Abstract
Objectives
We tested the hypothesis that chronic dietary nitrate supplementation protects against doxorubicin-induced cardiomyopathy through improving ventricular function and reducing mitochondrial respiratory chain damage.
Background
Doxorubicin is a powerful anthracycline antibiotic used to treat divergent human neoplasms. Its clinical use is limited due to severe cardiotoxic side-effects. Dietary nitrate/nitrite are essential nutrients for maintenance of the steady-state tissue levels of nitric oxide (NO) and may play a therapeutic role in diseases associated with NO insufficiency or dysregulation. Dietary nitrate/nitrite supplementation alleviates myocardial injury caused by ischemia-reperfusion and cardiac arrest-resuscitation.
Methods
Adult male CF-1 mice were given a single dose of doxorubicin (15 mg/kg, i.p.) and left ventricular contractile function was assessed 5 days later with both echocardiography and pressure-volume Millar catheter. Nitrate supplementation regimen (1 g/L NaNO3 in drinking water) was started 7 days before doxorubicin injection and continued thereafter. Cardiomyocyte necrosis/apoptosis, tissue lipid peroxidation, and plasma nitrate/nitrite levels were assessed. In addition, mitochondrial complex I activity, oxidative phosphorylation capacity, and H2O2 generation were determined in paralleled experiments.
Results
Doxorubicin caused impairment of ventricular contractility and cell death, which were significantly reduced by nitrate supplementation (P<0.05). These cardioprotective effects were associated with a significant decrease in tissue lipid peroxidation. Nitrate supplementation significantly preserved mitochondrial complex I activity, oxidative phosphorylation and attenuated H2O2 generation following doxorubicin treatment.
Conclusions
Chronic oral intake of inorganic nitrate attenuates doxorubicin-induced ventricular dysfunction, cell death, oxidative stress, and mitochondrial respiratory chain damage. Nitrate could be a promising therapeutic agent against doxorubicin-induced cardiotoxicity.
Keywords: Nitrate, Doxorubicin, Heart Failure, Cardioprotection, Mitochondria
Introduction
Doxorubicin (DOX; with trade names as Adriamycin® and Rubex®) is a broad-spectrum and potent anthracycline antibiotic that has been used to treat a variety of human neoplasms (1). Despite its anti-cancer efficacy, the clinical use of DOX is often limited by dose-dependent cardiotoxicity, which may lead to irreversible dilated cardiomyopathy and heart failure (2). The cardiotoxicity of DOX involves increased oxidative stress in cardiomyocytes, alteration of mitochondrial energetics and direct effect on DNA. However, the optimal therapeutic approaches for protection against DOX cardiotoxicity have not been identified. This is because most of the tested agents (such as anti-oxidants and β-adrenergic receptor blockers) have pronounced clinical disadvantages including decline in high-density lipoprotein levels, inability to prevent mortality, weight loss, and potentiation of myelosuppression (3).
Nitric oxide (NO) is essential for the integrity of cardiovascular system, and decreased production and/or bioavailability of NO leads to the development of cardiovascular disorders (4) and heart failure (5). NO protects against ischemia-reperfusion (I/R) injury induced by ischemic or pharmacological preconditioning (6), which could involve inducible and endothelial NO synthases (iNOS and eNOS) (7;8). Role of NO in protection against DOX-induced cardiotoxicity with phosphodiesterase-5 inhibitor, sildenafil was suggested by our group (9). Moreover, a possible role of NO in protection against DOX-induced cardiotoxicity was indicated by β-adrenergic blockers (10) or statin (11), because these drugs increase iNOS/eNOS mRNA/protein levels in myocardium (12) and cardiomyocytes (13).
In addition to the L-arginine-NOS-dependent NO synthesis, the NOS-independent mechanisms of NO generation also converts nitrate to nitrite to NO in vivo via both enzymatic reduction [e.g. xanthine oxidoreductase - (14)] and non-enzymatic acidic disproportionation (15). There are numerous colonies of bacteria in the mammalian oral cavity and intestine which can reduce nitrate to nitrite in vivo (16). The nitrate-nitrite-NO pathway is a complementary system to the NOS-dependent pathway for ensuring sufficient NO production, especially under pathophysiological situations including hypoxia and acidosis when the oxygen-dependent NOS activity is compromised (17;18). Since a substantial portion of nitrate/nitrite in the blood and tissue is derived from dietary sources, the dietary supplementation of nitrate or nitrite protects against myocardial I/R injury (19;20), cardiac arrest-resuscitation, reduces hypertension (21), and improves endothelial and platelet function as well as exercise performance (22).
