Abstract
We treated patients under age 50 years with 131I-anti-CD45 antibody combined with fludarabine and 2 Gy total body irradiation to create an improved hematopoietic cell transplantation (HCT) strategy for advanced acute myeloid leukemia or high-risk myelodysplastic syndrome patients. Fifteen patients received 332–1,561 mCi of 131I, delivering an average of 27 Gy to bone marrow, 84 Gy to spleen, and 21 Gy to liver. Although a maximum dose of 28 Gy was delivered to the liver, no dose-limiting toxicity was observed. Marrow doses were arbitrarily capped at 43 Gy to avoid radiation-induced stromal damage; however no graft failure or evidence of stromal damage was observed. Twelve patients (80%) developed Grade II graft-versus-host disease (GVHD), one patient developed Grade III GVHD, and no patients developed Grade IV GVHD during the first 100 days after HCT. Of the 12 patients with chronic GVHD data, 10 developed chronic GVHD, generally involving the skin and mouth. Six patients (40%) are surviving after a median of 5.0 years (range, 4.2 to 8.3 years). The estimated survival at 1 year was 73% among the 15 treated patients. Eight patients relapsed, 7 of whom subsequently died. The median time to relapse among these 8 patients was 54 days (range, 26 to 1364 days). No cases of non-relapse mortality were observed in the first year after transplant. However, two patients died in remission from complications of chronic GVHD and cardiomyopathy, at 18 months and 14 months after transplant, respectively. This study suggests that patients may tolerate myeloablative doses >28 Gy delivered to the liver using 131I-anti-CD45 antibody in addition to standard reduced intensity conditioning. Moreover, the arbitrary limit of 43 Gy to the marrow may be unnecessarily conservative, and continued escalation of targeted radioimmunotherapy doses may be feasible to further reduce relapse.
Keywords: radioimmunotherapy, AML, MDS, hematopoietic cell transplant, anti-CD45 antibody, Iodine-131
INTRODUCTION
Innovative therapeutic approaches are needed to reduce the morbidity and high-relapse rates of patients with advanced leukemia following myeloablative hematopoietic cell transplantation (HCT). Increasing radiation or chemotherapy doses of preparative regimens have historically decreased relapse rates, often at the cost of increased non-relapse mortality (NRM).1,2 We have has developed a strategy using 131I-labeled anti-CD45 antibody (Ab; BC8) to deliver targeted hematopoietic irradiation to the bone marrow, spleen, and lymph nodes in an effort to decrease relapse without increasing NRM.3–5 CD45 is a tyrosine phosphatase stably expressed on virtually all leukocytes at approximately 200,000 copies/cell and found on approximately 90% of acute leukemias, but absent from non-hematopoietic tissues.6–10 Pre-clinical and clinical studies have shown that 131I-BC8 Ab can deliver 2–3 times more radiation to sites of leukemic involvement compared to unaffected normal organs, and this targeted, highdose radiation does not significantly increase toxicity when added to myeloablative conditioning regimens.3,11–13
Reduced-intensity preparative regimens have been developed as an alternative approach for older patients and those with co-morbidities that might prevent them from undergoing a myeloablative HCT procedure. Standard reduced intensity regimens, however, have been commonly associated with higher relapse rates in patients with high burdens of active leukemia at the time of transplant. In view of the minimal added toxicity associated with targeted radiotherapy, we previously combined 131I-BC8 Ab with a reduced-intensity conditioning regimen in an effort to decrease relapse for older patients with advanced myeloid malignancies.14 Fifty-eight patients aged 50 years or older with advanced AML (beyond first remission) or high-risk MDS (≥5% marrow blasts at the time of HCT) were transplanted after delivery of 131I-BC8 Ab in conjunction with 90 mg/m2 of fludarabine (FLU) and 2 Gy total-body irradiation (TBI).14 In this study 86% of the patients had AML in active relapse or MDS with more than 5% blasts in their marrow by morphology at the time of HCT. Twenty patients (35%) with de novo AML had primary refractory disease (n=8) or relapsed refractory disease (n=12) at the time of conditioning and all 19 patients with secondary AML or MDS had greater than 5% blasts in the marrow at the time of conditioning. All patients achieved a complete remission, as well as 100% donor chimerism in the CD3 and CD33 compartments by day 28. The maximum tolerated dose (MTD) was estimated to be 24 Gy delivered by 131I-BC8 Ab to the normal organ receiving the highest dose, with renal insufficiency and cardiopulmonary toxicities being dose-limiting. This study suggested that 131I-anti-CD45 targeted radiotherapy could be safely integrated into a reduced-intensity conditioning regimen for older patients with advanced myeloid malignancies. We report here a similar strategy in younger patients (ages 16–50 years) with advanced AML or high-risk MDS with the goal of defining the MTD in this age group and to create an HCT approach with greater anti-tumor control and minimal added toxicities compared to standard ablative regimens.
