Abstract
Objective
To develop a method to rapidly stabilize the shape change process in peripheral slices of costal cartilage by using infrared laser irradiation in a porcine model.
Methods
Forty peripheral porcine costal cartilage specimens (40×10×2 mm) were harvested. Thirty of these specimens were immediately irradiated with an Nd: YAG laser (λ=1.32 μm; spot size, 2-mm diameter) using 1 of 3 exposure treatments: 6W, 2 seconds, and 4 spots; 8 W, 3 seconds, and 4 spots; or 6 W, 2 seconds, and 8 spots. Ten control specimens were only immersed in 0.9% saline solution. Angle of curvature was measured from photographs taken at 0 minutes, immediately after irradiation, and at 30 minutes, 1 hour, 5 hours, and 24 hours. Infrared imaging was used to measure surface temperatures during irradiation. Cell viability after irradiation was determined using a live/dead assay in conjunction with fluorescent confocal microscopy.
Results
Compared with the untreated controls, the irradiated grafts underwent accelerated shape change within the first 30 minutes to reach a stable geometry. Thereafter, irradiated grafts underwent little or no shape change, whereas the control group exhibited significant change in curvature from 30 minutes to 24 hours (P<.001). The average peak irradiated spot temperatures ranged from 76°C to 82°C. Cell viability measurements at the laser spot sites demonstrated a hemispherically shaped region of dead cells with a depth of 0.8 to 1.2 mm and a surface diameter of 1.9 to 2.7 mm.
Conclusions
Laser irradiation of peripheral costal cartilage slices provides an effective method for rapidly stabilizing acute shape change by accelerating the warping process. The temperature elevations necessary to achieve this are spatially limited and well within the limits of tolerable tissue injury.
Cartilage provides the structural framework for aesthetic features of the face and aids in maintaining patency of the upper airway. In reconstructive surgery, cartilage grafts are often needed to repair defects produced by trauma, by tumor resection, or from the correction of congenital malformations. Septal cartilage is an ideal graft material because it is suitably stiff, is largely homogeneous, and retains its shape after placement. If a curved graft is required, auricular cartilage is typically preferred. Unfortunately, the septal and auricular cartilage reservoir is limited.1–4
Costal cartilage is becoming an increasingly more common graft source, particularly in secondary rhinoplasty, because it can provide a relatively large source of autogenous material. In 1920, Gillies5 noted that grafts carved from the periphery of costal cartilage sections tend to warp toward the more peripheral side. Gibson and Davis6 theorized that the immediate and delayed warping of costal cartilage grafts is due to inherently increased tautness in peripheral regions and therefore advocated cutting balanced cross sections to minimize these forces, a heuristic that has become surgical dogma. Fry7,8 framed the warping process conceptually for this interplay of internal forces causing shape change by introducing the notion of interlocked stresses and the interactions of protein polysaccharides. The concept of balanced cross sections to prevent warping facilitates the use of costal cartilage in reconstructive surgery. However, harvesting balanced grafts can be challenging because cartilage is a viscoelastic tissue, and, furthermore, harvesting balanced grafts results in the wasting of significant amounts of viable peripheral cartilage tissue.
Given the disadvantages of relying solely on slicing perfectly balanced central cross sections, several additional techniques have been used or attempted to prevent or reduce warping. Adams et al9 irradiated cartilage with ionizing energy before cutting the grafts in an attempt to minimize warping but observed that both the irradiated and the nonirradiated samples warped to approximately the same degree. Erol10 introduced the “Turkish delight” method in which diced cartilage was wrapped in Surgicel, an oxidized regenerated cellulose agent (Ethicon, Inc, Johnson & Johnson, Somerville, New Jersey). Only a few years later, Daniel and Calvert11 discounted Erol’s method through histological methods that demonstrated a high rate of cartilage absorption and the presence of inflammatory infiltrates caused by the Surgicel. They resolved this problem by abandoning the use of Surgicel and instead wrapped the diced cartilage in temporalis fascia. The method of using diced cartilage provides volumetric augmentation but cannot provide grafts for structural purposes. In an attempt to mechanically arrest warping, Gunter et al12 placed Kirschner wire through longitudinally carved costal cartilage slices. However, this method can result in extrusion of the wire long after surgery.
The use of laser irradiation to reshape cartilage has been extensively studied. Helidonis et al13 first reported laser-assisted cartilage reshaping of rabbit ear cartilage grafts with the use of a carbon dioxide laser, and this technique was later demonstrated using other wavelengths and cartilage sources.14–18 Laser reshaping has also been used clinically in septoplasty operations and to perform otoplasty.19–22 However, laser technology has not been evaluated as a means to alter the dynamics of warping or to demonstrate stabilization of geometry in costal cartilage.
The purpose of this study was to determine whether laser irradiation can accelerate the warping process in porcine peripheral costal cartilage sections to allow rapid attainment of a stable steady-state geometry. This approach may potentially provide a reliable method for eliminating postoperative warping and distortion of costal cartilage grafts in reconstructive facial surgery.
