Abstract
Anabolic androgenic steroids (AAS) are illicitly administered to enhance athletic performance and body image. Although conferring positive actions on performance, steroid abuse is associated with changes in anxiety and aggression. AAS users are often keenly invested in understanding the biological actions of these drugs. Thus, mechanistic information on AAS actions is important not only for the biomedical community, but also for steroid users. Here we review findings from animal studies on the impact of AAS exposure on neural systems that are crucial for the production of anxiety and aggression, and compare the effects of the different classes of AAS and their potential signaling mechanisms, as well as context-, age- and sex-dependent aspects of their actions.
Keywords: anabolic steroid, anxiety, aggression, GABAA receptor, corticotropin releasing factor, extended amygdala
Introduction
Anabolic androgenic steroids (AAS [1]; Box 1) comprise a large class of synthetic derivatives of testosterone that are predominately taken in an illicit manner for ergogenic purposes and image enhancement [2]. An estimated 10 million Americans, some starting as early as 13 years of age, have self-administered AAS [3,4], a pattern that is mirrored in other countries [5]. Although most users are male, an appreciable percentage is female [4]. Thus, both age and sex are crucial parameters to consider when assessing the impact of illicit steroid use. AAS are taken in intricate and complex patterns, typified by concurrent (stacking) and intermittent (cycling) coadministration of different AAS in which doses may be increased then decreased (pyramiding) and coupled with coadministration of masking agents (Box 1) [6,7]. The rationalization for these sophisticated regimens is the belief that activation of multiple signaling pathways will result in the synergistic augmentation of anabolic (muscle-building) effects with minimization of unwanted side-effects [7]. All illicit regimens result in supratherapeutic levels of androgens and their metabolites: by an estimated 10–100-fold in men and by >1000-fold in women and adolescents [7–9]. Thus, this rationalization aside, illicit steroid use results in highly nonphysiological levels of endogenous steroids and comparably high levels of synthetic steroids, all of which have the capacity to alter not only peripheral physiology, but also central nervous system processing and behavioral output [1,10,11].
Box 1. Classification of AAS.
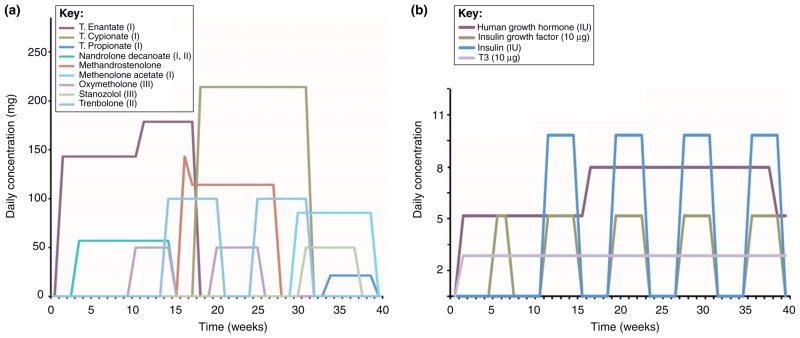
AAS are synthetic derivatives of testosterone, formed by chemical modification of the 4-ring 19-C androstane backbone into one of three classes (Figure I). The modifications of this fundamental chemical structure were made to endow new synthetic compounds with particular advantages as outlined below, and most modifications were derived to increase the anabolic (tissue-building) effects of the steroid while diminishing their androgenic (masculinizing) actions. Despite the explosion in the number of AAS that have been synthesized, all are active at the AR and thus have androgenic as well as anabolic properties [1]. The use-patterns of the AAS are typified by elaborate concurrent combinations of multiple AAS (stacking) with increasing (pyramiding) concentration believed by the users to limit the side effects of the compounds. A typical AAS schedule [7] is used to exemplify this (Figure II). Note the use of non-AAS compounds for energy replacement (insulin) and for fat loss (triiodothyronine; T3) (Figure IIb), in addition to a large number of AAS of various classes (Figure IIa).
Figure I.
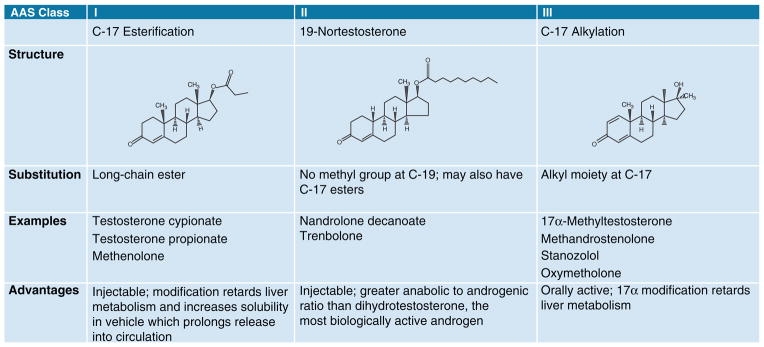
The three major classes of anabolic androgenic steroids.
Figure II.
Representative AAS regime. (a) Individual lines represent the increases and decreases in concentrations of concurrent use of multiple AAS, as well as interruptions in steroid use that, together, represent pyramiding, cycling and stacking. Parentheses indicate class of AAS (I, II, III). (b) Individual lines represent the patterns of self-administration of non-steroidal compounds that AAS users would take in concert with the AAS shown above.
The matrix of interactions of these different steroid regimes with key variables such as age, sex, intrinsic genetic makeup, and social context promotes an array of effects that may be quite disparate from one individual to the next. With regard to behavior, data from case reports, large population surveys, and experimental studies in humans have shown that AAS use can result in a plethora of actions that range from euphoria and increased self-confidence to impulsivity, irritability, and aggression [6,9,12,13]. A growing body of literature demonstrates that behavioral outcomes of chronic AAS use are recapitulated in animal models. As such, these models provide crucial mechanistic insight into the underlying bases for AAS-induced effects. Here, we review these recent studies and highlight the advances in our understanding of the neuronal mechanisms that underlie AAS-induced changes in anxiety and aggression.
The behavioral effects of AAS on anxiety
As indicated above, case studies in humans demonstrate that AAS impose a wide range of changes in behaviors in individuals who use these steroids [8,9,12]. For example, adult men have reported transient euphoria and hypomania early in the course of AAS administration [14], but long-term users may manifest increased anxiety and marked irritability with generalized and undirected anger [6,12]. Men who administer the highest doses of AAS have been shown to have elevated scores on the Symptom Check List-90, a self-report system that includes several different dimensions of anxiety, and on the Hostility and Direction of Hostility Questionnaire, which reflects both the willingness to respond to social situations with aggressive behavior and the tendency to evaluate people, including oneself, in negative terms [6]. Randomized clinical trials have been few owing to ethical constraints, and those that have been performed have been carried out using only moderate doses of a single AAS. However, men in these studies were also found to exhibit increased levels of hostility and anxiety [15,16]. Fewer studies have examined behavioral outcomes in women or in adolescents of either sex, but in such studies the data again suggest that chronic steroid use is associated with poor self-esteem, as well as eating and mood disorders, that may reflect a fundamental disruption in the neural circuitry that regulates anxiety [17,18].
Studies in laboratory rodents allow assessments of the behavioral effects of AAS exposure under conditions where the steroid regimen as well as the age and sex of the subjects can be controlled, and where direct observations of anxiety-like behaviors can be made by several independent means. Tests commonly used to assess AAS-induced anxiety in rodents include the acoustic startle response, the elevated plus-maze, the open-field test, the Vogel conflict test, shock–probe burying tests, and freezing behavior assays [19]. These studies indicate that the AAS have diverse effects on the expression of anxiety-like behaviors in rodents, exactly as they do in humans. For example, short-term exposure of adult male rats to low doses of the AAS, methenolone had anxiolytic actions as assessed by open-field behavior [20], a finding the authors suggest may be relevant to the reports of enhanced confidence and self-esteem that can be observed in human steroid users early in the course of self-administration. By contrast, chronic exposure to high doses of nandrolone decanoate increased anxiety-like behaviors in adult male rats on the elevated plus-maze [21] and in adult male mice, as assessed in both the open-field and elevated plus-maze tests [22].
Treatment of adult female mice with a high dose of 17α-methyltestosterone was reported to have no effect on anxiety-like behaviors on the elevated plus-maze, light–dark transitions or defensive behavior tests [23], whereas treatment of female mice during adolescence with a mixture of AAS (methandrostenolone, nandrolone decanoate and testosterone cypionate) significantly increased anxiety-like behavior as determined by the acoustic startle response and the elevated plus-maze [24,25]. Interestingly exposure to a chronic low dose of testosterone propionate increased contextual fear responses in female mice, but only if these mice had been previously subjected to social isolation [26].
