Abstract
Opiates are an important component for drug testing due to their high abuse potential. Proper urine opiate interpretation includes ruling out poppy seed ingestion; however, detailed elimination studies after controlled poppy seed administration with known morphine and codeine doses are not available. Therefore, we investigated urine opiate pharmacokinetics after controlled oral administration of uncooked poppy seeds with known morphine and codeine content. Participants were administered two 45g oral poppy seed doses 8h apart, each containing 15.7mg morphine and 3mg codeine. Urine was collected ad libitum up to 32h after the first dose. Specimens were analyzed with the Roche Opiates II immunoassay at 2,000 and 300μg/L cutoffs, and the ThermoFisher CEDIA® Heroin Metabolite (6-acetylmorphine, 6AM) and Lin-Zhi 6AM immunoassays with 10μg/L cutoffs to determine if poppy seed ingestion could produce positive results in these heroin marker assays. In addition, all specimens were quantified for morphine and codeine by GC/MS. Participants (N=22) provided 391 urine specimens over 32h following dosing; 26.6% and 83.4% were positive for morphine at 2,000 and 300μg/L GC/MS cutoffs, respectively. For the 19 subjects who completed the study, morphine concentrations ranged from <300 to 7,522μg/L with a median peak concentration of 5,239μg/L. The median first morphine-positive urine sample at 2,000μg/L cutoff concentration occurred at 6.6h (1.2-12.1), with the last positive from 2.6 to 18h after the second dose. No specimens were positive for codeine at a cutoff concentration of 2,000μg/L, but 20.2% exceeded 300μg/L, with peak concentrations of 658 μg/L (284-1540). The Roche Opiates II immunoassay had efficiencies greater than 96% for the 2000 and 300μg/L cutoffs. The CEDIA 6AM immunoassay had a specificity of 91%, while the Lin-Zhi assay had no false positive results. These data provide valuable information for interpreting urine opiate results.
Keywords: Poppy seeds, urine, morphine, codeine, controlled dose
1. Introduction
Opiates are important components of almost all judicial and clinical drug testing panels due to their high abuse potential. Positive opiate results require proper interpretation due to multiple potential sources of opiates in biological samples. For programs identifying heroin or morphine intake by analyzing urine for morphine and codeine, medical review officers must rule out ingestion of poppy seeds as a source for the positive opiate test. Urine morphine concentrations as high as 18,000μg/L were reported after ingestion of “Mon Kuchen”, a common German pastry containing poppy seeds [1]. A similar concentration was found in an infant’s urine after the mother accidentally poisoned the child with a homemade poppy seed milk mixture intended as a sleep aide [2]. Opiate potency varies greatly in different poppy seed lots and the spectrum of expected morphine and codeine urine concentrations following ingestion of poppy seed foods was recently reviewed [3]. These cited studies formed the scientific basis for the policy change in the USA federally mandated urine drug testing program to 2,000μg/L cutoff concentrations for morphine and codeine [4]. The intent of the policy was to reduce the number of donors who had positive urine drug test results due to ingested poppy seeds and codeine prescriptions, as most opiate-positive tests were reversed by Medical Review Officers and reported as negative results.
Identifying heroin abuse was improved by adding 6-acetylmorphine (6AM), a specific heroin marker, to testing menus, but this metabolite has a short half-life and often cannot be detected after the first urine void [5]. For this reason morphine and codeine continue to be important for identifying heroin use. Also, distinguishing morphine abuse from poppy seed ingestion remains a problem. Most of the past poppy seed studies were case reports where the morphine and codeine dose was unknown or controlled studies where food products were cooked, which is known to degrade both morphine and codeine [3]. Lotsch et al. provided a known oral morphine dose but did not determine a detailed elimination profile [6]. To the best of our knowledge, there are no sequential urine morphine elimination studies following controlled oral morphine administration to humans despite this being the analgesic of choice for treating cancer pain [7].
With the advent of new alternative matrices to detect opiates intake, it is important to establish detailed urine morphine pharmacokinetics following ingestion of known amounts of morphine and codeine in poppy seeds for comparison. This study examined controlled administration of 15.7mg morphine and 3mg codeine in each of two poppy seed doses separated by 8h to healthy normal volunteers. Morphine and codeine concentrations in each urine specimen voided over 32h were quantified by fully validated gas chromatography mass spectrometry (GC/MS) methods. In addition, each urine specimen also was analyzed by four commercially available opiate immunoassays.
