Abstract
Background
Higher plasma and pulmonary edema fluid levels of plasminogen activator inhibitor-1 (PAI-1) are associated with increased mortality in patients with pneumonia and acute lung injury. The 4G allele of the 4G/5G polymorphism of the PAI-1 gene is associated with higher PAI-1 levels and an increased incidence of hospitalizations for pneumonia. The authors hypothesized that the 4G allele would be associated with worse clinical outcomes (mortality and ventilator-free days) in patients with severe pneumonia.
Methods
The authors enrolled patients admitted with severe pneumonia in a prospective cohort. Patients were followed until hospital discharge. DNA was isolated from blood samples, and genotyping detection for the PAI-1 4G/5G polymorphism was carried out using Taqman-based allelic discrimination.
Results
A total of 111 patients were available for analysis. Distribution of genotypes was 4G/4G 26 of 111 (23%), 4G/5G 59 of 111 (53%), and 5G/5G 26 of 111 (23%). Of 111 patients, 32 (29%) died before hospital discharge and 105 patients (94%) received mechanical ventilation. Patients with the 4G/4G and the 4G/5G genotypes had higher mortality (35% vs. 8%, P = 0.007) and fewer ventilator-free days (median 4 vs. 13, P = 0.04) compared to patients with the 5G/5G genotype.
Conclusions
The 4G allele of the 4G/5G polymorphism in the PAI-1 gene is associated with fewer ventilator-free days and increased mortality in hospitalized patients with severe pneumonia. These findings suggest that PAI-1 may have a role in pathogenesis and that the 4G/5G polymorphism may be an important biomarker of risk in patients with severe pneumonia.
Procoagulant activity is increased and anticoagulant and fibrinolytic activities are decreased in the alveoli of patients with pneumonia. Intraalveolar fibrin deposition, which is the hallmark of many acute inflammatory lung diseases, including pneumonia, exerts beneficial effects by sealing leakage sites when the capillary endothelium and alveolar epithelial barrier are compromised. However, when this process of fibrin deposition is severe and persistent, it can have deleterious effects. Excessive fibrin deposition enhances inflammatory responses by activating endothelial cells to produce proinflammatory mediators and an increase in vascular permeability.
Fibrin is degraded by plasmin, a proteolytic enzyme that is present in the tissues in the form of an inactive precursor, plasminogen. The decreased fibrinolysis in patients with pneumonia is mainly attributed to elevation in plasminogen activator inhibitor–1 (PAI-1) activity.1– 8 PAI-1 is activated during infection and has been shown to be elevated in the air spaces of patients with ventilator-associated pneumonia, aspiration pneumonia, and acute lung injury.2–5,8 –11
Higher plasma and bronchoalveolar lavage (BAL) fluid levels of PAI-1 levels are associated with severe disease and adverse clinical outcomes both in patients with pneumonia and in patients with the acute respiratory distress syndrome. In patients with pneumonia, PAI-1 concentration in BAL fluid is higher in patients requiring mechanical ventilation than in those who do not require mechanical ventilation.9,12 In our earlier studies, we found that elevated plasma and BAL concentrations of PAI-1 were directly correlated with mortality and fewer ventilator-free days in patients with ventilator-associated pneumonia and acute respiratory distress syndrome.9,12–14 Also in a large multicenter trial of patients with acute lung injury, elevated PAI-1 was associated with higher mortality.14 It is not known whether the elevation of PAI-1 in patients with pneumonia is a result of genetic predisposition or occurs solely as a result of environmental factors (e.g., the severity of lung injury).
The regulation of PAI-1 is a complex process and is under control of metabolic, lifestyle, and genetic factors. Even though the genetic variation in the levels of PAI-1 under basal conditions is small, this difference becomes more amplified under conditions of stress.10 A common insertion/deletion polymorphism containing either four or five guanine bases (4G/5G) is located within the promoter region of the human PAI-1 gene 650 bases upstream from the start of transcription. The minor allele (4G allele) frequency is reported to be 45% in the Caucasian population. Both the alleles bind to a transcriptional activator, but the 5G allele reduces transcription by virtue of binding to a repressive protein and is associated with lower circulating PAI-1 levels.10 Artificial constructs have shown that the 4G alleles provide six times more PAI-1 messenger RNA than 5G alleles in response to interleukin.15
In children with meingococcemia, the 4G allele of the 4G/5G polymorphism in the PAI-1 gene has been associated with higher plasma PAI-1 levels and increased mortality.16 In a prospective cohort of adults, the 4G allele of the 4G/5G polymorphism has also has also been associated with higher PAI-1 levels and increased incidence of hospitalizations due to community-acquired pneumonia.17 Therefore, we hypothesized that genetic variation in the PAI-1 gene, specifically the 4G allele of the 4G/5G insertion/deletion polymorphism of the promoter region of the PAI-1 gene, would be associated with worse clinical outcomes (mortality and ventilator-free days) in patients with severe pneumonia.
