Abstract
Background & Aims
High-frequency gastric electrical stimulation (GES) is a relatively new treatment for medically-refractory gastroparesis. There have been a number of clinical studies based on the use of a high frequency stimulator (Enterra, Medtronic, Minneapolis, MN). A meta-analysis was performed to evaluate the evidence for improved clinical outcome with this device.
Methods
A literature search of major medical databases was performed for the period January 1992 to August 2008. Clinical studies involving an implanted high-frequency GES device were included and reported a range of clinical outcomes. Studies of external, temporary and/or low frequency GES were excluded.
Results
Of 13 included studies, 12 lacked controls and only one was blinded and randomized. Following GES, patients reported improvements in total symptom severity score (3/13 studies, mean difference 6.52 [CI: 1.32, 11.73], p=0.01), vomiting severity score (4/13, 1.45 [CI: 0.99, 1.91], p<0.0001), nausea severity score (4/13, 1.69 [CI: 1.26, 2.12], p<0.0001), SF-36 physical composite score (4/13, 8.05 [CI: 5.01, 11.10], p<0.0001), SF-36 mental composite score (4/13, 8.16 [CI: 4.85, 11.47], p<0.0001), requirement for enteral or parenteral nutrition (8/13, OR 5.53 [CI: 2.75, 11.13], p<0.001) and 4–hour gastric emptying (5/13, 12.7% [CI: 9.8, 15.6], p<0.0001). Weight gain did not reach significance (3/13, 3.68kg [CI: −0.23, 7.58], p=0.07). The device removal or reimplantation rate was 8.3%.
Conclusions
Results show substantial benefits for high frequency GES in the treatment of gastroparesis. However, caution is necessary in interpreting the results, primarily due to the limitations of uncontrolled studies. Further controlled studies are required to confirm the clinical benefits of high-frequency GES.
Keywords: Enterra, high-frequency GES, vomiting, gastric pacing, systematic review
Introduction
Gastroparesis is increasingly common, in part because of the rising prevalence of diabetes, and it presents a significant clinical challenge and economic burden1. The diagnosis of gastroparesis is made in patients with typical symptoms and evidence of delayed gastric emptying in the absence of mechanical obstruction2. In severe refractory cases, gastroparesis may be profoundly disabling, with intense and continuous symptoms including nausea, vomiting, bloating and abdominal pain. Patients may require long-term enteral or parenteral nutritional support, as well as frequent and prolonged hospital admissions, and may suffer potentially life-threatening metabolic derangements1.
Initial treatment strategies are directed at symptom relief, and include modifying dietary intake and administration of prokinetic and/or antiemetic medication3. Patients whose disease is refractory to these measures have few proven or effective alternatives4, although patients with severe gastroparesis are sometimes offered more aggressive therapies including enteral nutrition, gastrostomy, pyloric injection of botulinum toxin, pyloroplasty, and/or partial, sleeve or total gastric resection3.
In the last decade, high-frequency gastric electrical stimulation (GES) has emerged as a potential therapeutic option for patients with medically-refractory gastroparesis5,6. This therapy involves delivering low energy electrical stimuli into the muscularis propria of the stomach, at a frequency significantly higher than the normal three cycles per minute gastric slow wave activity7,8. High-frequency GES is therefore distinct from ‘gastric pacing’, in which high-energy stimuli are delivered at a frequency slightly above that of the intrinsic slow wave activity9. After early investigations demonstrated potential for the high-frequency approach10, a stimulation device was developed and commercially released (Enterra, Medtronic Inc, Minneapolis, MN). The Enterra was granted ‘Humanitarian Device Exemption’ by the Federal Drug Administration Agency (FDA) for use in diabetic and idiopathic gastroparesis, but this exemption does not imply clinical efficacy11.
A number of groups have reported positive results using high-frequency GES and have called for more widespread use12,13. However, no systematic reviews or meta-analyses of high-frequency GES outcomes have previously been reported. To summarise current evidence for the efficacy of high-frequency GES in the treatment of gastroparesis, we therefore conducted a comprehensive literature review and meta-analysis of selected published studies.
Materials and Methods
Literature Search
A literature search for clinical trials was undertaken for the period January 1992 to August 2008. Included sources were Medline, EMBASE, Google Scholar, ISI Proceedings, the Cochrane Library and online registers of controlled clinical trials. The search included the following terms: gastric electrical stimulation, GES, gastric electrostimulation, Enterra, gastric pacemaker, gastroparesis and vomiting. Reference lists of retrieved articles were also searched, and in addition, unpublished data was sought from a representative of the device manufacturer.
