Abstract
Inhibitory control and sensitivity to reward are relevant to the food choices individuals make frequently. An imbalance of these systems can lead to deficits in decision-making that are relevant to food ingestion. This study evaluated the relationship between dietary behaviors – binge eating and consumption of sweetened beverages and snacks - and behavioral control processes, among 198 ethnically diverse adolescents, ranging in age from 14 to 17, in Southern California. Neurocognitive control processes were assessed with the Iowa Gambling Task, a generic Go/No-Go task, and a food-specific Go/No-Go task. The food-specific Go/No-Go task directly ties the task to food cues that trigger responses, addressing an integral link between cue-habit processes. Dietary measures were assessed with self-administered food frequency and binge eating questionnaires. Results of latent variable models revealed marked gender differences. Inhibitory problems on the food-specific and generic Go/No-Go tasks were significantly correlated with binge eating only in females, whereas inhibitory problems measured with these tasks were the strongest correlates of sweet snack consumption in males. Higher BMI percentile and sedentary behavior also predicted binge eating in females and sweet snack consumption in males. Inhibitory problems on the generic Go/No-Go, poorer affective decision-making, assessed with the Iowa Gambling Task, and sedentary behavior were associated with sweetened beverage consumption in males, but not females. The food-specific Go/No-Go was not predictive in models evaluating sweetened beverage consumption, providing some initial discriminant validity for the task, which consisted of sweet/fatty snacks as no-go signals and no sugar-sweetened beverage signals. This research extends other study findings, revealing gender differences in inhibitory function relevant to behavioral control. Further, the findings contribute to research implicating the relevance of cues in habitual behaviors and their relationship to snack food consumption in an understudied population of diverse adolescents not receiving treatment for obesity or eating disorders.
Keywords: neurocognitive functions, inhibitory control, dietary behaviors, adolescents, habit, impulsivity, sweetened snacks, obesity, binge eating, cue effects
Introduction
Adolescent obesity has more than tripled over the past 30 years (Ogden, Carroll, Kit, & Flegal, 2012). In the United States, over 18% of youth between the ages of 12–19 are obese (Centers for Disease Control and Prevention; CDC 2013). Obesity in youth is associated with numerous negative health effects and an increased probability of being obese as an adult (CDC, 2013; Freedman, Mei, Srinivasan, Berenson, & Dietz, 2007; Jasik & Lustig, 2008; Li et al., 2009). While a range of snack foods contribute to this trend, this study focused on sugar-sweetened beverages (SSB) and sugary snack consumption in adolescents but also included a comparison of salty/fatty snacks. These sugar-sweetened snacks and SSBs are nutrient poor, have high sugar content, and are commonly consumed by adolescents (Harrington, 2008; Jahns, Siega-Riz, & Popkin, 2001; Keast, Nicklas, & O’Neil, 2010).
Although a range of influences affecting the rise in obesity among youth has been explored, differences in neurocognitive processes underlying behavioral regulation over sweet snack food consumption are understudied processes in a general adolescent population not currently receiving treatment for obesity or other eating disorders. The subsequent sections address some key neurocognitive processes relevant to diet behavior in this population, associative processes in habit formation, and resultant cue effects in behavioral regulation and decision-making.
Neurocognitive Processes and Dietary Behavior
The functioning of different but interacting neural systems and their influence on behavioral regulation of appetitive behaviors has been a recent focus of neuroscience. One realm of neural systems focuses on individual differences in prefrontally mediated inhibitory control and sensitivity to reward (mediated by subcortical systems), relevant to the food choices individuals make daily (Grigson, 2002; Kelley, Schiltz, & Landry, 2005). Some neurocognitive control functions are protective in regulating behavior when encountering such risks as high availability of sugary snacks and sugar-sweetened beverages. On the other hand, an imbalance of regulatory systems can lead to deficits in decision-making and control over impulses that may exacerbate food consumption (e.g., overeating or binge eating). Bechara and colleagues have argued for a distinction in functioning between response inhibition (mediated by prefrontal systems) and affective decision-making (mediated by prefrontal and subcortical systems), which are both relevant to behavioral control ability (Bechara, 2005; Bechara, Noel, & Crone, 2006; Bechara & Van der Linden, 2005). Both of these regulatory/inhibitory processes are important, specific aspects of higher order executive control functioning (Winstanley, Eagle, & Robbins, 2006).
Good inhibitory control functioning reflects the ability to actively stop a pre-potent behavioral response, such as binge eating or overeating, after it has been triggered (Braver & Ruge, 2006; Logan, Schachar, & Tannock, 1997). Individuals with weakened or overwhelmed regulatory control functions in prefrontal systems have a tendency to act more impulsively. The Go/No-Go, a valid test of response inhibition, is a commonly used task for assessing suppression of pre-potent behavioral responses (Aron & Poldrack, 2005) and has been used extensively among varied populations ranging from youth to adults (Casey, Giedd, & Thomas, 2000; Durston & Casey, 2006; Simmonds, Pekar, & Mostofsky, 2008). Several studies have shown that relative to female college students with better inhibitory control ability, female college students with poorer control consume more food (e.g., Guerrieri, Nederkoorn, & Jansen, 2007; Guerrieri et al., 2007; Guerrieri, Nederkoorn, Schrooten, Martijn & Jansen, 2009; Nederkoorn, Guerrieri, Havermans, Roefs & Jansen, 2009), and are more often overweight if they also have an implicit preference for snack foods (Nederkoorn, Houben, Hofmann, Roefs, & Jansen, 2010). Several studies that evaluated body weight differences in youth and response inhibition with Stop Signal Tasks found obese youth showed decreased response inhibition relative to leaner youth (e.g., Nederkoorn, Braet, Van Eijs, Tanghe, & Jansen, 2006; Nederkoorn, Jansen, Mulkens, & Jansen, 2007; Nederkoorn, Coelho, Guerrieri, Houben, & Jansen, 2012; Verbeken, Braet, Claus, Nederkoorn, & Oosterlaan, 2009). One study by Pauli-Pott et al. (2010) used the Go/No-Go task to evaluate response inhibition in overweight and obese youth ranging in age from 8 to 15. Inhibitory control was correlated with body weight in this study; that is, less control was observed in youth with higher body weight (Pauli-Pott, Albayrak, Hebebrand, & Pott, 2010).