Materials and Methods
Prospective Cohort Design
We recruited patients from the intensive care units at the University of California, San Francisco–affiliated Moffitt Hospital, San Francisco General Hospital, and the University of California San Franciso Fresno Medical Center. The study was reviewed and approved by the University of California, Committee on Human Research, Office of Research, University of California, San Francisco.
Patients over the age of 18 yr and diagnosed with pneumonia and meeting either the American Thoracic Society or the British Thoracic Society criteria for severe pneumonia were enrolled.18 The diagnosis of pneumonia was made on the basis of the appearance of a new infiltrate on the chest x-ray in the presence of cough or fever. The American Thoracic Society Criteria are: (1) need for mechanical ventilation or (2) septic shock or (3) two of following three minor criteria: (a) systolic blood pressure less than 90 mmHg, (b) multilobar pneumonia, and (c) PaO2/FiO2 less than 250. The British Thoracic Society criteria are two of four criteria: (1) respiratory rate greater than 30 breaths/min, (2) diastolic blood pressure less than 60 mmHg, (3) Blood urea nitrogen greater than 19 mg/dl, and (4) confusion.18
The study enrollment was carried out between November 2003 and December 2007. All patients meeting enrollment criteria were approached, and consent for enrollment in the study was obtained from either the patient or a surrogate.
Outcomes
The primary outcome was in-hospital mortality, and the secondary outcome was ventilator-free days (the number of days patient was alive with unassisted breathing during the first 28 days after enrollment).19
Shortly after enrollment, 10 ml of whole blood was collected in potassium EDTA tubes. Patient data were obtained from the patient’s charts at the time of enrollment and during hospital stay. The data collected included patient demographics, baseline comorbidities, and clinical and physiologic data, including the acute physiology and chronic health evaluation score (APACHE II), simplified acute physiologic score (SAPS II), the PaO2/FIO2 ratio, ventilator-associated variables, including tidal volume, peak, and positive end-expiratory pressure. Patients were followed daily for 28 days to determine their need for mechanical ventilation and until hospital discharge or death for other outcomes. Genomic DNA was isolated from the buffy coat, and the quantity and quality of the genomic DNA isolated was determined by 260/280 ultraviolet spectrophotometer and quantified using the picogreen method.
Genotyping
DNA was normalized to 10 ng/μl and plated in 384-well plates. Genotoyping for the PAI-1 4G/5G insertion/deletion polymorphism was carried out using the Taqman based allelic discrimination method on an Applied Biosystems 7900 Real-Time Polymerase Chain Reaction System (Foster City, CA), which combines thermal cycling, fluorescence detection, and application-specific software to measure the cycle-by-cycle accumulation of polymerase chain reaction products in a single-tube, homogeneous reaction.20
Power Estimation
We estimated that a sample size of 120 patients has power of 0.8 to detect an odds ratio of 3 or greater with P value of 0.05 with the following assumptions: (1) mortality 30%, (2) minimum allele frequency of 0.45, and (3) dominant genetic model.‡‡
Data Analysis
Normally distributed data are presented as mean and SD, and nonnormally data are presented as median and interquartile range. Nonnormally distributed continuous variables were compared across categories using the nonparametric Mann–Whitney test (two groups) or non-parametric trend test (three groups). Comparison of frequencies within the genotype categories was carried out using the chi-square test.
Odds ratios were calculated under a dominant genotypic model consistent with trends in our data and earlier studies. Logistic regression models were used to adjust for confounding due to race, age, and severity of illness on mortality. All P values were two-sided, and statistical significance was defined as a P value of 0.05 or less. The statistical analysis was carried out using Stata software (Stata Corporation, College Station, TX).
Results
A total of 121 patients were initially enrolled in the study. All patients were followed until hospital discharge. Eight patients were excluded due to the lack of availability of a suitable blood sample to extract the DNA. DNA was extracted from whole blood from 113 subjects and was genotyped for the 4G/5G polymorphism. Genotyping was unsuccessful in two patients. Therefore, a total of 111 patients were available for analysis. We ran 10% duplicates and no template controls during the genotyping. There was 100% agreement on the genotype calls among the duplicated samples.
