Abstract
Introduction
The recent emergence and widespread availability of many new synthetic cannabinoids support the need for an accurate and high-throughput urine screen for these new designer drugs. We evaluated performance of the immunalysis homogeneous enzyme immunoassay (HEIA) to sensitively, selectively, and rapidly identify urinary synthetic cannabinoids.
Methods
2443 authentic urine samples were analyzed with the HEIA that targets JWH-018 N-pentanoic acid, and a validated LC-MS/MS method for 29 synthetic cannabinoids and metabolites. Semiquantitative HEIA results were obtained, permitting performance evaluation at and around three cutoffs (5, 10 and 20 μg/L), and diagnostic sensitivity, specificity and efficiency determination. Performance challenges at ±25 and ±50% of each cutoff level, cross-reactivity and interferences also were evaluated.
Results
Sensitivity, specificity, and efficiency of the immunalysis HEIA K2 Spice kit with the manufacturer's recommended 10 μg/L cutoff were 75.6%, 99.6% and 96.8%, respectively, as compared to the reference LC-MS/MS method with limits of detection of 0.1 -10 μg/L. Performance at 5 μg/L was 92.2%, 98.1% and 97.4%, and for the 20 μg/L cutoff were 62.9%, 99.7% and 95.4%. Semi-quantitative results for in-house prepared standards were obtained from 2.5-30 μg/L, and documented acceptable linearity from 5-25 μg/L, with inter-day imprecision <30% (n = 17). Thirteen of 74 synthetic cannabinoids evaluated were classified as highly cross-reactive (≥50% at 10 μg/L); 4 showed moderate cross-reactivity (10–50% at 10 μg/L), 30 low cross-reactivity (<10% at 500 μg/L), and 27 <1% cross-reactivity at 500 μg/L. There was no interference from 102 investigated compounds. Only a mixture containing 1000 μg/L each of buprenorphine/norbuprenorphine produced a positive result above our proposed cutoff (5 μg/L) but below the manufacturer's recommended cutoff concentration (10 μg/L).
Conclusion
The Immunalysis HEIA K2 Spice kit required no sample preparation, had a high-throughput, and acceptable sensitivity, specificity and efficiency, offering a viable method for screening synthetic cannabinoids in urine that cross-react with JWH-018 N-pentanoic acid antibodies.
Keywords: Immunoassay, Synthetic cannabinoids, HEIA
1. Introduction
According to recent reports, cannabis is the most widely used illicit drug [1,2]. Its primary psychoactive compound, Δ9-tetrahydrocannabinol (THC), exhibits partial agonist activity at CB1 and CB2 receptors [3]. Numerous cannabimimetic compounds were synthesized to examine the endogenous cannabinoid system and evaluate the possibility of selectively activating individual cannabinoid receptors [4]. Selective CB2 receptor affinity could potentially provide analgesic properties without cannabis-induced psychoactive effects and may show promise for treating acute and chronic pain [5,6]. However, synthetic cannabinoids initially intended for research and clinical applications possess CB1 and/or CB2 receptor binding affinity up to 100 times more than THC and have rapidly become drugs of abuse [7,8]. Synthetic cannabinoids are now a popular alternative to cannabis for their psychoactive effects, availability, affordability and ability to avoid detection in routine monitoring programs [9].
Currently, synthetic cannabinoids are categorized into at least twelve classes based on chemical structure: adamantoylindole [10], benzoylindoles [10], cyclohexylphenols [11], classical dibenzopyrans [12], indazole based [13], naphthoylindoles and WIN 55,212-2 [10,14], naphthoylpyrroles [15,16], naphthylmethylindoles [17], naphthylmethylindenes [17], phenylacetylindoles [18], quinolinyl ester (indole based) [19] and tetramethylcyclopropyl ketone indoles [16], with new synthetic cannabinoids constantly emerging. Most synthetic cannabinoids are now structurally unrelated to traditional cannabinoids (Fig. 1).
Fig. 1.
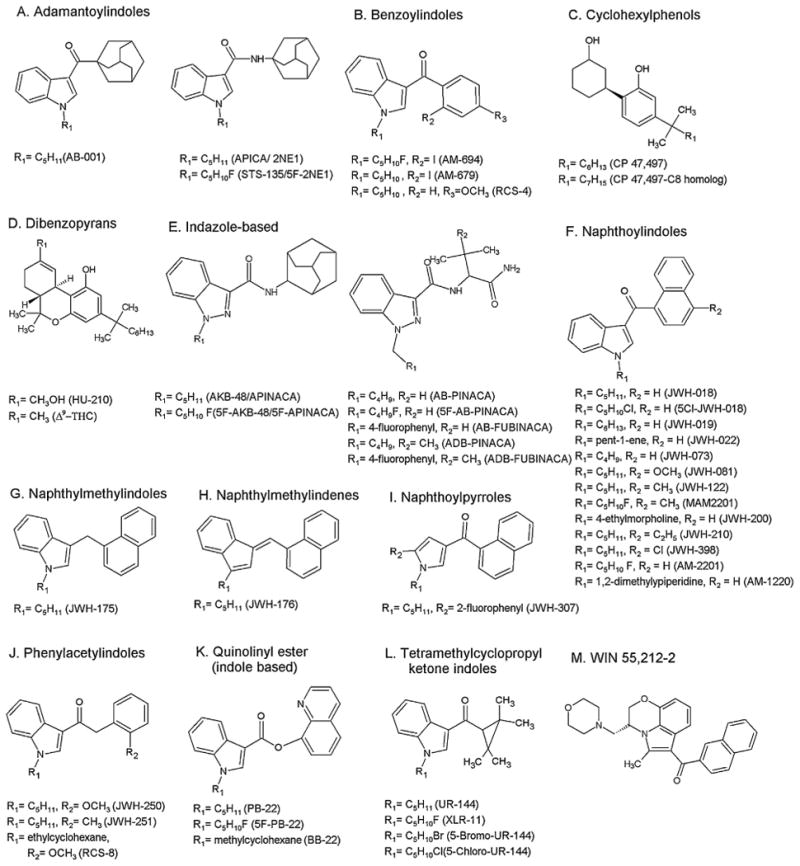
General structures of synthetic cannabinoids (A–M).