2. Materials and Methods
2.1. Participants
Subjects provided written informed consent to participate in this National Institute on Drug Abuse Intramural Research Program Institutional Review Board-approved study. Individuals were recruited by television, radio, and newspaper advertisements, flyers, and participant referrals. Participants received a comprehensive medical and psychological evaluation to verify compliance with eligibility criteria. Participants were 18-65 years of age with adequate peripheral venous access. Exclusion criteria included any current medical condition precluding safe participation, current dependence on any psychoactive substance other than nicotine, a positive screen for either opiates in urine or evidence of pregnancy, or inability to tolerate oral poppy seed doses.
2.2. Opiate dose
RTI International, Research Triangle Park, NC, USA, analyzed poppy seeds from Brugger’s Bagels, Raleigh, NC, USA, by liquid chromatography tandem mass spectrometry. Each participant ingested 45g of poppy seeds containing 15.7mg morphine and 3mg codeine at 0900h and 1700h on the first day. A dose consisted of 4 bottles each containing 11.25g raw poppy seeds suspended in 40mL Ora-Plus suspension vehicle and 10mL Ora-Sweet sweetener (both from Paddock Laboratories, Inc., MN, USA). After finishing the entire dose, each bottle was rinsed with 50mL water to collect residual poppy seeds and the participant consumed these rinses. Two additional 50mL rinses were permitted to collect any additional seeds; the total volume of liquid consumed per dose was less than 500mL. Participants were required to consume the entire dose in ≤15min.
2.3. Study design
Participants entered a secure research unit and provided a urine sample ≥2h before dosing. An aliquot was analyzed for opiates with an iScreen (Blue Grass Drug Screen, Inc.) and a urine pregnancy test was performed for females. Every urine sample was collected ad libitum for 32h after the first and 24h after the second opiate dose. The volume of each urine void was measured, and a 1mL aliquot for immunoassay testing and the remainder for GC/MS testing were stored at −20 °C prior to analysis. Analyses were performed by the United States Army Forensic Toxicology Drug Testing Laboratory, Fort Meade, MD 20755, a National Laboratory Certification Program (NLCP) certified laboratory. Samples were analyzed blind by the Army laboratory and blind quality control samples, prepared by the Chemistry and Drug Metabolism Section, IRP, NIDA, Baltimore, MD 21224, were included within each batch.
2.4. Immunoassays
Samples were thawed, transferred to barcode labeled screening vials and analyzed on a Hitachi P or D Module Immunoanalyzer (Roche Diagnostics). Four immunoassays were performed on each specimen according to manufacturers’ recommended instructions: KIMS Opiates II at 2,000 and 300μg/L cutoff concentrations (Roche Diagnostics); CEDIA® Heroin Metabolite (6-AM) Assay (ThermoFisher Scientific) at 10μg/L cutoff; and 6-AM Enzyme Immunoassay (Lin-Zhi International) at 10μg/L cutoff. The methods were validated in accordance with NLCP requirements [4]. Quality control samples in each batch were fortified at 0%, 75% and 125% of cutoff concentrations.
2.5. Gas chromatography mass spectrometry (GC/MS)
All presumptive positive and negative immunoassay samples were quantified by GC/MS for morphine and codeine. The validated method included small modifications of a previously published GC/MS selected ion monitoring procedure [8]. In brief, there was a single 2,000μg/L morphine and codeine calibrator and tri-deuterated internal standards (2,000μg/L). One mL urine was hydrolyzed and extracted with Cerex Polycrom Clin II™ tubes (SPEware). Trimethylsilyl derivatives were produced and ions monitored (quantification ions in bold) were codeine 234, 343, 371; d3-codeine 237, 374; morphine 196, 401, 429; and d3-morphine 199, 432. Quality control samples included in each batch were 0, 300, 500, 800 and 2,500 μg/L. When quantifications were above the upper limit of linearity (ULOL), specimens were re-aliquoted, diluted and analyzed to obtain results within the linear range. Modifications from the published method included hydrolysis with HCl 121°C for 30 min in place of enzyme hydrolysis, and addition of 500μL methoxylamine prior to extraction with incubation for 15 min at 70°C to reduce interference from 6-keto-opioids instead of hydroxylamine. Morphine accuracy, within run imprecision, between run imprecision, lower limit of quantification (LLOQ), and ULOL were >94%, 0.75-1.78%, 2.50-5.52%, 300μg/L and 6,000μg/L, and for codeine >95%, 1.2-2.2%, 3.2-5.7%, 300μg/L and 6,000μg/L, respectively.