In this context, the present study was designed to test the hypothesis that chronic nitrate supplementation may protect against ventricular dysfunction and cardiomyocyte loss from DOX-induced cardiomyopathy. We also investigated if mitochondria are protected by nitrate, because DOX mainly accumulates in mitochondria and cell nuclei (23). Since DOX enhances reactive oxygen species (ROS) generation in cardiac mitochondria (24;25), we studied the effect of nitrate/nitrite in reducing oxidative stress in the mitochondria. Because of the potential role of transient blockade of the mitochondrial complex I in nitrite-induced anti-ischemic protection (26), we also investigated the effect of nitrate on DOX-induced damage of mitochondrial electron transport chain (ETC).
Methods
Animals
Adult male CF-1 outbred mice were purchased from Harlan Sprague Dawley Inc. The animal experimental protocol was approved by the Institutional Animal Care and Use Committee of the Virginia Commonwealth University.
Experimental protocol
As illustrated in Online Fig. 1A, mice were administered a single dose of DOX intraperitoneally (15 mg/kg, dissolved in saline; DOX group; n=8) or volume-matched saline (0.2 mL; Control group; n=8). Another group of mice (Nitrate+DOX group; n=8) received NaNO3 added into their drinking water at a concentration of 1 g/L (12 mM) for 7 days before the DOX injection on Day 8. The nitrate treatment regimen was continued throughout the post-DOX period. A control group of mice were given nitrate alone (Nitrate group; n=8). This oral dose of NaNO3 has been shown to be cardioprotective against I/R injury (19). Five days later (i.e. on Day 13), left ventricular (LV) function was assessed with echocardiography under light anesthesia and subsequently with a Millar catheter inserted into the LV cavity under surgical anesthesia.
To determine the efficacy of non-oral nitrate, a subset of mice were subcutaneously implanted with a micro-osmotic pump (Alzet® model 1002), which continuously delivered NaNO3 solution (1 g/L concentration) or saline (as sham controls) at the rate of 0.25 μL/hour for 13 days. The nitrate as well as saline infused mice received DOX injection (15 mg/kg, i.p.) on Day 8 (n=7 per group).
Measurement of DOX cytotoxicity in vitro
As illustrated in Figure 1B, mice with or without 7 days of nitrate supplementation were sacrificed on Day 8 and cardiomyocytes were isolated and exposed to 1 μM DOX by adding into cell culture medium. Myocyte necrosis and apoptosis were quantified 18 hours later with trypan blue exclusion and TUNEL assays.
Figure 1. Echocardiographic assessment of left ventricular contractile function.
A significant decline in both ejection fraction (A) and fraction shortening (B) in the DOX-treated group was observed as compared with the control mice. Mice receiving nitrate supplementation had partial restoration of the contractile indices. Data are Mean±SEM (n=8 per group). * indicates P<0.05 versus all other groups; # indicates P<0.05 versus Control or Nitrate groups.
Echocardiographic assessment of ventricular contractile function
Echocardiography was performed using the Vevo770™ imaging system (VisualSonics Inc.).
Measurement of LV hemodynamics
Following the echocardiographic assessment, a micro-tip pressure-volume catheter transducer (Millar instruments Inc.) was inserted into the right carotid artery and advanced into LV cavity. LV systolic and end-diastolic pressures, maximal slope of systolic pressure increment (+dP/dtmax) and diastolic pressure decrement (−dP/dtmax), heart rate, and aortic blood pressure were recorded on a beat-by-beat basis.
Measurement of plasma nitrate and nitrite
The blood samples were collected from the mice that underwent four different treatment conditions (n=6 per group) and centrifuged to collect the plasma. The nitrate and nitrite were measured with a SIEVERS nitric oxide analyzer (model 280NOA).
Measurement of mitochondrial oxidative phosphorylation and enzyme activities
Oxidative phosphorylation was studied in the isolated mitochondria using glutamate+malate (complex I) and succinate (complex II) as substrates to localize defects within the ETC. Enzyme activities of complex I (NADH:duroquinone oxidoreductase) and NADH dehydrogenase (proximal segment of complex I) were determined.
Assessment of lipid peroxidation
Lipid peroxidation in the cardiac tissues was determined by measuring malondialdehyde and 4-hydroxyalkenals using a colorimetric assay kit (Bioxytech LPO-586, Oxis International).
Measurement of H2O2
The rate of H2O2 generation in mitochondria (n=5 per group) was determined using oxidation of fluorogenic indicator amplex red in the presence of horseradish peroxidase.
