Abstract
For over a century, biologists have strived to unravel the mechanisms that establish how cells are informed of their position in the embryo and differentiate to give rise to complex organs and structures. However, the historical idea that one predominant mode of ligand transport, largely accounted for by free diffusion, can explain how all signaling molecules, known as morphogens, control tissue patterning has greatly hindered our ability to fully appreciate the complexities driving the delivery and reception of signaling molecules at a distance. In reality, a cell’s shape, morphology, and location change continuously as development progresses. Thus, cellular context poses distinct challenges for morphogen transport in each unique cellular environment. Emerging studies reveal that some cells overcome such obstacles in an unexpected manner: via long, cellular projections, or specialized filopodia, that link distant cells and traffic signaling components. Here, we will review recent findings describing specialized filopodia and discuss the potential mechanisms and implications for filopodia-based long-range cell signaling and communication, particularly within the developing vertebrate embryo.
Introduction
During embryogenesis, all multicellular organisms face the astounding challenge of shaping a small population of seemingly uniform cells into complex, three-dimensional structures that typify the adult. In order to achieve this feat, cells in the embryo use molecular cues to establish their positional identity and, in turn, relay positional information to their neighbors to pattern surrounding tissues. Although we have long understood that signaling molecules, called morphogens, establish such embryonic tissue patterns [1–3], a number of outstanding questions remain. For example, how do signaling molecules travel through intricate embryonic tissues, over impressive distances, and along complex cellular landscapes to reach responding cells? How are embryonic cells able to precisely establish morphogen concentration gradients required to dictate unique cell fates and behaviors?
The simplest, and most historically accepted mode of morphogen movement is free diffusion, whereby a ligand secreted from producing cells travels passively through the extracellular space until arriving at the appropriate target cell [4,5]. A concentration gradient could be generated following the “clearance” of the morphogen at a distance, for example by cellular uptake or degradation, leading to activation of unique transcriptional responses specified by the concentration of ligand to which target cells are exposed [4]. Indeed, it is very likely that a ligand can be secreted from a cell and diffuse away, at least for a certain distance. However, free diffusion may not be an efficient and precise mechanism for the long-range distribution of signaling molecules across tissue and cell types. For a molecule to travel via free diffusion it must be soluble; however, many morphogens, including Hedgehog and Wnts, do not meet this criteria due to complex modifications (e.g. palmitoylation or cholesterol modification) necessary for their signaling capabilities [6–14]. Moreover, in a passive diffusion model, ligand-producing cells themselves exert little direct control over the path of ligand movement and thereby do not directly participate in shaping a signaling gradient over long distances [4,15].
An alternative, cellular-based mechanism for morphogen transport that has garnered recent attention is the direct delivery of ligands via actin-based, cellular extensions or specialized filopodia [4,16]. Early work indicating a potential role for filopodia in developmental cell signaling stemmed from the Kornberg lab during examination of the Drosophila wing disc. Nearly 15 years ago, they described remarkably long, straight cellular extensions, termed “cytonemes”, that orient in the direction of well-established signaling centers and are capable of reaching lengths of up to 700 µm [17]. These studies have been instrumental in shaping the context and conceptual framework for filopodia-based mechanisms for cell signaling. However, direct imaging of these wing disc filopodia in vivo has not been achieved. Moreover, preparation of microdissected wing discs for imaging, where even slight compression of tissue may significantly alter the endogenous cellular characteristics of filopodial extensions [18], has limited our understanding of their presence and function, leading to unresolved questions pertaining to a filopodia-based model for in vivo cellular communication. Are such structures a constitutive feature of all cells, and are they present in other organisms? If so, why have they not been observed more frequently? Moreover, can the initial formation of filopodial extensions and their behavior with respect to cell signaling be accurately and precisely visualized in vivo over time? What is the cellular make-up of these structures and how might they differ from typical filopodia that have been well characterized by cell biologists? Recent studies imaging whole embryos have brought new light to these questions, and illuminated the unexpected presence of fine cellular extensions in many diverse cell populations. In particular, recent studies using living vertebrate embryos and performed at high, single-cell and subcellular resolution, have opened new avenues for studying the cell biology of filopodial extensions from individual cells within their endogenous cellular context [19–24]. These studies allow for the direct and dynamic observation of filopodia behavior and cell signaling components (i.e. ligands and receptors), supporting a signaling function for filopodial extensions and leading to an exciting, new challenge to the historical doctrine of how cells communicate with one another over long distances. Here, we will review recent findings describing specialized filopodia, predominately within vertebrate embryos, discuss how these cellular extensions may be used for morphogen transport and cell-to-cell communication during embryonic tissue patterning, and highlight the questions that remain unanswered.
