiridol, because live/dead staining revealed similar chondrocyte viabilities between control and Flavopiridol treated bovine cartilage explants (Figure 5C). Bovine cartilage was used instead of human cartilage because normal, healthy human cartilage is not routinely available, and the live/dead ratio of arthritic human cartilage obtained from knee surgery is erratic even when adjacent areas were examined.
Flavopiridol is an ATP analog that preferentially inhibits CDK9 kinase activity (Ki=30 nM) by a high affinity interaction with its ATP-binding pocket (25). Although Flavopiridol can potentially inhibit other CDKs, numerous studies using a combination of specific inhibitors and siRNA have demonstrated that only CDK9 inhibition is responsible for the anti-inflammatory action of Flavopiridol (26, 27). We have also shown that both JQ-1-mediated inhibition of Brd4, which does not directly interact with other CDKs (10); as well as siRNA-mediated inhibition of CDK9, lead to the loss of IL-1β-mediated induction of iNOS in chondrocytes (Figure 1E & F). In addition, it is not documented that other CDKs involved in cell cycle regulation have a pronounced effect on the transcriptional activation of a broad range of PRG within the 5-hour time frame used in this study. Therefore, our results provide strong evidence that only CDK9 is responsible for the activation of PRG in chondrocytes.
Flavopiridol was originally known for its anti-proliferation properties by suppressing cell-cycle progression in rapidly dividing cells (e.g. cancers) or in cells with short life-span (e.g. neutrophils). Its pharmacological activity is well-documented over the last two decades because of its use in clinical trials as anti-proliferation/cancer agent (reviewed by (28)). Sekine et al have demonstrated that systemic administration of Flavopiridol reduces synovial hyperplasia, but does not induce apoptosis, and result in preventing the development of rheumatoid arthritis in a collagen-induced mouse model (29). However, it is not known whether the anti-arthritic activity of Flavopiridol is due to the systematic suppression of the leukocyte-mediated immune response to the injected collagen, or the localized suppression of the inflammatory response of chondrocytes in cartilage. Our group has developed a non-invasive knee injury mouse model for post-traumatic OA (PTOA) (30). Preliminary data indicate that systemic administration of Flavopiridol effectively suppresses the production of pro-inflammatory cytokines locally at the injured knee, thus confirming our in vitro finding detailed in this study. Future experiments are needed to determine whether Flavopiridol treatment will prevent or delay the development of PTOA in our mouse model, or in other existing PTOA models (31, 32).
In summary, our data for the first time demonstrate the absolute requirement of CDK9 activity in the activation of PRG in human chondrocytes. In addition, our data strongly indicate that Flavopiridol is an effective agent to prevent the activation of acute inflammatory response and catabolic pathways in cartilage. Our results thus may provide a new strategy to prevent or delay the onset of OA.
Supplementary Material
Acknowledgements
This study was supported by an Arthritis Foundation 2012 IRG award to DRH, a DOD PRMRP IIRA award #PR110507 to DRH, R21-AR063348 from NIAMS/NIH to DRH, Departmental Funds to BAC and DRH, and the National Natural Science Fund of China (81101378, 81271971) to ZH
No author received any financial support or other benefits from commercial sources for the work reported on in this manuscript, and no author has any other financial interest that could create a potential conflict of interest or the appearance of a conflict of interest with regard to the work.
Footnotes
Competing interest
The authors declare that they have no competing interest.
References
- 1.Lotz MK, Kraus VB. New developments in osteoarthritis. Posttraumatic osteoarthritis: pathogenesis and pharmacological treatment options. Arthritis Res Ther. 2007;12:211. doi: 10.1186/ar3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldring MB, Otero M, Tsuchimochi K, Ijiri K, Li Y. Defining the roles of inflammatory and anabolic cytokines in cartilage metabolism. Ann Rheum Dis. 2008;67(Suppl 3):iii75–iii82. doi: 10.1136/ard.2008.098764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hargreaves DC, Horng T, Medzhitov R. Control of inducible gene expression by signal-dependent transcriptional elongation. Cell. 2009;138:129–145. doi: 10.1016/j.cell.2009.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zippo A, Serafini R, Rocchigiani M, Pennacchini S, Krepelova A, Oliviero S. Histone crosstalk between H3S10ph and H4K16ac generates a histone code that mediates transcription elongation. Cell. 2009;138:1122–1136. doi: 10.1016/j.cell.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Q, Yik JH. The Yin and Yang of P-TEFb regulation: implications for human immunodeficiency virus gene expression and global control of cell growth and differentiation. Microbiol Mol Biol Rev. 2006;70:646–659. doi: 10.1128/MMBR.00011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krystof V, Baumli S, Furst R. Perspective of cyclin-dependent kinase 9 (CDK9) as a drug target. Current pharmaceutical design. 2012;18:2883–2890. doi: 10.2174/138161212800672750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H, Haudenschild DR, Posey KL, Hecht JT, Di Cesare PE, Yik JH. Comparative analysis with collagen type II distinguishes cartilage oligomeric matrix protein as a primary TGFbeta-responsive gene. Osteoarthritis Cartilage. 2011;19:1246–1253. doi: 10.1016/j.joca.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, et al. A third-generation lentivirus vector with a conditional packaging system. Journal of virology. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Z, Yik JH, Chen R, He N, Jang MK, Ozato K, et al. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Molecular cell. 2005;19:535–545. doi: 10.1016/j.molcel.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 11.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 12.Poole AR, Ionescu M, Fitzcharles MA, Billinghurst RC. The assessment of cartilage degradation in vivo: development of an immunoassay for the measurement in body fluids of type II collagen cleaved by collagenases. J Immunol Methods. 2004;294:145–153. doi: 10.1016/j.jim.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Fornier MN, Rathkopf D, Shah M, Patil S, O'Reilly E, Tse AN, et al. Phase I dose-finding study of weekly docetaxel followed by flavopiridol for patients with advanced solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:5841–5846. doi: 10.1158/1078-0432.CCR-07-1218. [DOI] [PubMed] [Google Scholar]