Synthetic cannabinoids have been documented in blood, oral fluid, serum and urine [20–23]. In urine, few parent synthetic cannabinoids are present, but more prevalent are their hydroxy and carboxy metabolites that are more readily excreted [24]. Sobolevsky et al. published the first report affirming JWH-018 metabolite detection in human urine [25]. Urine is the primary matrix for drug testing due to ample sample volume and extended detection windows.
The growing use of synthetic cannabinoids was reported in the United States [9], Europe [26], and Japan [27]. These new designer drugs are often advertised as incense, potpourris, and air fresheners and sold over the internet and in convenience stores, gas stations and “head shops” [9]. The constant emergence of new synthetic cannabinoids and their widespread availability make it difficult for regulatory agencies to stay abreast of this major public health problem. Due to the potential for abuse, five of the most widely abused synthetic cannabinoids were classified as Schedule I under a temporary ban by the US Department of Justice in March 2011 [28]. Following this initial ban, the Synthetic Drug Abuse Prevention Act was signed into US law in July 2012. This new law explicitly bans 15 synthetic cannabinoids under Schedule I of the Controlled Substances Act [29].
Synthetic cannabinoids are not detected in standard laboratory cannabinoid screening tests, making it an attractive alternative for users who must undergo drug testing. The current approach is to develop analytical methods for the qualitative identification and quantification of synthetic cannabinoids and metabolites in urine. To date, these methods include gas chromatography mass spectrometry (GC–MS) [30,31], gas chromatography tandem mass spectrometry (GC–MS/MS) [25], liquid chromatography tandem mass spectrometry (LC–MS/MS) [23,32–36] and liquid chromatography time-of-flight mass spectrometry (LC–TOF/MS) [37]. Many of these techniques require labor intensive sample preparation and long analysis times to achieve identification.
Immunoassays provide an inexpensive, sensitive and rapid screening alternative to hyphenated chromatographic techniques, offering high-throughput testing for targeted synthetic cannabinoids. Of course, a definitive mass spectrometry confirmation is required, but the number of specimens requiring confirmation will be much lower.
Although there are many evaluations of traditional cannabinoid immunoassays, there are limited synthetic cannabinoid immunoassay methods [38]. The optimized performance of the Immunalysis K2 HEIA (targeting the JWH-018 N-pentanoic acid metabolite) for identifying synthetic cannabinoids in urine was evaluated and compared to an LC–MS/MS assay for 29 synthetic cannabinoids markers.
2. Materials and methods
2.1. HEIA immunoassay
The HEIA technique is based on the principle of competitive antibody, and drug and drug-enzyme interactions. The polyclonal antibody and enzyme-drug conjugate are provided in ready-to-use solutions and do not require washing steps or long incubation periods common to enzyme-linked immunosorbent assays (ELISA). Advantages of this technique include low matrix effects, reduced sample preparation and high throughput. Semi-quantitative analysis was performed on an Olympus AU400e (Beckman Coulter) fully automated analyzer, capable of 400 tests per hour. Testing was performed using the Immunalysis HEIA K2 Spice kit, which determines the enzyme activity correlated with the concentration of the targeted JWH-018 N-pentanoic acid metabolite in urine. Enzyme activity was measured spectrophotometrically at 340 nm. Daily calibration was performed with synthetic urine fortified at 0, 10 and 20 μg/L of JWH-018 N-pentanoic acid metabolite. Blank urine and calibrators were pipetted (10 μL) into quartz cuvettes. After 18 s, the first reagent, containing the antibodies was added (100 μL) and the mixture incubated for 3 min. The second reagent, containing the conjugate was added (70 μL), an initial absorbance taken after 18 s and a final absorbance after 108 s. The Olympus software plotted the rate of absorbance (mAbs/min) against concentration (μg/L). The rate of absorbance difference (Δ mAbs/min) between 0 and 10 μg/L was compared to the rate of absorbance between 10 and 20 μg/L to check for consistency. A calibration curve from 0–20 μg/L JWH-018 N-pentanoic acid provided semi-quantitative results.
2.1.1. Specimens
In a project funded under inter-agency agreements between the Department of Defense (DoD) Drug Demand Reduction Initiative and the National Institutes on Drug Abuse, National Institutes of Health, 20,017 authentic urine specimens were analyzed for synthetic cannabinoids using the Randox Drugs of Abuse V biochip array technology. Specimens were collected over one year from around the world and stored at room temperature before initial DoD immunoassay analyses occurring within days of collection. Specimens screened negative for cannabinoids, cocaine, amphetamines, benzodiazepines, phencyclidine and opiates during routine urinalysis testing, and were transferred for synthetic cannabinoids analysis. Following biochip analysis for synthetic cannabinoids, presumptive positive (1389) and selected negative (1054) specimens were refrigerated at 4–7 °C before HEIA determination and LC–MS/MS confirmation.
2.2. Calibration and quality control
Analyses were performed utilizing a three point calibration (0,10 and 20 μg/L) from calibrators prepared in synthetic urine. The instrument was calibrated before each run and a positive in-house control (30 μg/L) was assayed throughout to monitor performance. Performanceat5, 10and20 μg/Lcutoffswereevaluatedbycomparing semi-quantitative screening results to qualitative LC–MS/MS confirmation results for 29 synthetic cannabinoids and metabolites.
2.3. External performance challenges
JWH-018 N-pentanoic acid was purchased from Cayman Chemicals (Ann Arbor, MI). Stock standard solutions were prepared by diluting contents with appropriate volumes of methanol. Working performance challenges at ±25% and ±50% of each cutoff concentration (5 μg/L, 10 μg/L and 20 μg/L) were prepared by fortifying drug free authentic urine with stock control solution. All external performance challenge samples were randomly assayed throughout the sequence of specimens for assessment of assay imprecision, and the ability to correctly classify concentrations around each cutoff.
2.3.1. Analyses
Approximately 1 mL of each authentic urine specimen, external quality control, interference, and cross-reactivity sample was aliquotted into 4 mL polypropylene tubes and analyzed by the HEIA. Specimens (2443), interference mixtures (32), cross-reactivity samples (148) and performance challenges (12) were analyzed on an Olympus AU400e automated analyzer.