Inclusion and Exclusion Criteria
Studies evaluating the treatment efficacy of high-frequency GES for medically-refractory gastroparesis were screened for inclusion. Because of the limited numbers of controlled clinical trials, non-controlled observational studies were also included. One prominent study has included a randomised controlled trial (RCT) phase, followed by a non-controlled observational phase7, from which RCT results were preferred over the uncontrolled results when possible. Studies reporting duplicate outcomes from a previously published study were excluded, to prevent multiple publication bias. Small case series (1–2 cases) were also excluded.
Only studies evaluating an implanted high-frequency GES device were included. Some studies have examined the concept of temporary GES. This is an experimental treatment in which stimulation wires are temporarily positioned, in order to define responders prior to selected implantation of a permanent GES device. Studies employing temporary GES were excluded from the current meta-analysis, as this methodology introduces a selection bias by only including patients who show a positive response to a trial of stimulation. The method, rationale, and results of temporary stimulation have been discussed elsewhere10,14,15.
Formal evaluation of study quality through the Jadad scale16 (for RCTs) or Newcastle-Ottawa Scale17 (for non-randomised studies) was intended, but not employed because of the paucity of published controlled clinical trials. Studies were instead defined by methodology, and the overall quality of evidence was assessed quantitatively.
Methods of Review
Studies were independently appraised by two reviewers. Four primary outcomes were assessed: symptom improvement, nutritional outcome, gastric emptying and device complications. Major symptom-related outcomes examined were total symptom severity score (TSS), vomiting severity score, and nausea severity score. Although unvalidated, these are commonly used scales. Severity scores were designated as: 0 = absent; 1 = mild (not influencing usual activities); 2 = moderate (causing diversions from usual activities); 3 = severe (urging modification of usual activities); 4 = extremely severe (markedly restricting usual activities). TSS is a sum of severity scores for six symptoms: vomiting, nausea, early satiety, bloating, postprandial fullness, and epigastric pain. Health Related Quality of Life Scores were retrieved when reported as SF36 physical (PCS) and mental (MCS) composite scores, which are validated norm-based measures (mean ± standard deviation (SD) in the United States population = 50 ± 10)18. Data on weekly vomiting frequency and requirement for antiemetics and prokinetics were also evaluated. Nutritional outcomes examined were weight change (kilograms) and the requirement for enteral or parenteral nutritional support. Rates of delayed gastric emptying at two and four hours were evaluated for studies reporting on standardised radionucleotide scans of a solid meal19. All studies were examined for types and rates of complications.
When outcomes were reported at multiple time points after device implantation, 12 month outcomes were preferred. Because of the limited availability of relevant data, detailed sub-group analyses (diabetic, idiopathic, postsurgical) were not attempted. The costs of treatments were not commonly reported and were not evaluated.
Statistical Methodology
Weighted mean differences with 95% confidence intervals (CI) were used in the analysis of continuous variables. Where studies had reported median and range, the mean and variance was estimated by the statistical methods described by Hozo20. For dichotomous variables, odds ratios (OR) with 95% CI were calculated. A random effects model was chosen for all calculations. All statistical calculations, including weighting, and forest plots were generated using Review Manager 5.0.14 (RevMan; Cochrane Collaboration, Copenhagen)21. A value of p<0.01 was chosen as the threshold for significance in outcome measures. Heterogeneity was calculated in RevMan, and values for I2 and the chi-squared test (p<0.10 significance level) are reported. In addition, the fail safe N statistic (Nfs) was calculated for all meta-analysis outcomes. The Nfs represents the number of new, unpublished or unretrieved null-result trials that would need to exist in order to reduce the significance of each meta-analysis outcome to a non-significant level. A larger Nfs indicates greater stability of the reported results to the addition of new findings.
Results
Search and Included Studies
A total of 26 possible studies were identified by the search criteria, of which 13 were found suitable for inclusion (Table 1). The other 13 studies were excluded (Supplementary Table 1) for one of three reasons: they either duplicated outcomes reported elsewhere (9 studies), employed temporary stimulation prior to device insertion (3 studies), or reported on two or fewer cases (1 study). No previously unpublished data was retrieved.
Table 1.