Statistical analysis
Data are presented as group mean±standard error (SEM). Statistical analysis was performed using one-way ANOVA with subsequent Student-Newman-Keuls post hoc test for pair-wise comparison among the four treatment groups. Unpaired t test was used for comparing two treatment groups in the study with subcutaneous infusion of nitrate. Probability value of P<0.05 was considered significant.
Please see the Online Appendix for a complete description of the methods.
Results
Nitrate supplementation ameliorates DOX-induced LV contractile dysfunction
LV ejection fraction and fractional shortening decreased significantly in the DOX group as compared with the control group after 5 days of treatment (Fig. 1), which improved with nitrate supplementation prior to and following DOX injection (i.e. P<0.05 Nitrate+DOX versus DOX; n=8 per group; Fig. 1). Similar results were obtained following invasive measurements with Millar catheter. The DOX-induced LV systolic (Figs. 2A, 2C) and diastolic (Figs. 2B, 2D) dysfunction was significantly improved by nitrate supplementation. In addition, heart rate (Fig. 2E) and mean aortic blood pressure (Fig. 2F) were decreased by DOX administration, which were partially attenuated by nitrate (i.e. P<0.05 Nitrate+DOX versus DOX; n=8 per group). Nitrate alone had no effect on these functional parameters (Figs. 2A to 2F).
Figure 2. Evaluation of left ventricular hemodynamic function with Millar micro-tip catheter.
A significant decline in systolic pressure (A), positive, negative dP/dtmax (C, D), heart rate (E), mean aortic blood pressure (F) and a significant increase in end-diastolic pressure (B) were observed in the DOX-treated mice as compared with the saline-treated controls. Nitrate supplementation restored the contractile function. Data are Mean±SEM (n=8 per group). * indicates P<0.05 versus all other groups; # indicates P<0.05 versus Control or Nitrate groups.
Nitrate supplementation enhances plasma levels of nitrate and nitrite
Oral nitrate supplementation enhanced plasma nitrate (Fig. 3A) and nitrite (Fig. 3B) as compared with Control group (P<0.05). DOX treatment also elevated plasma nitrate level (likely through the enhanced NOS-dependent NO synthesis triggered by DOX). In contrast, the nitrite levels did not increase in DOX-treated groups with or without nitrate supplementation (Figs. 3A, 3B), suggesting an impeded nitrate-to-nitrite conversion. The overall NO oxidation products (NOx) were increased in Nitrate or Nitrate+DOX group compared with Control or DOX group (Fig. 3C, P<0.05).
Figure 3. Assessment of plasma levels of nitrate (A), nitrite (B), and sum of nitrate and nitrite, i.e. NOx (C).
Data are Mean±SEM (n=6 per group). * indicates P<0.05 versus all other groups; # indicates P<0.05 versus DOX group.
Nitrate supplementation reduces DOX-induced cardiomyocyte death in vitro
The number of both typan blue-positive (necrotic) and TUNEL-positive (apoptotic) cardiomyocytes increased following 18 hours of exposure to 1 μM DOX in vitro (P<0.01 Control versus DOX; n=4 per group; Fig. 4). The basal cell viability was not altered with nitrate supplementation. However, cardiomyocytes from nitrate-treated mice were more resistant to DOX-induced cytotoxicity as indicated by reduction in necrosis (Fig. 4A) and apoptosis following 18 hours of DOX exposure (Fig. 4B) as compared with the DOX group (P<0.01 for necrosis and P<0.05 for apoptosis).
Figure 4. Effect of nitrate supplementation on DOX-induced cell death in isolated adult mouse cardiomyocytes.
Both necrosis (A) and apoptosis (B) were significantly increased after 18 hours of in vitro exposure to 1 μM DOX. The cardiomyocytes isolated from the mice receiving chronic nitrate supplementation were more resistant to the DOX-induced necrotic (A) and apoptotic (B) cell death. Data are Mean±SEM (n=4 per group). * P<0.01 versus DOX; # P<0.05 versus DOX; † P<0.05 versus Nitrate+DOX.