What are specialized filopodia?
Filopodia are cellular extensions composed of parallel bundles of actin filaments that protrude beyond the cell’s edge in a “finger-like” manner [25,26]. These dynamic structures extend and retract from the cell surface with the aid of mechanical forces provided by directed actin polymerization and F-actin retrograde flow [27]. This allows them to explore their extracellular environment, orient themselves based on guidance cues, and adhere to the extracellular matrix to provide a temporary “clutch” that helps propel motile cells forward [28,29]. The presence of filopodia on non-motile cell types suggests that some filopodia may serve specialized functions distinct from a role in cellular locomotion. Indeed, seminal studies nearly fifty years ago identified unique cellular extensions in early sea urchin embryos [30]. These projections, that span the fluid-filled blasotcoel of gastrula-stage embryos, were initially believed to guide migrating cells. Surprisingly, a later study showed that the location and timing of these specialized filopodia were consistent with a role in cell signaling [31], leading to the earliest proposal that one specialized function of filopodia may be long-range directed cell signaling (Fig. 1A). Thanks to recent advances in microscopy and live-imaging techniques, we now know that specialized filopodia are more common than initially perceived. Indeed, filopodia that appear to have specialized signaling functions are being increasingly characterized in many developmental model organisms including a broad-range of vertebrate embryos such as zebrafish, Xenopus, mouse, and chick [19–22,24], suggesting that filopodia may help alleviate some of the limitations for cell-cell communication that arise in complex cellular landscapes during embryonic development.
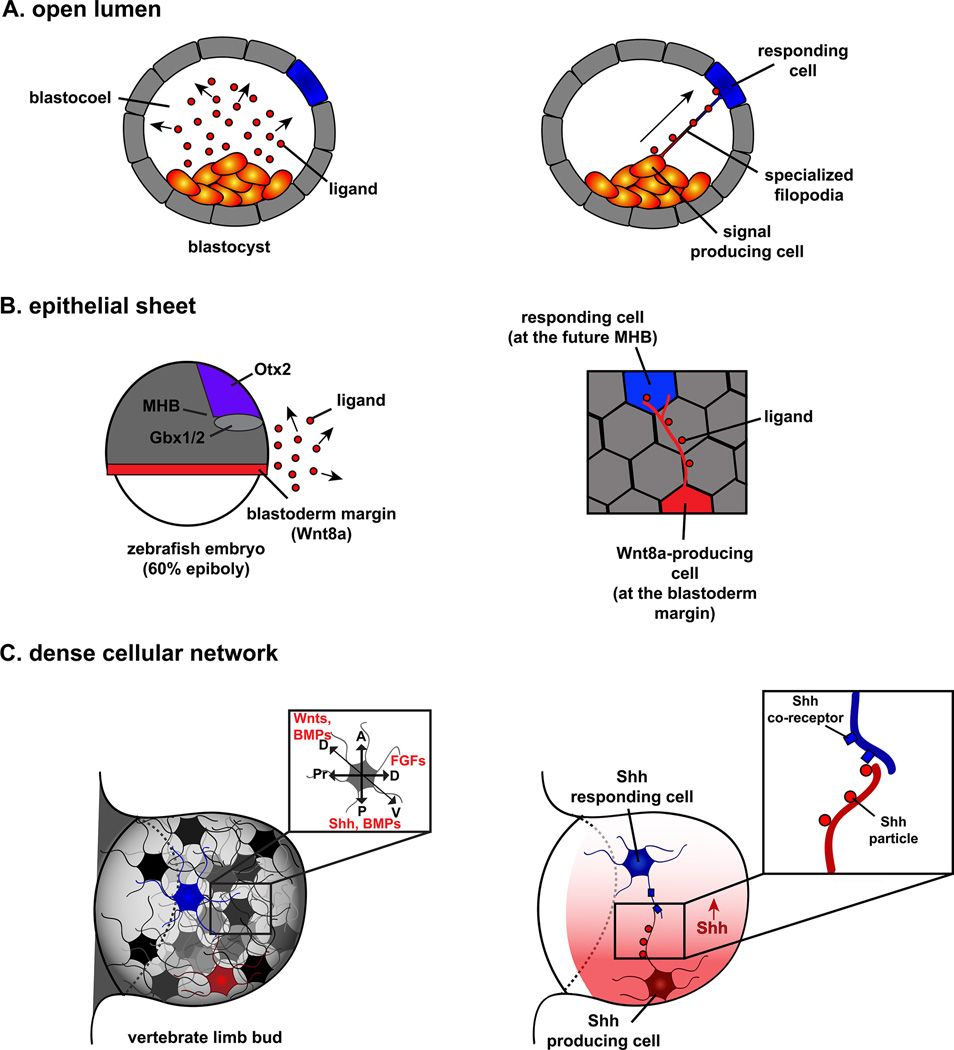
Figure 1. Specialized filopodia allow cells to overcome barriers to morphogen transport that arise from complex cellular landscapes.