METHODS
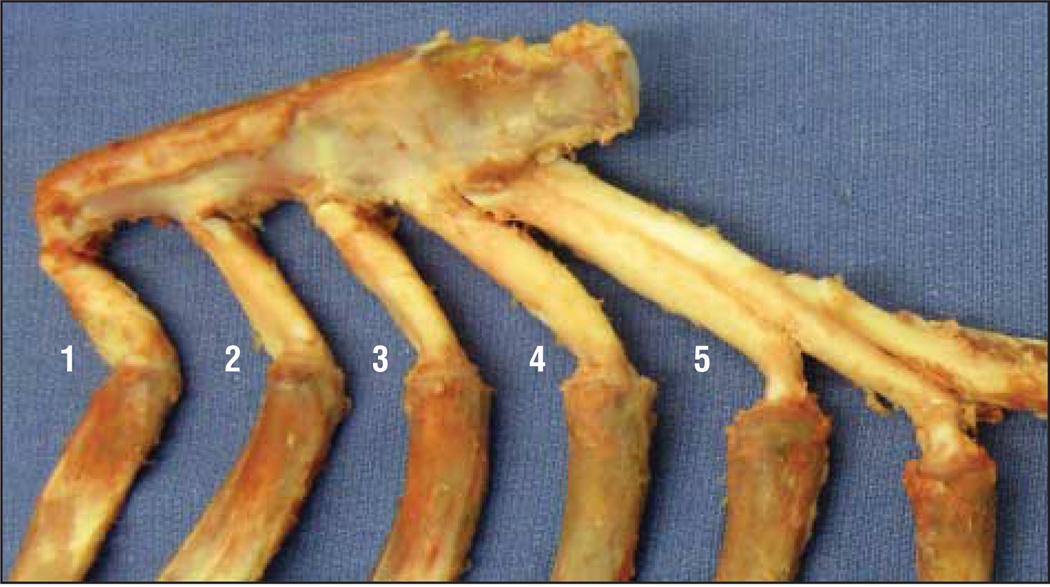
Porcine rib was obtained from a local packing house, and the costal cartilages from rib numbers 2 through 5 were used (Figure 1). These costal cartilage regions were selected because they provided enough length and diameter for our graft slices. The tissue surrounding the costal cartilage was roughly removed with a razor, and only a thin layer of perichondrium was preserved. A No. 10 scalpel was used to cut peripheral slab-shaped sections from the rib (40×10×2 mm). All sections were obtained from the more concave side of the rib. With the use of a random number table, a total of 40 peripheral sections were allocated among 1 control group and 3 experimental laser groups, yielding 10 slices per group.
Figure 1.
Costal cartilage was harvested from porcine ribs 2 through 5.
LASER IRRADIATION
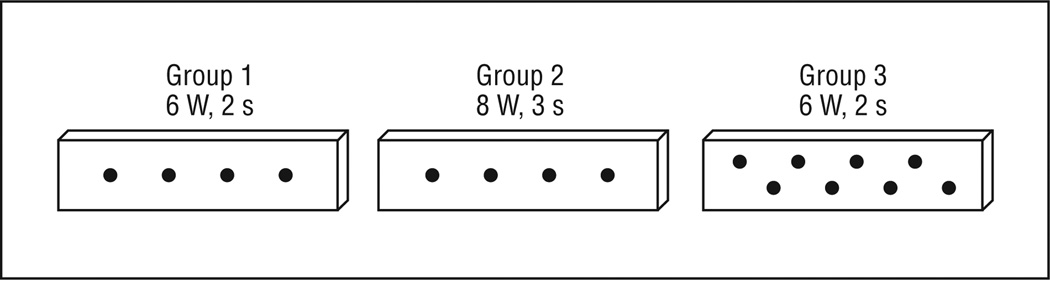
Immediately after section, each cartilage in the experimental groups was irradiated on the more peripheral side with light from an Nd:YAG laser (λ=1.32 μm, 50 Hz; New Star Lasers, Inc, Roseville, California) using 1 of 3 exposure treatments: group 1 (6 W, 191.0 W/cm2, 2 seconds, and 4 spots), group 2 (8 W, 254.6 W/cm2, 3 seconds, and 4 spots), and group 3 (6 W, 191.0 W/cm2, 2 seconds, and 8 spots). The laser energy was delivered via a 600-μm multimode silica fiber positioned perpendicular to the specimen to produce a 2-mm spot size, which was estimated using burn paper (Kentek Corporation, Pittsfield, New Hampshire). The 4 laser spots for groups 1 and 2 cartilage slices were evenly spaced down the central axis of the specimen; the 8 laser spots for group 3 cartilage slices were staggered along 2 parallel rows (Figure 2). Control cartilage slices were not irradiated. After irradiation, all samples were loosely wrapped in sterile gauze, immersed in individual containers of 0.9% saline, and stored at ambient temperature (22°C).
Figure 2.
Laser irradiation patterns for groups 1 through 3. Diagram drawn to scale.
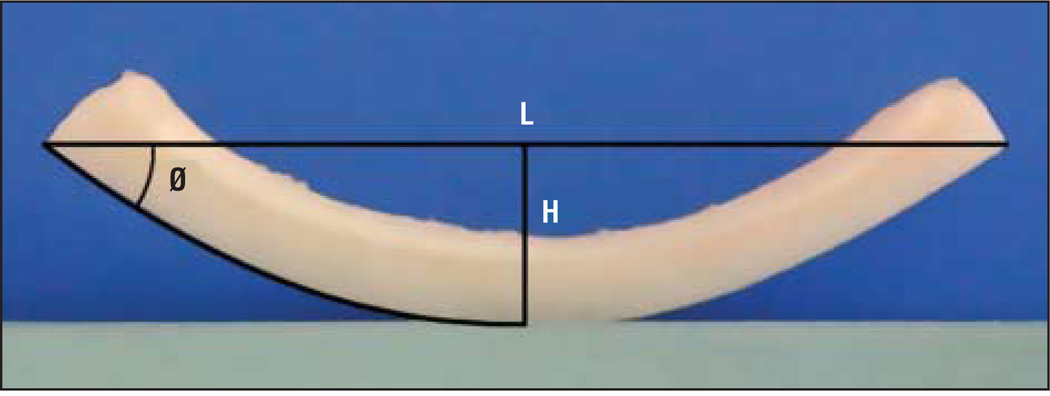
Digital photographs were taken for the control and laser groups at 0 minutes, 30 minutes, 1 hour, 5 hours, and 24 hours. For the laser groups, a photograph was also taken immediately after irradiation. Photographs were standardized by mounting a digital camera (COOLPIX 990; Nikon, Inc, Melville, New York) in a fixed position with the lens aimed perpendicular to a guide that provided for accurate and consistent alignment of the cartilage grafts. The position of the camera-and-guide apparatus was fixed without disturbance throughout the study. The warping angle was determined by measuring each photograph with the use of a graphics editing program (Photoshop CS3; Adobe Systems, Inc, San Jose, California). Following the methods described by Kim et al,23 the warping angle was calculated by the arctangent (2×[height/length]) as illustrated in Figure 3.