METHODS
Patient and Donor Selection
Patients between the age of 16 and 50 years were eligible if they had advanced AML (defined as beyond first remission, primary refractory, relapsed with >5% marrow blasts by morphology, or evolved from previous myeloproliferative neoplasm or MDS), MDS with >5% blasts in the marrow, or chronic myelomonocytic leukemia-2 (CMML-2), and if they had HLA-matched related or unrelated donors. Additional eligibility criteria were the same as those in our prior study among similar patients over the age of 50.14 Matching for related donors involved intermediate-resolution molecular typing for HLA-A, -B, -C, and -DQB1 and high-resolution typing for -DRB1, according to our Center’s standard practice guidelines. High-resolution typing of HLA-A, -B, -C, and -DRB1 and intermediate-resolution typing of DQB1 was used for allele matching of eligible unrelated donors. Both related and unrelated donors were allowed to have a single-allele mismatch at any of the HLA-A, -B, or –C loci. DNA sequencing or oligonucleotide hybridization was used to type the peripheral blood stem cell (PBSC) donors.15 HCT comorbidity indices (HCT-CI) were calculated for patients as previously described.16 All patients signed consent forms approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center (FHCRC). NCI Clinical Trials Network registration: NCT00119366.
Production of Radiolabeled Antibody, Biodistribution, and Dosimetry
The radiolabeled BC8 Ab (a murine IgG1 Ab to CD45) was produced, labeled with 131I (New England Nuclear, Boston, MA, specific activity ~8.0 Ci/mg) and tested in the Biologics Production Facility at the FHCRC as previously described.3 Patients were screened for human anti-mouse Ab (HAMA) using an enzyme-linked immunosorbent assay (ELISA) as previously described.14 Thyroid uptake of free 131I was blocked by the administration of oral Lugol’s solution (iodine/potassium iodide solution) starting two days prior to the biodistribution dose and continuing for three weeks following the therapeutic dose of 131I-BC8 Ab. A trace-labeled infusion of 5 mCi 131I-labeled BC8 Ab was first given to determine the biodistribution of Ab and to estimate radiation-absorbed doses to marrow, spleen, and non-target organs delivered per millicurie (mCi) of 131I as previously described.4,14,17–19 Methods consistent with those recommended by the Society of Nuclear Medicine’s and Molecular Imaging’s special committee on Medical Internal Radiation Dose (MIRD) were used to determine the radiation absorbed doses, as previously described.20
Therapy
Regardless of the biodistribution study results, all patients were eligible to receive a therapy dose of 131I-BC8 since the estimated radiation doses delivered to marrow and spleen in previous studies were greater than doses to lung, kidney and total body, even among the few patients whose marrow dose was slightly lower than liver dose.3,5 The therapeutic BC8 Ab was labeled with the amount of 131I calculated to deliver the desired dose to the normal organ (almost always liver) estimated to receive the highest radiation dose, unless that would result in an estimated marrow dose of >43 Gy, which was similar to our previous study of older patients transplanted for advanced myeloid malignancies.14 Briefly, patients were isolated in lead-lined rooms until radiation exposure was ≤7 mR/hour at 1 meter (median 6, range, 2–11 days). FLU 30 mg/kg/day was given intravenously (i.v.) on days −4, −3, and −2, followed by TBI (2 Gy; 0.06–0.07 Gy/min from a linear accelerator) and subsequent infusion of unmanipulated, mobilized PBSC on day 0. Cyclosporine (CSP) and mycophenolate mofetil (MMF) were used for graft-versus-host disease (GVHD) prophylaxis as previously described.14 All patients received prophylaxis against veno-occlusive disease with ursodiol.21
Dose-Adjustment Schema and Statistical Analysis
The primary objective of this study was to estimate the MTD of 131I-BC8 Ab used in combination with FLU/2 Gy TBI. The MTD was defined as the dose delivered to the normal organ receiving the highest radiation exposure that was associated with a dose-limiting toxicity (DLT) rate of 25% using the Bearman criteria, developed specifically for HCT patients. DLT was defined as a Bearman grade III or grade IV regimen-related toxicity occurring up to day 100 following HCT.22 A two-stage approach described by Storer et al. was planned for dose adjustment.23 In stage I, each successive patient received 2 Gy more radiation to the dose-limiting normal organ than the previous patient until the first DLT was observed. Stage II was then initiated at the next lower dose level, treating patients in cohorts of 4. Dose-escalation or de-escalation was carried out to allow for a maximum of 20 patients enrolled in the second stage as previously described.14 The first patient received 12 Gy to the organ receiving the highest radiation absorbed dose and did not experience a DLT. After analysis of patients older than 50 years treated with the same regimen revealed an estimated MTD of 24 Gy,14 the next patient in this trial was treated at a dose of 22 Gy. Due to the high-risk features of this patient population, we did not delay enrollment until all previously treated patients in a cohort could be evaluated for DLT. Therefore, additional patients could be enrolled at the current dose level and their outcome did not affect the dose adjustment decision, unless required for safety reasons. Accrual in some instances was fairly rapid, resulting in more than 1 patient enrolled at each dose level, despite the absence of observed DLTs.
Secondary objectives included an examination of potential efficacy in the context of a dose-finding study. For these purposes, overall survival (OS) and relapse-free survival (RFS) were estimated according to the Kaplan and Meier method, and relapse and NRM were summarized using cumulative incidence estimates. NRM was considered a competing risk for relapse, and relapse was treated as a competing risk for NRM.