The diversity of AAS actions on anxiety-like behaviors in rodents is likely to reflect the interactions of age, sex, type(s) of AAS, the regimen employed to administer them, and environmental context. Beyond these variables, differences in experimental methodologies employed to measure anxiety may also add to the disparities. Studies that employ multiple behavioral tests in conjunction with a single paradigm of drug administration will be particularly valuable in discerning the impact of criteria such as sex or age on the expression of AAS-induced anxiety, and in parsing out how the AAS alter the different facets of the repertoire of anxious behaviors.
What are the implications of AAS-dependent effects on anxiety? First, there are many physiological and psychological correlates that suggest an intriguing reverse-parallelism between AAS users who manifest muscle dysmorphia and the disorders of body image that characterize individuals who suffer from anorexia nervosa. As with anorexia, AAS-induced muscle dysmorphia is believed to reflect pathological anxiety, specifically a form of obsessive–compulsive disorder in which overly-muscled individuals perceive themselves as wimpy and weak [13]. Moreover, neuropeptides, such as corticotropin releasing factor (CRF), that are thought to play a key role in the animal models of anorexia [27], have also been shown to be dysregulated in animals exposed to supratherapeutic levels of AAS [24]. Second, AAS-dependent increases in anxiety may be important contributors to the impulsivity and hostility often reported in steroid users, effects that are mirrored in enhanced levels of aggression in male rodents chronically exposed to these synthetic steroids.
The behavioral effects of AAS on aggression
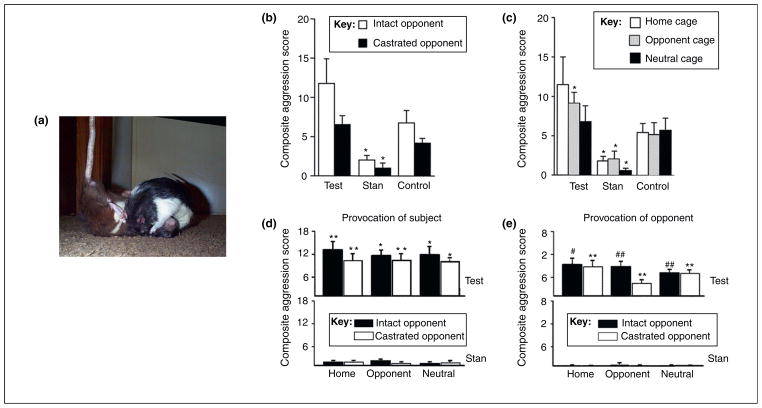
Steroid abuse in humans is associated with poor impulse-control, extreme mood-swings, and abnormal levels of aggressive actions [6,9,12]. Although acts of violence (so-termed ‘roid rage’) and hostility have been in the spotlight of the popular press, the evidence of a link between AAS and overt acts of violence is complex and equivocal. By contrast, data on AAS and aggression in animal models are not ambiguous: chronic exposure of either adult or adolescent male rodents has consistently been shown to promote elevated levels of offensive aggression directed against not only male (Figure 1a–e), but also female conspecifics [28]. Offensive aggression is typically assessed by the resident–intruder test during which the proportion of animals fighting, the latency and duration of the attack, and the display of other aggressive behaviors such as leaps, lunges and tail rattles when an intruder is introduced to the home-cage of a male rodent, are tallied [19]. The level of offensive aggression depends upon the social history of the home-cage resident, the sex and hormonal status of the intruder, the age of exposure, and the environmental context in which behaviors are tested (Figure 1a–e).
Figure 1.
AAS effects on aggression are compound- and context-specific. (a) Offensive inter-male aggression in rats. Reproduced, with permission, from http://www.ratbehavior.org/. (b,c) Social discrimination in AAS-induced aggression. Rats (n = 10 each group) were treated during adolescence with testosterone (Test; class I), stanozolol (Stan; class III) or vehicle (control). (b) Although aggressive behavior was greater in testosterone-treated than control rats, each group showed comparable social discrimination in aggression towards a gonadally-intact versus a castrated intruder. These experiments also demonstrate the importance of the chemical signature of the AAS because stanozolol inhibited offensive aggression compared to controls (*P <0.05) [31]. (c) Rats treated with testosterone were more aggressive than controls in the opponent’s cage, but rats treated with stanozolol showed decreased aggression relative to controls (P < 0.05) irrespective of environment [31]. Such findings demonstrate the importance of environmental cues, as well as inherent differences in behavioral responses to distinct AAS compounds. (d) Provocation (tail pinch) enhanced aggression in rats treated with testosterone propionate (but not stanozolol) in each environmental (home, opponent or neutral cage) and social (gonadally-intact or castrated) condition compared to controls (*P ≤ 0.0001; **P ≤ 0.001) [29]. Controls showed more aggression to an intact than a castrated male, even when provoked (data not shown). (e) Moreover, enhanced aggression was elicited in rats treated with testosterone propionate even when the provocation was given to the opponent male (**P ≤ 0.001; #P ≤ 0.01; ##P ≤ 0.05). Modified, with permission, from [31] (b,c) and [29] (d,e).
AAS-induced aggression is also dependent upon the type of AAS administered. Adult male rats subjected to a mild physical provocation (e.g. tail pinch) demonstrate increased inter-male offensive aggression when chronically treated with testosterone propionate (Figure 1d), but nandrolone decanoate was without effect, and long-term exposure to stanozolol actually decreased aggressive behavior (Figure 1d) [29]. The suppressive effect of stanozolol on aggression in the absence of provocation (Figure 1c) has been observed in both adolescent and adult rats, as well as in adult mice [1,30,31], underscoring the importance of the identity and chemical nature of any individual AAS in its behavioral actions. Although a correlation exists between the relative binding affinities of several AAS to the androgen receptor (AR) and their ability to elicit inter-male aggression, there is no simple relationship between AR affinity, AR binding and behavioral effects [29,32]; the suppressive effects of stanozolol may reflect the actions of this AAS at the estrogen receptor (ER) [33], as much as its low affinity at the AR.
Intriguingly, treatment of adolescent male rats, as opposed to adults, with stanozolol does not suppress, but instead elevates aggressive behaviors when they are subjected to provocation, and this heightened level of aggression is maintained after AAS treatment is discontinued [34]. Moreover, although adolescent male rats chronically treated with testosterone show elevated aggressive behaviors, they nonetheless respond appropriately to social and environmental cues [31] (Figure 1b,c). Enhanced levels of aggressive behaviors that last for weeks following AAS withdrawal have also been reported for adolescent hamsters [35,36]. These studies highlight not only the crucial importance of age in the behavioral effects of individual AAS, but also that enhanced levels of aggression in animals exposed during adolescence may persist for prolonged periods of time, even if steroid use is halted.
Such long-lasting effects of AAS, when coupled with the normal range of volatile behaviors that accompanies the transition through puberty, stand as a particularly important cautionary signal against AAS use in adolescence. The interactions of AAS with the hormonal milieu that characterizes adolescence may also contribute to the increases in risk-taking behavior that are observed in school-age children who use AAS [17,18]. Moreover, puberty is a developmental epoch during which endogenous hormones act through steroid receptors to organize the neural circuitries responsible for adult-like behaviors. Thus, neural circuits that have enhanced sensitivity to androgen exposure during adolescence may be permanently altered by AAS exposure during this time [37]. This concern is supported by studies showing that animals exposed to AAS during adolescence display higher levels of aggression than do animals who received AAS during adulthood [38,39]. It has also been shown that juvenile rats exposed to AAS had significantly smaller testes in adulthood following withdrawal from AAS than had rats exposed to AAS in adulthood, suggesting that early AAS exposure may impose a prolonged suppression of the hypothalamic/pituitary/gonadal (HPG) axis [38]. Such age-specific peripheral effects may, in turn, lead to long-term effects on aggression and anxiety through loss of the normal androgen-dependent regulation of cortisol/cortico-sterone synthesis and of stress-induced activation of the hypothalamic/pituitary/adrenal (HPA) axis [40,41].
Studies in rodents also provide insight into how AAS users may poorly process important social cues. Specifically, it is intriguing to note that AAS-treated adult male rats will respond more aggressively even when it is the intruder (rather than the AAS-treated animal himself) that is subjected to provocation [29] (Figure 1e). In addition, AAS treatment (nandrolone decanoate) of adult male rats can promote markedly-elevated levels of defensive aggression, even to an innocuous stimulus [42]. These studies in rodents may provide a window into understanding how persons who take AAS may misinterpret social interactions and respond with inappropriate levels of aggression when they themselves are not threatened.