Adequate affective decision-making reflects an integration of cognitive prefrontal and affective subcortical systems and the ability to optimally weigh short-term gains against long-term losses or probable outcomes of an action. This function is most commonly assessed with the Iowa Gambling Task (IGT), with higher scores revealing more adaptive affective decision-making (Bechara, Damasio, Damasio, & Anderson, 1994; Bechara et al., 2006). For example, a prediction related to food and the IGT is that overeating of foods high in sugar that are known to have short-term reinforcing effects (but longer-term negative consequences to health) should be less likely among individuals who score higher on the IGT; that is, they are more able to inhibit immediate gratification. The bulk of research investigating impaired decision-making (and sensitivity to reward) with the IGT and body weight has been in adult populations with eating disorders; however, one of the first studies to investigate affective decision-making in eating behavior in healthy adult females ranging in weight found the overweight females in the sample to have impaired decision-making. Further, the deficits in decision-making on the IGT were greater than those found in some studies with drug dependent individuals (Davis, Levitan, Muglia, Bewell, & Kennedy, 2004). In another study, Davis et al. (2010) found impaired decision-making, assessed with the IGT, in obese adult females as well as among females with binge eating disorders relative to normal weight females. Decision-making deficits did not differ between the females in the obese and binge eating disorder groups (Davis, Patte, Curtis, & Reid, 2010). Verbeken et al. (2013) evaluated inhibition or delayed gratification with the Hungry Donkey Task, a child version adaptation of the IGT, in children and younger adolescents ranging from healthy weight to overweight. They found impaired decision performance on the task among the overweight youth when compared to healthy weight youth (Verbeken, Braet, Bosmans, & Goossens, 2013).
The functional distinction between response inhibition and affective decision-making processes comes from extensive clinical observation and research with patient populations with damage in areas of the frontal lobe (Bechara & Van der Linden, 2005) as well as imaging studies that delineate key neural substrates of each function (Lawrence, Jollant, O’Daly, Zelaya, & Phillips, 2008; Simmonds et al., 2008). The importance of these neurocognitive processes in behavioral regulation has been demonstrated across numerous studies and a wide range of populations (Brand, Labudda, & Markowitsch, 2006; Dunn, Dalgleish, & Lawrence, 2006; Simmonds et al., 2008). The present study extends research on response inhibition and affective decision-making by evaluating these processes in a seldom studied, relatively older general adolescent population, ranging in weight from lean to obese.
Cue Effects on Behavioral Regulation and Decision Processes
The importance of cues in triggering automatic/habitual behaviors is well recognized in basic behavioral sciences spanning neuroscience (Knowlton, Mangels, & Squire, 1996; Yin & Knowlton, 2006a), memory (Nelson & Goodmon, 2003; Rescorla, 2008), and research on appetitive behaviors (LaBar et al., 2001). Yet, most approaches to understanding adolescent risk behavior do not incorporate cue effects and their link to habit formation. A framework for understanding the loss of ability to resist natural (e.g., sugary foods) as well as non-natural (e.g., drugs of abuse) rewards and the development of habitual behaviors can be explained by associative learning/memory models of appetitive behaviors (Stacy, 1997; Stacy, Ames, & Knowlton, 2004; Yin & Knowlton, 2006b). Similar key neural systems (dopamine dependent systems) are critical for motivational effects across a range of rewarding/reinforced behaviors (e.g., natural rewards like sugary foods; Kenny, 2011; Olsen, 2011: drugs of abuse; Chiara et al., 1999; Everitt & Robbins, 2005; Robbins & Everitt, 1999; Wise & Rompré, 1989). Dopaminergic activity reinforces the repetition of behaviors, such as the consumption of sugary snacks, and supports the encoding and processing of cues associated with the rewarding event (e.g. Cardinal & Everitt 2004; Everitt & Robbins 2005). As habitual behaviors develop, associative memory processes become increasingly stronger (Stacy, 1995, 1997). As associations in memory are strengthened, habitual behaviors then become increasingly under cue control and less under voluntary control, and are highly sensitive to predictive cues (Stacy et al., 2004; Stacy & Wiers, 2010; White, 1996; Wiers et al., 2007). Once a habit is formed, relevant cues appear to be able to elicit behavior with little need for executive control processes and regardless of anticipated outcomes (Wood & Neal, 2007; Yin & Knowlton, 2006a; Yin & Knowlton, 2006b).