RESULTS
Patient Characteristics
Of the sixteen patients enrolled on study, one patient developed gram-negative bacteremia after dosimetry and did not receive the therapeutic infusion of 131I-BC8 Ab. Fifteen patients with a median age of 39 years (range, 23–49) and an average HCT-CI score of 2.7 (range, 0–5) received a therapeutic infusion of 131I-BC8 Ab followed by FLU, 2 Gy TBI and allogeneic HCT. Five of these patients had primary refractory or relapsed refractory AML (all with circulating CD45+ blasts present at time of BC8 infusion), 9 patients had AML in second complete remission and 1 patient had CMML-2. Four patients had high-risk, 10 had intermediate-risk, and 1 had favorable-risk cytogenetics according to the Southwest Oncology Group criteria.24 Eight patients had HLA-matched related donors, and 7 patients had HLA-matched unrelated donors (Table 1).
Table 1.
Patient Characteristics
| Patient Number | Age/ Gender | Disease Status at Treatment | Cytogenetic Risk Stratification* | HCT-CI | Planned Dose to Liver (or Marrow) | Donor Status | Grade Acute GVHD | Current Status (days after HCT) |
|---|---|---|---|---|---|---|---|---|
| 1 | 34/F | AML-Primary Refractory | INT | 4 | 12 Gy | MUD | 2 | Relapse day 26: died day 42 |
| 2 | 48/M | AML-2nd Remission | HIGH | 0 | 22 Gy | MUD | 2 | Alive and disease-free: 1897 days |
| 3 | 49/M | AML-Relapsed Refractory | INT | 1 | 22 Gy | MUD | 2 | Alive and disease-free: 1863 days |
| 4 | 39/F | AML-2nd Remission | HIGH | 5 | 24 Gy | MRD | 2 | Alive and disease-free: 3025 days |
| 5 | 23/M | AML-2nd Remission | UNK | 5 | 24 Gy | MUD | 2 | Died of cardiomyopathy on day 437 (in remission) |
| 6 | 29/M | AML-2nd Remission | HIGH | 4 | 24 Gy | MUD | 2 | Relapse day 1364: Died day 1922 |
| 7 | 43/F | AML-2nd Remission | INT | 2 | 43 Gy | MRD | 2 | Died of Grade IV GVHD on day 544 (in remission) |
| 8 | 48/M | AML-2nd Remission | INT | 5 | 26 Gy | MRD | 2 | Relapse day 29: died on day 191 of fungal pneumonia |
| 9 | 48/M | MDS/AML– 2nd Remission | INT | 1 | 43 Gy | MRD | 2 | Relapse day 390: Died day 1620 |
| 10 | 30/F | AML-2nd Remission | INT | 1 | 43 Gy | MRD | 1 | Alive and disease-free: 1798 days |
| 11 | 37/M | AML-2nd Remission | INT | 1 | 28 Gy | MUD | 2 | Relapse day 1072: Died day 1110 |
| 12 | 40/F | CMML-2 | INT | 2 | 28 Gy | MUD | 3 | Alive and disease-free: 1709 days |
| 13 | 43/F | MDS/AML-Primary Refractory | HIGH | 3 | 43 Gy | MRD | 0 | Relapse day 27: Died day 307 Relapse day |
| 14 | 25/M | AML-Primary Refractory | HIGH | 4 | 28 Gy | MRD | 2 | Relapse day 78: Alive at day 1549 |
| 15 | 39/M | AML-Primary Refractory | HIGH | 3 | 28Gy | MRD | 2 | Relapse day 28: Died day 91 |
According to SWOG criteria.24 MRD = Matched Related Donor; MUD = Matched Unrelated Donor
Toxicities and GVHD
Despite pre-medication, Grade 2–3 Ab-related infusion reactions (i.e., fever and chills) were observed in eight of 15 patients, however, they resolved by the end of each infusion. Notably, no CTC Grade 4 Ab-related side effects were observed. Hepatic veno-occlusive disease (VOD) was not observed in any of the 15 patients treated, despite delivering an average of 21 Gy to the liver via 131I-BC8 Ab. No patient developed Grade IV GVHD during the first 100 days after HCT; however, 1 patient developed Grade III GVHD and 12 patients developed Grade II GVHD (Table 3).25,26 Two patients had elevated concentrations of TSH in the blood by day 100 after HCT, consistent with results from our prior study.14 Data regarding chronic GVHD was available for 12 of the 15 patients, since two patients died prior to day 100, and one patient was lost to follow-up. Of the 12 patients with chronic GVHD data, 10 of these patients had documented evidence of chronic GVHD, most commonly involving the skin and mouth. Six of the patients who developed chronic GVHD had matched unrelated donors and four of these patients had matched related donors.
Table 3.