The distribution of genotypes is shown in table 1 and is in agreement with the Hardy Weinberg equilibrium. Baseline demographic and physiologic data stratified by genotypes is depicted in table 2. Pathogens were isolated from blood culture in 37 (33%) patients, and from lavage obtained on the mini BAL in 47 patients (42%). Organisms isolated and the associated mortality is shown in table 3. Out of total of 111 patients, 23 patients (20%) met the Society of Critical Care Medicine criteria for sepsis, and 12 patients (10%) met the criteria for septic shock.21 At the time of diagnosis, 73% of the patients presented with a PaO2/FIO2 ratio of less than 300 and involvement of two or more quadrants on chest radiograph. During the course of hospitalization, 95% of the patients had at least a single blood gas with PaO2/FCO2 ratio of less than 300. Only one patient received activated protein C as part of treatment for sepsis.
Table 1.
Genotypes of Plasminogen Activator Inhibitor-1 4G/5G Polymorphism and Hospital Mortality among 111 Patients with Severe Pneumonia
| Genotype | n (%) | Hospital Mortality (%) |
|---|---|---|
| 4G/4G | 26 (23) | 9 (35%) |
| 4G/5G | 59 (53) | 21 (36%) |
| 5G/5G | 26 (23) | 2 (8%) |
Table 2.
Baseline Demographics and Physiologic Characteristics of 111 Patients with Severe Pneumonia
| 4G/4G and 4G/5G Genotypes | 5G/5G Genotype | P Value | |
|---|---|---|---|
| n | 85 | 26 | |
| Age, yr* | 56.5 ± 19.6 | 50.6 ± 18.4 | 0.15 |
| Gender, male (%) | 35 (41%) | 7 (27%) | 0.21 |
| Race | |||
| European descent | 64 (75%) | 20 (77%) | 0.15 |
| African descent | 5 (6%) | 4 (15%) | |
| Asian descent | 10 (12%) | 0 (0%) | |
| Other | 6 (7%) | 2 (8%) | |
| Ethnicity, Hispanic (%) | 8 (10%) | 10 (38%) | 0.001 |
| Physiological characteristics† | |||
| APACHE II | 20 (15–25) | 18 (16–26) | 0.56 |
| SAPS II | 40 (30–55) | 40 (30–47) | 0.39 |
| PF ratio | 165 (109–212) | 145 (119–183) | 0.35 |
| Minimum PF ratio < 300 | 82 (96%) | 24 (92%) | 0.88 |
| Comorbidities | |||
| Sepsis | 18 (21%) | 5 (19%) | 0.8 |
| Septic shock | 9 (11%) | 3 (12%) | 0.9 |
| NYHA category 3 or greater | 8 (9%) | 0 (0%) | 0.1 |
Mean ± SD.
Median (25th–75th percentile).
APACHE II = acute physiology and chronic health evalution score; NYHA = New York Heart Association; PF = PaO2/FIO2; SAPS = simplified acute physiology score.
Table 3.
Pathogens Isolated from 111 Patients* with Severe Pneumonia
| Pathogen Isolated | Number of Patients | Mortality, n (%) |
|---|---|---|
| Candida sp. | 23 | 5 (21.7%) |
| Staphylococcus epidermadis | 16 | 3 (18.8%) |
| Pseudomonas aeruginosa | 12 | 3 (25.0%) |
| Staphylococcus aureus | 12 | 3 (25.0%) |
| Enterococcus sp. | 12 | 5 (41.7%) |
| Klebsiella sp. | 11 | 2 (18.2%) |
| Escherichia coli | 6 | 2 (33.3%) |
| Yeast, other | 6 | 2 (33.3%) |
| Other | 34 | 5 (15%) |
| No organisms isolated | 43 | 16 (37.2%) |
Of the 111 patients, 39 had more than one organism isolated, and 18 patients had three or more organisms isolated.
Of 111 patients, 32 died before hospital discharge, yielding an overall mortality of 29%. The mortality rate was 34%, 36%, and 8% in patients with the 4G/4G, 4G/5G, and 5G/5G genotypes, respectively (table 1). Mortality rates varied among racial groups (European descent, 23%; African descent, 33%; Asian descent, 50%; others, 12%), but this difference was not statistically significant (P = 0.33).