2.4. Assay performance criteria
We evaluated imprecision, cross-reactivity, and interferences, and characterized sensitivity, specificity, and diagnostic efficiency. Linearity was assessed by graphing the semi-quantitative results of performance challenges (n = 17) with a quantile-quantile (Q–Q) plot, allowing a comparison of the distribution of two data sets (known concentrations vs. measured concentrations). Known concentrations were 2.5, 3.75, 5, 6.25, 7.5, 10, 12.5, 15, 20, 25 and 30 μg/L in urine.
2.4.1. Imprecision
Semi-quantitative values of performance challenges were monitored throughout the duration of analysis. Inter-day imprecision (n = 17) was evaluated from individual absorbance readings for samples prepared at ±25 and ±50% of each cutoff calibrator, collected over 5 separate days. For the 5 μg/L cutoff, samples were prepared at 2.5, 3.75, 6.25 and 7.5 μg/LJWH-018 N-pentanoic acid, for the 10 μg/L cutoff, 5, 7.5, 12.5, 15 μg/L were evaluated, and for the 20 μg/L cutoff we monitored performance at 10, 15, 25 and 30 μg/L Imprecision samples were randomly assayed each day, at a minimum of once each 8 h shift. The average percent difference of target and percent coefficient of variation were calculated as follows:
Average percent difference and coefficient of variation <30% and <15%, respectively, were considered acceptable degrees of imprecision for the semi-quantitative HEIA.
3. Cross-reactivity
Synthetic cannabinoids and metabolites structurally similar to the target JWH-018 N-pentanoic acid may cross-react with the antibody, increasing the effectiveness of monitoring. To evaluate cross-reactivity, low (10 μg/L) and high (500 μg/L) concentrations of 74 synthetic cannabinoid compounds (including the calibrator, JWH-018 N-pentanoic acid metabolite), were prepared by fortifying drug free urine with methanolic stock solutions. 148 blank urine cross-reactivity samples contained the following drugs: JWH-018, JWH-019, JWH-073, JWH-081, JWH-122, JWH-200, JWH-203, JWH-210, JWH-250, JWH-398, AM 2201, MAM2201, RCS-4, RCS-8, CP 47,497, AM 694, HU 210, UR-144, JWH-007, JWH-015, JWH-251, AM 1220, WIN 55,212-2 mesylate, AKB 48, URB 754, STS 135, XLR-11 and their metabolites. All synthetic cannabinoid parent and metabolite compounds used to evaluate cross-reactivity were obtained from Cayman Chemical (Ann Arbor, MI).
The percent cross-reactivity was calculated using the following formula:
4. Interferences
Common drugs of abuse and metabolites, co-administered drugs, over-the-counter medications and structurally similar compounds may interfere with drug-antibody interactions, altering assay response. Twenty-six interference mixtures, containing 96 compounds, were prepared at 1000 μg/L by fortifying 5 mL blank urine with methanolic stock drug solutions (10– 100 μg/mL). Individual interference challenges containing: methanol (72 μg/L), cannabigerol (100 μg/L), sodium chloride (40 g/L), ascorbic acid (4 g/L) and urine adjusted to pH > 8 with sodium hydroxide and urine also adjusted to pH < 4 with acetic acid samples also were prepared. Assay responses from 102 compounds evaluated potential interferences relative to the lowest investigated cutoff (5 μg/L).
5. Sensitivity, specificity, and diagnostic efficiency
Sensitivity, specificity, and efficiency of the immunoassay was determined by analyzing all presumptive positive and negative specimens by a previously published LC–MS/MS assay for 29 synthetic cannabinoids and metabolites [32]. Specimens with a good spectral library match (>60%), retention time within ±0.05 min of target, and presence of three characteristic masses and the molecular ion were confirmed positive. Immunoassay results were classified as positive or negative relative to each cutoff concentration (5, 10 and 20 μg/L). Specimens with immunoassay results greater or equal to the cutoff and positive LC–MS/MS results were true positives (TP). A true negative (TN) had negative immunoassay and LC-MS/MS results. A false negative (FN) had negative immunoassay results, but confirmed positive by LC–MS/MS, and a false positive (FP) had positive immunoassay results but were negative by LC–MS/MS. Percent sensitivity, specificity and efficiency for the assay at each cutoff were calculated as follows:
6. Results and discussion
Linearity (2.5–30 μg/L) was investigated with assay performance challenges above (+25 and +50%) and below (−25 and −50%) each proposed cutoff. A Q–Q plot compared distribution of the data sets. A perfect normal distribution exhibits a straight 45° line and the closer the data points are to this line, the closer the data set is to a normal distribution. Data that are not normally distributed display a systematic curve away from the straight line. For semi-quantitative purposes, the average percent difference between the target JWH-018 N-pentanoic acid concentration and the measured concentration each day (n = 17) was checked. Linearity was acceptable from 5–25 μg/L (Fig. 2), with % differences of actual and predicted concentrations <30.4% in this range. There appeared to be a trend of overestimating linearity concentrations <5 μg/L and differences from target concentrations below the limit of quantification were <68.0% (Table 1). Despite mean % differences of only −14.9% from target at 30 μg/L, the graphical representation appeared to indicate the beginning of a concentration plateau, and was excluded from the linear range. A full evaluation of linearity may benefit with more concentration levels; however, semi-quantitative screening results will ultimately require confirmation by another analytical technique. Despite the trend of overestimating results, the negative performance challenges (2.5 and 3.75 μg/L) for the 5 μg/L cutoff were always negative, and as expected the 6.25 and 7.5 μg/L were always positive. When evaluating the 10 μg/L cutoff, the 5 and 7.5 μg/L challenges were always negative while the 12.5 and 15 μg/L were positive 16 of 17 times. Positive and negative performance challenges (±25% and ±50%) for the 20 μg/L cutoff were always correctly classified. The differences among the performance challenges were always significant (p < 0.05), demonstrating the ability of the assay to distinguish between concentrations close (±25%) to each cutoff. Additionally, the performance challenges also examined immunoassay inter-day imprecision. Coefficients of variation (CV) ranged from 3.4% to 14.9% for each drug concentration (n = 17). Based on these results, we utilized a linear range from 5–25 μg/L when assessing semi-quantitative results from samples in the cross-reactivity evaluation.
Fig. 2.