All presumptive positive metabolite immunoassay samples were tested by GC/MS for 6AM. Specimens were thawed and precise aliquots transferred to barcode labeled tubes. The method was a modification of a previously published GC/MS selected ion monitoring procedure [9]. In brief, there was a single 10μg/L 6-AM calibrator and a tri-deuterated internal standard (10μg/L). 750μL saturated sodium bisulfite was added to 3mL urine and incubated 15 min at room temperature followed by addition of 100μL 1M phosphate buffer pH 6.0. Aliquots were extracted with SPEC Plus™ solid phase extraction tubes (Ansys Diagnostics, Inc.). Trimethylsilyl derivatives were produced and ions monitored (quantification ions in bold) were 6-AM 287, 340, 399; d3-6-AM 290, 402. Quality control samples included in each batch were 0, 3, 4 and 12.5μg/L 6-AM. The method was validated in accordance with requirements of the NLCP [4]. Accuracy, within run imprecision, between run imprecision, LLOQ, and ULOL were >95%, 1.2-2.0%, 1.1-2.6%, 3μg/L, and 100μg/L, respectively. There was no interference from 5,000μg/L codeine, morphine, hydrocodone, hydromorphone, oxycodone, oxymorphone or norcodeine in the blank or 4μg/L 6-AM sample.
2.6. Creatinine
Creatinine concentrations were measured on a Hitachi P or D Module Analyzer (Roche Diagnostics) by the modified Jaffe method (Sciteck). To remove variability due to dilution some results were normalized by dividing the urine concentration of drug by the creatinine concentration and reporting as μg drug/g creatinine.
3. Results and Discussion
Twenty-two healthy subjects (15 Male, 7 Female, 5 Caucasian, 13 Black, 1 American Indian/Alaska Native, and 3 more than one race) provided written informed consent to participate. Nineteen subjects received both doses and provided all urine samples for the duration of the study. One subject missed the second dose and another received an incomplete second dose. One subject did not meet all medical guidelines and was not included in pharmacokinetic calculations. A total of 391 urine samples were collected. One hundred and four (26.6%) and 326 (83.4%) of these were positive for morphine at cutoff concentrations of 2,000 and 300μg/L, respectively. No sample was positive for codeine at 2,000μg/L, and 79 (20.2%) were positive at 300μg/L. After removing data from the three subjects that had missing samples or incomplete doses, morphine concentrations ranged from less than the LLOQ to 7,522μg/L with a mean±SD peak concentration of 5,248±1154μg/L, range 2413 to 7522μg/L and median 5239μg/L (Table 1). This peak urine concentration falls within the range of other published concentrations of <500 to 18,000μg/L [3]. A mean of 9.0±1.6mg morphine was excreted within 32h; morphine could derive from morphine or codeine content in the poppy seeds. The highest concentration for codeine was 1,540μg/L with a mean±SD peak of 718±253μg/L, range 284 to 1540μg/L, and median 658μg/L. Mean codeine excreted unchanged over 32h was 1.1±0.2mg or 18.3% of the total 6mg dose.
Table 1.
Pharmacokinetics & positive prevalence results for morphine and codeine in urine samples collected over 32h from 19 subjects receiving two oral poppy seed doses each containing 15.7mg morphine and 3mg codeine separated by 8h.