Figure 3.
Warping angle (Ø) is calculated using the equation arctangent (2×H/L), in which H represents height and L indicates length.
TEMPERATURE MEASUREMENTS
An infrared imaging system (Inframetrics Model 600; FLIR Systems, Inc, Boston, Massachusetts) was used to determine the surface temperature in the laser spot during irradiation for each dosimetry setting as previously described.24
CELL VIABILITY
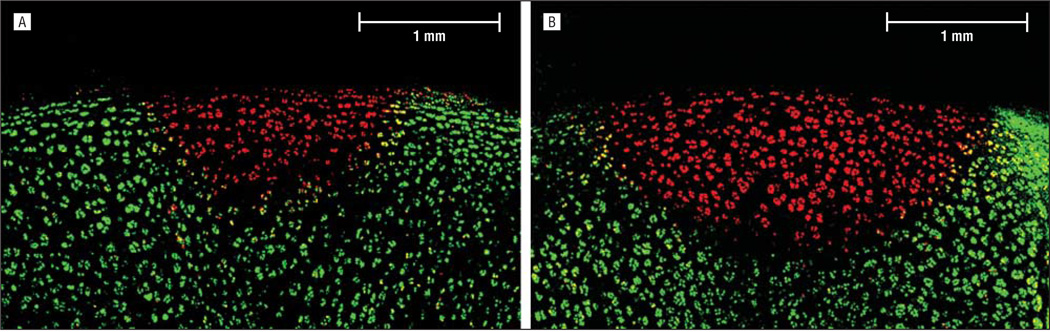
Thirty minutes after irradiation, a cross-sectional slice through the center of the laser spot was excised using microdissection methods. A cell viability assay (LIVE/DEAD; Molecular Probes, Eugene, Oregon) in conjunction with fluorescent confocal microscopy (Model LSM 510 META; Carl Zeiss, Jena, Germany) was performed on the cross-sectional slice by following the protocol described by Choi et al.25 In the resulting images, green fluorescence indicated live cells, whereas red fluorescence indicated dead cells with compromised cell membranes. The maximum area of thermal damage was determined by measuring the depth and surface diameter of the red fluorescing region for 3 samples per dosimetry setting.
ACCELERATED WARPING ANALYSIS
Analysis was performed with JMP 8 (SAS Institute, Inc, Cary, North Carolina) statistical software. After testing for equal variance and normality assumptions, comparisons between the groups were analyzed by analysis of variance and the nonparametric Kruskal-Wallis test as appropriate. The measurement variable was defined as the percentage of warping that occurred within the first 30 minutes of the total 24-hour period. Post hoc group comparisons were analyzed by the Dunnett test. Functional analysis using the linear slope estimate as a summary measure was then applied to compare the groups in terms of continued change over time beyond the first 30 minutes.26
RESULTS
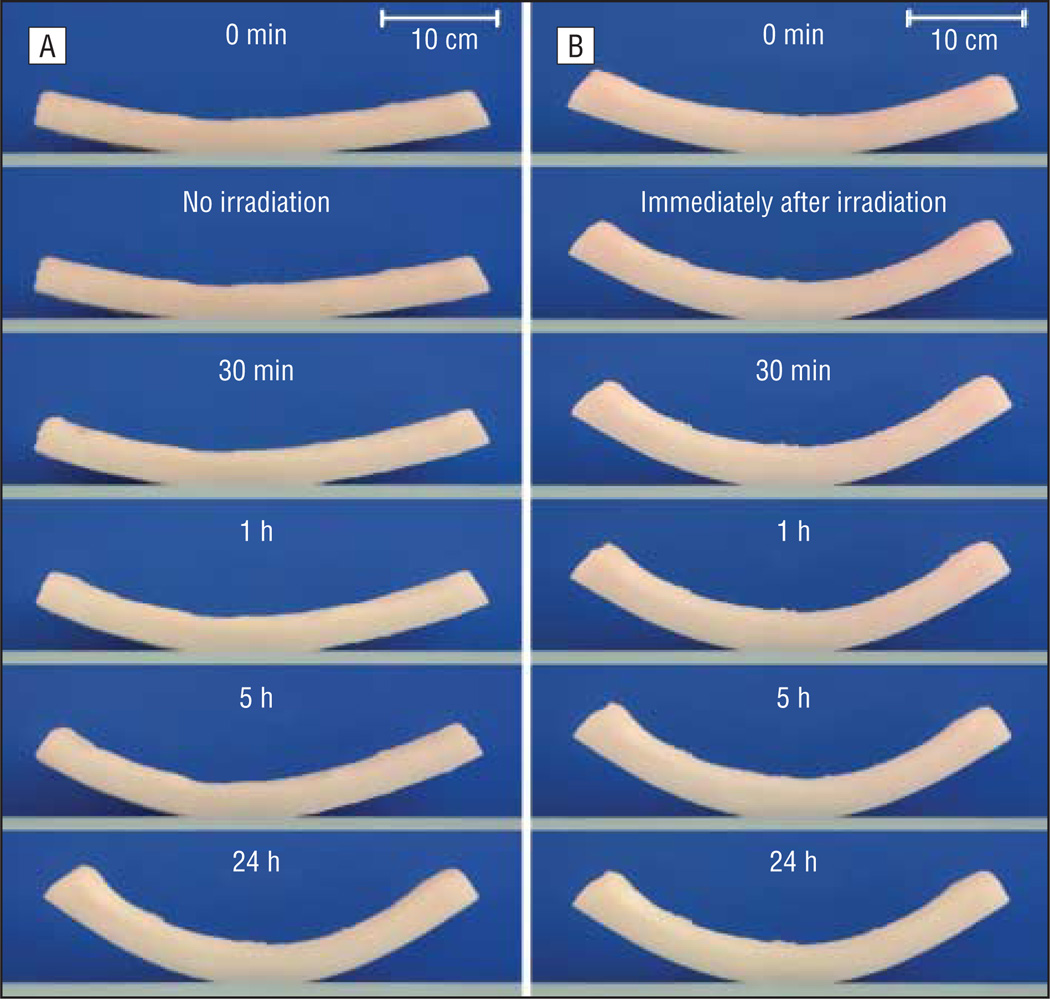
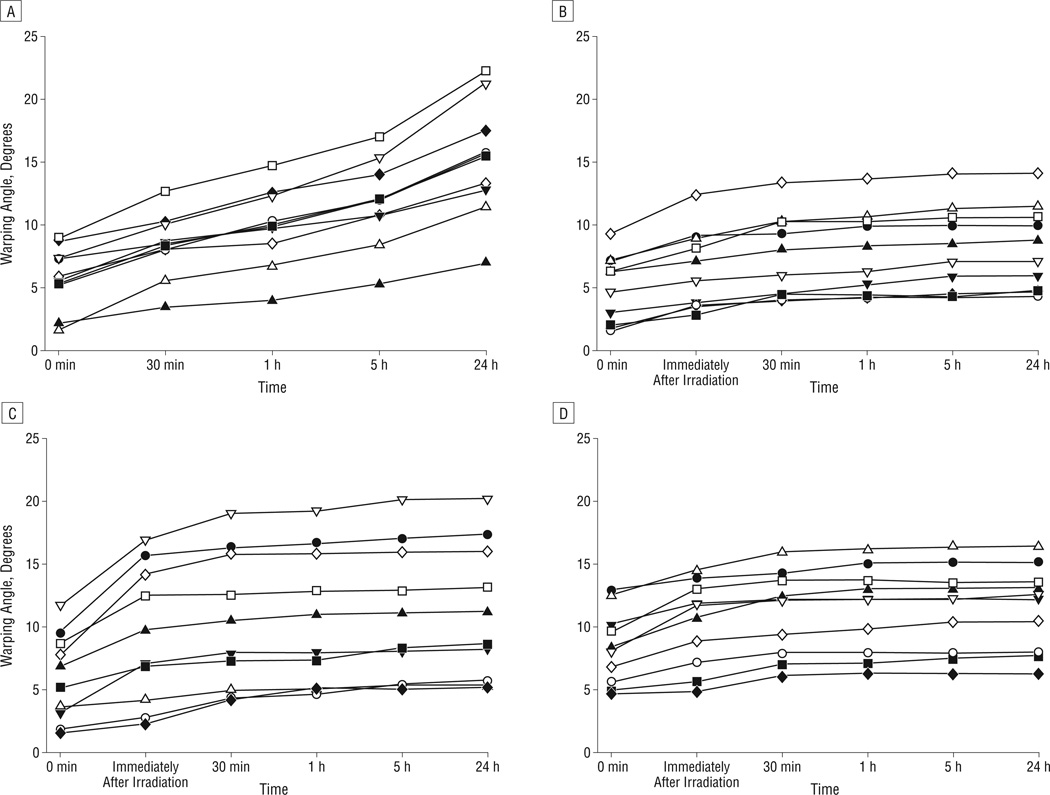
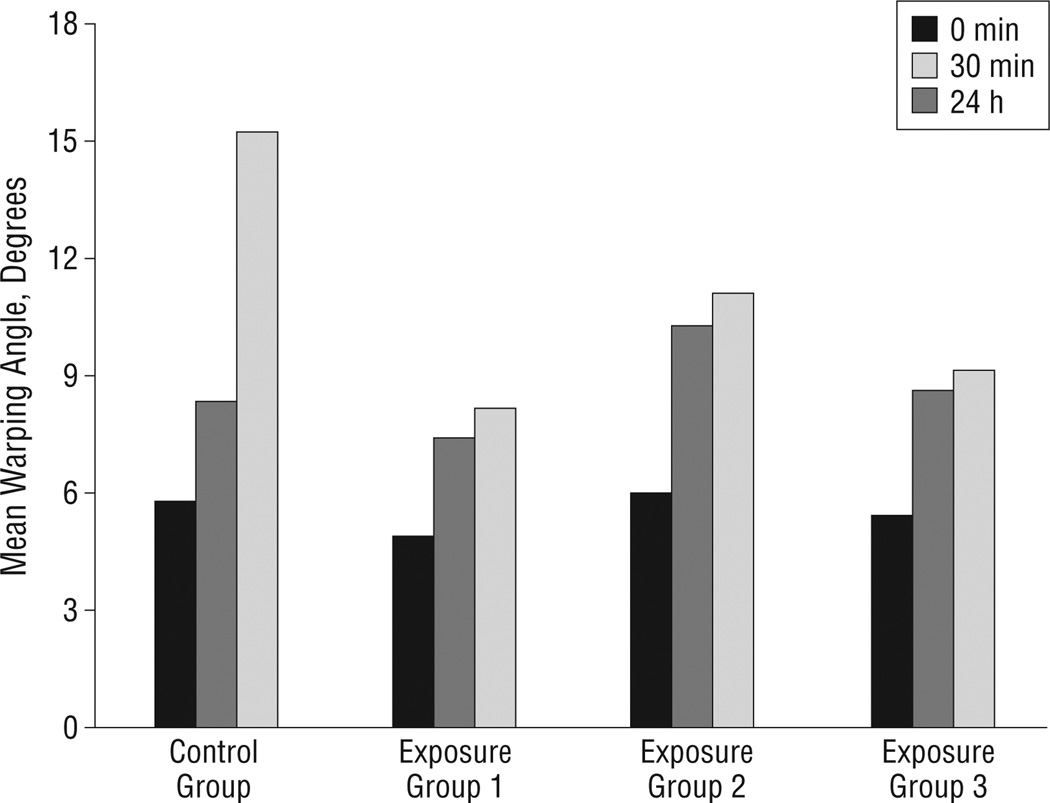
There was no significant difference between the baseline initial warping angles immediately after sectioning in each of the 4 groups (P=.86). Figure 4 is a photographic montage illustrating how the nonirradiated control grafts warped gradually across 24 hours (Figure 4A) in contrast to the laser-irradiated grafts that underwent most of their shape change during the first 30 minutes (Figure 4B). Figure 5 is a series of plots demonstrating warping angle over time for all specimens in the control and experimental groups. Note that the grafts in the control group continued to warp substantially beyond 30 minutes. In comparison, grafts in the laser exposure groups achieved