AAS effects on the underlying neural architecture of anxiety
The evidence to date suggests that AAS, similarly to endogenous steroids, impart interwoven and complex effects on multiple nodes of the neural networks that are involved in the generation of anxiety and aggression (Figure 2; Box 2). The task of fitting together the AAS-dependent effects on these molecular jigsaw pieces into a coherent behavioral picture has only started. However, recent studies have demonstrated that signaling mediated by both classical neurotransmitters and neuromodulatory peptides in these circuits is significantly altered in AAS-treated animals.
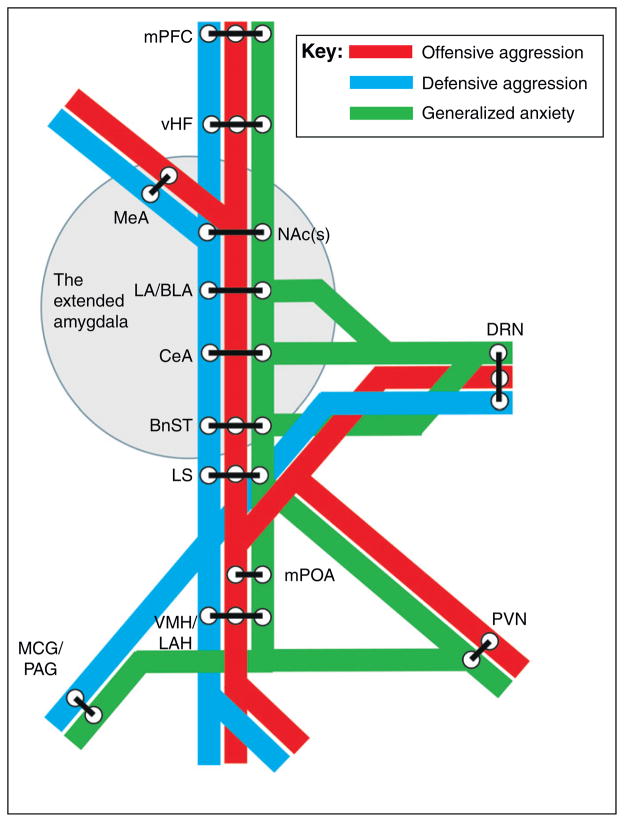
Figure 2.
The mind’s metro of anxiety and aggression. The neural circuitry implicated in the expression of both aggression and anxiety-like behaviors exhibits striking overlap. These neural areas are presented as train stations on different train lines of a subway or metro map, to illustrate the overlap and complexity of affective and aggressive circuitry. The medial pre-frontal cortex (mPFC) [87–89], the extended amygdala, and the dorsal raphe nuclei (DRN) [89,90] have been implicated independently and together by numerous studies in the modulation of both anxiety and aggressive behaviors. Sub-divisions of the extended amygdala [43], including the bed nucleus of the stria terminalis (BnST) [43,47,54,87,91] the shell of the nucleus accumbens [NAc(s)] [92–94], and the three parts of the amygdala proper {the lateral and basolateral (LA/BLA) nuclei [53,90,95], the medial nucleus (MeA) [73,87,91,96], and the central nucleus (CeA) [43,44,52,95,97]}, have been shown to be involved in aggression and anxiety, as have the ventral hippocampus (vHF) [87,88], the lateral septum (LS) [73,87,91,97], and parts of the hypothalamus [88,98] {the ventromedial nucleus and lateral anterior hypothalamic areas (VMH/LAH) [54,55,96,99], the paraventricular nucleus (PVN) [40,97,99] and the medial preoptic area (mPOA) [88,96]}. The lower brainstem {midbrain central grey (MCG) [88] and periaqueductal grey area (PAG) [43,88]} is also an integral part of the circuitry.
Box 2. Outstanding questions.
How does the extensive overlap of neural circuits involved in anxiety and aggression influence AAS actions? Does AAS-induced aggression arise from steroid-induced anxiety (Figure 2)? Are rodents that display inherently lower or higher levels of anxiety [53,118] more likely to show AAS-related aggression? Are there differential effects on anxiety in animals that have inherent differences in aggression [99]? Experimentally addressing such questions may point to important genetic determinants that could increase or decrease individual susceptibility to the negative behavioral effects associated with AAS use.
What role do anxiety-related circuits play in substance abuse among AAS users? Do AAS effects on the neural circuits that underlie the expression of anxiety play a role in the observed higher levels of administration of other drugs of abuse in AAS users versus non-users [4]? This is especially intriguing with regard to the actions of AAS and ethanol because the behavioral effects of both drugs depend on CRF-mediated changes in GABAergic transmission in the extended amygdala. Does an AAS-induced increase in anxiety lead to increased ethanol consumption in efforts to quell this behavioral effect (i.e. ‘self-medication’)? Could chronic AAS use lead to increased physiological tolerance to ethanol because of the convergence of effects on CRF signaling (Figure 3)?
What are the long-term effects of prior AAS use? With a large population of men who used AAS as teenagers and young adults now entering middle age, some data have suggested that these individuals are at higher risk of negative psychological outcomes [8]. Animal studies that model such long-term effects in former AAS users are warranted.
How do AAS-related actions in one brain region affect those in another? How do these changes result in behavioral modifications in both rodents and humans? There is ample evidence that AAS can alter the cellular biology of specific brain regions [11]. However, techniques employed by studies to date have not allowed assessments beyond isolated regions to determine how activity in whole circuits in a behaving animal is altered with AAS use. New advances in functional imaging of freely moving animals, as well as current optogenetic approaches [95,98], may provide answers to these questions.
How general are the underlying mechanisms of AAS-induced anxiety and aggression? Anxiety may be both a symptom and a cause of other psychiatric disorders. The experimental generalization of the underlying mechanisms of AAS-induced anxiety and aggression in rodents may yield significant clues into the etiology of these conditions within the human population.
What is the impact of exercise on AAS-induced aggression and anxiety? The effects of AAS on peripheral tissue clearly depend on exercise. Behaviorally, exercise is known to diminish anxiety and impulsivity in both humans [119] and in rodents [120]. No study to date has assessed the effects of chronic AAS on the expression of anxiety or aggression in animals that exercise. In particular, although moderate exercise might be expected to diminish AAS-induced anxiety, it will be important to examine the effects of excessive exercise, which may better reflect the conditions of professional athletes, to determine if it exacerbates AAS-induced anxiety and aggression.
A corridor of interrelated forebrain structures termed the extended amygdala, which includes the central amygdala (CeA), the shell of the nucleus accumbens [NAc(s)], and the adjoining bed nucleus of the stria terminalis (BnST), comprise the neural circuitry fundamental to the expression of anxious states [43,44] (Figure 3). The CeA is paramount in the acquisition and expression of phasic fear to specific threats whereas the BnST is crucial for sustained fear/anxiety [44], and the connection between these two regions is pivotal to the normal balance between adaptive, specific fear, and maladaptive, diffuse fear (i.e. anxiety). Changes to both specific threats and generalized anxiety are noted in steroid abusers, but the latter may be particularly important with respect to the expression of inappropriate reactions and increased negative perception of self and others [6].
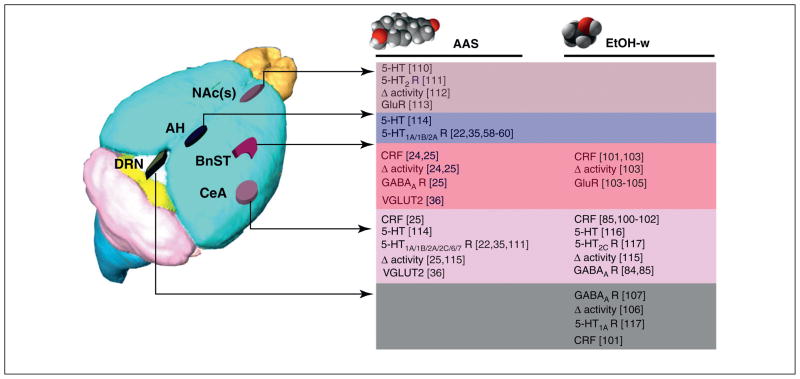
Figure 3.