| Minimum | Maximum | Median | Mean | SD1 | SEM2 | |
|---|---|---|---|---|---|---|
| Morphine peak concentration μg/L | 2413 | 7522 | 5239 | 5248 | 1154 | 288 |
| Codeine peak concentration μg/L | 284 | 1540 | 658 | 718 | 253 | 63 |
| Morphine 2000 μg/L cutoff | ||||||
| Time to first positive h | 1.2 | 12.1 | 6.6 | |||
| Time 2nd dose to last positive h | 2.6 | 18.3 | 12.2 | |||
| Number positive before 2nd dose | 0 | 4 | 1 | |||
| Number positive after 2nd dose | 1 | 6 | 3 | |||
| Morphine 300 μg/L cutoff | ||||||
| Time to first positive h | 0.80 | 2.4 | 1.4 | |||
| Codeine 300 μg/L | ||||||
| Time to first positive h | 1.6 | 19.5 | 6.6 | |||
| Time 2nd dose to last positive h | 2.6 | 14.0 | 8.8 | |||
| Number positive before 2nd dose | 0 | 3 | 1 | |||
| Number positive after 2nd dose | 0 | 5 | 3 |
Standard deviation
Standard error of the mean
Twelve (12) blind quality control samples containing 100μg/L morphine and codeine, and 12 containing 500μg/L morphine and codeine were analyzed. The 100μg/L blind samples all were < LLOQ. Morphine and codeine in the 500μg/L blind samples quantified within ±15% of target concentrations.
Presumptive immunoassay positive samples with the 2,000 or 300μg/L cutoffs were confirmed at the same GC/MS cutoffs (Table 2). Sensitivities, specificities and efficiencies at the 2,000μg/L cutoff were 91%, 99% and 97%, and at the 300μg/L cutoff 95%, 99% and 96%, respectively. Blind control samples were included in the immunoassay evaluation for a total of 415 specimens. Specimens contained no 6-AM, therefore, sensitivity was not determined for the heroin metabolite immunoassays. The CEDIA 6-AM method had 36 false positive results yielding 91% specificity. Each of these specimens had 6-AM <limit of detection (LOD) by GC/MS, but morphine concentrations greater than 1,000μg/L. According to the manufacturer’s cross-reactivity data, 9,000μg/L morphine or 500,000μg/L codeine is necessary to produce a positive result; however, our data indicated greater cross-reactivity for morphine than stated. There were no false positive results and 100% specificity for the Lin-Zhi 6-AM immunoassay.
Table 2.
Performance characteristics of immunoassays (IA) with GC/MS confirmation of urine samples containing morphine and codeine from subjects following ingestion of poppy seeds.
| Method | KIMS OPI1 | KIMS OPI1 | CEDIA 6AM2 | LinZhi 6AM3 |
|---|---|---|---|---|
| IA Cutoff μg/L | 300 | 2000 | 10 | 10 |
| GC/MS Cutoff μg/L | 300 | 2000 | 10 | 10 |
| True Negative | 76 | 309 | 379 | 415 |
| True Positive | 321 | 95 | 0 | 0 |
| False Negative | 17 | 9 | 0 | 0 |
| False Positive | 1 | 2 | 36 | 0 |
| Sensitivity | 95 | 91 | - | - |
| Specificity | 99 | 99 | 91 | 100 |
| Efficiency | 96 | 97 | 91 | 100 |
KIMS Opiates II immunoassay, Roche Diagnostics
CEDIA Heroin Metabolite (6-AM) immunoassay, Thermo Fisher Scientific
6-AM Enzyme Immunoassay, Lin-Zhi International
As expected from previous studies [3], there was great diversity in individual elimination pharmacokinetics. The appearance of the first positive morphine result (cutoff 2,000μg/L) ranged from the first void for some individuals (as short as 1.2 h) to 12.1h after the first dose (Table 1). Seven of 19 subjects did not produce a positive morphine result until after the second poppy seed dose, more than 8 h after the first dose. The median time to the first positive result was 6.6h. Following the second poppy seed dose, subjects produced their last morphine positive urine at about 12h (range 2.6-18.3h). Subjects had up to 4 positive urine samples after the first dose (median 1) and 1-6 (median 3) after the second poppy seed dose.
At a cutoff of 300μg/L morphine, all subjects produced their first positive urine test from the first or second void collected within the first 3h after dosing. Most subjects’ urine samples remained morphine positive when the study ended 24h after the second dose. Since no subject had a positive codeine result at the 2,000μg/L cutoff concentration, we examined positive results with a 300μg/L cutoff. Median times to the first (from the first dose) and last (from the last dose) positive were 6.6h and 8.8h, respectively. Most samples positive for codeine at 300μg/L were also positive for morphine at 2,000μg/L.