near-steady-state geometry after 30 minutes, as evidenced by the plateau of their warping angles during this period. Figure 6 provides a summary of the shape change dynamics for each group. The percentage of warping that occurred within the first 30 minutes of the total 24-hour period was significantly greater in the laser exposure groups compared with the controls (P<.001, R2=0.81). Specifically, within the first 30 minutes, the control group achieved only 27.5% of its total 24-hour warping, whereas the laser-irradiated groups had already attained 75.0% to 85.8% of their steady-state warping values. The average rate of change in warping angle during the time interval spanning 30 minutes to 24 hours was 0.21° per hour for controls. In contrast, the average rate of change for the 3 laser exposure groups varied from 0.015° to 0.008° per hour. This difference in rate of change between the control and exposure groups was statistically significant (P<.001). There was no significant difference in warping between the 3 laser exposure settings (P=.19).
Figure 4.
Warping over time for cartilage grafts from the control group (A) and laser exposure group 2 (B).
Figure 5.
Warping angle vs time for all grafts in the control and laser exposure groups. Grafts in the control group (A) continued to warp beyond 30 minutes. In comparison, laser-irradiated grafts for exposure group 1 (B), exposure group 2 (C), and exposure group 3 (D) achieved near-steady-state geometry after 30 minutes.
Figure 6.
Mean warping angles at 0 minutes, 30 minutes, and 24 hours for the control and laser exposure groups.
The average peak temperature within the laser spot varied from 76°C to 82°C, which is in agreement with numerical simulations.24,25,27–29 Confocal microscopy identified a sharply demarcated, hemispherically shaped region of nonviable cells at the laser target. After irradiation at 6 W for 2 seconds, the largest extent of nonviable cells was 0.8 mm deep with a 1.9-mm surface diameter (Figure 7A). After irradiation at 8 W for 3 seconds, the largest extent of nonviable cells was 1.2 mm deep with a 2.7-mm surface diameter (Figure 7B).
Figure 7.
Confocal images of cartilage stained with a cell viability assay (LIVE/DEAD; Molecular Probes, Eugene, Oregon). A, Cartilage after irradiation at 6 W for 2 seconds. B, Cartilage after irradiation at 8 W for 3 seconds.
COMMENT