(A) In early embryos, cells often become separated by the expansive, fluid-filled blastocoel; however, communication between distant cells is commonly required for proper development. Simple diffusion would result in the unrestricted travel of morphogens (red circles) in many directions, rather than directly towards the intended responding cell (blue; left panel). Specialized filopodia linking distant cells would provide a platform for the direct, long-range transport and delivery of signaling molecules (right panel). (B) In early zebrafish embryos, signals originating from distant organizers, such as Wnt8a present at the blastoderm margin (red line), influence distant cells in order to specify distinct anatomical structures. For example, Wnt8a patterns the future midbrain-hindbrain boundary (MHB), which forms between Otx2 and Gbx1/2 expression domains (left panel). Simple diffusion of a ligand (red circles) cannot explain the directed, lateral distribution of signaling molecules towards distant responding cells in the MHB. However, these epithelial cells extend specialized filopodia, to which Wnt8a ligand is localized (right panel), suggesting that Wnt8a can be directly delivered to responding cells multiple cell diameters away. (C) The developing vertebrate limb consists of a dense network of mesenchymal cells, each containing specialized filopodia extending in many directions (left panel) and individual cells must integrate many different signals (left panel insert), including gradients of Shh, FGF, and Wnt. Shh ligand (red circles, right panel) travels, in the form of distinct particles, along the extracellular surface of filopodia extending from Shh producing cells (red cell), while Shh co-receptors Cdo and Boc (blue rectangles) localize to filopodia of adjacent responding cells (blue cells; right panel and insert). These results suggest that contact-mediated release propagated by specialized filopodia contributes to the delivery of ligands at a distance.
All specialized filopodia described, to date, share a number of structural characteristics with typical filopodia, including their abundance of actin but absence of microtubules, diameter (lower limit ~ 200 nm), and dynamics [17,20,21,31]. However, specialized filopodia are found within diverse cellular contexts and they can show unique morphological differences including considerable differences in length, highly dynamic behaviors, and complex geometries (Fig. 1). The fact that such specialized cellular extensions are difficult to visualize, may not always survive fixation, and have only recently been imaged directly in-vivo has posed further challenges to their characterization. Interestingly, there are currently no unique molecular markers that distinguish specialized filopodia from typical filopodia, suggesting that their formation and structural characteristics are likely similar, albeit with certain specialized cytoskeletal properties. For example, the localization pattern of the actin-depolymerizing factor cofilin along specialized filopodia in the chick limb bud is very striking [21]. Moreover, the distal tips of these filopodia are not readily labeled by specific F-actin probes that do not bind to highly modified actin, such as LifeAct, which suggests distinguishing features [21]. Furthermore, it is yet unclear whether specialized filopodia are dedicated to cell signaling, or whether they may serve other non-exclusive roles in cell movement, adhesion or mechanical functions. For example, specialized filopodia have recently been observed in early mouse embryos, where they appear to directly impact cell shape changes during compaction in an E-cadherin dependent manner [32]. Meanwhile, migratory cells likely contain filopodia that may play a cell signaling function in addition to “path-finding”. Indeed, migrating cranial neural crest cells in vivo extend a diverse range of cellular processes, including extremely long filopodia that form stable connections with distant cells during long distance travel [33]. For these reasons, in this review, we veer away from giving filopodia unique names (i.e. cytonemes, nanotubes, tunneling nanotubes, invadopodia, cytoplasmic bridges) and instead review the most recent findings pertaining to how filopodia-based transport may impart cells with the ability to direct long-range cellular communication within unique cellular contexts.