Included Studies
| Authors | Study Type | Year Published | N* | Population | Study Quality† |
|---|---|---|---|---|---|
| Velanovich22 | Prospective Case Series | 2008 | 42 | Diabetic (24), Idiopathic (17), Post-surgical (1) | Low |
| Maranki et al23 | Prospective Case Series | 2008 | 28 | Diabetic (12), Idiopathic (16) | Low |
| Filichia & Cendan24 | Retrospective Case Series | 2008 | 13 | Post- transplant (13) | Low |
| Gourcerol et al12 | Prospective Case Series | 2007 | 15 | Diabetic (5), Idiopathic (6), Post-surgical (4) | Low |
| Gray & Fullarton25 | Retrospective Case Series | 2006 | 7 | Diabetic (5), Idiopathic (2) | Low |
| de Csepel26 | Prospective Case Series | 2006 | 16 | Diabetic (7), Idiopathic (7), Other (2) | Low |
| van der Voort et al27 | Prospective Case Series | 2005 | 17 | Diabetic (17) | Low |
| Mason et al28 | Retrospective Case Series | 2005 | 29 | Diabetic (24), Idiopathic (5) | Low |
| McCallum et al29 | Prospective Case Series | 2005 | 16 | Post-surgical (16) | Low |
| Lin et al30 | Prospective Case Series | 2004 | 48 | Diabetic (48) | Low |
| Abell et al7 | RCT (2 months), then Prospective (10 months) | 2003 | 33 | Diabetic (17), Idiopathic (16) | Moderate, then Low |
| Jones et al31 | Prospective Case Series | 2003 | 13 | Diabetic (12), Idiopathic (1) | Low |
| Forster et al32 | Prospective Case Series | 2001 | 25 | Diabetic (19), Idiopathic (3), Post-surgical (3) | Low |
N reports patients recruited into each study, outcomes were often available on fewer patients (refer Forest Plots).
Study quality judgement was based on the GRADE approach and definitions33.
The designs of the 13 included studies are detailed on Table 1. Only one study included a randomised, controlled (sham stimulation) and blinded experimental phase7. The remainder were solely observational studies without control populations.
Symptom Improvement
The methods used to quantify symptom and quality of life scores were found to vary between the included studies. These reporting methods were often irreconcilable, and therefore a number of results could not be incorporated into summary statistics. Total symptoms severity (TSS) score was available from three studies. GES demonstrated a significant benefit over sham GES or baseline (mean TSS difference 6.52 [CI: 1.32, 11.73], p=0.01, Nfs = 5), although with significant heterogeneity (I2=89%; p<0.001). Vomiting and nausea severity scores were available from four studies. Post-GES measures demonstrated a consistent and significant benefit over baseline measures for both vomiting severity score (mean difference 1.45 [CI: 0.99, 1.91], p<0.0001, Nfs = 87) and nausea severity score (mean difference 1.69 [CI: 1.26, 2.12], p<0.0001, Nfs = 138). Forest plots for these outcomes are presented in Figure 1. Change in weekly vomiting frequency following GES was available from three studies (57 patients)7,25,27, and was also found to improve significantly (mean difference 16.5 fewer vomiting episodes per week [CI: 6.17, 26.88], P=0.002, Nfs = 10).
Figure 1.
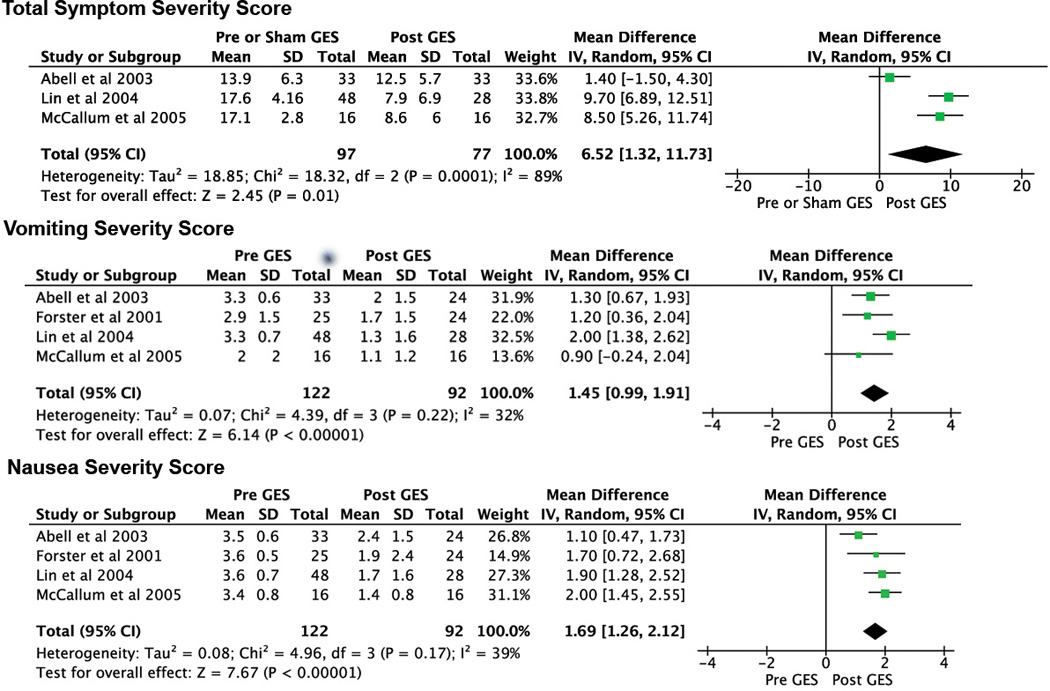
Forest plots of total symptom severity (TSS) score, vomiting severity score and nausea severity score. The included studies are listed for each outcome and their individual results are presented as corresponding squares on the adjacent plot. The size of each square correlates with study weighting. The horizontal bars represent confidence intervals, and the vertical line indicates no effect. The diamond indicates the meta-analysis
SF-36 PCS and MCS outcomes were each available from four studies. Both PCS (mean difference 8.05 [CI: 5.01, 11.10], p<0.0001, Nfs = 61) and MCS (mean difference 8.16 [CI: 4.85, 11.47], p<0.0001, Nfs = 52) (Figure 2) demonstrated consistent and highly significant improvements post-GES compared with baseline values. Because of insufficient data, prokinetic and antiemetic use before and after stimulation could not be evaluated.