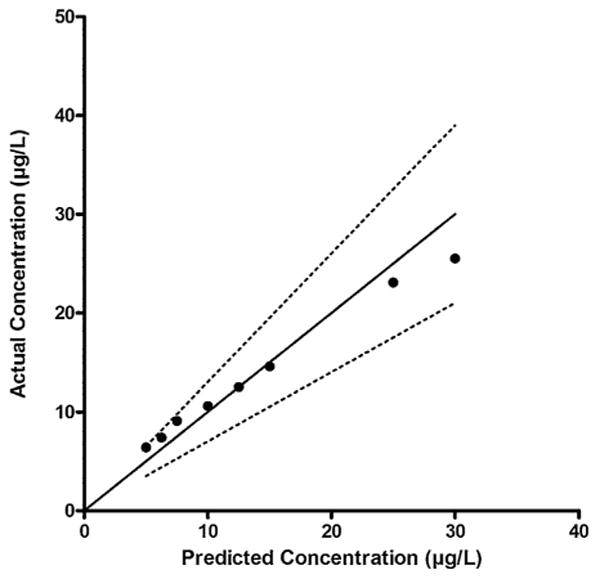
Linearity evaluation with the quantile-quantile plot for fortified performance challenge samples (5-30 μg/L) analyzed in 17 batches. Duplicate values were averaged in each run and mean concentrations were plotted vs. known concentrations. Straight line corresponds to 100% agreement with the predicted concentration. Dashed lines are ±30% of target concentrations.
Table 1.
Assay imprecision around 5, 10 and 20 μg/L cutoffs for Immunalysis K2 HEIA targeting JWH-018 N-pentanoic acid.
| Cutoff concentration (μg/L) | % Cutoff | Performance challenge concentration (μg/L) | Inter-day imprecision (n = 17) | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mean | SD | % CV | Min. | Max. | Average % difference of target | |||
| 5 | −50 | 2.5 | 4.2 | 0.63 | 14.9 | 3.3 | 6.2 | 68.0 |
| −25 | 3.75 | 5.2 | 0.58 | 11.2 | 4.2 | 6.7 | 37.4 | |
| +25 | 6.25 | 7.4 | 0.57 | 7.7 | 6.5 | 8.9 | 18.2 | |
| +50 | 7.5 | 9.5 | 1.13 | 11.9 | 8.1 | 12.7 | 26.7 | |
| 10 | −50 | 5 | 6.5 | 0.72 | 11.0 | 5.5 | 8.3 | 30.4 |
| −25 | 7.5 | 8.6 | 0.68 | 7.9 | 7.3 | 10.5 | 14.8 | |
| +25 | 12.5 | 12.5 | 0.88 | 7.0 | 10.7 | 14.5 | −0.1 | |
| +50 | 15 | 14.5 | 0.57 | 3.9 | 13.3 | 16.1 | −3.3 | |
| 20 | −50 | 10 | 10.6 | 0.72 | 6.8 | 9.2 | 12.0 | 5.8 |
| −25 | 15 | 14.7 | 1.58 | 10.8 | 9.0 | 16.6 | −1.9 | |
| +25 | 25 | 23.1 | 0.82 | 3.5 | 21.7 | 25.1 | −7.8 | |
| +50 | 30 | 25.5 | 0.85 | 3.3 | 23.1 | 27.2 | −14.9 | |
Three synthetic cannabinoid metabolites, JWH-073 N-(butanoic acid), JWH-073 N-(4-hydroxybutyl) and JWH-073 N-(3-hydroxybutyl) were the most cross-reactive (Table 2a) with a high affinity for the antibody, similar or better than the calibrator (JWH 018 N-pentanoic acid metabolite). Ten additional compounds were classified as highly reactive (≥50% cross-reactivity at 10 μg/L). Four compounds and metabolites showed moderate cross-reactivity (10–50%). Thirty compounds displayed low cross-reactivity (≥1% and <10% at 500 μg/L) (Table 2b), and 27 compounds had cross-reactivity <1% at the same tested concentration (Table 2c). For semi-quantitative purposes, only compounds with concentrations in the linear range of the assay (5– 25 μg/L) were evaluated in terms of percent cross-reactivity. These results indicate that the antibody is capable of recognizing a wide range of synthetic cannabinoids and metabolites.
Table 2a.
Synthetic cannabinoids with high and moderate levels of cross-reactivity. Cross-reactivity was calculated with concentrations of 10 μg/mL when initial measured concentrations exceeded the upper limit of linearity (25 μg/L).
| Synthetic cannabinoid | Concentration obtained for samples at 500 μg/La | Concentration obtained for samples at 10 μg/L | Cross reactivity (%) at 10 μg/L |
|---|---|---|---|
| JWH 073 N-(butanoic acid) metabolite | 32.1 | 11.8 | 118 |
| JWH 073 N-(4-hydroxybutyl) metabolite | 33.0 | 8.8 | 88 |
| JWH 073 N-(3-hydroxybutyl) metabolite | 33.7 | 8.3 | 83 |
| JWH 018 N-(5-pentanoic acid) metabolite | 33.1 | 8.3 | 83 |
| AM 2201 (4-hydroxypentyl) metabolite | 34.2 | 7. 5 | 75 |
| JWH 018 N-(5-hydroxypentyl) metabolite | 32.5 | 7. 4 | 74 |
| JWH 200 (6-hydroxyindole) metabolite | 30.6 | 7. 3 | 73 |
| JWH 200 | 31.6 | 7.1 | 71 |
| JWH 018 N-(4-hydroxypentyl) metabolite | 33.2 | 7.1 | 71 |
| AM 1220 | 31.6 | 6.9 | 69 |
| JWH 18 N-(5-hydroxypentyl)-β-D-glucuronide | 33.1 | 6.9 | 69 |
| JWH 200 (5-hydroxyindole) metabolite | 26.3 | 6.5 | 65 |
| JWH 019 N-(6-hydroxyhexyl) metabolite | 33.2 | 5.0 | 50 |
| MAM 2201 | 29.0 | 4.4 | 10–50%a |
| JWH 073 (6-hydroxyindole) metabolite | 27.7 | 3.8 | |
| AM 2201 | 30.5 | 2.7 | |
| JWH 073 | 26.9 | 1. 3 |
Measured concentrations out of the semi-quantitative range.