A cell’s context imparts distinct limitations on morphogen transport
Throughout morphogenesis, a cell’s shape, location and morphology are ever changing as complex tissues form and gain shape. As a result, embryonic cells encounter unique limitations that restrict cell signaling in different tissue types (Fig. 1). For example, in early embryos, embryonic cells are separated from one another by a fluid-filled cavity called the blastocoel, but communication between cells on opposite sides of the blastocoel is often required for proper development (Fig. 1A; [22,34]). How do signaling molecules travel precisely through a fluid18 filled space that contains no means for directed ligand guidance? Epithelia have a sheet-like organization and relatively “rigid” character leading to a situation where, in order to reach responding cells, ligands must travel laterally over great distances (Fig. 1B; [17,19,24]). In both of these cellular landscapes, free diffusion limits the manner in which a ligand travels, largely in an unrestricted fashion in multiple directions, rather than directed towards the proper responding cells. Moreover, many developmental tissues, such as the vertebrate limb bud [21], are comprised of densely packed cells that must incorporate readouts of many signaling centers, often many cell diameters away (Fig. 1C). Specialized filopodia would provide an important means of overcoming the cell-signaling limitations imposed by cellular context. Consistent with this idea, specialized filopodia that appear to play cell-signaling roles have recently been identified in all of the aforementioned cellular contexts: they have been observed spanning the blastocoel of early sea urchin, mouse and Xenopus embryos [20,30,31,34], in epithelial tissues of Drosophila, spider, zebrafish and mouse embryos [17–19,23,24,35–38] and in mesenchymal cells of the developing mouse and chick limb bud [21]. This suggests that specialized filopodia formation may be intimately tied to cellular context, and that their formation may overcome many of the physical barriers associated with ligand production and reception across specific cellular landscapes.
How do specialized filopodia transport cargo and signaling components?
What are the cellular mechanisms by which cell-signaling components travel along filopodia and thereby within intricate cellular landscapes? Early proposals suggested that signaling molecules could travel through filopodia by direct exchange of cytoplasmic material or via large vesicles [22,30,31]. Subsequent observations in Drosophila directly demonstrated that filopodia can transport signaling components laterally along epithelial sheets. Ligands such as Delta, a membrane-tethered activator of the Notch signaling pathway [18], as well as receptors involved in Decapentaplegic (Dpp), FGF and EGF signal transduction localize to specialized cellular extensions within the Drosophila wing imaginal disc [36,39]. Interestingly, receptors present along cytonemes form distinct puncta that move in both retro- and anterograde directions [36,39]. Recent studies of Drosophila tracheal cytonemes show that a phosphorylated form of Tkv, the Dpp receptor, localizes to cytonemes that extend towards the Dpp signaling center [40], suggesting that Dpp receptors present on filopodia can be activated by Dpp originating from the wing disc signaling center. However, it remains unknown whether and how such receptor activation is transmitted back to the cell soma.
As an exciting extension, recent studies reveal that vertebrate epithelial cells also possess specialized filopodia. For example, neuroepithelial cells in developing mouse embryos extend filopodia when opposing sides of the closing neural tube come into close contact, connecting the adjacent neuroepithelia [23] and epiblast cells of early zebrafish embryos extend very long processes (up to 300 µm) that span over 10 cell diameters [19]. Although these studies have not confirmed the transport of signaling molecules, a very recent study imaging zebrafish presumptive neuroectoderm cells in vivo shows that Wnt8a ligand forms distinct puncta that localize to, and travel along, specialized filopodia [24]. Interestingly, in rare instances, filopodia containing Wnt8a puncta retract, leaving Wnt8a in the vicinity of nearby cells [24], perhaps explaining how signaling molecules might be transferred to nearby responding cells.
The emerging studies in vertebrate embryos suggest that transport of morphogens along filopodial extensions might be a highly dynamic and active process. In further support of this idea, in the chick limb bud, imaging of Shh under native regulatory control, at subcellular resolution shows that Shh is produced in the form of a particle that travels in a net anterograde manner along specialized filopodia of Shh-producing cells to distant responding cells [21]. Interestingly, split-GFP experiments to examine the interaction of Shh particles with the outer or inner leaflet of the filopodial membrane have delineated the likely route of ligand movement along filopodial extensions. These findings strongly suggest that Shh-containing particles travel along the extracellular surface of filopodia, where they are competent to signal [21]. By simultaneously imaging Shh responding cells, the Shh co-receptors Cdo and Boc were found to co-localize to immobile microdomains within specialized filopodia that contact Shh-containing filopodia [21]. This finding highlights a novel mechanism for morphogen transport, by which Shh is secreted from the cell and travels along the outside of filopodia to reach responding cells that harbor Shh co-receptors. This allows for the precise and direct control of morphogen movement towards specific responding cells and establishes a new paradigm for how producing cells may control the formation of signaling gradients as morphogenesis progresses.
Our understanding of this mode of ligand movement along filopodia is still preliminary and a number of exciting questions remain to be answered. The puncta or particles that contain signaling components are poorly characterized and it remains largely unknown how these particles associate with filopodia membranes or whether they harbor clusters of proteins with or without a vesicular membrane. Might these particles contain cellular components in addition to signaling molecules, such as small RNAs or other macromolecular complexes? Moreover, it is intriguing that specific ligands or receptors do not localize to all filopodia extending from an individual cell [21,36], suggesting there may be a “gating” mechanism that controls what proteins enter select subsets of filopodia. Although we currently do not know the molecular players required for transport of particles along filopodia, the study by Sanders et al. suggests that non-conventional myosin motors, such as MyosinX (MyoX), may be involved in directed transport [21]. Future studies should focus on the identity of other myosins present along filopodia in embryonic tissues and quantify their dynamics along filopodial extensions in relation to known signaling molecules. Moreover, it is yet unknown how signaling components present on specialized filopodia at the tip of the cell confer downstream signals to the main cell body. Investigation into the nature of specific points of filopodial contacts will be an exciting area for future exploration.