Nitrate supplementation preserves oxidative phosphorylation in mitochondria
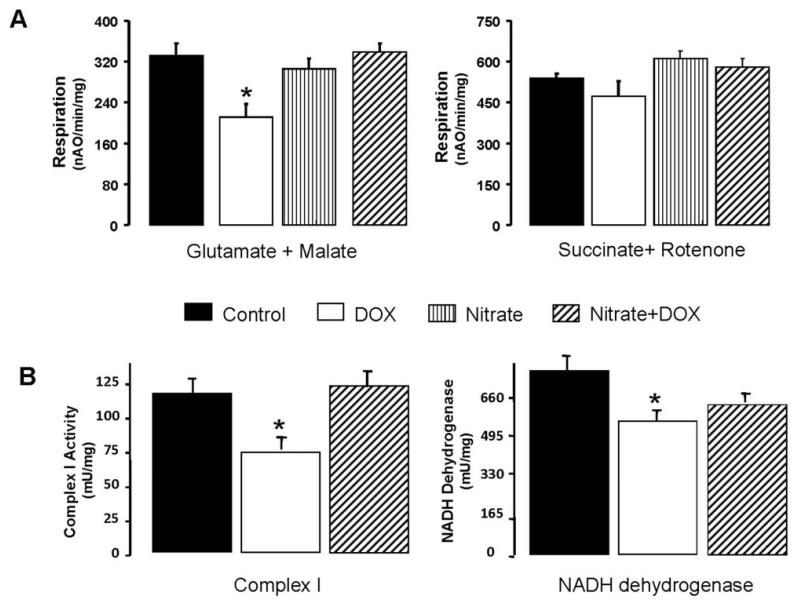
DOX interferes with mitochondrial respiration at several levels of the electron transport chain. It diverts electrons from complex I of the respiratory chain to generate the semiquinone free radical, initiating a futile redox cycle leading to a stimulation of oxygen free radical generation (27;28). To demonstrate the effect of nitrate supplementation on oxidative phosphorylation following DOX treatment, cardiac mitochondria were isolated from treated groups (see Online Fig. 1A). DOX treatment markedly decreased oxidative phosphorylation when glutamate and malate were used as complex I substrates. The rate of succinate oxidation was not altered in DOX treated group (Fig. 5A; n=6 per group). Nitrate supplementation restored oxidative phosphorylation with glutamate/malate as substrates, showing protection of complex I against DOX-induced damage (Fig. 5A).
Figure 5. Effect of nitrate supplementation on DOX-induced inhibition of mitochondrial complex I.
Graph A shows that DOX treatment decreased the rate of glutamate + malate oxidation (complex I substrate; Mean±SEM; * P<0.05 versus Saline; n=6 per group), but the succinate (complex II substrate) oxidation was not altered in the isolated mouse cardiac mitochondria. Nitrate supplementation completely prevented the impaired complex I-dependent oxidative rate. Graph B indicates that DOX treatment decreased the activities of complex I and NADH dehydrogenase in the heart tissue homogenate (Mean±SEM; * P<0.05 versus Saline; n=5 per group). Nitrate treatment maintained completely the complex I activity, whereas it only partially preserved NADH dehydrogenase activity († P<0.05 versus DOX).
Nitrate supplementation prevents DOX-induced damage of mitochondrial NADH dehydrogenase
To further localize the site of the defect in complex I, enzyme activities of complex I (NADH:duroquinone oxidoreductase) and NADH dehydrogenase (proximal part of complex I) were measured in the homogenates. DOX treatment decreased the activities of complex I and its NADH dehydrogenase (P<0.05 versus Saline; n=5 per group; Fig. 5B). Nitrate supplementation protected both complex I and its NADH dehydrogenase against DOX-induced damage.
Nitrate supplementation reduces lipid peroxidation
Lipid peroxidation in the DOX group was increased by 38% compared to control group (n=6, P<0.05, Fig. 6A), which was completely suppressed by nitrate supplementation (P>0.05 between Nitrate+DOX group and Control group; Fig. 6A). Nitrate supplementation alone had no effect on the oxidative markers.
Figure 6. Effect of nitrate supplementation on DOX-induced tissue lipid peroxidation (A) and H2O2 generation from isolated mitochondria (B).
Complex I substrate (glutamate + malate) and complex II substrate (succinate + rotenone) were utilized to identify the specific site(s) of H2O2 generation in the mitochondria. Data are Mean±SEM; n=6 per group for (A); n=5-8 per group for (B). * indicates P<0.05 versus all other groups; # indicates P<0.05 versus DOX group.
Nitrate supplementation limits DOX-induced H2O2 generation
H2O2 was increased in mitochondria isolated from DOX-treated mice as compared with the control group when glutamate + malate were used as complex I substrate (P<0.05; Fig. 6B). Nitrate significantly decreased H2O2 generation when combined with DOX. Nitrate alone had no effect on mitochondrial H2O2 generation. Similarly, nitrate supplementation attenuated DOX-induced H2O2 generation when succinate + rotenone were used as complex II substrate (Fig. 6B).