In one study, women with bulimia were found to be more impulsive than women who had a healthy weight on an adapted Go/No-Go that included food, body and object words as stimuli (Mobbs, Van der Linden, d’Acremont, & Perroud, 2008). This relationship was strongest with the food-related stimuli, highlighting the relevance of understanding the effects of cues and their link to more automatic/habitual eating behaviors. In another study, Muele et al. (2011) tested a generic Go/No-Go, flanked by food objects and neutral objects in restrained and unrestrained eaters. They found the food cues increased reaction time in the restrained eaters; that is, the restrained eaters were better able to control their response in the face of food cues compared to unrestrained eaters (Meule, Lukito, Vögele, & Kübler, 2011). Other studies have used an adapted Stop Signal task (SST) to examine food cue effects on response inhibition and found overweight children to have more difficulty inhibiting responses to food cues than lean children (Nederkoorn et al., 2012), and adult females with higher BMI to have more inhibitory problems on a food-specific SST than females with lower BMI (Houben, Nederkoorn & Jansen, 2014). Houben and colleagues did not find similar effects with a generic SST. Finally, Batterink et al (2010) evaluated both the behavioral and neural response to food cues on a Go/No-Go in lean to overweight adolescent girls. In this study, vegetable images served as go signals and desserts served as no-go signals during the task. On a neural level, they found that the overweight girls showed less recruitment of prefrontal regions responsible for inhibitory control during no-go signals, as well as increased activity in reward-related regions in response to food cues that correlated with BMI. On a behavioral level the overweight girls tended to be more impulsive (Batterink, Yokum, & Stice, 2010).
It should be noted that while the Stop Signal and Go/No-Go tasks are often interchanged as assessments of motor response inhibition, the Go/No-Go task is thought to involve “a decision-making component that is absent from the stop-signal task” (p. 441; Eagle, Bari Robbins, 2008). A meta-analysis of neural correlates of the two tasks revealed that although the tasks have overlapping neural systems, they also engage distinct systems (Swick, Ashley & Turken, 2011). Nevertheless, the studies reviewed above using both tasks highlight the importance of cue effects in behavioral regulation, and research findings with the Go/No-Go task reveal that it is sensitive to variations in cues at both behavioral and neural levels. Further, findings with both tasks reveal that performance tests of neurocognitive functioning provide valuable insight about cue effects and inhibitory functioning.
Overview
This study sampled youth ranging in age from 14 to 17 to evaluate the relationship between well-documented basic neurocognitive assessments of response inhibition and affective decision-making, both relevant to behavioral control ability (Bechara, 2005; Bechara et al., 2006; Bechara & Van der Linden, 2005) and dietary behavior. We expected youth with decision-making deficits assessed with the Iowa Gambling Task (IGT), as well as youth with poorer response inhibition assessed with the generic Go/No-Go task, to report more frequent intake of high sugar snacks, sugar-sweetened beverages (SSBs), and binge eating than youth with better control ability. The expected effect was similar for both performance tasks with no predicted difference in the magnitude.
Further, we expected a food-specific Go/No-Go to differentiate youth who have difficulty inhibiting automatic responses to certain food cues from youth with more inhibitory control. The magnitude of the effect, relative to the generic Go/No-Go, was expected to be stronger for binge eating and sugar-sweetened snack foods, since the food-specific Go/No-Go ties the task to specific food cues that trigger responses that should tap into both prefrontal and habit-based systems (Batterink et al., 2010), addressing the integral involvement of cue-habit processes (Wood & Neal, 2007). A similar prediction was not expected for sugar-sweetened beverages with the food-specific Go/No-Go since no beverages are used in the task, providing a test of discriminant validity. In addition, although the focus of the manuscript is on SSBs and sugary snacks, we also examined salty/fatty snacks to explore specificity of inhibitory effects without a priori hypotheses. Finally, we did not have gender specific hypotheses, but it is feasible that there could be gender differences in our adolescent population; therefore, gender was included in the analytic models as a covariate.
Methods
Participants
Participants were 198 adolescents recruited from regular public high schools in Southern California. Only schools with at least 25% of their student population enrolled in a free or reduced meal program were considered eligible for the study. Adolescents from these schools were eligible to participate if they were: (a) 14 to 17 years old, (b) able to speak and write English, (c) free of major illness (i.e., no heart disease, cancer, or diabetes), (d) not currently receiving treatment for obesity or other eating disorders, and (e) able to travel to the assessment site with a parent/guardian. No more than 21 students were recruited from each school in order to capture a representative sample of the adolescent population across a range of high schools in Southern California. Adolescents who took part in the study were required to have one parent or guardian who spoke English or Spanish. Spanish speaking data collectors were available to help recruit participants. Adolescents who spoke primarily Spanish were not included in the study. The majority of participants were Hispanic (77.8%, N=154). Eighty-seven (43.9%) were males and 111 (56.1%) were females, with a mean age of 15.84 (SD=.94).
With respect to body weight, the sample included a range of weight status categories based on the Centers for Disease Control (CDC) weight status category percentile range (CDC, 2010). Of the males in the study, 34.5% were obese, 3.4% were overweight, 55.2% were of healthy weight, 1.1% were underweight, and 5.7% were missing data. Of the females in the study, 20.7% were obese, 17.1% were overweight, 58.6% were of healthy weight, 1.8% were underweight and 1.8% were missing data.