Comparisons and similarities between AAS and ethanol actions. Chronic AAS exposure [24,25] and withdrawal from ethanol [85,100–102] both result in increased anxiety-like behavior. Molecular and cellular studies suggest that both of these drugs of abuse impose similar changes in neural signaling along a shared template that includes reciprocal connections between structures in the extended amygdala [43] {including the nucleus accumbens shell [NAc(s)], the bed nucleus of the stria terminalis (BnST), and the central nucleus of the amygdala (CeA) and the dorsal raphe (DRN)}, as well as their connections with structures, such as the anterior hypothalamus (AH), ventrolateral nucleus of the hypothalamus, hippocampus and medial prefrontal cortex, that are pivotal for the expression of both anxiety and aggression (Figure 2). Although some molecular targets have not been assessed in all of these key regions for both classes of drugs, the available data converge to suggest potential mechanisms by which both AAS exposure and ethanol withdrawal (EtOH-w) augment the expression of corticotropin releasing factor (CRF) in key regions of the anxiety circuitry, including the BnST [24,103], the CeA [25,84,85,100–102], and the DRN [102]. AAS and/or alcohol-dependent changes in CRF may, in turn, alter classical neurotransmission mediated by both GABAARs [25,84,85] and glutamate (as assessed by vesicular glutamate transporter 2, VGLUT2) [36] and glutamate receptors (GluRs) [56,103–105]. Finally, AAS and alcohol may significantly alter the activity of 5-HT neurons in the DRN [106] through GABAergic afferents to this region [90,107]. These DRN neurons may reflect a crucial focal point in the control of anxiety because they provide greater than 50% of the serotonergic innervation to the forebrain [108], including regions such as the BnST [61,109] and the CeA [52], implicated in the expression of anxious states, and regions such as the AH that are crucial for the expression of aggression [10]. Mouse brain adapted from an image in the Magnetic Resonance Microimaging Neurological Atlas (MRM NeAt) Mouse Brain Database (http://brainatlas.mbi.ufl.edu/ImageGallery.php).
The extended amygdala, as a component of the limbic system, integrates higher-order cognitive processes and homeostatic information, and neurons within these structures express a host of neuromodulatory peptides, including CRF [24,45], brain-derived neurotrophic factor [41], neuropeptide Y [46], pituitary adenylate cyclase activating peptide (PACAP) [47], nociceptin [48], and calcitonin gene-related peptide [49], as well as classical neurotransmitters including GABA, glutamate, serotonin (5-hydroxytryptamine; 5-HT) and dopamine [22,25,50–53] that regulate the expression of anxiety. Chronic AAS exposure has been shown to alter the expression of a wide range of these molecules and their cognate receptors within these regions (Figure 3). Knitting together AAS-dependent changes along the continuum from expression of signaling molecules to alterations in neurophysiology to behavior has not often been done, however recent work suggests an important functional mechanism by which AAS exposure gives rise to anxiety. Specifically, AAS treatment of female mice throughout adolescence resulted in an increase in anxiety-like behaviors (but not fear), as assessed by the acoustic startle response (Figure 4a) and the elevated plus-maze, and that this action of AAS could be blocked by intracerebroventricular infusion of the CRF receptor type 1 (CRF-R1) antagonist, antalarmin (Figure 4b) [25]. Although effects on other important signaling pathways cannot be ruled out, the increase in anxiety elicited by chronic AAS exposure was accompanied by increased activity in GABAergic neurons that project from the lateral CeA to the dorsolateral BnST, an increase in the frequency of GABAA receptor (GABAAR)-mediated spontaneous inhibitory synaptic currents (sIPSCs) in downstream BnST neurons (Figure 4c) and a concomitant decrease in their activity. The effects of chronic AAS treatment on inhibitory transmission to the BnST neurons could be blocked by the CRF-R1 antagonist, antalarmin (Figure 4d) or by the GABAAR antagonist, picrotoxin, and could be mimicked by direct application of CRF [25]. Furthermore, chronic treatment with AAS resulted in an increase of CRF mRNA in the CeA and of CRF peptide in processes in the BnST. Taken together, these data are consistent with a mechanism by which AAS promote an anxiety-like state by enhancing GABAergic inhibition in the CeA to BnST pathway via CRF-R1-dependent increases in presynaptic release of GABA – where the increased GABAergic tone could arise from the CeA afferents themselves or from nearby terminals of interneurons within the BnST [24,25].
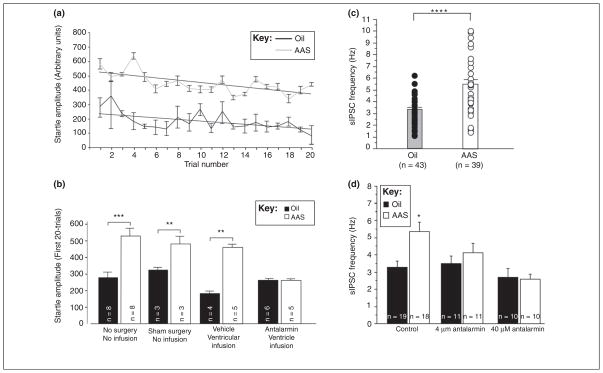
Figure 4.
AAS enhances anxiety and GABAergic transmission in the BnST by a CRF-dependent mechanism. (a) Data demonstrating that the amplitudes of the acoustic startle response were significantly greater on all trials in animals receiving a chronic mixture of three commonly used AAS (testosterone cypionate, nandrolone decanoate and methandrostenolone; 7.5 mg/kg/day) than in control animals receiving a comparable volume of sesame oil (vehicle) (n = 8 in each group) [25]. (b) Mean startle amplitudes over the first 20 trails for oil- and AAS-treated animals that received no infusions or surgery, sham surgery, ventricular infusion of saline, or ventricular infusion of the CRF-R1 antagonist, antalarmin. n values represent the number of animals per group/condition (**P <0.01; ***P <0.001) [25]. (c) sIPSC frequencies from individual dorsolateral BnST neurons from oil-injected (black circles) and AAS-treated (white circles) mice. Histograms display the same data as means with error bars (SEM); n values represent the number of cells (****P < 0.0001). (d) Average sIPSC frequency recorded from neurons in the dorsolateral BnST in saline alone (control) or in the presence of 4 or 40 uM of antalarmin. *The frequency of sIPSCs in these neurons of the dorsolateral BnST from AAS-treated animals under control (saline) recording conditions was significantly (P <0.05) different than the frequency recorded from neurons of oil-injected mice; n values represent the number of cells. Acute application of the CRF-R1 antagonist, antalarmin, abrogated the difference in sIPSC frequency in dorsolateral BnST neurons between oil- and AAS-treated mice [25]. Reproduced, with permission, from [25].
AAS effects on the underlying neural architecture of aggression
Although the neurophysiological interface has not been fully assessed in relating molecular changes to behavior in studies of aggression, a substantive body of literature demonstrates that chronic exposure to AAS alters the expression of molecules known to regulate the expression of aggression in those brain regions that subserve this behavior. For example, in many species the lateral hypothalamic area has been demonstrated to be important in attack behaviors [54] (Figure 2). Chronic AAS treatment of male hamsters during adolescence elicits significant changes in serotonergic, dopaminergic and glutamatergic signaling [10,55–57] in a network of regions that includes this part of the mediobasal hypothalamus, as well as its connections to other limbic structures termed the latero-anterior hypothalamus [10,56]. The importance of 5-HT in contributing to AAS-induced aggression is supported by work demonstrating decreased 5-HT1A receptor (5-HT1A R) and 5-HT1B Rs immunoreactivity in the latero-anterior hypothalamus of AAS-treated hamsters [58,59], but increased 5-HT2A R immunoreactivity in these same regions [60] (Figure 2). Interestingly, a shift in the balance of serotonergic actions from inhibition (5-HT1A Rs) to excitation (5-HT2A R) is hypothesized to be a major contributor to pathological anxiety states [61]. Similar AAS-induced shifts in the hypothalamus may also contribute to increased aggression. As has been shown with anxiety [61], the relationship between 5-HT and aggression, as it relates to AAS-initiated actions, is complex and may be highly state-dependent. For example, whereas depletion of 5-HT led to increased levels of aggression in AAS-treated rats [62,63], AAS treatment increased 5-HT levels in the frontal cortex in rats [63]. Moreover, the timing of changes in aggressive behaviors did not correlate with changes in expression of 5-HT, but did with another key regulator of aggression, arginine vasopressin (AVP) [64].