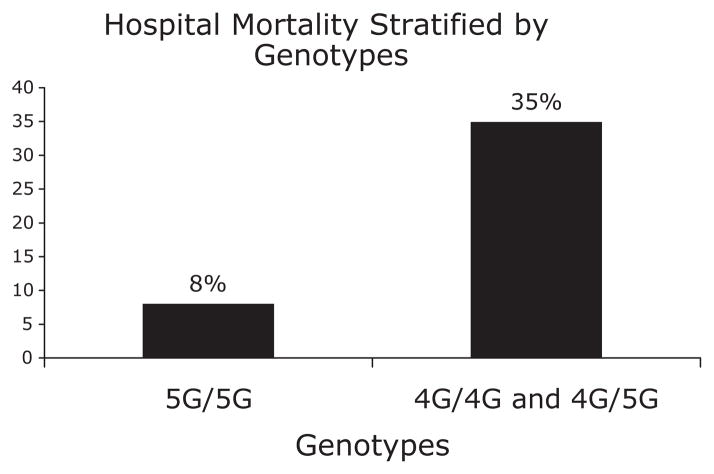
We examined a dominant genotypic model for association with mortality. Mortality in patients with the 5G/5G genotype was 8%, whereas the mortality in patients with 4G/4G and 4G/5G genotypes was 35% (P = 0.007) (fig. 1). Among those of European descent only, mortality in patients with the 5G/5G genotype was 10%, whereas the mortality in patients with 4G/4G and 4G/5G genotypes was 33% (P = 0.046). Overall, the odds ratio of mortality among patients with the 4G/4G and 4G/5G genotypes compared to patients with the 5G/5G genotype was 6.5 (1.4 –30) (P = 0.015). On univariate analysis, mortality was associated with age, APACHE II, and SAPS II at diagnosis, and with the genotypes, but it was not associated with race, gender, or the PaO2/FIO2 at the time of presentation. On multivariate logistic regression, the 4G/4G and 4G/5G genotype was independently associated with mortality after adjustment for age and severity of illness at presentation (table 4).
Fig. 1.
Mortality among 111 patients with severe pneumonia stratified by genotypes of PAI-1 4G/5G polymorphism (chi-square P = 0.007). PAI-1 = plasminogen activator inhibitor-1.
Table 4.
Univariate and Multivariate Analysis of the Effect of Selected Risk Factors on Hospital Mortality among Patients with Severe Pneumonia
| Outcome: Hospital Mortality
|
||
|---|---|---|
| OR (95% Confidence Interval) | P Value | |
| Predictors | ||
| Age, yr | 1.03 (1.002–1.05) | 0.03 |
| Gender, male | 1.3 (0.6–3.0) | 0.5 |
| Race_2 | 1.3 (0.3–5.0) | 0.7 |
| Race_3 | 2.6 (0.7–10) | 0.15 |
| Race_4 | .37 (.04–3.2) | 0.37 |
| APACHE II | 1.12 (1.05–1.20) | 0.001 |
| SAPS II | 1.07 (1.03–1.1) | 0.001 |
| 4G/4G and 4G/5G vs. 5G/5G genotypes | 6.4 (1.5–30) | 0.01 |
| Multivariate analysis | ||
| 4G/4G & 4G/5G vs. 5G/5G genotypes | 6.5 (1.3–34) | 0.026 |
| Age, yrs | 1.01 (0.97–1.03) | 0.61 |
| APACHE II | 1.13 (1.05–1.21) | 0.001 |
APACHE II = acute physiology and chronic health evaluation score; OR = odds ratio; Race_2 = Black; Race_3 = Asian; Race_4 = Other; SAPS = simplified acute physiology score.
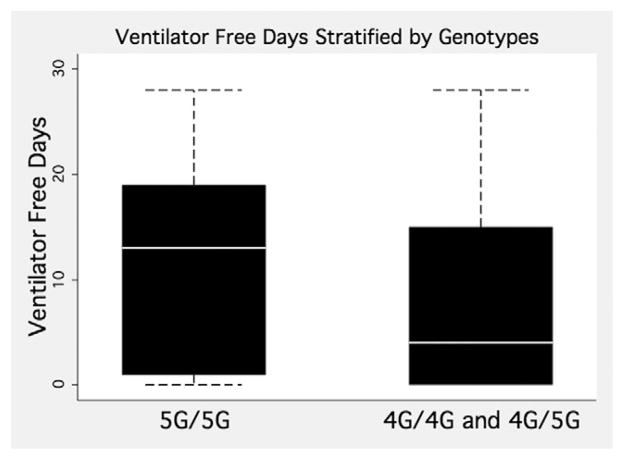
A total of 105 (94%) of the 111 patients required mechanical ventilation. We calculated ventilator-free days with the standard definition used in the Acute Respiratory Distress Syndrome network trials, specifically as the days patient was alive with unassisted breathing during the first 28 days after enrollment.19 Patients with the 5G/5G genotype had a median of 13 ventilator-free days, with an interquartile range of 1 to 19 days; the patients with the 4G/5G and 4G/5G genotypes had a median of 4 ventilator-free days, with an interquartile range of 0 to 15 (P < 0.04) (see fig. 2).