Procedures
Assessments were completed at a Claremont Graduate University facility. All parents/guardians read and signed a consent form and participants signed an assent form. The forms were available in Spanish for a parent or guardian as needed, and Spanish-speaking staff were available to answer any questions. Consented participants first had physical measurements of height and weight assessed by trained staff, following which they completed a computerized assessment battery of neurocognitive tasks, dietary intake and binge eating measures, a measure of sedentary behavior, and demographics, including measures of household size and parents’ education level as proxies for socioeconomic status (Grenard et al., 2013). Additionally, acculturation was assessed with the Acculturation, Habits, and Interests Multicultural Scale for Adolescents (Unger et al., 2002). Consistent with prior research on adolescent diet and health behaviors, the United States orientation subscale was evaluated (alpha =.79; see Table 2). Assessments were completed on mini-laptops programmed in Inquisit Experimental Software and the session took about 2 hours to complete. Participants were tested in small groups (8 to 10 at a time), as in previous studies using computerized assessments (Ames et al., 2007; Grenard et al., 2008). The assessments analyzed in this study are part of a large-scale research effort. Although additional measures were gathered as part of the larger study on adolescent behavior in a diverse community population, the issues addressed in this article are not addressed elsewhere. Additional measures not addressed in the present article included assessments of implicit cognition, general stress, and emotional, restrained, and external eating from the Dutch Eating Behavior Questionnaire (Van Strien, Frijters, Bergers, & Defares, 1986). All implicit cognition measures were assessed first and then other measures followed demographics. Although we did not anticipate order effects, the order of measurement across the inhibitory neurocognitive tasks was counterbalanced. Other measures were not counterbalanced. Subjects were paid $50 for their participation.
Table 2.
Descriptive Statistics for Modeled Variables by Gender.
| Variable | N | Mean (SD) | T-test for gender diff | ||
|---|---|---|---|---|---|
| Males | Females | t | p | ||
| Iowa Gambling Task Proportion of Good Deck Choices – Proportion of Bad Deck Choices | 186 | .027 (.26) | .020 (.25) | .176 | .49 |
| Generic Go/No-Go False Alarms | 197 | .033 (.035) | .019 (.022) a | 3.29** | < .001 |
| Food Go/No-Go False Alarms | 198 | .067 (.051) | .042 (.036) | 3.65** | < .001 |
| Sedentary Behavior | 193 | 57.27 (19.18) | 60.39 (17.47) | −1.17 | .24 |
| BMI percentile b | 191 | 70.08 (27.69) | 69.44 (27.26) | .159 | .87 |
| Sweet snacks | 172 | 1.37 (1.36) | 1.33 (1.54) | .175 | .86 |
| Sugar-Sweetened beverages | 190 | 1.19 (1.04) | .96 (.89) | 1.65 | .10 |
| Binge Eating Behavior c | 186 | −.472(6.35) | .428(7.21) | −.898 | .37 |
The number of females who performed the generic Go/No-Go task was 110. Otherwise, the N for analyses of female data was 111.
BMI percentiles were calculated using CDC child BMI formulas for age and gender.
Binge eating items were standardized (M=0, SD=1) and then summed in order to create a continuous variable, described in the Methods section.
Measures
Dietary intake outcome measures
Dietary intake was assessed with a self-administered food frequency questionnaire, Youth/Adolescent Questionnaire (YAQ), designed for youth ranging in age from 9 to 18 years (Rockett, Wolf, & Colditz, 1995). Prior research indicates that the YAQ is a valid (r = .54 when comparing YAQ with three 24-hour recalls; Rockett et al., 1997) and reproducible measure (Rockett et al., 1995). The questionnaire asks participants how often, on average, they consume each of 151 food items. Response categories for consumption vary on the basis of the food, with foods grouped as a serving unit. Consumption of sweet snacks was computed by summing the average consumption of 18 snack items high in sugar (e.g., fruit rollups, snack cakes, pop tarts, pastry, candy bars, sweet rolls), and consumption of sweetened beverages was computed by summing the average intake of six sugar-added or sugar-containing drink items (e.g., non-diet soda, lemonade or other non-carbonated fruit drinks). Consumption of salty/fatty snacks was computed by summing the average intake of three types of snacks (e.g., french fries, potato and corn chips, and pretzels or crackers).
Binge eating behavior
Binge eating was assessed using the 13-item binge-eating subscale of the Eating Disorder Diagnostic Scale (Stice, Telch, & Rizvi, 2000). The scale items assess binge eating behavior marked by loss of control, eating unusually large amounts of food, overeating, feelings of distress due to binge eating, and post binge counteracting behaviors. Three questions inquire about binge eating days and frequencies over the past 6 months (e.g., How many days per week on average over the past 6 months have you eaten an unusually large amount of food and experienced a loss of control?). Six yes/no items assess behavioral characteristics associated with binge eating (e.g., During these episodes of overeating and loss of control did you eat alone because you were embarrassed by how much you were eating?) and feelings associated with binge episodes (e.g., During these episodes of overeating and loss of control did you feel disgusted with yourself, depressed, or very guilty after overeating?). Four items assess the frequency of post-binge counteracting behaviors such as purging and fasting (e.g. How many times per week on average over the past 3 months have you made yourself vomit to prevent weight gain or counteract the effects of eating?). Thirteen standardized and averaged items were used to create a continuous measure of binge eating (for psychometrics see Stice, Fisher, & Martinez, 2004).