Figure 2.
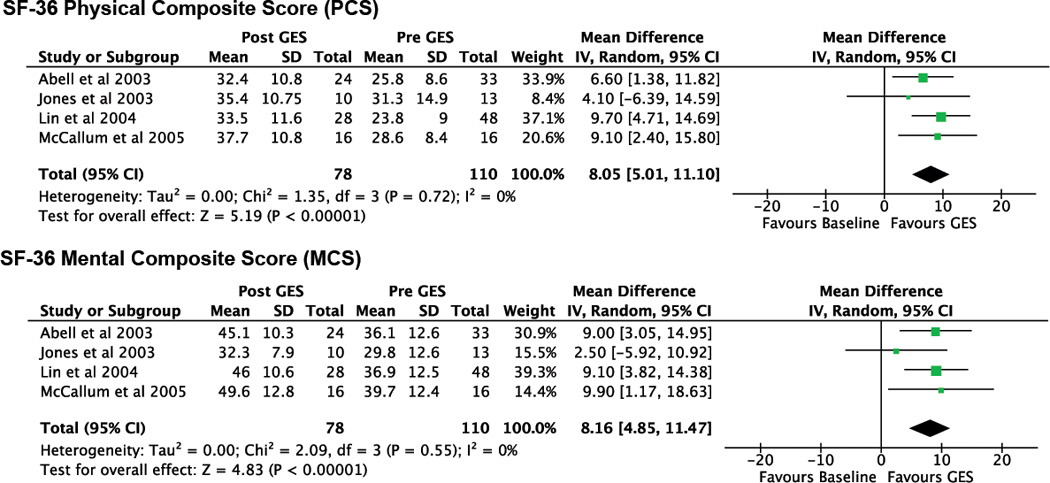
Forest plots of SF-36 physical composite score (PCS) and SF-36 mental composite score (MCS).
Nutritional support
The requirement for enteral or parenteral nutritional support was reported in 8 studies. Prior to GES, a total of 96 patients required enteral or parenteral nutritional support; however, after GES this was reduced to 21 patients (78% reduction; OR 5.53 [CI: 2.75,11.13], p<0.001, Nfs = 214) (Figure 3). Weight gain in kilograms following GES therapy was reported in four studies, but did not reach significance (mean difference 3.68kg [CI: −0.23, 7.58], p=0.07) (Figure 4).
Figure 3.
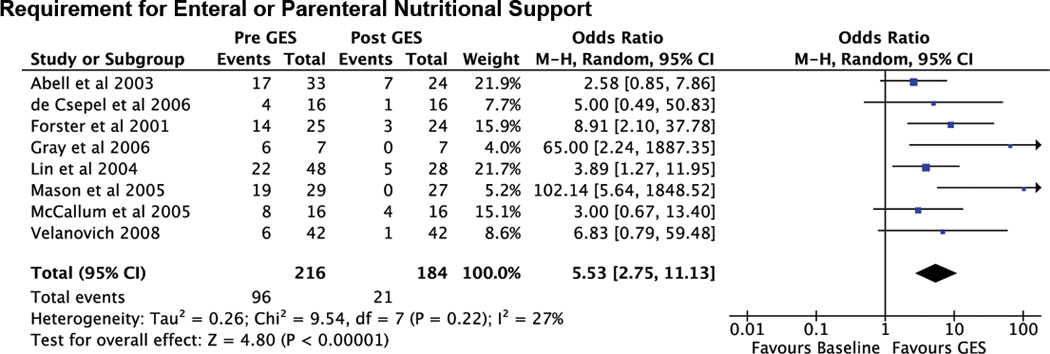
Forest plot of requirement for enteral or parenteral nutritional support.
Figure 4.

Forest plot of change in weight (kilograms).