- 14.Maier R, Bilbe G, Rediske J, Lotz M. Inducible nitric oxide synthase from human articular chondrocytes: cDNA cloning and analysis of mRNA expression. Biochim Biophys Acta. 1994;1208:145–150. doi: 10.1016/0167-4838(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 15.Ali S, Heathcote DA, Kroll SH, Jogalekar AS, Scheiper B, Patel H, et al. The development of a selective cyclin-dependent kinase inhibitor that shows antitumor activity. Cancer research. 2009;69:6208–6215. doi: 10.1158/0008-5472.CAN-09-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heath EI, Bible K, Martell RE, Adelman DC, Lorusso PM. A phase 1 study of SNS-032 (formerly BMS-387032), a potent inhibitor of cyclin-dependent kinases 2, 7 and 9 administered as a single oral dose and weekly infusion in patients with metastatic refractory solid tumors. Investigational new drugs. 2008;26:59–65. doi: 10.1007/s10637-007-9090-3. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, et al. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005;52:128–135. doi: 10.1002/art.20776. [DOI] [PubMed] [Google Scholar]
- 18.Wang M, Sampson ER, Jin H, Li J, Ke QH, Im HJ, et al. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res Ther. 2013;15:R5. doi: 10.1186/ar4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiocco U, Sfriso P, Oliviero F, Roux-Lombard P, Scagliori E, Cozzi L, et al. Synovial effusion and synovial fluid biomarkers in psoriatic arthritis to assess intraarticular tumor necrosis factor-alpha blockade in the knee joint. Arthritis Res Ther. 2010;12:R148. doi: 10.1186/ar3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNiff PA, Stewart C, Sullivan J, Showell HJ, Gabel CA. Synovial fluid from rheumatoid arthritis patients contains sufficient levels of IL-1 beta and IL-6 to promote production of serum amyloid A by Hep3B cells. Cytokine. 1995;7:209–219. doi: 10.1006/cyto.1995.1028. [DOI] [PubMed] [Google Scholar]
- 21.Deirmengian C, Hallab N, Tarabishy A, Della Valle C, Jacobs JJ, Lonner J, et al. Synovial fluid biomarkers for periprosthetic infection. Clinical orthopaedics and related research. 2010;468:2017–2023. doi: 10.1007/s11999-010-1298-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollander AP, Pidoux I, Reiner A, Rorabeck C, Bourne R, Poole AR. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest. 1995;96:2859–2869. doi: 10.1172/JCI118357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Attur M, Millman JS, Dave MN, Al-Mussawir HE, Patel J, Palmer G, et al. Glatiramer acetate (GA), the immunomodulatory drug, inhibits inflammatory mediators and collagen degradation in osteoarthritis (OA) cartilage. Osteoarthritis Cartilage. 2011;19:1158–1164. doi: 10.1016/j.joca.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Attur MG, Dave M, Cipolletta C, Kang P, Goldring MB, Patel IR, et al. Reversal of autocrine and paracrine effects of interleukin 1 (IL-1) in human arthritis by type II IL-1 decoy receptor. Potential for pharmacological intervention. J Biol Chem. 2000;275:40307–40315. doi: 10.1074/jbc.M002721200. [DOI] [PubMed] [Google Scholar]
- 25.Ni W, Ji J, Dai Z, Papp A, Johnson AJ, Ahn S, et al. Flavopiridol pharmacogenetics: clinical and functional evidence for the role of SLCO1B1/OATP1B1 in flavopiridol disposition. PLoS One. 2010;5:e13792. doi: 10.1371/journal.pone.0013792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang K, Hampson P, Hazeldine J, Krystof V, Strnad M, Pechan P, et al. Cyclin-dependent kinase 9 activity regulates neutrophil spontaneous apoptosis. PLoS One. 2012;7:e30128. doi: 10.1371/journal.pone.0030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmerwitz UK, Sass G, Khandoga AG, Joore J, Mayer BA, Berberich N, et al. Flavopiridol protects against inflammation by attenuating leukocyte-endothelial interaction via inhibition of cyclin-dependent kinase 9. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:280–288. doi: 10.1161/ATVBAHA.110.213934. [DOI] [PubMed] [Google Scholar]
- 28.Wang LM, Ren DM. Flavopiridol, the first cyclin-dependent kinase inhibitor: recent advances in combination chemotherapy. Mini Rev Med Chem. 2010;10:1058–1070. doi: 10.2174/1389557511009011058. [DOI] [PubMed] [Google Scholar]
- 29.Sekine C, Sugihara T, Miyake S, Hirai H, Yoshida M, Miyasaka N, et al. Successful treatment of animal models of rheumatoid arthritis with small-molecule cyclin-dependent kinase inhibitors. J Immunol. 2008;180:1954–1961. doi: 10.4049/jimmunol.180.3.1954. [DOI] [PubMed] [Google Scholar]
- 30.Christiansen BA, Anderson MJ, Lee CA, Williams JC, Yik JH, Haudenschild DR. Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2012;20:773–782. doi: 10.1016/j.joca.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 31.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Furman BD, Strand J, Hembree WC, Ward BD, Guilak F, Olson SA. Joint degeneration following closed intraarticular fracture in the mouse knee: a model of posttraumatic arthritis. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2007;25:578–592. doi: 10.1002/jor.20331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.