Fig. 2.
Ventilator-free days among 111 patients with severe pneumonia stratified by genotypes of plasminogen activator inhibitor-1 (PAI-1) 4G/5G polymorphism. The white line represents the median, box boundaries represent the 25th and 75th percentiles, and the whisker ends represent 10th and 90th percentile (Mann–Whitney P = 0.04).
Discussion
In this study, we found that the presence of 4G/4G and 4G/5G genotypes of the 4G/5G insertion/deletion polymorphism in the promoter region of the PAI-1 was independently associated with adverse clinical outcomes in adults with severe pneumonia. Patients with the 4G/4G or 4G/5G genotypes had higher hospital mortality and fewer ventilator-free days compared to the patients with the 5G/5G genotype.
These results are consistent with the results from a recently published study of 52 patients with acute lung injury, which reported higher 28-day mortality (70.6%) for patients with the 4G/4G genotype as compared to patients with the non-4G/4G genotype. (P = 0.06).22 In another recent study, Yende et al. reported an increased incidence of hospitalization for community-acquired pneumonia among subjects with the 4G/4G and 4G/5G genotypes of the 4G/5G polymorphism in a prospective cohort of healthy adults. They also reported higher plasma PAI-1 levels and increased expression of PAI-1 on whole blood stimulation assay among patients with the 4G/4G and 4G/5G genotypes.17 Several other studies that have reported that the 4G allele of the 4G/5G polymorphism is associated with greater production of PAI-1 under conditions of stress.15,16,23,24 Therefore, the association of 4G/4G and 4G/5G genotypes with adverse clinical outcomes in this study is concordant with the results from our earlier studies, in which we found that elevated plasma and alveolar fluid concentrations of PAI-1 were associated with adverse clinical outcomes in patients with acute respiratory distress syndrome and severe pneumonia.12,13
The alveolar compartment is an important site of PAI-1 production and activity. BAL fluid of patients with inflammatory pulmonary conditions, including interstitial pulmonary fibrosis, sarcoidosis, acute lung injury, and severe pneumonia have elevated levels of PAI-1, and higher levels of PAI-1 are associated with worse outcomes in patients with pneumonia and acute lung injury.1–3,5,9,12–14
PAI-1 is primarily an antifibrinolytic agent, but it can also dampen the inflammatory response. The urokinase plasminogen activator is important for an adequate immune response to respiratory tract infection through its role in migration of inflammatory cells. PAI-1 inhibits urokinase plasminogen activator and can, therefore, modulate the immune response in pneumonia by altering leukocyte trafficking by its inhibition of urokinase plasminogen activator.25–29 PAI-1 can also directly inhibit integrin-mediated cell migration.30
A significant strength of our study is the prospective cohort design. The cohort was chosen from mixed medical-surgical intensive care units in urban tertiary care health centers. The mortality in our cohort was associated with age, APACHE II, and SAPS II, but not with gender and race.31
Limitations of this study include the relatively small sample size and a mixed racial cohort. Our study population consisted of a mixed racial cohort that reflects the population served by the study centers. However, the difference in mortality among the various racial subgroups in our cohort was not statistically significant, as has been reported in previous studies.32 The association of genotypes with mortality was present when the analysis was restricted to those of European descent only. In addition, we included self-identified race as a covariate in the regression model to test for the effect of genotype on mortality. The effect of genotype on mortality was independent of race, and there was no significant change in the odds of death when we included race in the model. Therefore, we believe that, although we cannot rule it out with certainty, it is unlikely that our results were confounded by racial mixture in our cohort.