Subcutaneous infusion of nitrate fails to prevent DOX-induced LV contractile dysfunction
To determine if non-oral route of nitrate supplementation could protect against DOX cardiotoxicity, we implanted Alzet micro-osmotic pumps subcutaneously into two groups of DOX-treated mice (n=7 each group) receiving continuous infusion of either nitrate (NaNO3, 1 g/L concentration) or saline for the same 13-day duration of oral nitrate supplementation. The LV systolic (Online Figs. 2A, 2C) and diastolic (Online Figs. 2B, 2D) functional parameters were not improved by subcutaneous infusion of nitrate (P>0.05, DOX versus Nitrate+DOX). The subcutaneous infusion route increased plasma levels of nitrate (Online Fig. 2E; P<0.05, DOX versus Nitrate+DOX) while nitrite levels remained unchanged (Online Fig. 2F).
Discussion
DOX-induced cardiomyopathy remains a clinical dilemma in oncology and cardiology practices (3) and has severely limited the therapeutic potential of this potent anti-cancer drug (2). We examined the effects of chronic oral supplementation of nitrate against DOX-induced cardiomyopathy by a comprehensive approach to demonstrate protection at the level of intact organ, isolated cells and mitochondrion. Nitrate supplementation caused significant preservation of LV contractile function and decreased cardiomyocyte death through mechanisms involving inhibition of lipid peroxidation, reduction of mitochondrial complex I damage and attenuation of H2O2 generation in mice treated with DOX. These results suggest that nitrate supplementation could be a potentially novel treatment modality in alleviating DOX-induced cardiotoxicity in cancer patients.
Cardioprotective effects of nitrate supplementation: Role of NO and NOS
Nitrite and nitrate have been traditionally considered as the inert end-products of NO metabolism with limited intrinsic biological activity (29). Nitrate/nitrite have recently emerged at the forefront of NO biology because they represent a major storage form of NO in blood and tissues (30). It has been increasingly appreciated that nitrate/nitrite can be reduced to NO under tissue ischemia/hypoxia and acidosis (18;30-32). At extremely low tissue pH and PO2 during myocardial ischemia, nitrite may be reduced to NO by acidic disproportionation (15;33) or by the enzymatic reduction of xanthine oxidoreductase (34). The NOS-independent pathway of NO production is an important alternative system under pathological conditions such as myocardial ischemia, because NO production through this mechanism is critically influenced by various cofactors and presence of oxygen. Therefore, mobilization of the nitrate/nitrite/NO pathway is an important alternate means to produce NO, which in turn limits tissue injury under pathophysiological conditions. Particularly, dietary nitrate or nitrite supplementation was recently found to be beneficial against myocardial I/R injury (19;20), cardiac arrest-resuscitation, and hypertension (21). The present study has extended the potential use of nitrate supplementation in reducing myocardial damage caused by DOX. Our results provide conclusive evidence that chronic nitrate supplementation significantly improved DOX-induced LV contractile dysfunction (Figs. 1, 2), depressed central aortic pressure (Fig. 2F), and cardiomyocyte necrotic as well as apoptotic death (Fig. 4). Most importantly, these cardioprotective effects are associated with a concomitant increase in plasma levels of nitrate+nitrite (i.e. NOx, Fig. 3C) – an indicator of enhanced NO production.
The role of NOS-dependent versus NOS-independent NO generation in the nitrate-induced protection against DOX-induced cardiomyopathy remains largely elusive. Previous studies suggested a detrimental role of iNOS-derived NO in DOX-induced cardiomyopathy (35) (36). Conversely, NO is a ubiquitous signaling molecule in protection against myocardial I/R injury by ischemic or pharmacological preconditioning (6). Additionally, anthracyclines produce distinct and largely irreversible changes in levels of phosphate metabolites and substantial acidosis in the heart (37), which could potentially trigger NOS-independent protection induced by nitrite. Although the dietary nitrite supplementation induced cardioprotection against I/R injury has been reported to be eNOS independent (38), it is unclear whether this is the case with DOX-induced myocardial dysfunction. Further studies are necessary to delineate the potential role of NOS in nitrate-mediated protection against DOX cardiomyopathy.