Gastric Emptying
For of the studies that reported gastric emptying at 2 hours after standard testing by solid meal ingestion, and 5 other studies that reported gastric emptying at 4 hours. Change measured at 2 hours was highly heterogeneous between studies (I2=98%; p<0.0001), though demonstrated overall improvement after GES (mean difference in retention 23.2% [CI: 7.9, 38.4], p=0.003, Nfs = 17). Change measured at 4 hours was strongly consistent between studies (I2=0%), with a highly significant improvement demonstrated after GES (mean difference in retention 12.7% [CI: 9.8, 15.6], p<0.0001, Nfs = 270). Forest plots of gastric emptying outcomes are presented in Figure 5.
Figure 5.

Forest plot of change in 2 hour and 4 hour gastric emptying (% of gastric residual).
Complications
Complication rates were reported in 10 of the 13 studies. Device removal and/or replacement because of a complication was documented in 22 of 265 patients implants (8.3%). The reasons given for device removal were infection (8 cases), erosion through the skin (6 cases), pain at the implantation site (4 cases), perforation of the stomach by the stimulation lead (2 cases), device migration (1 case), and small bowel infarction related to volvulus around the device wires (1 case). Minor complications not requiring device removal were not reported frequently enough to summarise.
Discussion
This meta-analysis finds significant and clinically important benefits for patients receiving high-frequency GES for refractory gastroparesis. Vomiting, nausea and total symptom severity scores all decreased from severe to mild-moderate ranges, and validated quality of life scores also demonstrated significant improvement. The majority (78%) of patients on enteral and parenteral nutrition no longer required these invasive therapies. Importantly, heterogeneity was generally low between studies for the majority of outcomes. Study quality was generally low, as most included studies (12/13) were non-controlled case series.
This study has highlighted the range of complications associated with the use of an implantable high frequency GES device. Infection is the most frequent complication, occurring in around 3% of cases, and is expected with many patients are diabetic. Significant problems arise at the site of device implantation in around 4.9% of cases, specifically pain, lead perforation of the stomach, device migration and erosion through the skin. Data from this meta-analysis suggest that a removal rate of 8–9% can be expected. Severe life threatening complications are rare (<1%), and have included bowel volvulus and strangulation around stimulation leads30.
The role of high-frequency GES in clinical practice remains controversial and it is not universally accepted as the standard of care. This is because of concerns regarding the quality of the evidence4,5,34 and the cost of treatment, which ranges from US $40,000 to $60,0006. There are other investigators who consider that the evidence does justify more widespread use of high-frequency GES in cases of medically-refractory gastroparesis6. One small comparison study (18 patients) has suggested that high frequency GES might outperform intensive medical therapies for gastroparesis at 3 years, in relation to both symptom reduction and resource use35.
The results of this meta-analysis are limited by the design of the included studies. All of them with one exception7, were uncontrolled case series, reflecting worthwhile audits of GES outcomes conducted by researchers from a variety of different centres. Although this has provided a useful volume of evidence for evaluation, this study design potentially overestimates the efficacy of the treatment36. For example, a 50% reduction in vomiting has previously been reported following sham-GES alone5, and in an uncontrolled series this improvement would likely be attributed to the intervention. Furthermore, the natural history of gastroparesis remains poorly understood37, and even patients with longstanding disease may spontaneously improve with traditional medical care alone.
One study included an initial randomised controlled trial (RCT) phase (the Worldwide Anti-Vomiting and Electrical Stimulation Study (WAVESS)7) involving sham stimulation. This study has been included in this meta-analysis even though concerns have previously been raised regarding this study, principally because it has been considered that statistical significance was only reached for the major outcome (decrease in weekly vomiting frequency) after a post-hoc re-evaluation of the study design5. Moreover, this study was powered for 80 patients, but only 33 patients could be recruited7 because of the withdrawal of sponsor funding32. After two months the study was changed to an open-label observational design. As a result, few results were published from the RCT phase, from which only weekly vomiting frequency and the TSS were included in this meta-analysis. The TSS scores were not significantly improved during the brief RCT phase, but did improve significantly during the open-label phase.
Another issue in relation to study design is the potential confounding effect of concurrent pharmacological therapy, and in particular opiate use, which is an issue in some of the studies included in this meta-analysis. Opiates may induce complex gastric dysrhythmias38, slow gastric emptying, and exacerbate gastroparesis-related symptoms4. One included study reported that 45% of the patients were narcotic dependent prior to device implantation and were actively weaned, with variable success, after the institution of high-frequency GES28. Thus symptom improvement may have resulted from the reduction in narcotic dosage rather than the implementation of high-frequency GES. Prokinetic and antiemetic drug use was not routinely controlled in the included studies.