Predictor measures
Body Mass Index Percentile (BMI)
BMI was computed as [weight (kg)/height (m)2] by measuring height and weight three times using a calibrated stadiometer and a digital scale, and then taking the mean of the three measurements. Trained study staff measured participants’ height and weight. BMI percentile ranking is then calculated by plotting the BMI on the Center for Disease Control and Prevention BMI-for-age growth charts (http://www.cdc.gov/growthcharts/). BMI percentile is the most commonly used indicator assessing the body fatness of children and adolescents in the United States and indicates the relative weight status of a youth among other youth of the same sex and age. A BMI less than the 5th percentile is categorized as underweight, 5th percentile to less than the 85th percentile as healthy weight, 85th to less than the 95th percentile as overweight, and equal to or greater than the 95th percentile as obese.
Sedentary Behavior
Sedentary behavior served as a proxy measure for physical activity and consisted of a continuous index of six items that assess how many hours an individual engages in a sedentary behavior during a regular week (scale range: 0 to 5 hours per day56). Three items refer to weekday behaviors, and three identical items refer to weekend behaviors. Each participant’s score of sedentary hours per week was computed by calculating a weighted sum of weekday and weekend day using the formula: (week-day response x 5) + (week-end day response x 2) = hours per week doing each activity, and then summing the hours for each activity.
Neurocognitive measures
Iowa Gambling Task (IGT)
The IGT is a neuropsychological task that simulates real-life affective decision-making (Bechara et al., 1994). It has been shown to tax aspects of decision-making that are guided by affect and emotions and it has been used to assess individuals exhibiting poor decision skills. The task detects whether people learn from experiences with negative outcomes and make appropriate choices. Consistent with the original task paradigm, participants were presented with 4 virtual decks of cards labeled A, B, C, and D on computer screens. On-screen audible instructions told participants to choose cards from any of the four decks to try to win as much money as possible. The card decks differed in the number of trials over which losses were distributed. In this study, the C and D decks were considered “good decks” because choosing cards from these decks led to gains over the long-run, while A and B decks were considered “bad decks” because choosing from these decks led to losses over the long-run. The computer tracked participants’ selections of cards from both “good” and “bad” decks over trials. The task consisted of 100 trials presented in five blocks. The overall score is the sum of the good card selections minus the sum of the bad card selections, which results in a score across trials.
Cued Go/No-Go tasks
Response inhibition was assessed with two versions of the Go/No-Go task: a generic cued and a food cued task. Cues provided information on the type of target stimulus. On this task, participants were instructed to react as rapidly as possible to go signals on a computer screen by pressing a specified response key and withhold a response to no-go signals by inhibiting the response to press a key, without making mistakes (Fillmore, 2009; Fillmore, Kelly, & Martin, 2005; Simmonds et al., 2008). Failing to withhold a response to no-go stimuli is a false alarm, and is a measure of response inhibition failure that indicates problems with impulsivity (Fillmore, 2009; Fillmore & Rush, 2006; Fillmore, Ostling, Martin, & Kelly, 2009; Patterson & Newman, 1993).
The generic version of the cued Go/No-Go was based on the work of Fillmore, Marczinski and Bowman (2005). During this task, a green rectangle represented “go signals”, and a blue rectangle represented no-go signals. The food-cued Go/No-Go used snack food items as signals and ties the task to eating behavior (Mobbs et al., 2008). This task was evaluated as an alternative measure of response inhibitory processes. During the food-specific task go signals consisted of low fat/low sugar snacks (e.g., apples, carrots), and no-go signals consisted of high fat or sugary snacks (e.g., candy bars). The food items were comprised of the highest frequency food cues elicited during focus groups among adolescents similar to those studied.
Both the generic and food cued tasks consisted of 200 trials each presented in four blocks of 50 trials. The orientation of the cue (e.g., a horizontal or vertical rectangle) signaled the probability that a go or no-go signal would be displayed. Cues presented horizontally preceded go signals on 80% of the trials and preceded the no-go signal on 20% of the trials. Cues presented vertically preceded the no-go signal on 80% of the trials and preceded the go signal on 20% of the trials. Fifty percent of the trials were go signals and 50% were no-go signals. Previous research has demonstrated that this level of cue validity produces pre-potent responding (Abroms, Jorgensen, Southwell, Geller, & Emmons, 2003; Marczinski & Fillmore, 2003). Individual trials with mean reaction times greater than 1000ms or less than 100ms were excluded from statistical analyses (Fillmore & Rush, 2006; Fillmore et al., 2009). Outlier reaction times occurred on average less than 0.5% of the trials on the generic and food-cued tasks. The proportion of false alarms (i.e., failure to withhold a response to no-go stimuli) across trials was used in our analyses.
Analytic Procedure
Confirmatory factor analysis (CFA) and structural equation modeling (SEM) were used to estimate model parameters with MPlus 6 software (Muthén & Muthén, 1998–2011). This analytic approach provides more power and helps to adjust for measurement error compared with alternative strategies. In general, this analytic approach allows for more accurate estimates of the relationships among variables (Bollen, 1989; Bollen & Long, 1993; Hoyle, 1995). Initial confirmatory factor analysis (CFA) models were evaluated to determine whether the created indicators adequately reflected the proposed latent factors for the Generic-Cued False Alarms, Food-Cued False Alarms, and Affective Decision-Making constructs. For brevity, only CFA models for the generic-cued False Alarms construct were reported. Full information maximum likelihood estimation methods were used for estimating models. This method handles both missing data and the estimation of model parameters under the missing at random assumption. The interpretation of the maximum likelihood estimates is the same as in a linear least squares regression analysis with complete data. Multiple indices were used to assess the fit of the models (Bentler, 1990; Hu & Bentler, 1998; Hu & Bentler, 1999).