The use of costal cartilage grafts for facial reconstruction is challenging because cut costal cartilage tends to warp over time. Warping is unacceptable in facial reconstructive surgery, particularly in rhinoplasty, because even slight changes in postoperative graft shape may lead to noticeable deformity or functional deficiency. Given that costal cartilage is the only autogenous cartilage source for grafts when the supply of septal and auricular cartilage is insufficient or exhausted, much research has focused on attempting to effectively use costal cartilage grafts by minimizing postoperative warping.
To date, the only method to minimize costal cartilage graft warping without introducing foreign objects or destroying structural integrity (ie, morselization or Turkish delight methods) is to obtain balanced cross sections from the center of the costal cartilage specimen.6 However, obtaining perfectly balanced grafts is not only often challenging but sectioning costal cartilage through central regions of the rib leads to wasting of otherwise valuable peripheral costal cartilage tissue during the harvest and graft-fashioning process. Even though it is accepted that central slices warp significantly less than peripheral slices, in a practical setting, grafts harvested from central regions are not completely resistant to warping.30
The initial motivation for this study was to determine whether laser reshaping methods and techniques could be adapted to prevent warping in costal cartilage grafts. In preliminary experiments, it became clear that preventing warping immediately after graft harvesting was not feasible even when using extreme laser dosimetry settings with our device and experimental setup. However, we observed that focal and targeted laser heating accelerated the warping process and that near-steady-state shape change could be attained in a relatively short period. It was during our preliminary experiments when we also discovered that, whether we irradiated the external or internal side of the graft with an Nd:YAG laser, the dynamics and direction of warping were not altered. This finding is reasonable because 1.32-μm light is poorly absorbed by cartilage and is highly scattered; thus, the axial fluence rate distribution is essentially uniform.31,32 The Nd:YAG laser operating at 1.32 μm was selected because of its near-uniform heating through the entire cartilage thickness and because the interaction between cartilage and this laser has been studied extensively by our group.16,18,24,27,33 Although the Nd:YAG laser is an expensive precision instrument, dozens of commercially available infrared and near-infrared lasers exist that would produce essentially the same tissue effect at a fraction of the cost. Hence, the use of a laser for optimizing the warping process in surgery is economically feasible because many of these lasers are less expensive than electrocautery units.