There are two further limitations to the quality of this meta-analysis. Firstly, a number of patients in some studies were lost to follow up, possible leading to an attrition bias. The non-responders to GES may have had their devices removed, or they may have declined to participate in post-treatment evaluations, leading to a greater representation of responders in the summary statistics. Secondly, the included studies used a variety of different scoring systems to evaluate for changes in symptoms and quality of life following device implantation. This meant that a number of important studies could not be incorporated into the summary statistics, which were based on only the most commonly-used scoring systems available. Greater standardisation in the use of health scoring systems would be beneficial to allow a more comprehensive comparison of the results of future studies.
Based on these stated methodological concerns, the overall quality of evidence for high-frequency GES evaluated in this meta-analysis must be considered weak. Using the Oxford Centre for Evidence-Based Medicine Levels of Evidence Criteria39, for example, high-frequency GES only currently achieves a weak “C” Grade of Recommendation, from four possible grades (A defining strong evidence, to D defining poor and inconclusive evidence). To improve the quality of evidence it would be necessary for future studies to include a control or sham stimulation arm. Our results therefore reaffirm the American Gastroenterology Association position statement on gastroparesis treatment from 2004, which concluded that further blinded and controlled studies are still required to confirm the effectiveness of high-frequency GES therapy, and also to define which patients are most likely to respond2.
A technical review of gastric stimulation research has previously cast doubt on the possibility that high-frequency GES can directly improve gastric emptying8. Furthermore, the relationship between delayed gastric emptying and symptoms in gastroparesis remains controversial, as poor emptying is not a consistent finding in affected patients19. Although the present meta-analysis finds consistent and significant benefits for high-frequency GES, it does not prove a causal relationship.
At present there is no consensus regarding the mechanism of action of high-frequency GES. Unlike gastric pacing (also called low-frequency, high-energy stimulation)40, high-frequency GES is unlikely to entrain gastric slow waves or revert gastric dysrhythmias41,8. Current research focuses on the possible beneficial effects of high-frequency GES on gastric tone, fundal relaxation and accommodation, enteric nervous system function and central neuronal pathways8. Until the mechanism of action is better understood, it will be difficult to further improve the efficacy of high-frequency GES.
Meanwhile, research continues into other methods of gastric stimulation, and these also hold some promise for the treatment of gastroparesis. For example, multi-channel gastric pacing has been shown to accelerate gastric emptying in a canine model42, an outcome that single-channel gastric pacing could not reliably achieve9. Another group of researchers has successfully invoked gastric contractions using an ultra-high frequency stimulation protocol that can induce the intramural cholinergic fibres to release acetylcholine (called neural gastric electrical stimulation or NGES)43. There is a need to improve the power efficiency and protocol designs of such treatments before they become a clinical reality8,9. There is also considerable scope for innovation based on an improved understanding of gastric electropathophysiology44. For example, Lammers et al have recently used high-resolution electrical mapping to define the origin and propagation of gastric arrhythmias for the first time38. Complex slow wave focal activities and re-entrant circuits have been revealed, like those known to occur during cardiac arrhythmias. It is yet to be adequately determined what role these abnormalities might play in gastroparesis, and what effect gastric stimulation may have in treating these abnormal rhythms.
In conclusion, this meta-analysis of the current best available evidence demonstrates significant benefits for high-frequency GES in the treatment of refractory gastroparesis. The most frequent benefits appear to be a substantial reduction in nausea and vomiting, a reduction in the requirement for enteral and parenteral nutritional support, and an improvement in gastric emptying. The relatively poor quality of study design means that this evidence must be interpreted with some caution. Nevertheless, the results provide strong grounds for the further development and evaluation of high frequency GES.
Supplementary Material
Acknowledgement
This work is partially supported by grants from the NIH (R01 DK64775), NZ Society of Gastroenterology, and the NZ Health Research Council. We thank Professor Wim Lammers, for his comments on the manuscript.