Each neurocognitive factor was conceptualized as a latent construct with repeated trials as indicators. Indicators consisted of sums of multiple items, that is, parcel scores. The parcel method is based on the work of Little, Cunningham, Shahar, and Widaman (2002). Parcels create an accurate representation of the data when the measure is unidimensional in theory and measurement, and the measurement does not systematically vary (e.g., no experimental effects such as fatigue). In this study, this method was used to combine trials to create more reliable and valid indicators for the latent constructs (Little, Rhemtulla, Gibson, & Schoemann, 2013). Individual trials were randomly assigned to one of three parcels, and then standardized scores were computed within each parcel.
Affective Decision-Making indicators were created from parcels consisting of trials from the Iowa Gambling Task (IGT). IGT trials are a selection from a good card deck (C or D) or a bad card deck (A or B). Each of the three parcels were composed of 33 trials randomly selected. Within the 33 trials the total times decks C or D (good decks) were selected was subtracted from the total times decks A or B (bad decks) were selected to derive a single value for each parcel (max range −33 to +33). A positive value indicated participants selected the good decks more often than the bad decks. This is consistent with overall scoring of the IGT (i.e., the sum of the good card selections minus the sum of the bad card selections across trials). Similarly, the False Alarms factors for the Generic-Cued Go/No-Go and the Food-Cued Go/No-Go were comprised of three indicators or parcels of 33 false alarm (No-Go) trials randomly assigned to each parcel. The proportion of false alarms (i.e., failure to withhold a response to no-go stimuli) across trials was used to create the parcel indicators for latent variable models
The dietary outcome behaviors (i.e., binge eating, servings of sweet snacks, and servings of sugar-sweetened beverages) were modeled as manifest variables consisting of composite scores, described above. Sedentary behavior and BMI percentile were also modeled as manifest variables. Structural models were evaluated by examining the associations between the neurocognitive latent constructs, sedentary behavior, BMI percentile, and the dietary outcomes.
The overall goodness of fit of the models was evaluated through use of the chi-square goodness-of-fit test, comparative fit index (CFI), and root-mean-square error of approximation (RMSEA) and its confidence interval (MacCallum, Browne, & Sugawara, 1996), and standardized root mean square residual (SRMR). Values of CFI above .90 or .95 indicate an adequate or good fit to the data, and values of RMSEA and SRMR less than .08 indicate adequate fit (Hu & Bentler, 1999).
Separate models were evaluated for the Generic and Food-Cued False Alarms latent construct models, because estimating both constructs in the same model led to multicollinearity among parameter estimates (see Tables 4 & 5 for intercorrelations). Alternatively, affective decision-making was not strongly correlated with either False Alarms constructs and was retained as a distinct construct in all models evaluated. Additionally, multi-group models were estimated stratified by gender in order to test for gender differences in the association between the neurocognitive and behavioral factors and eating behaviors.
Table 4.
Significant Bivariate Correlations for Male Models
| Male Models | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.IGT parcel 1 | 1 | ||||||||||||
| 2. IGT parcel 2 | .618** | 1 | |||||||||||
| 3. IGT parcel 3 | .710** | .740** | 1 | ||||||||||
| 4. False alarm parcel 1 | 1 | ||||||||||||
| 5. False alarm parcel 2 | .688** | 1 | |||||||||||
| 6. False alarm parcel 3 | −.266* | .691** | .659** | 1 | |||||||||
| 7. Food false alarm parcel 1 | .564** | .694** | .588** | 1 | |||||||||
| 8. Food false alarm parcel 2 | .390** | .403** | .419** | .693** | 1 | ||||||||
| 9. Food false alarm parcel 3 | .379** | .448** | .386** | .588** | .644** | 1 | |||||||
| 10. Sedentary behavior | 1 | ||||||||||||
| 11. BMI percentile | 1 | ||||||||||||
| 12. Sweet snacks | .282* | .309* | .261* | .351** | 1 | ||||||||
| 13. Sweet drinks | −.401** | .320* | .287* | .516** | 1 | ||||||||
| 14. Binge eating | .271* | .292* |
Note.
p<.01,
p<.05. Listwise N=58.
Table 5.
Significant Bivariate Correlations for Female Models
| Female Models | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. IGT parcel 1 | 1 | ||||||||||||
| 2. IGT parcel 2 | .624** | 1 | |||||||||||
| 3. IGT parcel 3 | .768** | .572** | 1 | ||||||||||
| 4. False alarm parcel 1 | .229* | 1 | |||||||||||
| 5. False alarm parcel 2 | .674** | 1 | |||||||||||
| 6. False alarm parcel 3 | .460** | .439** | 1 | ||||||||||
| 7. Food false alarm parcel 1 | .617** | .649** | .454** | 1 | |||||||||
| 8. Food false alarm parcel 2 | .496** | .362** | .354** | .577** | 1 | ||||||||
| 9. Food false alarm parcel 3 | .471** | .533** | .234* | .629** | .462** | 1 | |||||||
| 10. Sedentary behavior | 1 | ||||||||||||
| 11. BMI percentile | 1 | ||||||||||||
| 12. Sweet snacks | .376** | 1 | |||||||||||
| 13. Sweet drinks | 1 | ||||||||||||
| 14. Binge eating | .269* | .243* | .345** |
Note.
p<.01,
p<.05. Listwise N=80.