The fact that AAS treatment can alter the levels of AVP, as well as of substance P [65], another peptide known to play an important role in the expression of aggression, begs caution with respect to attributing behavioral actions based on histochemical correlations alone. The interplay between neurons in the hypothalamus expressing neuromodulators such as AVP and 5-HT neurons in the dorsal raphe have been postulated to converge on a single population of neurons that facilitates aggression, although the presumptive identity of these neurons is not known [10], and no functional studies have been performed to test this mechanism. Furthermore, the ability of the AAS to alter the biosynthesis of endogenous neurosteroids, such as allopregnanolone [66–68], must not be overlooked when searching for molecular correlates of AAS-induced changes in anxiety and aggression. In fact, for both these behaviors, the changes imposed by AAS are likely to reflect an integration of a myriad of effects on classical neurotransmitter systems and their modulators.
Mechanisms of AAS signaling
Steroid receptor signaling
The mechanisms by which chronic AAS exposure modifies the expression of anxiety and aggressive behaviors are only beginning to be uncovered, but any discussion of AAS action in the brain must begin with steroid receptors. All AAS bind to ARs, albeit with significantly different activities. AAS can also be aromatized to estrogens with the capability to interact with both ERα and ERβ AAS can also signal through ERα and ERβ and the progesterone receptor without metabolic conversion [11]. The regions of the mammalian forebrain that regulate anxiety and aggression are rich in the expression of these steroid receptors and the enzymes that both synthesize and metabolize steroids [68–72]. It is likely that AAS impart their effects through interactions with all of these receptors, as well as by altering the activity of steroid-metabolizing enzymes.
The importance of AR and ER signaling on the expression of aggression has been demonstrated through several elegant experiments using knockout mice [73–75] and naturally occurring AR-deficient (tfm) mice [76]. These studies suggest that both AR-dependent and AR-independent signaling are important in the ability of AAS to increase aggression in mice. AAS can also have important effects by indirectly altering signaling via ER by their ability to allosterically inhibit aromatase, thus limiting the availability of endogenous estrogens[77]. Furthermore, they may havekey non-receptor-mediated effects on both aggression and anxiety through their ability to decrease the biosynthesis of endogenous neurosteroids [66,68,77].
Allosteric modulation of GABAA receptors (GABAARs)
Although the chronic use of supratherapeutic levels of AAS is most commonly associated with negative behavioral outcomes, including decreased libido, increased aggression, irritability, anxiety, and impulsivity [2,6,8,12,15,16,78], humans who use AAS, both therapeutically and illicitly, also report positive mood symptoms, including increased libido, increased self-confidence, diminished anxiety, a sense of euphoria, and hypomania [15,16,78]. Increases in anxiety and aggression are evident after chronic exposure to AAS, and these effects are likely to arise from genomic mechanisms. However, the positive effects of AAS in diminishing anxiety may arise from more rapid, non-genomic mechanisms. Indeed, significant, rapid anxiolytic effects (within 30 min) have been reported for adult male rats and mice given acute doses of testosterone or androgenic neurosteroids [79,80], and these actions could be antagonized by concomitant administration of bicuculline or picrotoxin [80], implicating an allosteric action of these androgens at the GABAAR. As recently summarized [81], GABAAR activation is the key mediator in regulating the balance of anxiolytic and anxiogenic tone.
The native GABAAR is a pentameric ionotropic transmembrane protein for which sixteen different receptor subunit genes (α1–α6, β1–β3, γ1–γ3, δ, ε, π, and θ) have been identified in mammals [82]. Receptors containing the α2 subunit are highly expressed in the extended amygdala and are implicated as being the key regulators of the expression of anxiety [81]. The AAS, 17α-methyltestosterone, was shown to augment currents through α2-containing receptors elicited by GABA under synapse-like conditions, suggesting direct allosteric modulation of α2-containing receptors in the extended amygdala may promote acute anxiolytic actions [82]. Although these studies are consistent with a putative mechanism by which acute AAS administration promotes transient positive mood symptoms, perhaps through enhancement of GABAAR function, behavioral studies have failed to observe decreases in anxiety-like behaviors on the acoustic startle response in adult female mice given a single injection of a mixture of testosterone cypionate, methandrostenolone and nandrolone decanoate [25]. The disparity in these studies may reflect a sex-specific difference, or an inherent difference in allosteric modulation induced solely by naturally occurring neurosteroids, such as 5α-androstane-3α,17β-diol (3α-diol) [79,80] and the chemically modified, synthetic AAS [82].
Concluding remarks
Illicit use of AAS is associated with behavioral effects that promote social dysfunction, depression, and an increased incidence of abuse of other addictive substances. Despite these reports from human subjects, steroid users dismiss behavioral effects of these drugs (‘Anabolic androgenic steroids can increase aggression, but they cannot alter your mind’; http://www.steroid.com), in part because mechanistic results from animal studies have not been sufficiently disseminated. Work summarized here demonstrates that the AAS have significant effects in rodents on the neural circuits that underlie the expression of anxiety and aggression. It is interesting to speculate that AAS effects on aggression and anxiety are intertwined because they involve overlapping neural systems (Figure 2; Box 2). Thus, enhanced generalized anxiety and irritability may contribute to increased levels of aggression and the expression of poor social behaviors observed in steroid users.
Intriguingly, data from animal models also demonstrate a convergence in behavioral and neurophysiological effects of AAS and other illicit drugs whose use is known to covary with AAS [83]. For example, both AAS [25] and ethanol [84,85] impose significant effects on CRF-dependent modulation of GABAergic transmission in the extended amygdala, raising the possibility that AAS use may predispose individuals to alcohol abuse by altering the sensitivity of these circuits to the effects of alcohol. Similarly, AAS effects on dopaminergic transmission have been shown to alter the rewarding properties of cocaine [86]. These data highlight that the neuropsychological complications associated with illicit AAS use should not be considered solely in isolation, but also as a crucial factor in the treatment and counseling for other abused drugs.
Acknowledgments
The authors’ work was supported by the National Institutes of Health (grants DA022716 and DA014137 to L.P.H. and training grant DK07508 to J.G.O.).
References
- 1.Clark AS, Henderson LP. Behavioral and physiological responses to anabolic-androgenic steroids. Neurosci Biobehav Rev. 2003;27:413–436. doi: 10.1016/s0149-7634(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 2.Kanayama G, et al. Illicit anabolic–androgenic steroid use. Horm Behav. 2010;58:111–121. doi: 10.1016/j.yhbeh.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.vandenBerg P, et al. Steroid use among adolescents: longitudinal findings from Project EAT. Pediatrics. 2007;119:476–486. doi: 10.1542/peds.2006-2529. [DOI] [PubMed] [Google Scholar]
- 4.Johnston LD, et al. Monitoring the Future National Survey Results on Drug Use, 1975–2008, Secondary School Students. I. National Institute on Drug Abuse; 2009. [Google Scholar]
- 5.Nilsson S, et al. Trends in the misuse of androgenic anabolic steroids among boys 16–17 years old in a primary health care area in Sweden. Scand J Prim Health Care. 2001;19:181–182. doi: 10.1080/028134301316982423. [DOI] [PubMed] [Google Scholar]
- 6.Pagonis TA, et al. Psychiatric side effects induced by supraphysiological doses of combinations of anabolic steroids correlate to the severity of abuse. Eur Psychiatry. 2006;21:551–562. doi: 10.1016/j.eurpsy.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Llewellyn W. Anabolics 2007: Anabolic Steroids Reference Manual. Body of Science. 2007:47–69. [Google Scholar]