Acute GVHD in Patients Receiving 131I-BC8/FLU/TBI and HLA-matched Related and Unrelated Allogeneic HCT
| Patient Number | Acute GVHD Grade | Acute GVHD Skin Stage | Acute GVHD Gut Stage | Acute GVHD Liver Stage |
|---|---|---|---|---|
| 1 | 2 | 1 | 1 | 0 |
| 2 | 2 | 3 | 1 | 0 |
| 3 | 2 | 3 | 0 | 0 |
| 4 | 2 | 0 | 1 | 0 |
| 5 | 2 | 3 | 0 | 0 |
| 6 | 2 | 2 | 1 | 0 |
| 7 | 2 | 0 | 1 | 0 |
| 8 | 2 | 3 | 1 | 0 |
| 9 | 2 | 3 | 1 | 0 |
| 10 | 1 | 2 | 0 | 0 |
| 11 | 2 | 1 | 1 | 0 |
| 12 | 3 | 3 | 0 | 2 |
| 13 | 0 | 0 | 0 | 0 |
| 14 | 2 | 3 | 1 | 0 |
| 15 | 2 | 2 | 1 | 0 |
Biodistribution, Dosimetry, and Engraftment
Approximately 9 days after the biodistribution study, patients received BC8 Ab labeled with the amount of 131I activity calculated to deliver an estimated 12–28 Gy to the normal organ receiving the highest dose (i.e., typically the liver). The mean absorbed dose per unit administered activity (cGy/mCi ± S.D.) for the 15 treated patients was 5.1 ± 4 to the bone marrow, 3.5 ± 1.0 to liver, 15.8 ± 7.2 to spleen, 0.5 ± 0.2 to kidney, 0.3 ± 0.1 to lung, and 0.4 ± 0.1 to the total body (Fig. 1). The mean absorbed doses to the bone marrow and spleen were 24 ± 15 and 76 ± 22 Gy for patients with recurrent AML after HCT, respectively, compared to 27 ± 14 and 91 ± 39 Gy for those without recurrent disease, respectively. The 131I activity administered at each dose level and calculated absorbed doses to marrow and spleen are summarized in Table 2.
Fig. 1.
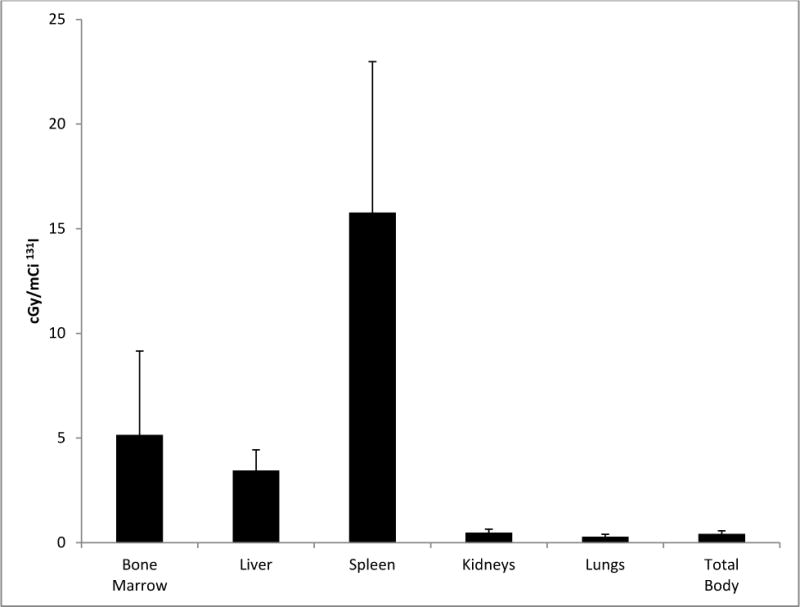
Estimated radiation absorbed doses per millicurie of 131I administered for all 15 patients.
Table 2.
131I Activity Administered, Total Absorbed Radiation Doses, and Grade 3 or 4 Regimen-Related Toxicities.
| Dose to liver (Gy)* | mCi 131Ia [MBq] | Dose to marrow (Gy) | Dose to spleen (Gy) | DLT/no. of patients treated |
|---|---|---|---|---|
| 12 | 345 [12765] | 12.0 | 15.3 | 0/1 |
| 22 | 461.0 ± 9.9 (454–468) [17057 ± 366.3 (16798–17316)] | 19.2 ± 0.4 (18.9–19.5) | 121.5 ± 38.8 (94.1–148.9) | 0/2 |
| 24 | 981.3 ± 510.6 (598–1561) 39309.3 ± 18893.7 (22126–57757)] | 15.3 ± 5.3 (9.3–18.9) | 69.82 ± 39 (32–109.9) | 0/3 |
| 26 | 479 ± 80.6 (422–536) [17723 ± 2982.6 (15614–19832)] | 24.6 ± 26.4 (6.0–43.3) | 88.6 ± 1.2 (87.8–89.5) | 0/2 |
| 28 | 655.7 ± 277.1 (332–1029) 24259.8 ± 10250.8 (12284–38073)] | 34.0 ± 13.1 (14.8–43.1) | 79.5 ± 21.5 (56.4–111.2) | 0/7 |
Mean ± SD (range).
Protocol modified to increase starting dose to 22 Gy to organ receiving highest dose after 24 Gy found to MTD in patients older than 50 treated with the same regimen.14
Despite the lack of any Grade III/IV DLTs observed among the 15 patients treated, including 7 treated at the highest dose, the BC8 Ab was not labeled with higher amounts of 131I that would deliver >28 Gy to any critical normal organ due to concerns of potential damage to the Abavidity and function. Dosimetry showed that the planned amount of 131I-BC8 would deliver more than 43 Gy to the marrow in 1 of the 2 patients treated at the 26 Gy dose level (liver) and 3 of the 7 patients treated at the 28 Gy dose level (liver). Due to theoretical concerns for graft failure the radiation absorbed dose from 131I-BC8 Ab in these 4 patients was capped at the highest dose that would deliver the equivalent of 43 Gy to the marrow.