Table 2b.
Synthetic cannabinoids with a low level of cross-reactivity. Measured concentrations of fortified challenges were within the semi-quantitation range (5–25 μg/mL) at known concentration of 500 μg/L.
| Synthetic cannabinoid | Concentration obtained for samples at 500 μg/L | Cross-reactivity (%) |
|---|---|---|
| JWH 073 (7-hydroxyindole) metabolite | 24.9 | 5.0 |
| JWH 018 (6-hydroxyindole) metabolite | 23.7 | 4.7 |
| JWH 398 N-(pentanoic acid) metabolite | 23 | 4.6 |
| JWH 398 N-(5-hydroxypentyl) metabolite | 22 | 4.4 |
| JWH 022 | 21.4 | 4.3 |
| MAM2201 N-(pentanoic acid) metabolite | 20.3 | 4.1 |
| JWH 122 N-(5-hydroxypentyl) metabolite | 20.4 | 4.1 |
| AM694 | 20.5 | 4.1 |
| JWH 018 (7-hydroxyindole) metabolite | 19.8 | 4.0 |
| JWH 018 | 19.7 | 3.9 |
| JWH 019 | 18.7 | 3.7 |
| JWH 073 (5-hydroxyindole) metabolite | 17 | 3.4 |
| WIN 55,212-2 (mesylate) | 16.3 | 3.3 |
| JWH 018 (5-hydroxyindole) metabolite | 12.6 | 2.5 |
| AM 2201 (6-hydroxyindole) metabolite | 12.6 | 2.5 |
| RCS-4 2-methoxyisomer | 12.1 | 2.4 |
| JWH-015 | 12.2 | 2.4 |
| AM2201 N-(4-fluoropentyl isomer) | 11.4 | 2.3 |
| JWH 081 N-(5-hydroxypentyl) metabolite | 11.1 | 2.2 |
| JWH 210 N-(5-hydroxypentyl) metabolite | 9 | 1.8 |
| JWH 019 (5-hydroxyindole) metabolite | 9.2 | 1.8 |
| JWH 210 N-(5-pentanoic acid) metabolite | 8.7 | 1.7 |
| JWH 398 | 7.5 | 1.5 |
| JWH 250 N-(4-hydroxypentyl) metabolite | 6.8 | 1.4 |
| JWH 250 N-(5-hydroxypentyl) metabolite | 6.8 | 1. 4 |
| JWH 210 N-(4-hydroxypentyl metabolite) | 6.9 | 1.4 |
| JWH 122 | 7.1 | 1.4 |
| JWH-007 | 6.3 | 1.3 |
| JWH 250 N-(pentanoic acid) metabolite | 6.4 | 1.3 |
| JWH 073 (4-hydroxyindole) metabolite | 6.5 | 1.3 |
Table 2c.
Synthetic cannabinoids with cross-reactivity <1%.
| Synthetic cannabinoid |
|---|
| AKB 48 |
| CP 47, 497 |
| JWH 018 adamantyl carboxamide |
| URB 754 |
| JWH-251 |
| STS-135 |
| UR144 |
| JWH 108 adamantyl analog |
| JWH 250 (5-hydroxyindole) metabolite |
| RCS-4 |
| UR144 N-(pentanoic acid) metabolite |
| JWH 250 |
| HU210 |
| XLR-11 |
| CP 47, 497 C8 homolog |
| CP 47, 497 (C7-hydroxy) metabolite |
| RCS-8 |
| UR144 N-(5-hydroxypentyl) metabolite |
| JWH 203 |
| JWH 018 (2-hydroxyindole) metabolite |
| RCS-4 N-(5-pentanoic acid) metabolite |
| JWH 210 |
| JWH 210 (5-hydroxyindole) metabolite |
| JWH 073 (2-hydroxyindole) metabolite |
| RCS-4 N-(5-hydroxypentyl) metabolite |
| JWH 081 |
JWH-073 N-butanoic acid had the highest cross-reactivity of the 74 compounds evaluated. The metabolite is a naphthoylindole with a carboxylated 4-carbon N-alkyl chain, differing from the target structure only by a shorter alkyl side chain length. It appears that decreasing the side chain length by one carbon increased cross-reactivity. Comparing the number of carbons on the side chain, JWH-073 (4C), JWH-018 (5C) and JWH-019 (6C), cross-reactivity for parent drugs was JWH-073 >JWH-018 >JWH-019. This order of reactivity appeared to be the same considering the terminal hydroxyl metabolites or the carboxyl metabolites for each. In general, the n-terminal carboxyl metabolites were more reactive than the correspondent hydroxyl metabolite and both cross-reacted more than the parent. Hydroxylation, carboxylation and fluorination of the alkyl side chain appeared to have a strong positive affect on cross-reactivity. Similar responses were observed for hydroxyl metabolites that differ in the location of the hydroxyl on the side chain, as evident when comparing JWH-018 N-(4-hydroxy) vs. N-(5-hydroxy) and JWH-073 N-(4-hydroxy) vs. N-(3-hydroxy). Synthetic cannabinoids having a hydroxyl on the indole ring displayed lower cross-reactivity than the parent compound. These structural characteristics are useful in anticipating cross-reactivities of new synthetic cannabinoid variations as they emerge on the market.
Additionally, other commonly abused drugs, over-the-counter and prescription medications, and structurally similar compounds that could produce false positive results or discrepant absorbance readings were investigated. One hundred of the 102 interference samples, 97 of which were tested at 1000 μg/L, yielded a concentration less than the lowest cutoff evaluated (5 μg/L) (Table 3). Only the buprenorphine and norbuprenorphine mixture (1000 μg/L each) produced a value of 7.3 μg/L, above our recommended cutoff, yet lower than the manufacturer's recommended cutoff (10 μg/L). These results indicate that although the antibody recognizes drugs other than synthetic cannabinoids, there is negligible contribution to the concentration of the analyte of interest (at 10 and 20 μg/L).
Table 3.