- 8.Kanayama G, et al. Long-term psychiatric and medical consequences of anabolic-androgenic steroid abuse: a looming public health concern? Drug Alcohol Depend. 2008;98:1–12. doi: 10.1016/j.drugalcdep.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trenton AJ, Currier GW. Behavioural manifestations of anabolic steroid use. CNS Drugs. 2005;19:571–595. doi: 10.2165/00023210-200519070-00002. [DOI] [PubMed] [Google Scholar]
- 10.Melloni RH, Jr, Ricci LA. Adolescent exposure to anabolic/androgenic steroids and the neurobiology of offensive aggression: a hypothalamic neural model based on findings in pubertal Syrian hamsters. Horm Behav. 2010;58:177–191. doi: 10.1016/j.yhbeh.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Oberlander JG, et al. Anabolic androgenic steroid abuse: multiple mechanisms of regulation of GABAergic synapses in neuroendocrine control regions of the rodent forebrain. J Neuroendocrinol. 2012;24:202–214. doi: 10.1111/j.1365-2826.2011.02151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall RC, Chapman MJ. Psychiatric complications of anabolic steroid abuse. Psychosomatics. 2005;46:285–290. doi: 10.1176/appi.psy.46.4.285. [DOI] [PubMed] [Google Scholar]
- 13.Rohman L. The relationship between anabolic androgenic steroids and muscle dysmorphia: a review. Eat Disord. 2009;17:187–199. doi: 10.1080/10640260902848477. [DOI] [PubMed] [Google Scholar]
- 14.Thiblin I, Petersson A. Pharmacoepidemiology of anabolic androgenic steroids: a review. Fundam Clin Pharmacol. 2005;19:27–44. doi: 10.1111/j.1472-8206.2004.00298.x. [DOI] [PubMed] [Google Scholar]
- 15.Pope HG, Jr, et al. Effects of supraphysiologic doses of testosterone on mood and aggression in normal men: a randomized controlled trial. Arch Gen Psychiatry. 2000;57:133–140. doi: 10.1001/archpsyc.57.2.133. [DOI] [PubMed] [Google Scholar]
- 16.Su TP, et al. Neuropsychiatric effects of anabolic steroids in male normal volunteers. JAMA. 1993;269:2760–2764. [PubMed] [Google Scholar]
- 17.Irving LM, et al. Steroid use among adolescents: findings from Project EAT. J Adolesc Health. 2002;30:243–252. doi: 10.1016/s1054-139x(01)00414-1. [DOI] [PubMed] [Google Scholar]
- 18.Elliot DL, et al. Cross-sectional study of female students reporting anabolic steroid use. Arch Pediatr Adolesc Med. 2007;161:572–577. doi: 10.1001/archpedi.161.6.572. [DOI] [PubMed] [Google Scholar]
- 19.Crawley JN. What’s Wrong With My Mouse? Behavioral Phenotyping of Transgenic and Knockout Mice. Wiley-Liss; 2000. [Google Scholar]
- 20.Agren G, et al. Behavioural anxiolytic effects of low-dose anabolic androgenic steroid treatment in rats. Physiol Behav. 1999;66:503–509. doi: 10.1016/s0031-9384(98)00323-0. [DOI] [PubMed] [Google Scholar]
- 21.Rocha VM, et al. Influence of anabolic steroid on anxiety levels in sedentary male rats. Stress. 2007;10:326–331. doi: 10.1080/10253890701281344. [DOI] [PubMed] [Google Scholar]
- 22.Ambar G, Chiavegatto S. Anabolic–androgenic steroid treatment induces behavioral disinhibition and downregulation of serotonin receptor messenger RNA in the prefrontal cortex and amygdala of male mice. Genes Brain Behav. 2009;8:161–173. doi: 10.1111/j.1601-183X.2008.00458.x. [DOI] [PubMed] [Google Scholar]
- 23.Barreto-Estrada JL, et al. Modulation of affect after chronic exposure to the anabolic steroid 17α-methyltestosterone in adult mice. Behav Neurosci. 2004;118:1071–1079. doi: 10.1037/0735-7044.118.5.1071. [DOI] [PubMed] [Google Scholar]
- 24.Costine BA, et al. Chronic anabolic androgenic steroid exposure alters corticotropin releasing factor expression and anxiety-like behaviors in the female mouse. Psychoneuroendocrinology. 2010;35:1473–1485. doi: 10.1016/j.psyneuen.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oberlander JG, Henderson LP. Corticotropin releasing factor modulation of forebrain GABAergic transmission plays a pivotal role in the expression of anabolic steroid induced anxiety in the female mouse. Neuropyschopharmacology. 2012 doi: 10.1038/npp.2011.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agis-Balboa RC, et al. Enhanced fear responses in mice treated with anabolic androgenic steroids. Neuroreport. 2009;20:617–621. doi: 10.1097/WNR.0b013e32832a2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hotta M, et al. Corticotropin-releasing factor receptor type 1 mediates emotional stress-induced inhibition of food intake and behavioral changes in rats. Brain Res. 1999;823:221–225. doi: 10.1016/s0006-8993(99)01177-4. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham RL, McGinnis MY. Factors influencing aggression toward females by male rats exposed to anabolic androgenic steroids during puberty. Horm Behav. 2007;51:135–141. doi: 10.1016/j.yhbeh.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 29.McGinnis MY, et al. Physical provocation potentiates aggression in male rats receiving anabolic androgenic steroids. Horm Behav. 2002;41:101–110. doi: 10.1006/hbeh.2001.1742. [DOI] [PubMed] [Google Scholar]
- 30.Breuer ME, et al. Aggression in male rats receiving anabolic androgenic steroids: effects of social and environmental provocation. Horm Behav. 2001;40:409–418. doi: 10.1006/hbeh.2001.1706. [DOI] [PubMed] [Google Scholar]
- 31.Farrell SF, McGinnis MY. Effects of pubertal anabolic-androgenic steroid (AAS) administration on reproductive and aggressive behaviors in male rats. Behav Neurosci. 2003;117:904–911. doi: 10.1037/0735-7044.117.5.904. [DOI] [PubMed] [Google Scholar]
- 32.Wesson DW, McGinnis MY. Stacking anabolic androgenic steroids (AAS) during puberty in rats: a neuroendocrine and behavioral assessment. Pharmacol Biochem Behav. 2006;83:410–419. doi: 10.1016/j.pbb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Whitney AC, Clark AS. Effects of acute stanozolol treatment on puberty in female rats. Biol Reprod. 2001;64:1460–1465. doi: 10.1095/biolreprod64.5.1460. [DOI] [PubMed] [Google Scholar]
- 34.Farrell SF, McGinnis MY. Long-term effects of pubertal anabolic-androgenic steroid exposure on reproductive and aggressive behaviors in male rats. Horm Behav. 2004;46:193–203. doi: 10.1016/j.yhbeh.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Grimes JM, Melloni RH., Jr Prolonged alterations in the serotonin neural system following the cessation of adolescent anabolic-androgenic steroid exposure in hamsters (Mesocricetus auratus) Behav Neurosci. 2006;120:1242–1251. doi: 10.1037/0735-7044.120.6.1242. [DOI] [PubMed] [Google Scholar]
- 36.Carrillo M, et al. Developmental and withdrawal effects of adolescent AAS exposure on the glutamatergic system in hamsters. Behav Neurosci. 2011;125:452–464. doi: 10.1037/a0023475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato SM, et al. Adolescents and androgens, receptors and rewards. Horm Behav. 2008;53:647–658. doi: 10.1016/j.yhbeh.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salas-Ramirez KY, et al. Anabolic androgenic steroids differentially affect social behaviors in adolescent and adult male Syrian hamsters. Horm Behav. 2008;53:378–385. doi: 10.1016/j.yhbeh.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salas-Ramirez KY, et al. Anabolic steroids have long-lasting effects on male social behaviors. Behav Brain Res. 2010;208:328–335. doi: 10.1016/j.bbr.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gray M, et al. A comparison of two repeated restraint stress paradigms on hypothalamic-pituitary-adrenal axis habituation, gonadal status and central neuropeptide expression in adult male rats. J Neuroendocrinol. 2010;22:92–101. doi: 10.1111/j.1365-2826.2009.01941.x. [DOI] [PubMed] [Google Scholar]
- 41.Matrisciano F, et al. Repeated anabolic androgenic steroid treatment causes antidepressant-reversible alterations of the hypothalamic–pituitary–adrenal axis, BDNF levels and behavior. Neuropharmacology. 2010;58:1078–1084. doi: 10.1016/j.neuropharm.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 42.Johansson P, et al. Anabolic androgenic steroids affects alcohol intake, defensive behaviors and brain opioid peptides in the rat. Pharmacol Biochem Behav. 2000;67:271–279. doi: 10.1016/s0091-3057(00)00365-8. [DOI] [PubMed] [Google Scholar]