Another interesting observation was that DOX elevated plasma nitrate levels without significant change in the nitrite levels (with or without nitrate supplementation). The reason is not clear but it could presumably have resulted from the enhanced NOS-dependent NO synthesis by DOX treatment (Fig. 3A, 3B). This would suggest an impeded nitrate-to-nitrite conversion, possibly due to the antibiotic property of DOX, which may have hindered the bacterial nitrate-to-nitrite conversion following DOX treatment. Nevertheless, the overall NO oxidation products (NOx) were significantly increased in Nitrate+DOX group as compared with DOX group (Fig. 3C; P<0.05), suggesting an association between enhanced NO production and nitrate-induced cardioprotection. It is noteworthy that even though nitrate supplementation resulted in a relatively small change in nitrite level (Fig. 3B), but it caused large increase in NOx level (Fig. 3C). The reason is not clear although it is possible that a substantial amount of NO could be generated via NOS-dependent pathway, because both iNOS and eNOS were upregulated in the heart by nitrate supplementation (unpublished data). Future studies are needed to examine the role of each of the NOS isoforms (i.e. iNOS/eNOS/nNOS) in nitrate-induced cardioprotection against DOX cardiotoxicity.
Role of nitrate in protection of mitochondrial complex I
DOX-induced cardiomyopathy occurs primarily via generation of ROS in mitochondria (24;25), a mechanism that is separate from its anti-neoplastic activity (39). Complex I and complex III of the mitochondria are the key sites for ROS generation (40) and DOX enhances ROS generation through its bioreductive activation that converts DOX to a semiquinone radical via univalent reduction (24). Complex I, especially the NADH dehydrogenase (the initial segment of complex I), is the key site for DOX bioreductive activation in cardiac cells (27;28). The increased generation of ROS from the ETC in turn damages mitochondria and induces cell injury (40;41). The cardiotoxicity of DOX is apparently dose-dependent, i.e. a low concentration of DOX selectively damages NADH dehydrogenase by increasing oxidative stress, whereas high concentration of DOX induces non-oxidative inactivation of the complexes at ETC through the formation of a DOX-cardiolipin complex (24;42). The present study showed that DOX impairs complex I by decreasing NADH dehydrogenase activity (Fig. 5), which is protected by nitrate supplementation. These data suggest that the attenuated damage at mitochondrial complex I level may at least partially explain the nitrate-induced protection against cardiotoxicity. Moreover, these results are conceptually consistent with the nitrosylation of complex I through NO generation by nitrate/nitrite (26;43). Complex I nitrosylation results in the decrease of its activity (44) thereby providing an acute, protective partial blockade of electron transport (41;44). The transient blockade of complex I during stress, including I/R, protects complex I activity and reduces cardiomyocyte injury (26). We propose that analogous blockade of electron transport during DOX exposure protects against mitochondrial and cardiac damage.
Nitrate supplementation suppresses DOX-induced oxidative stress
DOX-enhanced tissue lipid peroxidation (Fig. 6A) as well as mitochondrial H2O2 generation (Fig. 6B) were effectively diminished by chronic nitrate intake. These novel observations may represent a key mechanistic explanation for the reduction of DOX-induced cardiotoxicity by nitrate supplementation.
Oral administration of nitrate is critical for cardioprotection
An interesting observation in the current study is the necessity of the oral route for nitrate-induced protection against DOX cardiotoxicity. The chronic subcutaneous infusion of nitrate failed to significantly improve DOX-induced LV dysfunction (Online Figs. 2A-2D), despite the comparable plasma nitrate levels between the oral and subcutaneous routes of administration (Fig. 3A and Online Fig. 2E). These results suggest that the bacterial bioconversion and reduction of nitrate in the mouth and lower gastrointestinal tract is critical for initiation and/or maintenance of the cardioprotective signals induced by nitrate supplementation. Therefore oral route of inorganic nitrate administration is more effective in protection against DOX-induced cardiomyopathy.
Conclusions
We have provided the first evidence for the efficacy of chronic dietary nitrate supplementation to attenuate DOX-induced ventricular dysfunction, cell death, oxidative stress, and mitochondrial respiratory chain damage. These results are potentially important in designing clinical trial on nitrate supplementation as a prophylactic pharmacological intervention to attenuate DOX-induced cardiomyopathy in cancer patients.
Supplementary Material
Acknowledgments
This study was supported in part by the grants from the National Institutes of Health (HL51045, HL79424, HL93685 to Dr. Kukreja; AG15885 to Dr. Lesnefsky), the American Heart Association (National Scientist Development Grant #0530157N to Dr. Xi; Mid-Atlantic Affiliate Beginning Grant-in-Aid #0765273U to Dr. Das), and Medical Research Service, Department of Veterans Affairs (Merit Review Award to Dr. Lesnefsky).