How might filopodia orientation, length, and selectivity form a precise signaling gradient?
Given that cellular landscapes in the developing embryo are highly complex, the accurate formation and orientation of specialized filopodial extensions would be an essential pre-requisite for proper and directed morphogen transport. Indeed, a recent study has shown that the length and dynamics of specialized filopodia imaged in vivo correlate with the formation of a Hedgehog gradient in the Drosophila abdominal epidermis [38]. Filopodia imaged in isolated wing discs of Drosophila embryos, appear remarkably straight and orientated in the direction of well8 established signaling centers [17]. Indeed, these filopodia appear to grow in the direction of context-dependent guidance cues including Dpp, FGF, and EGF [17,36,41], suggesting that they may respond to, and acquire their directionality from, the source of the ligand itself.
Using live imaging, recent studies in vertebrate embryos have now shed new light on how specialized filopodia may establish their correct length and directionality. These studies have highlighted complex mechanisms that reflect the “history” of cell-to-cell contacts. For example, in the zebrafish epiblast, intercellular connections are formed when epithelial cells divide but maintain membrane tethers with daughter cells despite active cell movements [19]. Likewise, Danilchik et al. showed by live, 3-D confocal imaging within cleavage-stage Xenopus embryos in vivo that dynamic filopodia make stable contacts with nearby cells early during development, prior to blastocoel expansion [20]. Remarkably, these connections, once formed, are maintained through multiple rounds of cleavage despite the strong mechanical forces generated by ongoing cell divisions, eventually leading to long projections that span impressive distances across the blastocoel cavity [20]. This finding suggests that specialized filopodia can form when cells are close together and continue to extend as the embryo grows and cellular landscapes undergo dramatic changes, thus providing a constant means of long-range communication at the advent and subsequently throughout the early steps of morphogenesis.
Although the filopodia of mesenchymal cells in the vertebrate limb bud appear to have some directional bias towards signaling centers, all cells extend filopodia in multiple orientations, suggesting that a single cell may dynamically “probe” the extracellular environment [21]. By visualizing filopodial dynamics at single cell resolution, over extended periods of time in-vivo, it is clear that these extensions are highly dynamic and only a subset form more stabilized, prolonged contacts with distant cells [21]. Even these interactions are not permanent and a cell can retract and re-form new filopodial contacts repeatedly. This may confer to a cell the ability to respond or transmit multiple signals, and further suggests a complex landscape by which specific subsets of filopodia are “specified” for the transport or transmission of explicit signals [21]. Thereby, filopodia-based control of signaling gradients is likely to reflect a highly dynamic and fluid, yet directed process. These innate characteristics may impart signaling cells with the ability of tightly controlling cell signaling, while simultaneously allowing cells to adapt to a changing environment.
Concluding remarks and future perspectives
There is accumulating evidence that, in addition to free diffusion, specialized filopodia constitute a novel, additional, means of overcoming many of the physical barriers arising as morphogenesis causes cellular landscapes to become increasingly complex. Recent studies have highlighted the presence of specialized filopodia in a number of organisms, cell types, and cellular contexts, and begun to probe their dynamics of formation within living vertebrate embryos. These studies also provide additional explanations for certain surprising observations pertaining to the long-range activity of some signaling molecules. For example, wing discs expressing only a membrane-tethered Wingless (Wg), the Drosophila Wnt homolog, develop normally [42]. Although the authors take this finding as evidence that long-range Wg activity is not required for proper wing development, it may also support that Wg can signal over greater distances through an association with the membrane of specialized filopodial. Recent work has greatly increased our understanding for how specialized filopodia drive the direct delivery of signaling molecules to responding cells, however, a number of key questions remain to be answered. First, it is essential to explore the cellular and molecular characteristics of specialized filopodia to provide novel insight into their formation and how they establish precise orientations and lengths within diverse cellular landscapes. This information is crucial, given that specialized filopodia must navigate complex, ever-changing cellular contexts in order to properly establish accurate networks for cell-cell communication. Furthermore, we know little about the molecular players involved in the transport of signaling molecules along specialized filopodia, including control of their speeds and directional movement. Delineating the molecular composition of specialized filopodia will help to answer these questions. Moreover, specific genetic and functional perturbation of these molecules will help to determine their roles in the control of filopodia formation, orientation, or selectivity. Secondly, we do not know how the localization of signaling molecules along filopodia establishes the formation of a concentration gradient. A graded cellular response to a morphogen is central to specifying the distinct cellular behaviors responsible for patterning embryonic tissues. Understanding this process has long been a central focus of developmental biologists, and specialized filopodia provide a novel platform for establishing such gradients while allowing ligand-producing cells to maintain direct control of the directionality, distance, and speed at which signaling molecules travel. The use of live-cell imaging to visualize this dynamic process is essential, and combining recent advances in live-cell imaging techniques with optogenetics could provide further understanding of how the speed at which signaling molecules travel along filopodia, and their subsequent delivery, contribute to the establishment of precise concentration gradients. Together, these ongoing studies suggest that direct communication among cells through finger-like extensions extends the range of possible cell-to-cell interactions. Moving forward, specialized filopodia may reflect a new frontier in control of information among cells during embryonic development, regeneration, and even pathological processes such as cancer metastasis.