Immunoassay interference during the detection of synthetic cannabinoids.
| Compound/Mix | Analyte concentration (μg/L) | Immunoassay detected concentration (μg/L) |
|---|---|---|
| Methanol | 72 | 0.0 |
| Buprenorphine, norbuprenorphine | 1000 | 7.3 |
| Cocaine, benzoylecgonine, norcocaine, nor-benzoylecgonine | 1000 | 2.4 |
| Ecgonine ethyl ester, ecgonine methyl ester, anhydroecgonine methyl ester, ecgonine | 1000 | 1.8 |
| Cocaethylene, norcocaethylene | 1000 | 1.7 |
| m-OH-cocaine, p-OH-cocaine, m-OH-benzoylecgonine, p-OH-benzoylecgonine | 1000 | 1.5 |
| Morphine, normorphine, M3G, M6G | 1000 | 1.9 |
| Codeine, norcodeine, 6-acetylmorphine, 6-acetylcodeine | 1000 | 3.0 |
| Hydrocodone, hydromorphone, oxycodone | 1000 | 0.9 |
| Noroxycodone, oxymorphone, noroxymorphone | 1000 | 1.0 |
| Propoxyphene, 3,4-methylenedioxyphenyl-2-butanamine (BDB), N-methyl BDB | 1000 | 2.2 |
| R-cath, EAMP, 2-CB (4-bromo-2,5-dimethoxiphenethylamine) | 1000 | 2.2 |
| Diazepam, lorazepam, oxazepam, alprazolam | 1000 | 2.2 |
| Imipramine, clomipramine, fluoxetine, norfluoxetine | 1000 | 1.2 |
| Paroxetine, 7-aminoclonazepam, 7-aminoflunitrazepam, 7-aminonitrazepam | 1000 | 3.3 |
| Clonidine, ibuprofen, pentazocine, caffeine | 1000 | 0.7 |
| Diphenhydramine, chlorpheniramine, brompheniramine | 1000 | 1.9 |
| Salicylic acid, acetaminophen, ibuprofen, caffeine | 1000 | 0.4 |
| Bromazepam, clonazepam, flurazepam | 1000 | 1.4 |
| Nitrazepam, flunitrazepam, temazepam, nordiazepam | 1000 | 2.7 |
| Nicotine, cotinine, OH-cotinine, norcotinine | 1000 | 2.1 |
| Methadone, 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP) | 1000 | 2.5 |
| Paramethoxyamphetamine, paramethoxymetamphetamine | 1000 | 0.4 |
| Amphetamine (AMP), Methamphetamine (MAMP), MDMA, MDA, MDEA, HMMA, HMA, OH-AMP, OH-MAMP | 1000 | 1.2 |
| Ephedrine, pseudoephedrine | 1000 | 2.2 |
| Ketamine, dextromethorphan, phentermine | 1000 | 2.4 |
| Cannabigerol | 100 | 2.2 |
| THC, 11-OH-THC, THCOOH, CBD, CBN | 1000 | 2.7 |
| NaCl | 40a | −2.6 |
| Ascorbic acid | 4a | −0.1 |
| Urine pH > 8 (NaOH) | – | 1. 0 |
| Urine pH < 4 (acetic acid) | – | −1.0 |
g/L.
The sensitivity, specificity and efficiency of the Immunalysis HEIA for detection of JWH-018 N-pentanoic acid were assessed. Semi-quantitative results from authentic urine specimens were compared to LC–MS/MS results. As there are currently no mandated screening cutoffs for synthetic cannabinoids by any regulatory body, assay performance at 5, 10 and 20 μg/L cutoffs was evaluated. Sensitivity, specificity, and efficiency were 92.2%, 98.1% and 97.4% for the 5, 75.6%, 99.6% and 96.8% for the 10, and 62.9%, 99.7% and 95.4% for the 20 μg/L cutoff, respectively (Table 4). As expected, the lowest cutoff (5 μg/L) exhibited the highest sensitivity (92.2%) and efficiency (97.4%) while the highest (20 μg/L) cutoff gave the greatest specificity (99.7%). Using the 5 μg/L cutoff produced fewer FN specimens (22), but more false positive tests (42). With the 10 μg/L cutoff, there were 69 FN and 9 FP results, whereas the 20 μg/L cutoff had 105 FN and 7 FP. Although there is only a marginal difference between the specificity and efficiency of the assay using the 5, 10 and 20 μg/L cutoffs, improvements in sensitivity by using a cutoff of 5 μg/L should be considered. However, suitable negative immunoassay controls must be employed and established confirmation methods must be able to verify these presumptive positive screening results.
Table 4.
Immunalysis HEIA performance for detection of synthetic cannabinoids in urine (n = 2443).
| HEIA cutoff, μg/L | True positive | True negative | False positive | False negative | Sensitivity (%) | Specificity (%) | Efficiency (%) |
|---|---|---|---|---|---|---|---|
| 5 | 261 | 2118 | 42 | 22 | 92.2 | 98.1 | 97.4 |
| 10 | 214 | 2151 | 9 | 69 | 75.6 | 99.6 | 96.8 |
| 20 | 178 | 2153 | 7 | 105 | 62.9 | 99.7 | 95.4 |
This evaluation of the Immunalysis HEIA synthetic cannabinoid assay demonstrated good performance at 5 and 10 μg/L cutoffs as compared to an LC–MS/MS assay for 29 synthetic cannabinoids and metabolites (Table 5). Immunoassays have many advantages, making them attractive, cost-effective options for workplace drug testing or high-throughput military laboratories. Simple and rapid sample preparation, short analysis times and the potential for automation are advantages compared to the expense of screening samples by LC–MS/MS that include the high cost of instrumentation, need for highly trained personnel and reduced throughput. However, there are limitations as well, especially in the ever-changing synthetic cannabinoid market. As manufacturers constantly create subtle differences in chemical compounds to avoid existing laws, laboratories are tasked with identifying these newer synthetic compounds. Unfortunately, the antibodies in the Immunalysis K2 HEIA that are capable of identifying first generation synthetic cannabinoids based on the targeted napthoylindole structure may not cross react with the most recent compounds on the market. Confounding this problem is the length of time required to produce, develop and validate new immunoassay methods that will be able to detect second generation synthetic cannabinoids. Despite these limitations, the current antibody is capable of recognizing a wide range of synthetic cannabinoids and metabolites, although it will be critical to evaluate cross-reactivities of newly emerging compounds.
Table 5.