Results
Confirmatory factor analysis models
First, a full model with gender as a covariate was evaluated. Preliminary tests of measurement invariance were not statistically significant for both Generic-Cued False Alarms and Affective Decision-Making factors showing measurement invariance (i.e., invariant factor loadings and parcel intercepts) across gender, χ2(4) = 7.33 (ns), CFI = .98, RMSEA = .09 (90% CI: .00, .19), SRMR = .06, and χ2(4) = 7.26 (ns), CFI = .99, RMSEA = .09 (90% CI: .00, .20), SRMR = .06, respectively. Although we had no rationale or a priori hypotheses regarding gender differences, significant differences in gender were observed in the analyses of the full model. Therefore, all further analyses consisted of only multi-group (i.e., across gender) models. The fit indices for the multi-group CFA analyses with correlated factors including binge eating and YAQ outcomes were χ2(48) = 71.81 (p=.02), CFI = .95, RMSEA = .07 (90% CI: .03, .10), SRMR = .06, and χ2(56) = 85.68 (p=.01), CFI = .94, RMSEA = .07 (90% CI: .04, .10), SRMR = .06, respectively.
Mean differences by gender across variables are provided in Table 2, Go/No-Go performance scores are provided in Table 3, and correlations among the model indicators and variables by gender are provided in Table 4 & 5. There were no significant correlations between BMI percentile and the neurocognitive tasks as a whole group or by gender (all ps>.05).
Table 3.
Food Go/No-Go Descriptives by Gender
| Males (N=87) | Females (N=111) | |||
|---|---|---|---|---|
|
| ||||
| Mean (SD) | Range | Mean (SD) | Range | |
|
| ||||
| Mean proportion False Alarm across blocksa | .067* (.051) | 0.0 –.24 | .042* (.036) | 0.0 – .16 |
| Mean proportion Missed Go across blocks | .004 (.006) | 0.0 –.03 | .005 (.012) | 0.0 – .11 |
| D′ c | .430* (.054) | .26 –.50 | .453* (.044) | .30 – .50 |
| Mean reaction time for Correct Go Hits | 429.72 (40.35) | 288.11–540.58 | 439.48 (41.86) | 360.21–556.07 |
| Generic Go/No-Go Descriptives by Gender | ||||
|---|---|---|---|---|
| Males (N=87) | Females (N=110) | |||
|
| ||||
| Mean (SD) | Range | Mean (SD) | Range | |
|
| ||||
| Mean proportion False Alarm across blocksb | .033* (.035) | 0.0 – .21 | .019* (.022) | 0.0 – .10 |
| Mean proportion Missed Go across blocks | .002 (.007) | 0.0 – .05 | .004 (.009) | 0.0 – .08 |
| D′ c | .464* (.038) | .30 –.50 | .477* (.028) | .40 – .50 |
| Mean reaction time for Correct Go Hits | 331.71 (35.81) | 252.51–449.05 | 349.64 (41.33) | 274.09 – 507.34 |
1 female and 2 males had zero false alarms on food Go/No-Go task.
17 females and 7 males had zero false alarms on generic Go/No-Go task.
D′ (z scored mean proportion of correct Go’s – z scored mean proportion of False alarms).
p < .05; Significant difference between males and females.
Structural models
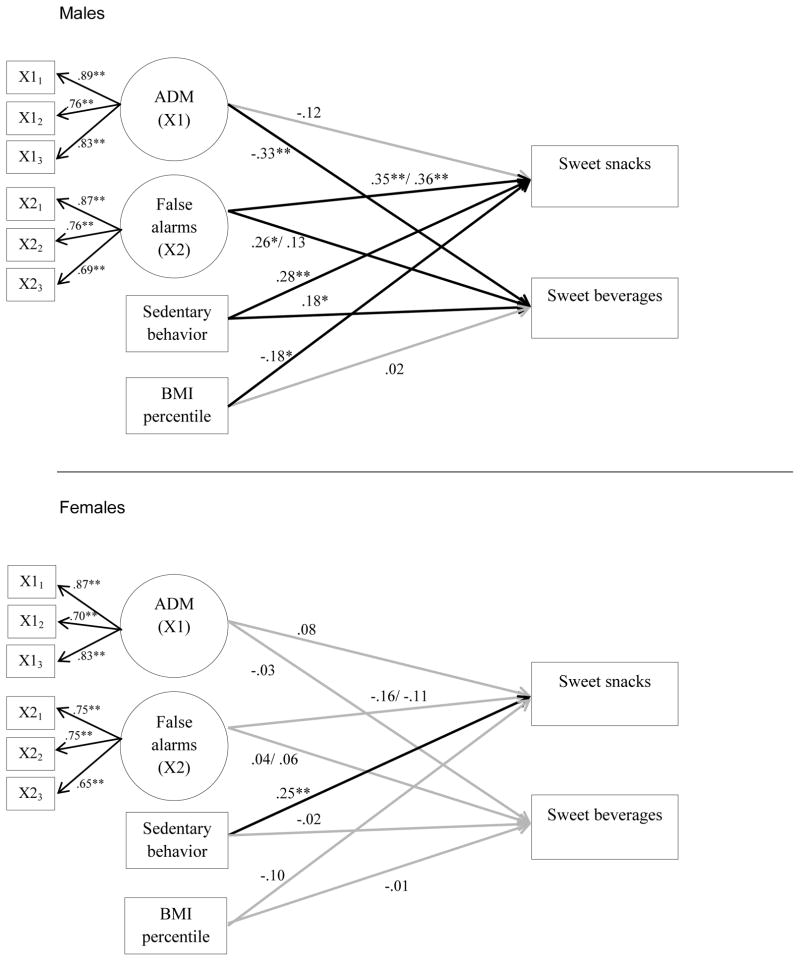