The present study demonstrates a method that leads to the stabilization of shape change in peripheral costal cartilage sections: infrared laser irradiation generated focal regions of heat in the specimen, which led to acceleration of the warping process and attainment of near-steady-state shape change in the first 30 minutes. Thereafter, the slices remained in a largely stable geometry until the end of our trial (24 hours) compared with the control, which warped continuously during the same time interval. The 30 minutes that elapse while the laser-irradiated specimens are immersed in 0.9% saline solution is an acceptable period in the operating room during most facial surgery.
Although our main objective was to stabilize the warping process, it was also important to minimize irradiation of the cartilage specimens and spatially limit the potential extent of any tissue injury produced by heating. The 3 laser exposure groups differed in dosimetry and spatial arrangement but yielded very similar results in that all sections reached near-steady-state geometry after 30 minutes compared with the control. Upon visual and manual inspection of all specimens, there were no discernable differences noted between irradiated and control slices except for minor dimpling at the irradiated sites. Furthermore, the average peak temperatures (76°C–82°C) within the laser spots for the 3 exposure groups suggest that the specimens were not being overheated. The lowest dosimetry settings consisted of irradiating only 4 spots per graft, with each spot receiving 191.0 W/cm2 of power for 2 seconds. Given a laser spot size of 2 mm (as determined by burn paper) and a cartilage surface area of 400 mm2, the specimens in this experimental group had less than 4% of their total surface area exposed to laser heating. In support of this measurement, our cell viability results obtained using a live-dead assay demonstrated cell injury that spanned a surface diameter of 1.9 mm and a depth of 0.8 mm per irradiated spot. The viability assay that we used is a well-established approach for determining cell death in cartilage after laser irradiation.24,25 However, the amount of thermal damage demonstrated by our viability analysis was lower than predicted. This is because the viability staining was performed shortly after irradiation and thus did not account for the cells that will undergo cell death via apoptosis across a longer period.34 These observations suggest that shape stabilization can be achieved with minimal thermal injury to the specimen or loss of structural integrity. 24,33,35 What is interesting is that the spatial distribution of laser spots across the specimen had modest if any effect on the dynamics of warping, which suggests that only a certain threshold value of total tissue volume must be heated to accelerate the release of Fry’s interlocked stresses.7,8
The use of laser irradiation to reshape cartilage has been extensively studied in head and neck surgery. In laser cartilage reshaping, a mold is typically used to hold the cartilage in a desired shape during or immediately after laser irradiation. After a period of time, the mold is removed. Although the final geometry of the irradiated cartilage is similar to the shape of the mold, there may be a memory effect that causes some reversion to the original cartilage shape over time.16 In clinical settings, laser irradiation has been used to successfully reshape human auricular cartilage to correct excessive ear protrusion. 21,22 Furthermore, Ovchinnikov et al19 used laser irradiation to reshape the nasal septum and correct the nasal airway.
The present study used laser irradiation in a distinctly different application compared with laser cartilage reshaping. We irradiated costal cartilage, which is structurally anisotropic. This type of cartilage falls under neither elastic nor hyaline categories like those of septal and auricular cartilage, which have been extensively studied with respect to laser irradiation. Furthermore, our emphasis is on obtaining a stable geometry within a length of time that is acceptable for use in the operating room before graft placement.
Although the exact mechanism is not known, appreciation for the current theories of laser cartilage reshaping may aid understanding of laser-mediated accelerated warping of costal cartilage to a steady state. Cartilage is composed of a collagen framework with negatively charged proteoglycan molecules that interact with polarized water molecules. It has been suggested that shape change is caused by the disruption of the intermolecular forces within the cartilage, as well as by collagen denaturation. 36–38 Additional studies describe the role of stress relaxation and phase transformation between free and bound water during laser heating.39,40 These investigations have demonstrated that the shape change process during laser heating occurs between 70°C and 80°C. Our dosimetry settings resulted in similar maximum spot temperatures (mean temperature, 76°C–82°C), so it is likely that similar mechanisms are at play.
Regardless of technique, every peripheral costal cartilage section warps immediately after being cut. In this study, the initial curvature ranged from 1.1° to 11.7°. This large range may be a consequence of obtaining slices from different anatomical rib locations, differently shaped ribs, and error from shaping with the use of a traditional scalpel, albeit we randomized specimen selection to reduce bias toward a single group. Although trends were visible between individual laser exposure groups, there was no statistical significance. This may have resulted from relatively large standard deviations, likely accounted for by the wide variation in initial curvatures and errors throughout the slicing and measuring process.
Although curved specimens are not ideal for use in most rhinoplasty maneuvers, the stabilized laser-irradiated grafts have a shape that may be used for alar grafts, tip and rim contouring, and auricular reconstruction. Future work optimizing laser dosimetry and establishing a rationale for laser target selection will enable more precise fashioning of the final specimen shape. Our experimental design used a porcine model because it is affordable, readily available, and easy to work with. Porcine costal cartilage behaves similarly to its human counterpart, with peripheral regions warping more than central regions. Adams et al9 observed that mean warping in the peripheral human costal cartilage doubles in the first 24 hours, from about 5° to 12°. Although slice dimensions and the method of angle measurements should be considered, these values follow the same general trend along the time points we used in this study of porcine tissue.
This is the first study, to our knowledge, that successfully accelerates the peripheral costal cartilage warping process to a steady state. This is done by using laser irradiation in a reliable and convenient method within a short period, which may render it practical for use in the operating room. The next step is to rigorously interrogate the impact of laser dosimetry and cartilage thickness on the shape change process and then proceed to in vivo studies.