- 43.Alheid GF. Extended amygdala and basal forebrain. Ann N Y Acad Sci. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- 44.Davis M, et al. Phasic vs sustained fear in rats and humans: role of theextended amygdala infear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Asan E, et al. The corticotropin-releasing factor (CRF)-system and monoaminergic afferents in the central amygdala: investigations in different mouse strains and comparison with the rat. Neuroscience. 2005;131:953–967. doi: 10.1016/j.neuroscience.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 46.Kash TL, Winder DG. Neuropeptide Y and corticotropin-releasing factor bi-directionally modulate inhibitory synaptic transmission in the bed nucleus of the stria terminalis. Neuropharmacology. 2006;51:1013–1022. doi: 10.1016/j.neuropharm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 47.Hammack SE, et al. Roles for pituitary adenylate cyclase-activating peptide (PACAP) expression and signaling in the bed nucleus of the stria terminalis (BNST) in mediating the behavioral consequences of chronic stress. J Mol Neurosci. 2010;42:327–340. doi: 10.1007/s12031-010-9364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Green MK, et al. Roles of the bed nucleus of stria terminalis and of the amygdala in N/OFQ-mediated anxiety and HPA axis activation. Neuropeptides. 2007;41:399–410. doi: 10.1016/j.npep.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 49.Sink KS, et al. Calcitonin gene-related peptide in the bed nucleus of the stria terminalis produces an anxiety-like pattern of behavior and increases neural activation in anxiety-related structures. J Neurosci. 2011;31:1802–1810. doi: 10.1523/JNEUROSCI.5274-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kash TL, et al. Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1-dependent process. J Neurosci. 2008;28:13856–13865. doi: 10.1523/JNEUROSCI.4715-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McElligott ZA, Winder DG. Modulation of glutamatergic synaptic transmission in the bed nucleus of the stria terminalis. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1329–1335. doi: 10.1016/j.pnpbp.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spannuth BM, et al. Investigation of a central nucleus of the amygdala/dorsal raphe nucleus serotonergic circuit implicated in fear-potentiated startle. Neuroscience. 2011;179:104–119. doi: 10.1016/j.neuroscience.2011.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tasan RO, et al. Altered GABA transmission in a mouse model of increased trait anxiety. Neuroscience. 2011;183:71–80. doi: 10.1016/j.neuroscience.2011.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toth M, et al. Neural inputs of the hypothalamic ‘aggression area’ in the rat. Behav Brain Res. 2010;215:7–20. doi: 10.1016/j.bbr.2010.05.050. [DOI] [PubMed] [Google Scholar]
- 55.Schwartzer JJ, et al. Interactions between the dopaminergic and GABAergic neural systems in the lateral anterior hypothalamus of aggressive AAS-treated hamsters. Behav Brain Res. 2009;203:15–22. doi: 10.1016/j.bbr.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 56.Carrillo M, et al. Glutamate and the aggression neural circuit in adolescent anabolic steroid-treated Syrian hamsters (Mesocricetus auratus) Behav Neurosci. 2011;125:753–763. doi: 10.1037/a0025127. [DOI] [PubMed] [Google Scholar]
- 57.Carrillo M, et al. The effect of increased serotonergic neurotransmission on aggression: a critical meta-analytical review of preclinical studies. Psychopharmacology (Berl) 2009;205:349–368. doi: 10.1007/s00213-009-1543-2. [DOI] [PubMed] [Google Scholar]
- 58.Grimes JM, Melloni RH., Jr Serotonin-1B receptor activity and expression modulate the aggression-stimulating effects of adolescent anabolic steroid exposure in hamsters. Behav Neurosci. 2005;119:1184–1194. doi: 10.1037/0735-7044.119.5.1184. [DOI] [PubMed] [Google Scholar]
- 59.Ricci LA, et al. Serotonin-1A receptor activity and expression modulate adolescent anabolic/androgenic steroid-induced aggression in hamsters. Pharmacol Biochem Behav. 2006;85:1–11. doi: 10.1016/j.pbb.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 60.Schwartzer JJ, et al. Adolescent anabolic-androgenic steroid exposure alters lateral anterior hypothalamic serotonin-2A receptors in aggressive male hamsters. Behav Brain Res. 2009;199:257–262. doi: 10.1016/j.bbr.2008.11.048. [DOI] [PubMed] [Google Scholar]
- 61.Hammack SE, et al. The response of neurons in the bed nucleus of the stria terminalis to serotonin: implications for anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1309–1320. doi: 10.1016/j.pnpbp.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keleta YB, et al. Behavioral effects of pubertal anabolic androgenic steroid exposure in male rats with low serotonin. Brain Res. 2007;1132:129–138. doi: 10.1016/j.brainres.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 63.Kubala KH, et al. The effects of an anabolic androgenic steroid and low serotonin on social and non-social behaviors in male rats. Brain Res. 2008;1232:21–29. doi: 10.1016/j.brainres.2008.07.065. [DOI] [PubMed] [Google Scholar]
- 64.Grimes JM, et al. Alterations in anterior hypothalamic vasopressin, but not serotonin, correlate with the temporal onset of aggressive behavior during adolescent anabolic–androgenic steroid exposure in hamsters (Mesocricetus auratus) Behav Neurosci. 2007;121:941–948. doi: 10.1037/0735-7044.121.5.941. [DOI] [PubMed] [Google Scholar]
- 65.Hallberg M, et al. Anabolic-androgenic steroids affect the content of substance P and substance P1–7 in the rat brain. Peptides. 2000;21:845–852. doi: 10.1016/s0196-9781(00)00218-7. [DOI] [PubMed] [Google Scholar]
- 66.Pinna G, et al. Changes in brain testosterone and allopregnanolone biosynthesis elicit aggressive behavior. Proc Natl Acad Sci USA. 2005;102:2135–2140. doi: 10.1073/pnas.0409643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Agis-Balboa RC, et al. Down-regulation of neurosteroid biosynthesis in corticolimbic circuits mediates social isolation-induced behavior in mice. Proc Natl Acad Sci USA. 2007;104:18736–18741. doi: 10.1073/pnas.0709419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pinna G, et al. Neurosteroid biosynthesis regulates sexually dimorphic fear and aggressive behavior in mice. Neurochem Res. 2008;33:1990–2007. doi: 10.1007/s11064-008-9718-5. [DOI] [PubMed] [Google Scholar]
- 69.Simerly RB, et al. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 70.Ostlund H, et al. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Ann N Y Acad Sci. 2003;1007:54–63. doi: 10.1196/annals.1286.006. [DOI] [PubMed] [Google Scholar]
- 71.Scordalakes EM, Rissman EF. Aggression and arginine vasopressin immunoreactivity regulation by androgen receptor and estrogen receptor α. Genes Brain Behav. 2004;3:20–26. doi: 10.1111/j.1601-183x.2004.00036.x. [DOI] [PubMed] [Google Scholar]
- 72.Tsuruo Y. Topography and function of androgen-metabolizing enzymes in the central nervous system. Anat Sci Int. 2005;80:1–11. doi: 10.1111/j.1447-073x.2005.00098.x. [DOI] [PubMed] [Google Scholar]
- 73.Scordalakes EM, Rissman EF. Aggression in male mice lacking functional estrogen receptor α. Behav Neurosci. 2003;117:38–45. [PubMed] [Google Scholar]
- 74.Sato T, et al. Brain masculinization requires androgen receptor function. Proc Natl Acad Sci USA. 2004;101:1673–1678. doi: 10.1073/pnas.0305303101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Choleris E, et al. Involvement of estrogen receptor α, β and oxytocin in social discrimination: a detailed behavioral analysis with knockout female mice. Genes Brain Behav. 2006;5:528–539. doi: 10.1111/j.1601-183X.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- 76.Robinson S, et al. The role of the androgen receptor in anabolic androgenic steroid-induced aggressive behavior in C57BL/6J and Tfm mice. Horm Behav. 2012;61:67–75. doi: 10.1016/j.yhbeh.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 77.Penatti CA, et al. Chronic exposure to anabolic androgenic steroids alters neuronal function in the mammalian forebrain via androgen receptor- and estrogen receptor-mediated mechanisms. J Neurosci. 2009;29:12484–12496. doi: 10.1523/JNEUROSCI.3108-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bahrke MS, et al. Psychological and behavioural effects of endogenous testosterone and anabolic-androgenic steroids. An update. Sports Med. 1996;22:367–390. doi: 10.2165/00007256-199622060-00005. [DOI] [PubMed] [Google Scholar]
- 79.Bing O, et al. High doses of testosterone increase anticonflict behaviour in rat. Eur Neuropsychopharmacol. 1998;8:321–323. doi: 10.1016/s0924-977x(97)00095-3. [DOI] [PubMed] [Google Scholar]
- 80.Aikey JL, et al. Testosterone rapidly reduces anxiety in male house mice (Mus musculus) Horm Behav. 2002;42:448–460. doi: 10.1006/hbeh.2002.1838. [DOI] [PubMed] [Google Scholar]