Another limitation is that we only tested for one polymorphism in the PAI-1 gene. However, we studied the role of the 4G/5G insertion deletion polymorphism because it is a well-characterized polymorphism in the PAI-1 gene that has been studied both in normal individuals and in disease states. The 4G/5G polymorphism is associated with PAI-1 levels both in health and disease, and it is also associated with clinical outcomes in disease states. Kathiresan et al. studied the relationship of 18 single nucleotide polymorphisms in the PAI-1 gene to plasma PAI-1 levels among 1,328 individuals enrolled in the Framingham cohort and reported that the 4G/5G polymorphism accounts for most of the genetic variation in PAI-1 levels in normal individuals and that individuals with the 4G allele have higher PAI-1 levels.33 Similarly, in a cohort of patients with myocardial infraction, Errikson et al. reported higher PAI-1 levels among subjects with the 4G allele of the 4G/5G polymorphism.23 In a study of children with meningococcal sepsis, Hermans et al. reported that the 4G allele of the 4G/5G polymorphism is associated with higher PAI-1 levels and increased mortality.16
The results of this study suggest that the 4G/5G polymorphism may serve as a useful biomarker of prognosis in patients with severe pneumonia. It may help identify patients who are at the greatest risk for poor clinical outcomes so that therapeutic and preventive interventions could be directed towards the susceptible patients. In addition, genetic markers are present before the development of illness, and they cannot be the result of the pneumonia. Therefore, the elevation of PAI-1 in patients with severe pneumonia, which is associated with clinical outcomes, is not exclusively the result of environmental factors (such as the extent and severity of pneumonia), but may be determined by genetic predisposition of the individual. Therefore, this study provides further evidence of the role of PAI-1 in the pathogenesis of acute lung injury and its relationship to clinical outcomes in patients with severe pneumonia that has been reported both by our group and other investigators.9,12–14
In conclusion, the results from our study suggest that the 4G/4G and 4G/5G genotypes of the 4G/5G polymorphism of the PAI-1 gene are associated with higher mortality and fewer ventilator-free days among patients hospitalized with severe pneumonia and may, therefore, be useful as a biomarker of risk in patients with severe pneumonia. These results also suggest that PAI-1 may have a role in the pathogenesis of severe pneumonia and adverse clinical outcomes.
Acknowledgments
Supported by grants HL074005 NHLBISCCOR for Acute Lung Injury Translational Research, NHLBI 1K23HL085526-01, and NICHD HD047349 from the National Institutes of Health (Bethesda, Maryland). Presented in part at the Annual Meeting of the American Thoracic Society, San Francisco, California, May 20, 2007.
Footnotes
Gauderman J, Morrison J: Quanto, Version 0.5, 2004. Available at: http://hydra.usc.edu/gxe. Accessed: June 12, 2007.
References
- 1.Bertozzi P, Astedt B, Zenzius L, Lynch K, LeMaire F, Zapol W, Chapman HA. Depressed bronchoalveolar urokinase activity in patients with adult respiratory distress syndrome. N Engl J Med. 1990;322:890–7. doi: 10.1056/NEJM199003293221304. [DOI] [PubMed] [Google Scholar]
- 2.Choi G, Schultz MJ, van Till JW, Bresser P, van der Zee JS, Boermeester MA, Levi M, van der Poll T. Disturbed alveolar fibrin turnover during pneumonia is restricted to the site of infection. Eur Respir J. 2004;24:786–9. doi: 10.1183/09031936.04.00140703. [DOI] [PubMed] [Google Scholar]
- 3.Determann RM, Millo JL, Garrard CS, Schultz MJ. Bronchoalveolar levels of plasminogen activator inhibitor-1 and soluble tissue factor are sensitive and specific markers of pulmonary inflammation. Intensive Care Med. 2006;32:946–7. doi: 10.1007/s00134-006-0167-9. [DOI] [PubMed] [Google Scholar]
- 4.Gunther A, Mosavi P, Heinemann S, Ruppert C, Muth H, Markart P, Grimminger F, Walmrath D, Temmesfeld-Wollbruck B, Seeger W. Alveolar fibrin formation caused by enhanced procoagulant and depressed fibrinolytic capacities in severe pneumonia. Comparison with the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000;161:454–62. doi: 10.1164/ajrccm.161.2.9712038. [DOI] [PubMed] [Google Scholar]