Acknowledgements
We thank members of the Barna lab for active discussion and critical review of this manuscript. This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Disease, part of NIH, under award number NIH R21AR062262 (M.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference annotations
* of special interest
** of outstanding interest
- 1.Stumpf HF. Mechanism by which cells estimate their location within the body. Nature. 1966;212:430–431. doi: 10.1038/212430a0. [DOI] [PubMed] [Google Scholar]
- 2.Wolpert L. Positional information and the spatial pattern of cellular differentiation. J Theor Biol. 1969;25:1–47. doi: 10.1016/s0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]
- 3.Rogers KW, Schier AF. Morphogen gradients: from generation to interpretation. Annu Rev Cell Dev Biol. 2011;27:377–407. doi: 10.1146/annurev-cellbio-092910-154148. [DOI] [PubMed] [Google Scholar]
- 4.Muller P, Rogers KW, Yu SR, Brand M, Schier AF. Morphogen transport. Development. 2013;140:1621–1638. doi: 10.1242/dev.083519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu AJ, Scott MP. Incredible journey: how do developmental signals travel through tissue? Genes Dev. 2004;18:2985–2997. doi: 10.1101/gad.1233104. [DOI] [PubMed] [Google Scholar]
- 6.Galli LM, Burrus LW. Differential palmit(e)oylation of Wnt1 on C93 and S224 residues has overlapping and distinct consequences. PLoS One. 2011;6:e26636. doi: 10.1371/journal.pone.0026636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grover VK, Valadez JG, Bowman AB, Cooper MK. Lipid modifications of Sonic hedgehog ligand dictate cellular reception and signal response. PLoS One. 2011;6:e21353. doi: 10.1371/journal.pone.0021353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miura GI, Treisman JE. Lipid modification of secreted signaling proteins. Cell Cycle. 2006;5:1184–1188. doi: 10.4161/cc.5.11.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Resh MD. Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci STKE. 2006;2006:re14. doi: 10.1126/stke.3592006re14. [DOI] [PubMed] [Google Scholar]
- 10.Zhai L, Chaturvedi D, Cumberledge S. Drosophila wnt-1 undergoes a hydrophobic modification and is targeted to lipid rafts, a process that requires porcupine. J Biol Chem. 2004;279:33220–33227. doi: 10.1074/jbc.M403407200. [DOI] [PubMed] [Google Scholar]
- 11.Dennis JF, Kurosaka H, Iulianella A, Pace J, Thomas N, Beckham S, Williams T, Trainor PA. Mutations in Hedgehog acyltransferase (Hhat) perturb Hedgehog signaling, resulting in severe acrania-holoprosencephaly-agnathia craniofacial defects. PLoS Genet. 2012;8:e1002927. doi: 10.1371/journal.pgen.1002927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gradilla AC, Guerrero I. Hedgehog on the move: a precise spatial control of Hedgehog dispersion shapes the gradient. Curr Opin Genet Dev. 2013;23:363–373. doi: 10.1016/j.gde.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Pepinsky RB, Zeng C, Wen D, Rayhorn P, Baker DP, Williams KP, Bixler SA, Ambrose CM, Garber EA, Miatkowski K, et al. Identification of a palmitic acid-modified form of human Sonic hedgehog. J Biol Chem. 1998;273:14037–14045. doi: 10.1074/jbc.273.22.14037. [DOI] [PubMed] [Google Scholar]
- 14.Mann RK, Beachy PA. Novel lipid modifications of secreted protein signals. Annu Rev Biochem. 2004;73:891–923. doi: 10.1146/annurev.biochem.73.011303.073933. [DOI] [PubMed] [Google Scholar]
- 15.Kerszberg M, Wolpert L. Mechanisms for positional signalling by morphogen transport: a theoretical study. J Theor Biol. 1998;191:103–114. doi: 10.1006/jtbi.1997.0575. [DOI] [PubMed] [Google Scholar]
- 16.Briscoe J, Vincent JP. Hedgehog threads to spread. Nat Cell Biol. 2013;15:1265–1267. doi: 10.1038/ncb2878. [DOI] [PubMed] [Google Scholar]
- 17. Ramirez-Weber FA, Kornberg TB. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell. 1999;97:599–607. doi: 10.1016/s0092-8674(00)80771-0. One of the reports credited with pioneering the field of filopodia-based morphogen transport. This study describes remarkably long, unique cellular processes (termed “cytonemes”) that extend from cells in the Drosophila wing disc. Their consistent orientation towards the signaling center at the anterior-posterior boundary of the wing disc epithelium suggested that they play important role in cell signaling. *