Footnotes
Disclosure of the Authors’ Relationship with Industry: None
References
- 1.Bristow MR, Mason JW, Billingham ME, Daniels JR. Doxorubicin cardiomyopathy: evaluation by phonocardiography, endomyocardial biopsy, and cardiac catheterization. Ann Intern Med. 1978;88:168–75. doi: 10.7326/0003-4819-88-2-168. [DOI] [PubMed] [Google Scholar]
- 2.Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900–5. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 3.Liu X, Chen Z, Chua CC, et al. Melatonin as an effective protector against doxorubicin-induced cardiotoxicity. Am J Physiol Heart Circ Physiol. 2002;283:H254–H263. doi: 10.1152/ajpheart.01023.2001. [DOI] [PubMed] [Google Scholar]
- 4.Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: a historical overview. J Physiol Pharmacol. 2002;53:503–14. [PubMed] [Google Scholar]
- 5.O’Murchu B, Miller VM, Perrella MA, Burnett JC., Jr. Increased production of nitric oxide in coronary arteries during congestive heart failure. J Clin Invest. 1994;93:165–71. doi: 10.1172/JCI116940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol. 2001;33:1897–918. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- 7.Xi L, Jarrett NC, Hess ML, Kukreja RC. Essential role of inducible nitric oxide synthase in monophosphoryl lipid A-induced late cardioprotection: evidence from pharmacological inhibition and gene knockout mice. Circulation. 1999;99:2157–63. doi: 10.1161/01.cir.99.16.2157. [DOI] [PubMed] [Google Scholar]
- 8.Guo Y, Jones WK, Xuan YT, et al. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc Natl Acad Sci U S A. 1999;96:11507–12. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher PW, Salloum F, Das A, Hyder H, Kukreja RC. Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation. 2005;111:1601–10. doi: 10.1161/01.CIR.0000160359.49478.C2. [DOI] [PubMed] [Google Scholar]
- 10.de Nigris F, Rienzo M, Schiano C, Fiorito C, Casamassimi A, Napoli C. Prominent cardioprotective effects of third generation beta blocker nebivolol against anthracycline-induced cardiotoxicity using the model of isolated perfused rat heart. Eur J Cancer. 2008;44:334–40. doi: 10.1016/j.ejca.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Riad A, Bien S, Westermann D, et al. Pretreatment with statin attenuates the cardiotoxicity of Doxorubicin in mice. Cancer Res. 2009;69:695–9. doi: 10.1158/0008-5472.CAN-08-3076. [DOI] [PubMed] [Google Scholar]
- 12.Atar S, Ye Y, Lin Y, et al. Atorvastatin-induced cardioprotection is mediated by increasing inducible nitric oxide synthase and consequent S-nitrosylation of cyclooxygenase-2. Am J Physiol Heart Circ Physiol. 2006;290:H1960–H1968. doi: 10.1152/ajpheart.01137.2005. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda U, Shimpo M, Ikeda M, Minota S, Shimada K. Lipophilic statins augment inducible nitric oxide synthase expression in cytokine-stimulated cardiac myocytes. J Cardiovasc Pharmacol. 2001;38:69–77. doi: 10.1097/00005344-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Jansson EA, Huang L, Malkey R, et al. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat Chem Biol. 2008;4:411–7. doi: 10.1038/nchembio.92. [DOI] [PubMed] [Google Scholar]
- 15.Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med. 1995;1:804–9. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 16.Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med. 2004;37:395–400. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 17.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci U S A. 2004;101:13683–8. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cosby K, Partovi KS, Crawford JH, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 19.Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2007;104:19144–9. doi: 10.1073/pnas.0706579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duranski MR, Greer JJ, Dejam A, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–40. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuchiya K, Kanematsu Y, Yoshizumi M, et al. Nitrite is an alternative source of NO in vivo. Am J Physiol Heart Circ Physiol. 2005;288:H2163–H2170. doi: 10.1152/ajpheart.00525.2004. [DOI] [PubMed] [Google Scholar]
- 22.Gilchrist M, Winyard PG, Benjamin N. Dietary nitrate--good or bad? Nitric Oxide. 2010;22:104–9. doi: 10.1016/j.niox.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Nicolay K, Fok JJ, Voorhout W, Post JA, De Kruijff B. Cytofluorescence detection of adriamycin-mitochondria interactions in isolated, perfused rat heart. Biochim Biophys Acta. 1986;887:35–41. doi: 10.1016/0167-4889(86)90119-9. [DOI] [PubMed] [Google Scholar]