- 5.Idell S, James KK, Levin EG, Schwartz BS, Manchanda N, Maunder RJ, Martin TR, McLarty J, Fair DS. Local abnormalities in coagulation and fibrinolytic pathways predispose to alveolar fibrin deposition in the adult respiratory distress syndrome. J Clin Invest. 1989;84:695–705. doi: 10.1172/JCI114217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakstad B, Lyberg T, Skjonsberg OH, Boye NP. Local activation of the coagulation and fibrinolysis systems in lung disease. Thromb Res. 1990;57:827–38. doi: 10.1016/0049-3848(90)90150-b. [DOI] [PubMed] [Google Scholar]
- 7.Vadasz I, Morty RE, Olschewski A, Konigshoff M, Kohstall MG, Ghofrani HA, Grimminger F, Seeger W. Thrombin impairs alveolar fluid clearance by promoting endocytosis of Na+, K+-ATPase. Am J Respir Cell Mol Biol. 2005;33:343–54. doi: 10.1165/rcmb.2004-0407OC. [DOI] [PubMed] [Google Scholar]
- 8.Schultz MJ, Millo J, Levi M, Hack CE, Weverling GJ, Garrard CS, van der Poll T. Local activation of coagulation and inhibition of fibrinolysis in the lung during ventilator associated pneumonia. Thorax. 2004;59:130–5. doi: 10.1136/thorax.2003.013888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Solh AA, Bhora M, Pineda L, Aquilina A, Abbetessa L, Berbary E. Alveolar plasminogen activator inhibitor-1 predicts ARDS in aspiration pneumonitis. Intensive Care Med. 2006;32:110–5. doi: 10.1007/s00134-005-2847-2. [DOI] [PubMed] [Google Scholar]
- 10.Horrevoets AJ. Plasminogen activator inhibitor 1 (PAI-1): In vitro activities and clinical relevance. Br J Haematol. 2004;125:12–23. doi: 10.1111/j.1365-2141.2004.04844.x. [DOI] [PubMed] [Google Scholar]
- 11.Gunther A, Mosavi P, Ruppert C, Heinemann S, Temmesfeld B, Velcovsky HG, Morr H, Grimminger F, Walmrath D, Seeger W. Enhanced tissue factor pathway activity and fibrin turnover in the alveolar compartment of patients with interstitial lung disease. Thromb Haemost. 2000;83:853–60. [PubMed] [Google Scholar]
- 12.Song Y, Lynch SV, Flanagan J, Zhuo H, Tom W, Dotson RH, Baek MS, Rubio-Mills A, Singh G, Kipnis E, Glidden D, Brown R, Garcia O, Wiener-Kronish JP. Increased plasminogen activator inhibitor-1 concentrations in bronchoalveolar lavage fluids are associated with increased mortality in a cohort of patients with Pseudomonas aeruginosa. Anesthesiology. 2007;106:252–61. doi: 10.1097/00000542-200702000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Prabhakaran P, Ware LB, White KE, Cross MT, Matthay MA, Olman MA. Elevated levels of plasminogen activator inhibitor-1 in pulmonary edema fluid are associated with mortality in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2003;285:L20–8. doi: 10.1152/ajplung.00312.2002. [DOI] [PubMed] [Google Scholar]
- 14.Ware LB, Matthay MA, Parsons PE, Thompson BT, Januzzi JL, Eisner MD. Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2007;35:1821–8. doi: 10.1097/01.CCM.0000221922.08878.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawson SJ, Wiman B, Hamsten A, Green F, Humphries S, Henney AM. The two allele sequences of a common polymorphism in the promoter of the plasminogen activator inhibitor-1 (PAI-1) gene respond differently to interleukin-1 in HepG2 cells. J Biol Chem. 1993;268:10739–45. [PubMed] [Google Scholar]
- 16.Hermans PW, Hibberd ML, Booy R, Daramola O, Hazelzet JA, de Groot R, Levin M. 4G/5G promoter polymorphism in the plasminogen-activator-inhibitor-1 gene and outcome of meningococcal disease. Meningococcal Research Group. Lancet. 1999;354:556–60. doi: 10.1016/s0140-6736(99)02220-5. [DOI] [PubMed] [Google Scholar]
- 17.Yende S, Angus DC, Ding J, Newman AB, Kellum JA, Li R, Ferrell RE, Zmuda J, Kritchevsky SB, Harris TB, Garcia M, Yaffe K, Wunderink RG. 4G/5G plasminogen activator inhibitor-1 polymorphisms and haplotypes are associated with pneumonia. Am J Respir Crit Care Med. 2007;176:1129–37. doi: 10.1164/rccm.200605-644OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niederman MS, Mandell LA, Anzueto A, Bass JB, Broughton WA, Campbell GD, Dean N, File T, Fine MJ, Gross PA, Martinez F, Marrie TJ, Plouffe JF, Ramirez J, Sarosi GA, Torres A, Wilson R, Yu VL. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163:1730–54. doi: 10.1164/ajrccm.163.7.at1010. [DOI] [PubMed] [Google Scholar]