- 18.De Joussineau C, Soule J, Martin M, Anguille C, Montcourrier P, Alexandre D. Delta-promoted filopodia mediate long-range lateral inhibition in Drosophila. Nature. 426:555–559. doi: 10.1038/nature02157. [DOI] [PubMed] [Google Scholar]
- 19. Caneparo L, Pantazis P, Dempsey W, Fraser SE. Intercellular bridges in vertebrate gastrulation. PLoS One. 2011;6:e20230. doi: 10.1371/journal.pone.0020230. This study describes long cellular projections that extend from epithelial cells in the early zebrafish epiblast. The authors describe the characteristics and dynamics of the cellular projections and provide novel insights into their formation as cells divide. *
- 20. Danilchik M, Williams M, Brown E. Blastocoel-spanning filopodia in cleavage-stage Xenopus laevis: Potential roles in morphogen distribution and detection. Dev Biol. 2013;382:70–81. doi: 10.1016/j.ydbio.2013.07.024. The first report of long cellular processes that span the blastocoel of early Xenopus embryos. Using live cell imaging of intact embryos, this study provides novel insights into how these structures form early on, and are maintained for many cell divisions, despite the mechanical forces that arise as development progresses. **
- 21. Sanders TA, Llagostera E, Barna M. Specialized filopodia direct long-range transport of SHH during vertebrate tissue patterning. Nature. 2013;497:628–632. doi: 10.1038/nature12157. This study reveals the dense, complex network of filopodia extending from mesenchymal cells within the developing vertebrate limb bud. By imaging live embryos at single-cell resolution, the authors detail the dynamics and cellular characteristics of these specialized filopodia. Furthermore, it is the first study that shows the dynamic transport of a cell signaling molecule (Shh), in the form of distinct particles, along the extracellular surface of specialized filopodia, which supports a novel mechanism for morphogen transport via direct delivery of signaling molecules to responding cells. **
- 22.Salas-Vidal E, Lomeli H. Imaging filopodia dynamics in the mouse blastocyst. Dev Biol. 2004;265:75–89. doi: 10.1016/j.ydbio.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Pyrgaki C, Trainor P, Hadjantonakis AK, Niswander L. Dynamic imaging of mammalian neural tube closure. Dev Biol. 2010;344:941–947. doi: 10.1016/j.ydbio.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luz M, Spannl-Muller S, Ozhan G, Kagermeier-Schenk B, Rhinn M, Weidinger G, Brand M. Dynamic Association with Donor Cell Filopodia and Lipid-Modification Are Essential Features of Wnt8a during Patterning of the Zebrafish Neuroectoderm. PLoS One. 2014;9:e84922. doi: 10.1371/journal.pone.0084922. The first study to describe the dynamic movement of a signaling molecule (Wnt8a) along filopodia extending from epithelial cells in the zebrafish presumptive neuroectoderm. Live imaging at single-cell resolution shows that ligand-containing filopodia retract, leaving Wnt8a ligand in the vicinity of nearby cells, suggesting how filopodia deliver ligand to responding cells.**
- 25.Gupton SL, Gertler FB. Filopodia: the fingers that do the walking. Sci STKE. 2007;2007:re5. doi: 10.1126/stke.4002007re5. [DOI] [PubMed] [Google Scholar]
- 26.Faix J, Breitsprecher D, Stradal TE, Rottner K. Filopodia: Complex models for simple rods. Int J Biochem Cell Biol. 2009;41:1656–1664. doi: 10.1016/j.biocel.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Mallavarapu A, Mitchison T. Regulated actin cytoskeleton assembly at filopodium tips controls their extension and retraction. J Cell Biol. 1999;146:1097–1106. doi: 10.1083/jcb.146.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davenport RW, Dou P, Rehder V, Kater SB. A sensory role for neuronal growth cone filopodia. Nature. 1993;361:721–724. doi: 10.1038/361721a0. [DOI] [PubMed] [Google Scholar]