Notably, however, evidence of delayed engraftment or marrow stromal damage was not observed among the 15 treated patients. Transfusion-independent platelet counts surpassed 20,000/μL after a median of 12 days (range, 10–27) and sustained neutrophil counts surpassed 500/μL after a median of 15 days (range, 13–22) after HCT. Chimerism studies documented ≥93% donor-derived cells in the CD33+ and CD3+ compartments of the blood by day 28 after HCT in 13 patients. One patient lacked day 28 chimerism data but had 100% donor chimerism in both compartments by day 56. The only patient who did not achieve ≥93% donor chimerism by day 28 was transplanted for primary refractory AML with high-risk cytogenetics and relapsed on day 27 after HCT.
Overall and Relapse-free Survival, Non-Relapse Mortality, and Relapse
Of the 15 patients who received a therapeutic infusion of 131I-BC8 Ab, 6 patients are still alive, with a median follow-up among them of 5.0 years (range, 4.2 to 8.3 years). Median OS and RFS (the time at which the Kaplan-Meier estimates cross 50%) among these 15 patients was 4.4 years and 1.5 years, respectively. The estimated survival at 1 year was 73% among the 15 treated patients (Fig. 2). The estimated probabilities of relapse and relapse-free survival at 1 year were 33% and 67%, respectively. Figure 2 summarizes the probabilities of OS, RFS, relapse, and NRM among all 15 patients. Eight patients relapsed, 7 of whom subsequently died. The median time to relapse among these 8 patients was 54 days (range, 26 to 1364 days). Of the 5 patients with relapsed/refractory disease and circulating CD45+ blasts at time of HCT, four patients relapsed at a median of 26.5 days (range, 26 to 78 days), with one patient remaining disease-free at 1,863 days after HCT (Table. 1). No cases of NRM were observed in the first year after transplant; however, two patients died in remission, one from complications of chronic GVHD 18 months after HCT and one from complications of cardiomyopathy 14 months after transplant.
Fig. 2.
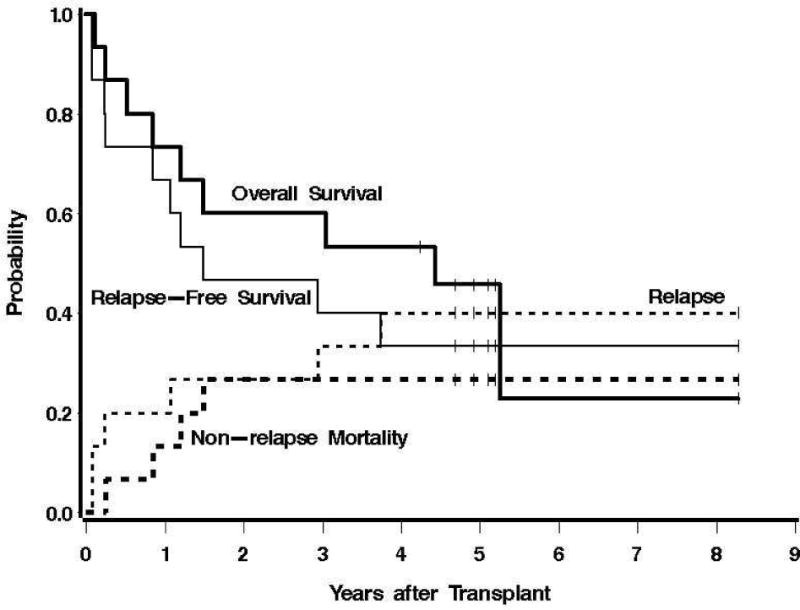
Estimates of the probability of OS, RFS, NRM, and relapse among all patients who received a therapeutic dose of 131I-BC8 Ab followed by TBI/FLU.
DISCUSSION
Our group has previously shown encouraging results evaluating the safety and feasibility of combining 131I-BC8 Ab with the non-myeloablative conditioning regimen using 2 Gy TBI and FLU in older patients with advanced myeloid malignancies.14 The trial described here extends the applicability of 131I-anti-CD45 Ab added to FLU/2 Gy TBI as part of allogeneic HCT conditioning to younger patients with advance myeloid malignancies. Administration of 131I-BC8 Ab delivered an estimated average of 26 Gy to the marrow and 82 Gy to the spleen, which were 1.2 and 3.9 times greater than the absorbed dose delivered to the liver, respectively. Due to a theoretical concern for stromal damage and marrow failure this protocol capped the maximum dose delivered to the marrow at 43 Gy. Similar to results in our previous trials, there was a wide range of organ specific radiation delivered by 131I-BC8 Ab, with hepatic doses ranging from 1.5 to 4.8 cGy/mCi 131I in order to deliver 12–28 Gy to the liver.3,5 Although we previously estimated a MTD of 24 Gy in our study with older patients,14 no Grade III/IV DLTs were observed in this trial despite targeted doses of up to 28 Gy to the liver. This suggests that younger patients may tolerate higher hepatic radiation absorbed doses from 131I-anti-CD45 Ab in addition to a standard reduced intensity conditioning regimen.
There were no cases of NRM in the first year following transplant among the 15 patients treated, suggesting that this transplant conditioning strategy may enhance the efficacy of reduced-intensity allogeneic HCT for patients with AML or MDS. Nonetheless, the most frequent cause of death in these high-risk patients was relapse, with 47% of patients dying from recurrent disease. In addition, 4 of the 5 patients transplanted with active disease relapsed within the first 100 days after HCT. A dose limit of 43 Gy delivered to the marrow was included in the trial based on results of studies using standard radiotherapy showing that doses above 45 Gy results in stromal damage that lead to long-term local marrow hypoplasia.39 The absence of any evidence of delayed hematopoietic stem cell engraftment or marrow failure in this trial suggests that the arbitrary limit of 43 Gy to the marrow delivered by low-dose rate radioimmunotherapy (RIT) imposed by this protocol may have been unnecessarily conservative and that further escalation of the systemic dose of targeted RIT with a much higher marrow dose cap may be advisable to further reduce the relapse rate.