- 81.Mohler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology. 2012;62:42–53. doi: 10.1016/j.neuropharm.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 82.Henderson LP. Steroid modulation of GABAA receptor-mediated transmission in the hypothalamus: effects on reproductive function. Neuropharmacology. 2007;52:1439–1453. doi: 10.1016/j.neuropharm.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dodge T, Hoagland MF. The use of anabolic androgenic steroids and polypharmacy: a review of the literature. Drug Alcohol Depend. 2011;114:100–109. doi: 10.1016/j.drugalcdep.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roberto M, et al. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roberto M, et al. Corticotropin releasing factor-induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010;67:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kailanto S, et al. Subchronic steroid administration induces long lasting changes in neurochemical and behavioral response to cocaine in rats. Steroids. 2011;76:1310–1316. doi: 10.1016/j.steroids.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 87.Gregg TR, Siegel A. Brain structures and neurotransmitters regulating aggression in cats: implications for human aggression. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:91–140. doi: 10.1016/s0278-5846(00)00150-0. [DOI] [PubMed] [Google Scholar]
- 88.Canteras NS, et al. Neuroanatomy of anxiety. Curr Top Behav Neurosci. 2010;2:77–96. doi: 10.1007/7854_2009_7. [DOI] [PubMed] [Google Scholar]
- 89.Takahashi A, et al. Behavioral and pharmacogenetics of aggressive behavior. Curr Top Behav Neurosci. 2012 doi: 10.1007/7854_2011_1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hammack SE, et al. Overlapping neurobiology of learned helplessness and conditioned defeat: Implications for PTSD and mood disorders. Neuropharmacology. 2012;62:565–575. doi: 10.1016/j.neuropharm.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cheng SY, et al. Neural circuitry of play fighting in golden hamsters. Neuroscience. 2008;156:247–256. doi: 10.1016/j.neuroscience.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 92.Reynolds SM, Berridge KC. Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J Neurosci. 2001;21:3261–3270. doi: 10.1523/JNEUROSCI.21-09-03261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kuhn S, et al. Structural correlates of trait anxiety: reduced thickness in medial orbitofrontal cortex accompanied by volume increase in nucleus accumbens. J Affect Disord. 2011;134:315–319. doi: 10.1016/j.jad.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 94.Chen YW, et al. Corticotropin-releasing factor in the nucleus accumbens shell induces swim depression, anxiety, and anhedonia along with changes in local dopamine/acetylcholine balance. Neuroscience. 2012;206:155–166. doi: 10.1016/j.neuroscience.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 95.Tye KM, et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haller J, et al. The activation of raphe serotonergic neurons in normal and hypoarousal-driven aggression: a double labeling study in rats. Behav Brain Res. 2005;161:88–94. doi: 10.1016/j.bbr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 97.Veenema AH, Neumann ID. Central vasopressin and oxytocin release: regulation of complex social behaviours. Prog Brain Res. 2008;170:261–276. doi: 10.1016/S0079-6123(08)00422-6. [DOI] [PubMed] [Google Scholar]
- 98.Lin D, et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–226. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Veenema AH, et al. Low inborn anxiety correlates with high intermale aggression: link to ACTH response and neuronal activation of the hypothalamic paraventricular nucleus. Horm Behav. 2007;51:11–19. doi: 10.1016/j.yhbeh.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 100.Funk CK, Koob GF. A CRF(2) agonist administered into the central nucleus of the amygdala decreases ethanol self-administration in ethanol-dependent rats. Brain Res. 2007;1155:172–178. doi: 10.1016/j.brainres.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang MM, et al. Corticotropin-releasing factor (CRF) sensitization of ethanol withdrawal-induced anxiety-like behavior is brain site specific and mediated by CRF-1 receptors: relation to stress-induced sensitization. J Pharmacol Exp Ther. 2010;332:298–307. doi: 10.1124/jpet.109.159186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Knapp DJ, et al. Effects of a stressor and corticotrophin releasing factor on ethanol deprivation-induced ethanol intake and anxiety-like behavior in alcohol-preferring P rats. Psychopharmacology (Berl) 2011;218:179–189. doi: 10.1007/s00213-011-2366-5. [DOI] [PubMed] [Google Scholar]
- 103.Francesconi W, et al. Protracted withdrawal from alcohol and drugs of abuse impairs long-term potentiation of intrinsic excitability in the juxtacapsular bed nucleus of the stria terminalis. J Neurosci. 2009;29:5389–5401. doi: 10.1523/JNEUROSCI.5129-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kash TL, et al. Alcohol exposure alters NMDAR function in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2009;34:2420–2429. doi: 10.1038/npp.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Conrad KL, Winder DG. Altered anxiety-like behavior and long-term potentiation in the bed nucleus of the stria terminalis in adult mice exposed to chronic social isolation, unpredictable stress, and ethanol beginning in adolescence. Alcohol. 2011;45:585–593. doi: 10.1016/j.alcohol.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pistis M, et al. Effects of acute, chronic ethanol and withdrawal on dorsal raphe neurons: electrophysiological studies. Neuroscience. 1997;79:171–176. doi: 10.1016/s0306-4522(96)00643-4. [DOI] [PubMed] [Google Scholar]
- 107.Takahashi A, et al. GABAA receptors in the dorsal raphe nucleus of mice: escalation of aggression after alcohol consumption. Psychopharmacology (Berl) 2010;211:467–477. doi: 10.1007/s00213-010-1920-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Waselus M, et al. Collateralized dorsal raphe nucleus projections: a mechanism for the integration of diverse functions during stress. J Chem Neuroanat. 2011;41:266–280. doi: 10.1016/j.jchemneu.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guo JD, et al. Bi-directional modulation of bed nucleus of stria terminalis neurons by 5-HT: molecular expression and functional properties of excitatory 5-HT receptor subtypes. Neuroscience. 2009;164:1776–1793. doi: 10.1016/j.neuroscience.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kurling-Kailanto S, et al. Subchronic nandrolone administration reduces cocaine-induced dopamine and 5-hydroxytryptamine outflow in the rat nucleus accumbens. Psychopharmacology (Berl) 2010;209:271–281. doi: 10.1007/s00213-010-1796-9. [DOI] [PubMed] [Google Scholar]
- 111.Kindlundh AM, et al. The anabolic-androgenic steroid nandrolone induces alterations in the density of serotonergic 5HT1B and 5HT2 receptors in the male rat brain. Neuroscience. 2003;119:113–120. doi: 10.1016/s0306-4522(03)00120-9. [DOI] [PubMed] [Google Scholar]
- 112.Johansson-Steensland P, et al. The anabolic androgenic steroid, nandrolone decanoate, increases the density of Fos-like immunoreactive neurons in limbic regions of guinea-pig brain. Eur J Neurosci. 2002;15:539–544. doi: 10.1046/j.0953-816x.2001.01877.x. [DOI] [PubMed] [Google Scholar]
- 113.Le Greves P, et al. Effects of an anabolic-androgenic steroid on the regulation of the NMDA receptor NR1, NR2A and NR2B subunit mRNAs in brain regions of the male rat. Neurosci Lett. 1997;226:61–64. doi: 10.1016/s0304-3940(97)00244-9. [DOI] [PubMed] [Google Scholar]
- 114.Grimes JM, Melloni RH., Jr Serotonin modulates offensive attack in adolescent anabolic steroid-treated hamsters. Pharmacol Biochem Behav. 2002;73:713–721. doi: 10.1016/s0091-3057(02)00880-8. [DOI] [PubMed] [Google Scholar]
- 115.Feng HJ, Faingold CL. The effects of chronic ethanol administration on amygdala neuronal firing and ethanol withdrawal seizures. Neuropharmacology. 2008;55:648–653. doi: 10.1016/j.neuropharm.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Carlson JN, Drew Stevens KD. Individual differences in ethanol self-administration following withdrawal are associated with asymmetric changes in dopamine and serotonin in the medial prefrontal cortex and amygdala. Alcohol Clin Exp Res. 2006;30:1678–1692. doi: 10.1111/j.1530-0277.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- 117.Overstreet DH, et al. Reduction in repeated ethanol-withdrawal-induced anxiety-like behavior by site-selective injections of 5-HT1A and 5-HT2C ligands. Psychopharmacology (Berl) 2006;187:1–12. doi: 10.1007/s00213-006-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Landgraf R, Wigger A. High vs low anxiety-related behavior rats: an animal model of extremes in trait anxiety. Behav Genet. 2002;32:301–314. doi: 10.1023/a:1020258104318. [DOI] [PubMed] [Google Scholar]
- 119.Lavie CJ, et al. Impact of exercise training on psychological risk factors. Prog Cardiovasc Dis. 2011;53:464–470. doi: 10.1016/j.pcad.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 120.Binder E, et al. Regular voluntary exercise reduces anxiety-related behaviour and impulsiveness in mice. Behav Brain Res. 2004;155:197–206. doi: 10.1016/j.bbr.2004.04.017. [DOI] [PubMed] [Google Scholar]