References
- 1.Wang YR, Fisher RS, Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics and outcomes, 1995–2004. Am J Gastroenterol. 2008;103:313–322. doi: 10.1111/j.1572-0241.2007.01658.x. [DOI] [PubMed] [Google Scholar]
- 2.Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association medical position statement: diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1589–1591. doi: 10.1053/j.gastro.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 3.Abell TL, Bernstein RK, Cutts T, et al. Treatment of gastroparesis: a multidisciplinary clinical review. Neurogastroenterol Motil. 2006;18:263–283. doi: 10.1111/j.1365-2982.2006.00760.x. [DOI] [PubMed] [Google Scholar]
- 4.Tack J. The difficult patient with gastroparesis. Best Pract Res Clin Gastroenterol. 2007;21:379–391. doi: 10.1016/j.bpg.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Jones MP. Is gastric electrical stimulation an effective therapy for patients with drug-refractory gastroparesis? Nat Clin Pract Gastroenterol Hepatol. 2008;5:368–370. doi: 10.1038/ncpgasthep1157. [DOI] [PubMed] [Google Scholar]
- 6.Abrahamsson H. Treatment options for patients with severe gastroparesis. Gut. 2007;56:877–883. doi: 10.1136/gut.2005.078121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–428. doi: 10.1016/s0016-5085(03)00878-3. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Chen JD. Systematic review: applications and future of gastric electrical stimulation. Aliment Pharmacol Ther. 2006;24:991–1002. doi: 10.1111/j.1365-2036.2006.03087.x. [DOI] [PubMed] [Google Scholar]
- 9.Bortolotti M. The "electrical way" to cure gastroparesis. Am J Gastroenterol. 2002;97:1874–1883. doi: 10.1111/j.1572-0241.2002.05898.x. [DOI] [PubMed] [Google Scholar]
- 10.Abell TL, Van Cutsem E, Abrahamsson H, et al. Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion. 2002;66:204–212. doi: 10.1159/000068359. [DOI] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration. H990014 - EnterraTM Therapy System (formerly named Gastric Electrical Stimulation (GES) system) [accessed Sept. 2008]; Issued March 31, 2000. [url: http://www.fda.gov/cdrh/ode/H990014sum.html]
- 12.Gourcerol G, Leblanc I, Leroi AM, Denis P, Ducrotte P. Gastric electrical stimulation in medically refractory nausea and vomiting. Eur J Gastroenterol Hepatol. 2007;19:29–35. doi: 10.1097/01.meg.0000250584.15490.b4. [DOI] [PubMed] [Google Scholar]
- 13.Anand C, Al-Juburi A, Familoni B, et al. Gastric electrical stimulation is safe and effective: a long-term study in patients with drug-refractory gastroparesis in three regional centers. Digestion. 2007;75:83–89. doi: 10.1159/000102961. [DOI] [PubMed] [Google Scholar]
- 14.Abell TL, Thompson J, Johnson WD, Minocha A. Double blinded randomized study of temporary gastric electrical stimulation (GES): preliminary results of the endostim study (endoscopic stimulation temporarily implanted mucosally) (Abstract; IEGGS Proceedings) Neurogastroenterol Motil. 2007;19(6):527. [Google Scholar]
- 15.Ayinala S, Batista O, Goyal A, et al. Temporary gastric electrical stimulation with orally or PEG-placed electrodes in patients with drug refractory gastroparesis. Gastrointest Endosc. 2005;61:455–461. doi: 10.1016/s0016-5107(05)00076-3. [DOI] [PubMed] [Google Scholar]
- 16.Jadad AR, Moore RA, Carroll D, Jenkinson C, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 17.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Accessed: Sept 2008];Ottawa Health Research Institute. Available online: [URL: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm] [Google Scholar]
- 18.Ware JE., Jr . SF-36 Health Survey: manual and interpretation guide. Boston, MA: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 19.Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol. 2008;103:753–763. doi: 10.1111/j.1572-0241.2007.01636.x. [DOI] [PubMed] [Google Scholar]
- 20.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Review Manager (RevMan) [Computer program]. Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2008. [Google Scholar]
- 22.Velanovich V. Quality of life and symptomatic response to gastric neurostimulation for gastroparesis. J Gastrointest Surg. 2008;12:1656–1663. doi: 10.1007/s11605-008-0655-z. [DOI] [PubMed] [Google Scholar]
- 23.Maranki JL, Lytes V, Meilahn JE, et al. Predictive factors for clinical improvement with Enterra gastric electric stimulation treatment for refractory gastroparesis. Dig Dis Sci. 2008;53:2072–2078. doi: 10.1007/s10620-007-0124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filichia LA, Cendan JC. Small case series of gastric stimulation for the management of transplant-induced gastroparesis. J Surg Res. 2008;148:90–93. doi: 10.1016/j.jss.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 25.Gray J, Fullarton GM. Gastric electrical stimulation in severe gastroparesis - the Scottish experience. Neurogastroenterol Motil. 2006;18:493. [Abstract]. [Google Scholar]