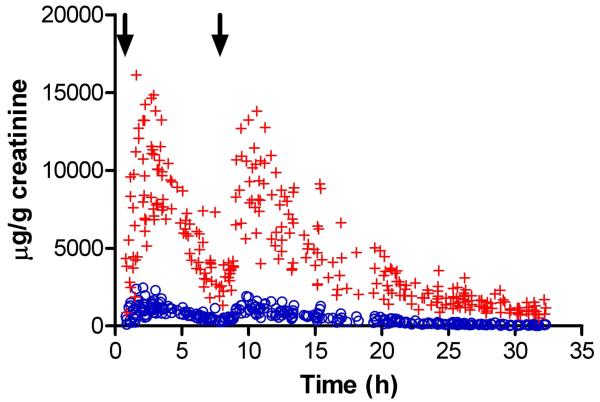
The figure displays the creatinine-normalized urine morphine and codeine concentrations (μg/g creatinine) vs. time for the 19 subjects who completed the study. Concentrations were normalized to reduce the variability caused by dilution. Two peaks are apparent, each following a poppy seed dose. The mean estimated elimination half-lives were 7 and 6h, respectively, for morphine and codeine over 32h. These results are comparable to total codeine and morphine elimination kinetics reported previously [10,11].
Lachenmeier et al. noted that the morphine and codeine content of poppy seeds decreased over the years due to manufacturer self-regulation [3]. Our results demonstrate that individuals consuming poppy seeds currently available for purchase as a food product can produce urine specimens positive for morphine at the USA federally regulated drug testing cutoff concentration of 2000μg/L. These results support continued efforts to distinguish heroin from poppy seed ingestion eliminating the so-called “poppy seed defense” in treatment programs and litigation. The search for specific markers of heroin consumption continues and pitfalls remain [3]. One set of approaches examined poppy seeds for other alkaloids such as narcotine, papaverine and thebaine, but their appearance in different varieties of seeds and in urine following poppy seed ingestion was inconsistent [12,13]. Others reported markers in heroin, such as acetylcodeine, with a similar outcome [3,14]. A recent report by Chen et al. appears more promising [15]. These investigators employed a sensitive liquid chromatography tandem mass spectrometric method to identify an acetylated thebaine glucuronide (3,6-dimethoxy-4-acetoxy-8-[2-(N-methylacetamido)ethyl]phenanthrene; abbreviated ATM4G) in 16 of 22 urine specimens produced by 22 heroin addicts who had 6-AM <10μg/L in each specimen and total morphine (morphine-3-glucuronide and morphine) concentrations >300μg/L in 17 of the specimens. They did not find this glucuronidated phenanthrene in urine from six participants who ingested thebaine-rich poppy seeds.
4. Conclusion
After 19 subjects ingested a total of 31.4mg morphine and 6mg codeine in two poppy seed doses, urine samples had morphine results ≥2,000μg/L, but were negative by 19h after the second dose; no urine sample was positive for codeine at the same cutoff. The Roche Opiates II immunoassay had efficiencies greater than 96% for the 2,000 and 300μg/L cutoffs. The CEDIA 6-AM immunoassay had a specificity of 91% while the Lin-Zhi 6-AM immunoassay had no false positive results in the presence of morphine concentrations as high as 7,500μg/L. We demonstrated that ingestion of 45g of poppy seeds produced 26.6% and 83.4% urine morphine concentrations above 2000μg/L and 300μg/L, respectively, peaking at a median of 6.6h and with an elimination half-life of 7h.
Highlights.
Controlled administration of uncooked poppy seeds with known opiate content
Each urine void collected for 32h 26.6% specimens with morphine >2000μg/L
Median (range) peak morphine 5239μg/L (2413-7522)
Last morphine >2000μg/L 2.6-18h after last dose
Figure 1.
Creatinine normalized opiate concentrations in urine following 2 ingestions of poppy seeds containing 15.7mg morphine (+) and 3mg codeine (o) each dose. Time of doses indicated by arrows. (n = 19)
Acknowledgements
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Drug Abuse and the Division of Workplace Programs, Substance Abuse Mental Health Services Administration, USA. The opinions in this article are those of the authors and do not necessarily reflect the views of the United States Department of Army or Department of Defense.