Limits of detection for the LC–MS/MS qualitative screening reference method.
| Analyte | Limit of detection (μg/L) |
|---|---|
| JWH-200 5-hydroxyindole | 5 |
| JWH-200 6-hydroxyindole | 2.5 |
| RCS-4 pentanoic acid | 10 |
| RCS-4 5-hydroxypentyl | 5 |
| JWH-250 pentanoic acid | 0.5 |
| JWH-250 4/5-hydroxypentyl | 1 |
| JWH-073 butanoic acid | 5 |
| JWH-073 4-hydroxybutyl | 2.5 |
| JWH-018 pentanoic acid | 2.5 |
| JWH-018 5-hydroxypentyl | 0.5 |
| AM2201 4-hydroxypentyl | 2.5 |
| AM2201 6-hydroxyindole | 5 |
| JWH-081 5-hydroxypentyl | 2.5 |
| JWH-122 5-hydroxypentyl | 0.5 |
| JWH-073 5/6-hydroxyindole | 2.5 |
| JWH-250 5-hydroxyindole | 2.5 |
| JWH-210 pentanoic acid | 2.5 |
| JWH-210 4/5-hydroxypentyl | 1 |
| JWH-018 5/6-hydroxyindole | 1 |
| AM2201 | 1 |
| RCS-4 | 2.5 |
| JWH-210 5-hydroxyindole | 10 |
| MAM2201 | 2.5 |
| JWH-250 | 5 |
| JWH-073 | 5 |
| JWH-018 | 1 |
| JWH-081 | 10 |
| JWH-122 | 2.5 |
| JWH-210 | 10 |
Evaluation of synthetic cannabinoid use by sensitive and specific drug screens is necessary for clinical, forensic, drug treatment and workplace drug screening programs. After investigating 2443 authentic urine specimens, performance challenges, cross-reactivity and mixtures of potentially interfering compounds, the Immunalysis K2 HEIA was demonstrated to be a highly sensitive and selective method for rapidly monitoring synthetic cannabinoids in urine by targeting the JWH-018 N-pentanoic acid metabolite at a cutoff concentration of 5 μg/L.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health. We also thank Immunalysis, Inc. for their support during the conduct of this research including providing the assays, calibrators and controls for urine analyses.
References
- 1.Bosker WM, Huestis MA. Oral fluid testing for drugs of abuse. Clin Chem. 2009;55:1910–1931. doi: 10.1373/clinchem.2008.108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United Nations Office on Drugs Crime. World Drug Report 2012. United Nations Office on Drugs and Crime; Vienna: 2012. [Google Scholar]
- 3.Ameri A. The effects of cannabinoids on the brain. Progr Neurobiol. 1999;58:315–348. doi: 10.1016/s0301-0082(98)00087-2. [DOI] [PubMed] [Google Scholar]
- 4.Seely KA, Prather PL, James LP, Moran JH. Marijuana-based drugs: innovative therapeutics or designer drugs of abuse? Mol Interv. 2011;11:36–51. doi: 10.1124/mi.11.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malan TP, Jr, Ibrahim MM, Deng H, Mata HP, Vanderah T, Porreca F, et al. CB2 cannabinoid receptor agonists: pain relief without psychoactive effects? Curr Opin Pharmacol. 2003;3:62–67. doi: 10.1016/s1471-4892(02)00004-8. [DOI] [PubMed] [Google Scholar]
- 6.Porter AC, Felder CC. The endocannabinoid nervous system: unique opportunities for therapeutic intervention. Pharmacol Ther. 2001;90:45–60. doi: 10.1016/s0163-7258(01)00130-9. [DOI] [PubMed] [Google Scholar]
- 7.Huffman JW, Zengin G, Wu MJ, Lu J, Hynd G, Bushell K, et al. Structure-activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB(1) and CB(2) receptors: steric and electronic effects of naphthoyl substituents. New highly selective CB(2) receptor agonists. Bioorg Med Chem. 2005;13:89–112. doi: 10.1016/j.bmc.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 8.Pertwee RG, Howlett AC, Abood ME, Alexander SPH, Di Marzo V, Elphick MR, et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fattore L, Fratta W. Beyond THC: The new generation of cannabinoid designer drugs. Front Behav Neurosci. 2011;5:60. doi: 10.3389/fnbeh.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makriyannis A, Hongfeng D. Cannabimimetic Indole Derivatives. US Patent 6900236 B1. 2000
- 11.Huffman JW, Thompson AL, Wiley JL, Martin BR. Synthesis and pharmacology of 1-deoxy analogs of CP-47,497 and CP-55,940. Bioorg Med Chem. 2008;16:322–335. doi: 10.1016/j.bmc.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mechoulam R. Looking back at cannabis research. Curr Pharm Des. 2000;6:1313–1322. doi: 10.2174/1381612003399509. [DOI] [PubMed] [Google Scholar]
- 13.Buchler IP, Hayes MJ, Hegde SG, Hockerman SL, Jones DE, Kortum SW, et al. Indazole Derivatives. US Patent 2011/0028447 A1. 2009
- 14.Aung MM, Griffin G, Huffman JW, Wu MJ, Keel C, Yang B, et al. Influence of the JV-1 alkyl chain length of cannabimimetic indoles upon CB1 and CB2 receptor binding. Drug Alcohol Depen. 2000;60:133–140. doi: 10.1016/s0376-8716(99)00152-0. [DOI] [PubMed] [Google Scholar]
- 15.Wiley JL, Compton DR, Dai D, Lainton JA, Phillips M, Huffman JW, et al. Structure-activity relationships of indole- and pyrrole-derived cannabinoids. J Pharmacol Exp Ther. 1998;285:995–1004. [PubMed] [Google Scholar]
- 16.Wiley JL, Marusich JA, Huffman JW. Moving around the molecule: relationship between chemical structure and in vivo activity of synthetic cannabinoids. Life Sci. 2014;97:55–63. doi: 10.1016/j.lfs.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huffman JW, Padgett LW. Recent developments in the medicinal chemistry of cannabimimetic indoles, pyrroles and indenes. Curr Med Chem. 2005;12:1395–1411. doi: 10.2174/0929867054020864. [DOI] [PubMed] [Google Scholar]