- 24.Berthiaume JM, Wallace KB. Adriamycin-induced oxidative mitochondrial cardiotoxicity. Cell Biol Toxicol. 2007;23:15–25. doi: 10.1007/s10565-006-0140-y. [DOI] [PubMed] [Google Scholar]
- 25.Chaiswing L, Cole MP, Ittarat W, Szweda LI, St Clair DK, Oberley TD. Manganese superoxide dismutase and inducible nitric oxide synthase modify early oxidative events in acute adriamycin-induced mitochondrial toxicity. Mol Cancer Ther. 2005;4:1056–64. doi: 10.1158/1535-7163.MCT-04-0322. [DOI] [PubMed] [Google Scholar]
- 26.Shiva S, Sack MN, Greer JJ, et al. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089–102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies KJ, Doroshow JH. Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by NADH dehydrogenase. J Biol Chem. 1986;261:3060–7. [PubMed] [Google Scholar]
- 28.Doroshow JH, Davies KJ. Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J Biol Chem. 1986;261:3068–74. [PubMed] [Google Scholar]
- 29.Lauer T, Preik M, Rassaf T, et al. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci U S A. 2001;98:12814–9. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gladwin MT, Raat NJ, Shiva S, et al. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. Am J Physiol Heart Circ Physiol. 2006;291:H2026–H2035. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- 31.Bryan NS, Rassaf T, Maloney RE, et al. Cellular targets and mechanisms of nitros(yl)ation: an insight into their nature and kinetics in vivo. Proc Natl Acad Sci U S A. 2004;101:4308–13. doi: 10.1073/pnas.0306706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tiravanti E, Samouilov A, Zweier JL. Nitrosyl-heme complexes are formed in the ischemic heart: evidence of nitrite-derived nitric oxide formation, storage, and signaling in post-ischemic tissues. J Biol Chem. 2004;279:11065–73. doi: 10.1074/jbc.M311908200. [DOI] [PubMed] [Google Scholar]
- 33.Zweier JL, Samouilov A, Kuppusamy P. Non-enzymatic nitric oxide synthesis in biological systems. Biochim Biophys Acta. 1999;1411:250–62. doi: 10.1016/s0005-2728(99)00018-3. [DOI] [PubMed] [Google Scholar]
- 34.Godber BL, Doel JJ, Sapkota GP, et al. Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J Biol Chem. 2000;275:7757–63. doi: 10.1074/jbc.275.11.7757. [DOI] [PubMed] [Google Scholar]
- 35.Weinstein DM, Mihm MJ, Bauer JA. Cardiac peroxynitrite formation and left ventricular dysfunction following doxorubicin treatment in mice. J Pharmacol Exp Ther. 2000;294:396–401. [PubMed] [Google Scholar]
- 36.Pacher P, Liaudet L, Bai P, et al. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation. 2003;107:896–904. doi: 10.1161/01.cir.0000048192.52098.dd. [DOI] [PubMed] [Google Scholar]
- 37.Ng TC, Daugherty JP, Evanochko WT, Digerness SB, Durant JR, Glickson JD. Detection of antineoplastic agent induced cardiotoxicity by 31P NMR of perfused rat hearts. Biochem Biophys Res Commun. 1983;110:339–47. doi: 10.1016/0006-291x(83)91301-3. [DOI] [PubMed] [Google Scholar]
- 38.Bryan NS, Calvert JW, Gundewar S, Lefer DJ. Dietary nitrite restores NO homeostasis and is cardioprotective in endothelial nitric oxide synthase-deficient mice. Free Radic Biol Med. 2008;45:468–74. doi: 10.1016/j.freeradbiomed.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyers PA, Gorlick R, Heller G, et al. Intensification of preoperative chemotherapy for osteogenic sarcoma: results of the Memorial Sloan-Kettering (T12) protocol. J Clin Oncol. 1998;16:2452–8. doi: 10.1200/JCO.1998.16.7.2452. [DOI] [PubMed] [Google Scholar]
- 40.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–44. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Q, Camara AK, Stowe DF, Hoppel CL, Lesnefsky EJ. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol. 2007;292:C137–C147. doi: 10.1152/ajpcell.00270.2006. [DOI] [PubMed] [Google Scholar]
- 42.Bergh J. Best use of adjuvant systemic therapies II, chemotherapy aspects: dose of chemotherapy-cytotoxicity, duration and responsiveness. Breast. 2003;12:529–37. doi: 10.1016/s0960-9776(03)00162-0. [DOI] [PubMed] [Google Scholar]
- 43.Selemidis S, Dusting GJ, Peshavariya H, Kemp-Harper BK, Drummond GR. Nitric oxide suppresses NADPH oxidase-dependent superoxide production by S-nitrosylation in human endothelial cells. Cardiovasc Res. 2007;75:349–58. doi: 10.1016/j.cardiores.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 44.Burwell LS, Nadtochiy SM, Tompkins AJ, Young S, Brookes PS. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem J. 2006;394:627–34. doi: 10.1042/BJ20051435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.