Acknowledgments
Funding/Support: This study was supported in part by grant DR090349 from the Department of Defense Deployment Related Medical Research Program and grant 1R21DE019026 from the National Institute for Dental and Craniofacial Research.
Role of the Sponsors: The Department of Defense Deployment Related Medical Research Program and the National Institute for Dental and Craniofacial Research played no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Mr Foulad had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Foulad, Ghasri, Garg, and Wong. Acquisition of data: Foulad and Ghasri. Analysis and interpretation of data: Foulad. Drafting of the manuscript: Foulad, Ghasri, and Wong. Critical revision of the manuscript for important intellectual content: Foulad, Ghasri, and Garg. Statistical analysis: Foulad. Obtained funding: Wong. Administrative, technical, and material support: Foulad, Garg, Ghasri, and Wong. Study supervision: Wong.
Financial Disclosure: None reported.
Previous Presentation: This article was presented in part, and won the Residency Travel Award, at the fall meeting of the American Academy of Facial Plastic and Reconstructive Surgery; October 3, 2009; San Diego, California.
REFERENCES
- 1.Tardy ME, Jr, Denneny J, III, Fritsch MH. The versatile cartilage autograft in reconstruction of the nose and face. Laryngoscope. 1985;95(5):523–533. doi: 10.1288/00005537-198505000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Lovice DB, Mingrone MD, Toriumi DM. Grafts and implants in rhinoplasty and nasal reconstruction. Otolaryngol Clin North Am. 1999;32(1):113–141. doi: 10.1016/s0030-6665(05)70118-3. [DOI] [PubMed] [Google Scholar]
- 3.Brent B. The versatile cartilage autograft: current trends in clinical transplantation. Clin Plast Surg. 1979;6(2):163–180. [PubMed] [Google Scholar]
- 4.Romo T, III, Kwak ES. Nasal grafts and implants in revision rhinoplasty. Facial Plast Surg Clin North Am. 2006;14(4):373–387. doi: 10.1016/j.fsc.2006.06.006. vii. [DOI] [PubMed] [Google Scholar]
- 5.Gillies HD. Plastic Surgery of the Face. London, England: Oxford University Press; 1920. [Google Scholar]
- 6.Gibson T, Davis WB. The distortion of autogenous cartilage grafts: its cause and prevention. Br J Plast Surg. 1958;10:257–274. [Google Scholar]
- 7.Fry HJ. Interlocked stresses in human nasal septal cartilage. Br J Plast Surg. 1966;19(3):276–278. doi: 10.1016/s0007-1226(66)80055-3. [DOI] [PubMed] [Google Scholar]
- 8.Fry H. Cartilage and cartilage grafts: the basic properties of the tissue and the components responsible for them. Plast Reconstr Surg. 1967;40(5):526–539. [PubMed] [Google Scholar]
- 9.Adams WP, Jr, Rohrich RJ, Gunter JP, Clark CP, Robinson JB., Jr The rate of warping in irradiated and nonirradiated homograft rib cartilage: a controlled comparison and clinical implications. Plast Reconstr Surg. 1999;103(1):265–270. doi: 10.1097/00006534-199901000-00042. [DOI] [PubMed] [Google Scholar]
- 10.Erol OO. The Turkish delight: a pliable graft for rhinoplasty. Plast Reconstr Surg. 2000;105(6):2229–2243. doi: 10.1097/00006534-200005000-00051. [DOI] [PubMed] [Google Scholar]
- 11.Daniel RK, Calvert JW. Diced cartilage grafts in rhinoplasty surgery. Plast Reconstr Surg. 2004;113(7):2156–2171. doi: 10.1097/01.prs.0000122544.87086.b9. [DOI] [PubMed] [Google Scholar]
- 12.Gunter JP, Clark CP, Friedman RM. Internal stabilization of autogenous rib cartilage grafts in rhinoplasty: a barrier to cartilage warping. Plast Reconstr Surg. 1997;100(1):161–169. doi: 10.1097/00006534-199707000-00026. [DOI] [PubMed] [Google Scholar]
- 13.Helidonis E, Sobol E, Kavvalos G, et al. Laser shaping of composite cartilage grafts. Am J Otolaryngol. 1993;14(6):410–412. doi: 10.1016/0196-0709(93)90115-n. [DOI] [PubMed] [Google Scholar]
- 14.Mordon S, Wang T, Fleurisse L, Creusy C. Laser cartilage reshaping in an in vivo rabbit model using a 1.54 μm Er:Glass laser. Lasers Surg Med. 2004;34(4):315–322. doi: 10.1002/lsm.20029. [DOI] [PubMed] [Google Scholar]
- 15.Ayhan M, Deren O, Görgü M, Erdoğan B, Dursun A. Cartilage shaping with the Er:YAG laser: an in vivo experimental study. Ann Plast Surg. 2002;49(5):527–531. doi: 10.1097/00000637-200211000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Gray DS, Kimball JA, Wong BJ. Shape retention in porcine-septal cartilage following Nd:YAG (λ=1.32 μm) laser-mediated reshaping. Lasers Surg Med. 2001;29(2):160–164. doi: 10.1002/lsm.1104. [DOI] [PubMed] [Google Scholar]
- 17.Jones N, Sviridov A, Sobol E, Omelchenko A, Lowe J. A prospective randomised study of laser reshaping of cartilage in vivo. Lasers Med Sci. 2001;16(4):284–290. doi: 10.1007/pl00011365. [DOI] [PubMed] [Google Scholar]
- 18.Wong BJ, Milner TE, Harrington A, et al. Feedback-controlled laser-mediated cartilage reshaping. Arch Facial Plast Surg. 1999;1(4):282–287. doi: 10.1001/archfaci.1.4.282. [DOI] [PubMed] [Google Scholar]