- 19.Schoenfeld D. Statistical evaluation of ventillator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30:1772–7. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ. Allelic discrimination using fluorogenic probes and the 5′ nuclease assay. Genet Anal. 1999;14:143–9. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 21.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 22.Tsangaris I, Tsantes A, Bonovas S, Lignos M, Kopterides P, Gialeraki A, Rapti E, Orfanos S, Dimopoulou I, Travlou A, Armaganidis A. The impact of the PAI-1 4G/5G polymorphism on the outcome of patients with ALI/ARDS. Thromb Res. 2008 Sep 19; doi: 10.1016/j.thromres.2008.07.018. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Eriksson P, Kallin B, van ’t Hooft FM, Bavenholm P, Hamsten A. Allele-specific increase in basal transcription of the plasminogen-activator inhibitor 1 gene is associated with myocardial infarction. Proc Natl Acad Sci U S A. 1995;92:1851–5. doi: 10.1073/pnas.92.6.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoekstra T, Geleijnse JM, Schouten EG, Kluft C. Plasminogen activator inhibitor-type 1: Its plasma determinants and relation with cardiovascular risk. Thromb Haemost. 2004;91:861–72. doi: 10.1160/TH03-08-0546. [DOI] [PubMed] [Google Scholar]
- 25.Chapman HA. Plasminogen activators, integrins, and the coordinated regulation of cell adhesion and migration. Curr Opin Cell Biol. 1997;9:714–24. doi: 10.1016/s0955-0674(97)80126-3. [DOI] [PubMed] [Google Scholar]
- 26.Gyetko MR, Chen GH, McDonald RA, Goodman R, Huffnagle GB, Wilkinson CC, Fuller JA, Toews GB. Urokinase is required for the pulmonary inflammatory response to Cryptococcus neoformans. A murine transgenic model. J Clin Invest. 1996;97:1818–26. doi: 10.1172/JCI118611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rijneveld AW, Florquin S, Bresser P, Levi M, De Waard V, Lijnen R, Van Der Zee JS, Speelman P, Carmeliet P, Van Der Poll T. Plasminogen activator inhibitor type-1 deficiency does not influence the outcome of murine pneumococcal pneumonia. Blood. 2003;102:934–9. doi: 10.1182/blood-2003-01-0227. [DOI] [PubMed] [Google Scholar]
- 28.Sitrin RG, Shollenberger SB, Strieter RM, Gyetko MR. Endogenously produced urokinase amplifies tumor necrosis factor-alpha secretion by THP-1 mononuclear phagocytes. J Leukoc Biol. 1996;59:302–11. doi: 10.1002/jlb.59.2.302. [DOI] [PubMed] [Google Scholar]
- 29.Sitrin RG, Todd RF, 3rd, Albrecht E, Gyetko MR. The urokinase receptor (CD87) facilitates CD11b/CD18-mediated adhesion of human monocytes. J Clin Invest. 1996;97:1942–51. doi: 10.1172/JCI118626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waltz DA, Natkin LR, Fujita RM, Wei Y, Chapman HA. Plasmin and plasminogen activator inhibitor type 1 promote cellular motility by regulating the interaction between the urokinase receptor and vitronectin. J Clin Invest. 1997;100:58–67. doi: 10.1172/JCI119521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tejerina E, Frutos-Vivar F, Restrepo MI, Anzueto A, Palizas F, Gonzalez M, Apezteguia C, Abroug F, Matamis D, Bugedo G, Esteban A. Prognosis factors and outcome of community-acquired pneumonia needing mechanical ventilation. J Crit Care. 2005;20:230–8. doi: 10.1016/j.jcrc.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Pippins JR, Fitzmaurice GM, Haas JS. Hospital characteristics and racial disparities in hospital mortality from common medical conditions. J Natl Med Assoc. 2007;99:1030–6. [PMC free article] [PubMed] [Google Scholar]
- 33.Kathiresan S, Gabriel SB, Yang Q, Lochner AL, Larson MG, Levy D, Tofler GH, Hirschhorn JN, O’Donnell CJ. Comprehensive survey of common genetic variation at the plasminogen activator inhibitor-1 locus and relations to circulating plasminogen activator inhibitor-1 levels. Circulation. 2005;112:1728–35. doi: 10.1161/CIRCULATIONAHA.105.547836. [DOI] [PubMed] [Google Scholar]