- 29.Lidke DS, Lidke KA, Rieger B, Jovin TM, Arndt-Jovin DJ. Reaching out for signals: filopodia sense EGF and respond by directed retrograde transport of activated receptors. J Cell Biol. 2005;170:619–626. doi: 10.1083/jcb.200503140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gustafson T, Wolpert L. Studies on the cellular basis of morphogenesis in the sea urchin embryo. Gastrulation in vegetalized larvae. Exp Cell Res. 1961;22:437–449. doi: 10.1016/0014-4827(61)90120-3. [DOI] [PubMed] [Google Scholar]
- 31. Miller J, Fraser SE, McClay D. Dynamics of thin filopodia during sea urchin gastrulation. Development. 1995;121:2501–2511. doi: 10.1242/dev.121.8.2501. One of the first studies to image filopodial structures in the gastrulating sea urchin embryo. The authors determine that the dynamics of these structures are consistent with a signaling role, in contrast to the previously suggested role in cell guidance. *
- 32.Fierro-Gonzalez JC, White MD, Silva JC, Plachta N. Cadherin-dependent filopodia control preimplantation embryo compaction. Nat Cell Biol. 2013;15:1424–1433. doi: 10.1038/ncb2875. [DOI] [PubMed] [Google Scholar]
- 33.Teddy JM, Kulesa PM. In vivo evidence for short- and long-range cell communication in cranial neural crest cells. Development. 2004;131:6141–6151. doi: 10.1242/dev.01534. [DOI] [PubMed] [Google Scholar]
- 34.Ducibella T, Albertini DF, Anderson E, Biggers JD. The preimplantation mammalian embryo: characterization of intercellular junctions and their appearance during development. Dev Biol. 1975;45:231–250. doi: 10.1016/0012-1606(75)90063-9. [DOI] [PubMed] [Google Scholar]
- 35.Rojas-Rios P, Guerrero I, Gonzalez-Reyes A. Cytoneme-mediated delivery of hedgehog regulates the expression of bone morphogenetic proteins to maintain germline stem cells in Drosophila. PLoS Biol. 2012;10:e1001298. doi: 10.1371/journal.pbio.1001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roy S, Hsiung F, Kornberg TB. Specificity of Drosophila cytonemes for distinct signaling pathways. Science. 2011;332:354–358. doi: 10.1126/science.1198949. By imaging cytonemes at high resolution, the authors show that Decapentaplegic, FGF, and EGF receptors localize to cytonemes. Furthermore, this report suggests that cytonemes display “specificity”; such that receptors are localized to specific cytonemes corresponding to nearby signaling pathways. *
- 37.Akiyama-Oda Y, Oda H. Early patterning of the spider embryo: a cluster of mesenchymal cells at the cumulus produces Dpp signals received by germ disc epithelial cells. Development. 2003;130:1735–1747. doi: 10.1242/dev.00390. [DOI] [PubMed] [Google Scholar]
- 38. Bischoff M, Gradilla AC, Seijo I, Andres G, Rodriguez-Navas C, Gonzalez-Mendez L, Guerrero I. Cytonemes are required for the establishment of a normal Hedgehog morphogen gradient in Drosophila epithelia. Nat Cell Biol. 2013;15:1269–1281. doi: 10.1038/ncb2856. First live imaging of the dynamics of Drosophila cytonemes. The length and dynamics of cytonemes in the Drosophila abdominal epidermis correspond with formation of a Hedgehog gradient.*
- 39.Hsiung F, Ramirez-Weber FA, Iwaki DD, Kornberg TB. Dependence of Drosophila wing imaginal disc cytonemes on Decapentaplegic. Nature. 2005;437:560–563. doi: 10.1038/nature03951. [DOI] [PubMed] [Google Scholar]
- 40.Roy S, Huang H, Liu S, Kornberg TB. Cytoneme-Mediated Contact-Dependent Transport of the Drosophila Decapentaplegic Signaling Protein. Science. 2014 doi: 10.1126/science.1244624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato M, Kornberg TB. FGF is an essential mitogen and chemoattractant for the air sacs of the drosophila tracheal system. Dev Cell. 2002;3:195–207. doi: 10.1016/s1534-5807(02)00202-2. [DOI] [PubMed] [Google Scholar]
- 42.Alexandre C, Baena-Lopez A, Vincent JP. Patterning and growth control by membrane-tethered Wingless. Nature. 2014;505:180–185. doi: 10.1038/nature12879. [DOI] [PMC free article] [PubMed] [Google Scholar]