- 26.de Csepel J, Goldfarb B, Shapsis A, et al. Electrical stimulation for gastroparesis: gastric motility restored. Surg Endosc. 2006;20:302–306. doi: 10.1007/s00464-005-0119-4. [DOI] [PubMed] [Google Scholar]
- 27.van der Voort IR, Becker JC, Dietl KH, et al. Gastric electrical stimulation results in improved metabolic control in diabetic patients suffering from gastroparesis. Exp Clin Endocrinol Diabetes. 2005;113:38–42. doi: 10.1055/s-2004-830525. [DOI] [PubMed] [Google Scholar]
- 28.Mason RJ, Lipham J, Eckerling G, et al. Gastric electrical stimulation: an alternative surgical therapy for patients with gastroparesis. Arch Surg. 2005;140:841–846. doi: 10.1001/archsurg.140.9.841. discussion 847-8. [DOI] [PubMed] [Google Scholar]
- 29.McCallum R, Lin Z, Wetzel P, Sarosiek I, Forster J. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49–54. doi: 10.1016/s1542-3565(04)00605-6. [DOI] [PubMed] [Google Scholar]
- 30.Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071–1076. doi: 10.2337/diacare.27.5.1071. [DOI] [PubMed] [Google Scholar]
- 31.Jones MP, Ebert CC, Murayama K. Enterra for gastroparesis. Am J Gastroenterol. 2003;98(11):2578. doi: 10.1111/j.1572-0241.2003.08681.x. [DOI] [PubMed] [Google Scholar]
- 32.Forster J, Sarosiek I, Delcore R, et al. Gastric pacing is a new surgical treatment for gastroparesis. Am J Surg. 2001;182:676–681. doi: 10.1016/s0002-9610(01)00802-9. [DOI] [PubMed] [Google Scholar]
- 33.Grades of Recommendation, Assessment, Development and Evaluation (GRADE) Working Group. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490–1494. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ang D, Tack J. Gastric electrical stimulation for the treatment of gastroparesis: ready for prime time? Digestion. 2007;75:80–82. doi: 10.1159/000102960. [DOI] [PubMed] [Google Scholar]
- 35.Cutts TF, Luo J, Starkebaum W, Rashed H, Abell TL. Is gastric electrical stimulation superior to standard pharmacologic therapy in improving GI symptoms, healthcare resources, and long-term health care benefits? Neurogastroenterol Motil. 2005 Feb;17(1):35–43. doi: 10.1111/j.1365-2982.2004.00609.x. [DOI] [PubMed] [Google Scholar]
- 36.Guyatt GH, Sackett DL, Cook DJ. Users' guides to the medical literature. II. How to use an article about therapy or prevention. A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA. 1993;270:2598–2601. doi: 10.1001/jama.270.21.2598. [DOI] [PubMed] [Google Scholar]
- 37.Vittal H, Farrugia G, Gomez G, Pasricha PJ. Mechanisms of disease: the pathological basis of gastroparesis--a review of experimental and clinical studies. Nat Clin Pract Gastroenterol Hepatol. 2007;4:336–346. doi: 10.1038/ncpgasthep0838. [DOI] [PubMed] [Google Scholar]
- 38.Lammers WJEP, Ver Donck LV, Stephen B, et al. Focal activities and re-entrant propagations as mechanisms of gastric tachyarrhythmias. Gastroenterology. 2008 doi: 10.1053/j.gastro.2008.07.020. Articles in Press (Available on-line). [DOI] [PubMed] [Google Scholar]
- 39.Phillips B, Ball C, Sackett D, Badenoch D, Straus S, Haynes B, Dawes M. Oxford Centre for Evidence-based Medicine Levels of Evidence. 2001 May; Available online: [URL: http://www.cebm.net/index.aspx?o=1025] [Google Scholar]
- 40.McCallum RW, Chen JDZ, Lin Z, Schirmer BD, Williams RD, Ross RA. Gastric pacing improves emptying and symptoms in patients with gastroparesis. Gastroenterology. 1998;114:456–461. doi: 10.1016/s0016-5085(98)70528-1. [DOI] [PubMed] [Google Scholar]
- 41.Lin Z, Forster J, Sarosiek I, McCallum RW. Effect of high-frequency gastric electrical stimulation on gastric myoelectric activity in gastroparetic patients. Neurogastroenterol Motil. 2004;16:205–212. doi: 10.1111/j.1365-2982.2004.00503.x. [DOI] [PubMed] [Google Scholar]
- 42.Chen JD, Xu X, Zhang J, et al. Efficiency and efficacy of multi-channel gastric electrical stimulation. Neurogastroenterol Motil. 2005;17:878–882. doi: 10.1111/j.1365-2982.2005.00688.x. [DOI] [PubMed] [Google Scholar]
- 43.Jalilian E, Onen D, Neshev E, Mintchev MP. Implantable neural electrical stimulator for external control of gastrointestinal motility. Med Eng Phys. 2007;29:238–252. doi: 10.1016/j.medengphy.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Du P, O’Grady G, Egbuji J, et al. High-resolution mapping of in-vivo gastrointestinal slow wave activity using flexible printed circuit board electrodes: methodology and validation. Ann Biomed Eng. 2009;37:839–846. doi: 10.1007/s10439-009-9654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