Although this study treated a limited number of high-risk patients under the age of 50, the results appear encouraging considering the high-risk cohort enrolled, including 33% of patients with circulating blasts at the time of transplant conditioning. We therefore conducted a review of FHCRC historical data among similar patients. Among 94 patients aged 18–50 with AML beyond first complete remission who were transplanted using myeloablative regimens at our Center over the past 10 years, the estimated 1 year OS and RFS were 64% and 53%, respectively, with an estimated relapse rate at 1 year of 32%. We note that 8 of the 15 patients treated on our study had HCT-CI scores ≥3 and all patients received HCT for AML that was beyond first remission. Additional retrospective data estimates the 1 year outcomes for comparable patients treated without radiolabeled Ab, with an NRM of approximately 40%, a relapse rate of 35% and an OS of approximately 32%.27 The NRM and survival data from this radiolabeled Ab trial suggest that patients with high-risk myeloid malignancies may have similar (or perhaps better) outcomes combining RIT with FLU/2 Gy TBI compared to those obtained using standard myeloablative regimens.
To more widely advance 131I-anti-CD45 Ab based therapy, an upcoming, multi-institution, randomized phase III trial for older patients with advanced myeloid malignancies (http://www.actiniumpharmaceuticals.com) is in development. Nonetheless, the optimal antigen, radiation moiety, and delivery method to optimize an RIT regimen for HCT remain under investigation. Previous clinical experience demonstrated the feasibility of integrating radioimmunoconjugates targeting CD66, CD45, or CD33 antigens into treatment regimens for leukemias.28 Our study employed the therapeutic radionuclide 131I, which emits beta particles with a 0.8 mm path length to CD45 antigen-positive cells as well as surrounding antigen-negative cells in hematolymphoid tissue. However, the high-energy gamma component of 131I poses a radiation risk for staff and family, requiring patients to be treated in radiation isolation. RIT studies have now advanced to employ pure beta-emitters such as 90Y that delivers high energy (2.3 MeV) with a relatively short half-life (2.7 days) that lacks gamma emissions, thus eliminating the need for radiation isolation.29–32 To further intensify therapy and reduce relapse, we most recently focused on pre-clinical studies using anti-CD45 Ab labeled with alpha-emitters (213Bi, 211At) in HCT conditioning regimens.33–35 These alpha-emitters deposit higher energy over a much shorter distance compared to beta-emitters, providing single-cell kill while sparing normal surrounding tissue.29 We anticipate that this increased cell-specific potency will provide less off-target toxicity, resulting in an approach that decreases relapse rates and is better tolerated. Alpha-emitters may prove particularly useful for minimal-residual disease or extramedullary disease, as well as in the non-HCT setting if conjugated to select target antigens. To this end, RIT employing the alpha-emitter 225Ac conjugated to a CD33 Ab remains under evaluation as a single-agent or in combination for MDS and AML patients of various ages and disease states (NCI Clinical Trials Network registration NCT01756677 and NCT00672165).
As noted in our study there are limitations, however, to the amount of radiation delivered by conventional RIT. In particular, the long circulating half-life of a radiolabeled Ab increases normal organ radiation exposure, limiting further dose-escalation. To address this issue, one new pretargeted approach under investigation involves administration of the monoclonal Ab followed by separate administration of the radiolabeled moiety. Our pretargeted strategy capitalizes on the well-established streptavidin-biotin affinity and allows for rapid localization of radiolabeled biotin after maximal accumulation of streptavidin-bound Ab at target sites. Preclinical studies using both beta- or alpha-emitters have shown that a pretargeted strategy can deliver more than three times the target-specific absorbed radiation dose compared to conventional RIT.36–38 We have translated these results to the clinic, where a pretargeted RIT trial is on-going for patients with advanced leukemia or MDS requiring HCT (NCI Clinical Trials Network registration: NCT00988715). Future integration of alpha-emitters with a pre-targeted strategy will likely increase the potency of leukemia-specific cytotoxicity, possibly with an improved toxicity profile. We anticipate the expansion of RIT feasibility and maximization of its effect through the use of alpha-emitters and pretargeted technology in future studies, with the ultimate goal of improving the tolerability and outcomes following allogeneic HCT for patients with advanced leukemia.
Acknowledgments
The authors are indebted to Nathan Holm for his expert data management, Jennifer Davies and Monina Almeda for their comprehensive management of regulatory affairs, Sujit Pal and Carolyn Thostensen for their radiolabeling technical assistance and to Sally Lundberg, RN and Robyn Haaf, RN for their outstanding patient directed care. We also acknowledge the excellent care provided to these patients by the physicians and nurses of the HCT teams, as well as the work of the staff in the Long Term Follow-up office.