- 19.Ovchinnikov Y, Sobol E, Svistushkin V, Shekhter A, Bagratashvili V, Sviridov A. Laser septochondrocorrection. Arch Facial Plast Surg. 2002;4(3):180–185. doi: 10.1001/archfaci.4.3.180. [DOI] [PubMed] [Google Scholar]
- 20.Bourolias C, Prokopakis E, Sobol E, Moschandreas J, Velegrakis GA, Helidonis E. Septal cartilage reshaping with the use of an Erbium doped glass fiber laser: preliminary results. Rhinology. 2008;46(1):62–65. [PubMed] [Google Scholar]
- 21.Trelles MA, Mordon SR. Correction of ear malformations by laser-assisted cartilage reshaping (LACR) Lasers Surg Med. 2006;38(7):659–665. doi: 10.1002/lsm.20372. [DOI] [PubMed] [Google Scholar]
- 22.Leclère FM, Petropoulos I, Mordon S. Laser-assisted cartilage reshaping (LACR) for treating ear protrusions: a clinical study in 24 patients. Aesthetic Plast Surg. 2010;34(2):141–146. doi: 10.1007/s00266-009-9353-9. [DOI] [PubMed] [Google Scholar]
- 23.Kim DW, Shah AR, Toriumi DM. Concentric and eccentric carved costal cartilage: a comparison of warping. Arch Facial Plast Surg. 2006;8(1):42–46. doi: 10.1001/archfaci.8.1.42. [DOI] [PubMed] [Google Scholar]
- 24.Li C, Protsenko DE, Zemek A, Chae YS, Wong B. Analysis of Nd:YAG laser-mediated thermal damage in rabbit nasal septal cartilage. Lasers Surg Med. 2007;39(5):451–457. doi: 10.1002/lsm.20514. [DOI] [PubMed] [Google Scholar]
- 25.Choi IS, Chae YS, Zemek A, Protsenko DE, Wong B. Viability of human septal cartilage after 1.45 μm diode laser irradiation. Lasers Surg Med. 2008;40(8):562–569. doi: 10.1002/lsm.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300(6719):230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Protsenko DE, Zemek A, Wong BJ. Temperature dependent change in equilibrium elastic modulus after thermally induced stress relaxation in porcine septal cartilage. Lasers Surg Med. 2008;40(3):202–210. doi: 10.1002/lsm.20611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Protsenko DE, Wong BJ. Engineering of a straighter septum: numerical model of mechanical stress relaxation in laser-heated septal cartilage. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:5399–5402. doi: 10.1109/IEMBS.2007.4353563. [DOI] [PubMed] [Google Scholar]
- 29.Díaz SH, Nelson JS, Wong BJ. Rate process analysis of thermal damage in cartilage. Phys Med Biol. 2003;48(1):19–29. doi: 10.1088/0031-9155/48/1/302. [DOI] [PubMed] [Google Scholar]
- 30.Lopez MA, Shah AR, Westine JG, O’Grady K, Toriumi DM. Analysis of the physical properties of costal cartilage in a porcine model. Arch Facial Plast Surg. 2007;9(1):35–39. doi: 10.1001/archfaci.9.1.35. [DOI] [PubMed] [Google Scholar]
- 31.Youn JI, Telenkov SA, Kim E, et al. Optical and thermal properties of nasal septal cartilage. Lasers Surg Med. 2000;27(2):119–128. doi: 10.1002/1096-9101(2000)27:2<119::aid-lsm3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 32.Díaz SH, Aguilar G, Lavernia EJ, Wong BJ. Modeling the thermal response of porcine cartilage to laser irradiation. IEEE J Sel Top Quantum Electron. 2001;7(6):944–951. [Google Scholar]
- 33.Karam AM, Protsenko DE, Li C, et al. Long-term viability and mechanical behavior following laser cartilage reshaping. Arch Facial Plast Surg. 2006;8(2):105–116. doi: 10.1001/archfaci.8.2.105. [DOI] [PubMed] [Google Scholar]
- 34.Grogan SP, Aklin B, Frenz M, Brunner T, Schaffner T, Mainil-Varlet P. In vitro model for the study of necrosis and apoptosis in native cartilage. J Pathol. 2002;198(1):5–13. doi: 10.1002/path.1169. [DOI] [PubMed] [Google Scholar]
- 35.Sviridov AP, Sobol EN, Jones N, Lowe J. Effect of holmium laser radiation on stress, temperature and structure in cartilage. Lasers Med Sci. 1998;13(1):73–78. [Google Scholar]
- 36.Helidonis E, Volitakis M, Naumidi I, Velegrakis G, Bizakis J, Christodoulou P. The histology of laser thermo-chondro-plasty. Am J Otolaryngol. 1994;15(6):423–428. doi: 10.1016/0196-0709(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 37.Sobol E, Bagratashvili V, Sviridov A, et al. Phenomenon of laser-induced stress relaxation and reshaping of cartilage. Proc SPlE. 1996;2623:544–552. [Google Scholar]
- 38.Sobol EN, Sviridov A, Bagratashvili VV, et al. Stress relaxation and cartilage shaping under laser radiation. Proc SPIE. 1996;2681:358–363. [Google Scholar]
- 39.Bagratashvili VN, Sobol EN, Sviridov AP, Popov VK, Omel’chenko AI, Howdle SM. Thermal and diffusion processes in laser-induced stress relaxation and reshaping of cartilage. J Biomech. 1997;30(8):813–817. doi: 10.1016/s0021-9290(97)00028-6. [DOI] [PubMed] [Google Scholar]
- 40.Chae Y, Aguilar G, Lavernia EJ, Wong BJ. Characterization of temperature dependent mechanical behavior of cartilage. Lasers Surg Med. 2003;32(4):271–278. doi: 10.1002/lsm.10167. [DOI] [PubMed] [Google Scholar]