Abbreviations
- 6AM
6-Acetylmorphine
- LLOQ
lower limit of quantification
- NLCP
National Laboratory Certification Program
- LOD
limit of detection
- ULOL
upper limit of linearity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fritschi G, Prescott WR., Jr Morphine levels in urine subsequent to poppy seed consumption. Forensic Sci. Int. 1985;27:111–117. doi: 10.1016/0379-0738(85)90173-2. [DOI] [PubMed] [Google Scholar]
- 2.Hahn A, Michalak H, Begemann K, et al. Severe health impairment of a 6-week-old infant related to the ingestion of boiled poppy seeds. Clin. Toxicol. 2008;46:607. [Google Scholar]
- 3.Lachenmeier DW, Sproll C, Musshoff F. Poppy seed foods and opiate drug testing--where are we today? Ther. Drug Monit. 2010;32:11–18. doi: 10.1097/FTD.0b013e3181c0eee0. [DOI] [PubMed] [Google Scholar]
- 4.Department of Health and Human Services Mandatory Guidelines for Federal Workplace Drug Testing Programs. Fed. Regist. 2008;73:71858–71907. [Google Scholar]
- 5.Smith ML, Shimomura ET, Summers J, Paul BD, Jenkins AJ, Darwin WD, Cone EJ. Urinary excretion profiles for total morphine, free morphine, and 6-acetylmorphine following smoked and intravenous heroin. J. Anal. Toxicol. 2001;25:504–514. doi: 10.1093/jat/25.7.504. [DOI] [PubMed] [Google Scholar]
- 6.Lötsch J, Weiss M, Ahne G, Kobal G, Geisslinger G. Pharmacokinetic modeling of M6G formation after oral administration of morphine in healthy volunteers. Anesthesiology. 1999;90:1026–1038. doi: 10.1097/00000542-199904000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Wiffen PJ, Wee B, Moore RA. Oral morphine for cancer pain. Cochrane Database Syst. Rev. 2013 Jul 22;:7. doi: 10.1002/14651858.CD003868.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Broussard LA, Presley LC, Pittman T, Clouette R, Wimbish GH. Simultaneous identification and quantitation of codeine, morphine, hydrocodone, and hydromorphone in urine as trimethylsilyl and oxime derivatives by gas chromatography-mass spectrometry. Clin. Chem. 1997;43:1029–1032. [PubMed] [Google Scholar]
- 9.Paul BD, Shimomura ET, Smith ML. A practical approach to determine cutoff concentrations for opiate testing with simultaneous detection of codeine, morphine, and 6-acetylmorphine in urine. Clin. Chem. 1999;45:510–519. [PubMed] [Google Scholar]
- 10.Cone EJ, Welch P, Paul BD, Mitchell JM. Forensic drug testing for opiates, III. Urinary excretion rates of morphine and codeine following codeine administration. J. Anal. Toxicol. 1991;15:161–166. doi: 10.1093/jat/15.4.161. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell JM, Paul BD, Welch P, Cone EJ. Forensic drug testing for opiates. II. Metabolism and excretion rate of morphine in humans after morphine administration. J. Anal. Toxicol. 1991;15:49–53. doi: 10.1093/jat/15.2.49. [DOI] [PubMed] [Google Scholar]
- 12.Trafkowski J, Madea B, Musshoff F. The significance of putative urinary markers of illicit heroin use after consumption of poppy seed products. Ther. Drug Monit. 2006;28:552–558. doi: 10.1097/00007691-200608000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Paul BD, Dreka C, Knight ES, Smith ML. Gas chromatography/mass spectrometric detection of narcotine, papaverine, and thebaine in seeds of Papaver somniferum. Planta Med. 1996;62:544–547. doi: 10.1055/s-2006-957966. [DOI] [PubMed] [Google Scholar]
- 14.O’Neal CL, Poklis A. The detection of acetylcodeine and 6-acetylmorphine in opiate positive urines. Forensic Sci. Int. 1998;95:1–10. doi: 10.1016/s0379-0738(98)00074-7. [DOI] [PubMed] [Google Scholar]
- 15.Chen P, Braithwaite RA, George C, Hylands PJ, Parkin MC, Smith NW, Kicman AT. The poppy seed defense: a novel solution. Drug Test. Anal. 2014;6:194–201. doi: 10.1002/dta.1590. [DOI] [PubMed] [Google Scholar]