FINANCIAL SUPPORT
This work was supported by National Institute of Health grants PO1CA44991, CA138720 CA109663, CA78902, CA15704, HL36444, CA18029, a SCOR grant from the Leukemia and Lymphoma Society of America, the Edson Foundation, and the Frederick Kullman Memorial Fund. JMP is a recipient of Career Development Awards form the Lymphoma Research Foundation and ASCO, and is a Scholar of the Damon Runyon Cancer Research Foundation. AKG is a Scholar in Clinical Research from the Leukemia and Lymphoma Society.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
Conception and Design: RM, TAG, JGR, DRF, AKG, FRA, OWP, and JMP
Data collection: RM, DRF, JGR, MLS, and JMP
Data Analysis: RM, TAG, JGR, DRF, MLS, OWP, and JMP
Data Interpretation: All authors
Manuscript writing: All authors
Approval of final manuscript: All authors
CONFLICT OF INTEREST DISCLOSURES
The authors declare no competing financial interests related to this work.
References
- 1.Clift RA, Buckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with acute myeloid leukemia in first remission: a randomized trial of two irradiation regimens. Blood. 1990;76(9):1867–1871. [PubMed] [Google Scholar]
- 2.Clift RA, Buckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with chronic myeloid leukemia in the chronic phase: a randomized trial of two irradiation regimens. Blood. 1991;77(8):1660–1665. [PubMed] [Google Scholar]
- 3.Matthews DC, Appelbaum FR, Eary JF, et al. Phase I study of (131)I-anti-CD45 antibody plus cyclophosphamide and total body irradiation for advanced acute leukemia and myelodysplastic syndrome. Blood. 1999;94(4):1237–1247. [PubMed] [Google Scholar]
- 4.Pagel J, Gooley T, Rajendran J, et al. Targeted Radiotherapy Using 131I-anti-CD45 Antibody Followed By Allogeneic Hematopoietic Cell Transplantation (HCT): The Relationships Among Dosimetry, Bone Marrow Uptake, and Relapse. European Journal of Nuclear Medicine and Molecular Imaging. 2006;33(Supp 2):S193. [Google Scholar]
- 5.Pagel JM, Appelbaum FR, Eary JF, et al. 131I-anti-CD45 antibody plus busulfan and cyclophosphamide before allogeneic hematopoietic cell transplantation for treatment of acute myeloid leukemia in first remission. Blood. 2006;107(5):2184–2191. doi: 10.1182/blood-2005-06-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andres TL, Kadin ME. Immunologic markers in the differential diagnosis of small round cell tumors from lymphocytic lymphoma and leukemia. Am J Clin Pathol. 1983;79(5):546–552. doi: 10.1093/ajcp/79.5.546. [DOI] [PubMed] [Google Scholar]
- 7.Omary MB, Trowbridge IS, Battifora HA. Human homologue of murine T200 glycoprotein. J Exp Med. 1980;152(4):842–852. doi: 10.1084/jem.152.4.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Press OW, Howell-Clark J, Anderson S, Bernstein I. Retention of B-cell-specific monoclonal antibodies by human lymphoma cells. Blood. 1994;83(5):1390–1397. [PubMed] [Google Scholar]
- 9.van der Jagt RH, Badger CC, Appelbaum FR, et al. Localization of radiolabeled antimyeloid antibodies in a human acute leukemia xenograft tumor model. Cancer Res. 1992;52(1):89–94. [PubMed] [Google Scholar]
- 10.Taetle R, Ostergaard H, Smedsrud M, Trowbridge I. Regulation of CD45 expression in human leukemia cells. Leukemia. 1991;5(4):309–314. [PubMed] [Google Scholar]
- 11.Scheinberg DA, Lovett D, Divgi CR, et al. A phase I trial of monoclonal antibody M195 in acute myelogenous leukemia: specific bone marrow targeting and internalization of radionuclide. J Clin Oncol. 1991;9(3):478–490. doi: 10.1200/JCO.1991.9.3.478. [DOI] [PubMed] [Google Scholar]
- 12.Appelbaum F, Matthews D, Eary JF, et al. The use of radiolabeled anti-CD33 antibody to augment marrow irradiation prior to marrow transplantation for acute myelogenous leukemia. Transplantation. 1992;54(5):829–833. doi: 10.1097/00007890-199211000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz MA, Lovett DR, Redner A, et al. Dose-escalation trial of M195 labeled with iodine 131 for cytoreduction and marrow ablation in relapsed or refractory myeloid leukemias. J Clin Oncol. 1993;11(2):294–303. doi: 10.1200/JCO.1993.11.2.294. [DOI] [PubMed] [Google Scholar]
- 14.Pagel JM, Gooley TA, Rajendran J, et al. Allogeneic hematopoietic cell transplantation after conditioning with 131I-anti-CD45 antibody plus fludarabine and low-dose total body irradiation for elderly patients with advanced acute myeloid leukemia or high-risk myelodysplastic syndrome. Blood. 2009;114(27):5444–5453. doi: 10.1182/blood-2009-03-213298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersdorf EW, Anasetti C, Martin PJ, et al. Limits of HLA mismatching in unrelated hematopoietic cell transplantation. Blood. 2004;104(9):2976–2980. doi: 10.1182/blood-2004-04-1674. [DOI] [PubMed] [Google Scholar]
- 16.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eary JF, Press OW, Badger CC, et al. Imaging and treatment of B-cell lymphoma. J Nucl Med. 1990;31(8):1257–1268. [PubMed] [Google Scholar]