- 18.Huffman JW, Szklennik PV, Almond A, Bushell K, Selley DE, He H, et al. 1-pentyl-3-phenylacetylindoles, a new class of cannabimimetic indoles. Bioorg Med Chem Lett. 2005;15:4110–4113. doi: 10.1016/j.bmcl.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Uchiyama N, Matsuda S, Kawamura M, Kikura-Hanajiri R, Goda Y. Two new-type cannabimimetic quinolinyl carboxylates, QUPIC and QUCHIC, two new cannabimimetic carboxamide derivatives, ADB-FUBINACA and ADBICA, and five synthetic cannabinoids detected with a thiophene derivative a-PVT and an opioid receptor agonist AH-7921 identified in illegal products. Forensic Toxicol. 2013;31:223–240. [Google Scholar]
- 20.Ammann J, McLaren JM, Gerostamoulos D, Beyer J. Detection and quantification of new designer drugs in human blood: part 1 - synthetic cannabinoids. J Anal Toxicol. 2012;36:372–380. doi: 10.1093/jat/bks048. [DOI] [PubMed] [Google Scholar]
- 21.Coulter C, Garnier M, Moore C. Synthetic cannabinoids in oral fluid. J Anal Toxicol. 2011;35:424–430. doi: 10.1093/anatox/35.7.424. [DOI] [PubMed] [Google Scholar]
- 22.Dresen S, Kneisel S, Weinmann W, Zimmermann R, Auwarter V. Development and validation of a liquid chromatography-tandem mass spectrometry method for the quantitation of synthetic cannabinoids of the aminoalkylindole type and methanandamide in serum and its application to forensic samples. J Mass Spectrom. 2011;46:163–171. doi: 10.1002/jms.1877. [DOI] [PubMed] [Google Scholar]
- 23.Moran CL, Le VH, Chimalakonda KC, Smedley AL, Lackey FD, Owen SN, et al. Quantitative measurement of JWH-018 and JWH-073 metabolites excreted in human urine. Anal Chem. 2011;83:4228–4236. doi: 10.1021/ac2005636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer MR, Peters FT. Analytical toxicology of emerging drugs of abuse—an update. Ther Drug Monit. 2012;34:615–621. doi: 10.1097/FTD.0b013e31826d0915. [DOI] [PubMed] [Google Scholar]
- 25.Sobolevsky T, Prasolov I, Rodchenkov G. Detection of JWH-018 metabolites in smoking mixture post-administrationurine. ForensicSci Int. 2010;200:141–147. doi: 10.1016/j.forsciint.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 26.European Monitoring Centre for Drugs ans Drug Addiction. Understanding the ‘Spice’ Phenomenon. EMCDDA; Luxemborug: 2009. [Google Scholar]
- 27.Kikura-Hanajiri R, Uchiyama N, Kawamura M, Ogata J, Goda Y. Prevalence of new designer drugs and their legal status in Japan. Yakugaku Zasshi. 2013;133:31–40. doi: 10.1248/yakushi.12-00247-6. [DOI] [PubMed] [Google Scholar]
- 28.Drug Enforcement Administration. Department of Justice. Schedules of controlled substances: temporary placement of three synthetic cathinones into Schedule I. Fed Regist. 2011;76:11075–11078. [PubMed] [Google Scholar]
- 29.Drug Enforcement Administration. Department of Justice. Establishment of drug codes for 26 substances. Final rule Fed Regist. 2013;78:664–666. [PubMed] [Google Scholar]
- 30.Grigoryev A, Melnik A, Savchuk S, Simonov A, Rozhanets V. Gas and liquid chromatography-mass spectrometry studies on the metabolism of the synthetic phenylacetylindole cannabimimetic JWH-250, the psychoactive component of smoking mixtures. J Chromatogr B. 2011;879:2519–2526. doi: 10.1016/j.jchromb.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Kavanagh P, Grigoryev A, Melnik A, Simonov A. The identification of the urinary metabolites of 3-(4-methoxybenzoyl)-1-pentylindole (RCS-4), a novel cannabimimetic, by gas chromatography-mass spectrometry. J Anal Toxicol. 2012;36:303–311. doi: 10.1093/jat/bks032. [DOI] [PubMed] [Google Scholar]
- 32.Wohlfarth A, Scheidweiler KB, Chen X, Liu HF, Huestis MA. Qualitative confirmation of 9 synthetic cannabinoids and 20 metabolites in human urine using LC-MS/MS and library search. Anal Chem. 2013;85:3730–3738. doi: 10.1021/ac3037365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dowling G, Regan L. A method for CP 47, 497 a synthetic non-traditional cannabinoid in human urine using liquid chromatography tandem mass spectrometry. J Chromatogr B. 2011;879:253–259. doi: 10.1016/j.jchromb.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Elsohly MA, Gul W, Elsohly KM, Murphy TP, Madgula VLM, Khan SI. Liquid chromatography-tandem mass spectrometry analysis of urine specimens for K2 (JWH-018) metabolites. J Anal Toxicol. 2011;35:487–495. doi: 10.1093/anatox/35.7.487. [DOI] [PubMed] [Google Scholar]
- 35.Wissenbach DK, Meyer MR, Remane D, Philipp AA, Weber AA, Maurer HH. Drugs of abuse screening in urine as part of a metabolite-based LC-MSn screening concept. Anal Bioanal Chem. 2011;400:3481–3489. doi: 10.1007/s00216-011-5032-1. [DOI] [PubMed] [Google Scholar]
- 36.de Jager AD, Warner JV, Henman M, Ferguson W, Hall A. LC-MS/MS method for the quantitation of metabolites of eight commonly-used synthetic cannabinoids in human urine - an Australian perspective. J Chromatogr B. 2012;897:22–31. doi: 10.1016/j.jchromb.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Guale F, Shahreza S, Walterscheid JP, Chen HH, Arndt C, Kelly AT, et al. Validation of LC-TOF-MS screening for drugs, metabolites, and collateral compounds in forensic toxicology specimens. J Anal Toxicol. 2013;37:17–24. doi: 10.1093/jat/bks084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arntson A, Ofsa B, Lancaster D, Simon JR, McMullin M, Logan B. Validation of a novel immunoassay for the detection of synthetic cannabinoids and metabolites in urine specimens. J Anal Toxicol. 2013;37:284–290. doi: 10.1093/jat/bkt024. [DOI] [PubMed] [Google Scholar]