- 18.Siegel JA, BW W, Watson EE, et al. Bone marrow dosimetry and toxicity for radioimmunotherapy. Antibody Immunoconj Radiopharm. 1990;3:213. [Google Scholar]
- 19.Sgouros G. Bone marrow dosimetry for radioimmunotherapy: theoretical considerations. J Nucl Med. 1993;34(4):689–694. [PubMed] [Google Scholar]
- 20.Fisher DR, Badger CC, Brietz H, et al. Internal radiation dosimetry for clinical testing of radiolabeled monoclonal antibodies. Antibody Immunoconj Radiopharm. 1991;4:655. [Google Scholar]
- 21.Tay J, Tinmouth A, Fergusson D, Huebsch L, Allan DS. Systematic review of controlled clinical trials on the use of ursodeoxycholic acid for the prevention of hepatic veno-occlusive disease in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13(2):206–217. doi: 10.1016/j.bbmt.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Bearman SI, Appelbaum FR, Buckner CD, et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol. 1988;6(10):1562–1568. doi: 10.1200/JCO.1988.6.10.1562. [DOI] [PubMed] [Google Scholar]
- 23.Storer BE. Small-sample confidence sets for the MTD in a phase I clinical trial. Biometrics. 1993;49(4):1117–1125. [PubMed] [Google Scholar]
- 24.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96(13):4075–4083. [PubMed] [Google Scholar]
- 25.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 26.Martin PJ, McDonald GB, Sanders JE, et al. Increasingly frequent diagnosis of acute gastrointestinal graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2004;10(5):320–327. doi: 10.1016/j.bbmt.2003.12.304. [DOI] [PubMed] [Google Scholar]
- 27.Sorror ML, Sandmaier BM, Storer BE, et al. Comorbidity and disease status based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J Clin Oncol. 2007;25(27):4246–4254. doi: 10.1200/JCO.2006.09.7865. [DOI] [PubMed] [Google Scholar]
- 28.Pagel JM. Radioimmunotherapeutic approaches for leukemia: the past, present and future. Cytotherapy. 2008;10(1):13–20. doi: 10.1080/14653240701679881. [DOI] [PubMed] [Google Scholar]
- 29.Orozco JJ, Zeller J, Pagel JM. Radiolabeled antibodies directed at CD45 for conditioning prior to allogeneic transplantation in acute myeloid leukemia and myelodysplastic syndrome. Ther Adv Hematol. 2012;3(1):5–16. doi: 10.1177/2040620711422265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jurcic J. Potential for myeloablation with yttrium-90-HuM195 (anti-CD33) in myeloid leukemia. J Clin Oncol. 2000;19:8a. [Abstract] [Google Scholar]
- 31.Nemecek ER, Hamlin DK, Fisher DR, et al. Biodistribution of yttrium-90-labeled anti-CD45 antibody in a nonhuman primate model. Clin Cancer Res. 2005;11(2 Pt 1):787–794. [PubMed] [Google Scholar]
- 32.Glatting G, Müller M, Koop B, et al. Anti-CD45 monoclonal antibody YAML568: A promising radioimmunoconjugate for targeted therapy of acute leukemia. J Nucl Med. 2006;47(8):1335–1341. [PubMed] [Google Scholar]
- 33.Sandmaier BM, Bethge WA, Wilbur DS, et al. Bismuth 213-labeled anti-CD45 radioimmunoconjugate to condition dogs for nonmyeloablative allogeneic marrow grafts. Blood. 2002;100(1):318–326. doi: 10.1182/blood-2001-12-0322. [DOI] [PubMed] [Google Scholar]
- 34.Kornblit B, Chen Y, Sandmaier BM. Conditioning with alpha-emitter based radioimmunotherapy in canine allogeneic hematopoietic cell transplantation. Chimerism. 2012;3(2):40–42. doi: 10.4161/chim.20726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Kornblit B, Hamlin DK, et al. Durable donor engraftment after radioimmunotherapy using alpha-emitter astatine-211-labeled anti-CD45 antibody for conditioning in allogeneic hematopoietic cell transplantation. Blood. 2012;119(5):1130–1138. doi: 10.1182/blood-2011-09-380436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pagel JM, Hedin N, Drouet L, et al. Eradication of disseminated leukemia in a syngeneic murine leukemia model using pretargeted anti-CD45 radioimmunotherapy. Blood. 2008;111(4):2261–2268. doi: 10.1182/blood-2007-06-097451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pagel JM, Matthews DC, Kenoyer A, et al. Pretargeted radioimmunotherapy using anti-CD45 monoclonal antibodies to deliver radiation to murine hematolymphoid tissues and human myeloid leukemia. Cancer Res. 2009;69(1):185–192. doi: 10.1158/0008-5472.CAN-08-2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pagel JM, Kenoyer AL, Bäck T, et al. Anti-CD45 pretargeted radioimmunotherapy using bismuth-213: high rates of complete remission and long-term survival in a mouse myeloid leukemia xenograft model. Blood. 2011;118(3):703–711. doi: 10.1182/blood-2011-04-347039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knopse WH, Blom J, Crosby WH. Regeneration of Locally Irradiated Bone Marrow: I. Dose Dependent, and Stromal Reaction, Long-Term Changes in the Rat, with Particular Emphasis upon Vascular and Stromal Reaction. Blood. 1966;28(3):398–415. [PubMed] [Google Scholar]