The first structural model evaluated focused on outcomes of sugar-sweetened beverages and sweet snack consumption. The fit of the structural models was good for 1) Generic False Alarms; χ2(56) = 84.29 (p=.01), CFI = .94, RMSEA = .07, (90% CI: .04, .10), SRMR = .06, and 2) Food-Cued False Alarms χ2(56) = 83.03 (p=.01), CFI = .95, RMSEA = .07 (90% CI: .04, .10), SRMR = .06. Standardized path estimates are shown in Figure 1 for the model including generic false alarms with path estimates for the food-cued false alarms provided after the slash mark. The effects of affective decision-making and false alarms on YAQ outcomes were only statistically significant for males. Male participants scoring higher on the IGT reported lower sugar-sweetened beverage (SSB) consumption, and male participants who had a greater proportion of false alarms on both Go/No-Go tasks reported more sweet snack consumption, as predicted. The food specific false alarms did not predict SSB consumption. Additionally, sedentary behavior was associated with greater sweet snack consumption for both males and females, and higher SSB consumption for males.1
Figure 1.
Standardized path estimates for YAQ outcomes.
Note. Standardized path estimates are shown for the model including generic false alarms or inhibitory failures. IGT and false alarms are latent variables with three parcel indicators each. The path estimates for the food false alarms are given after the slash mark. Predictors were allowed to correlate. Outcomes were allowed to correlate. P-values are one-tailed at **p<0.01, *p<0.05. Paths that were statistically significant at p < .05 are depicted in bold. N=193 (males=84, females=109). ADM: Affective Decision Making.
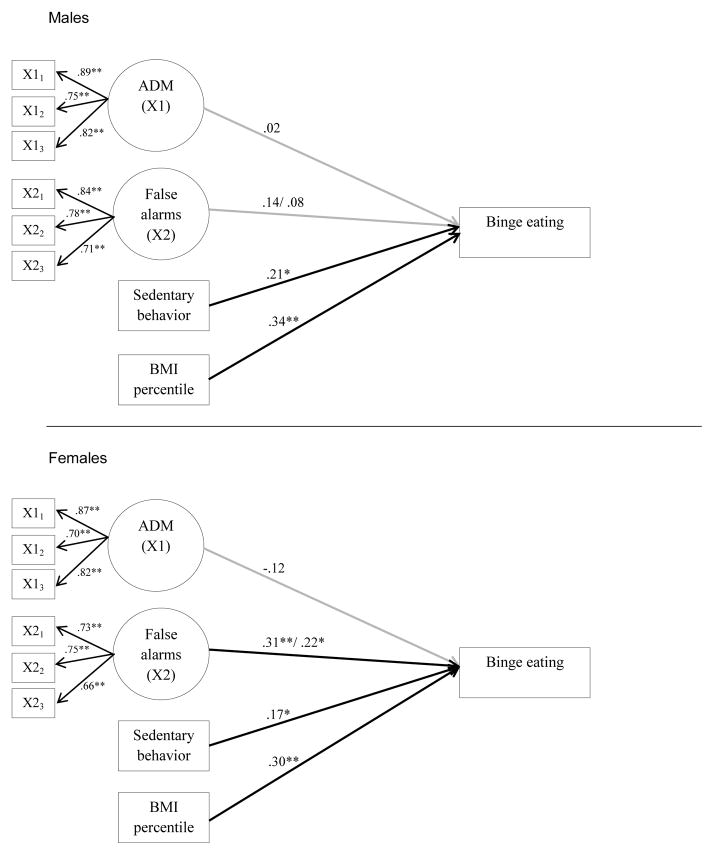
Next, we tested models with the same predictors using binge eating as the outcome variable. The models for males and females fit the data well. The model fit indices for models focusing on generic false alarms and food false alarms were χ2(48) = 70.47 (p=.02), CFI = .95, RMSEA = .07(90% CI: .03, .10), SRMR = .06 and χ2(48) = 71.96 (p=.01), CFI = .95, RMSEA = .07(90% CI: .03, .10), SRMR = .06 respectively. Standardized path estimates are shown in Figure 2 for the models. False alarms on both Go/No-Go tasks were associated with more binge eating in females only. Sedentary behavior and BMI percentile were associated with more binge eating in both males and females.
Figure 2.
Standardized path estimates for binge eating outcome.
Note. Standardized path estimates are shown for the model including generic false alarms. IGT and false alarms are latent variables with three parcel indicators each. The path estimates for the food false alarms are given after the slash mark. Predictors were allowed to correlate. P-values are one-tailed at **p<0.01, *p<0.05. Paths that were statistically significant at p < .05 are depicted in bold. N=193 (males=84, females=109). ADM: Affective Decision Making.
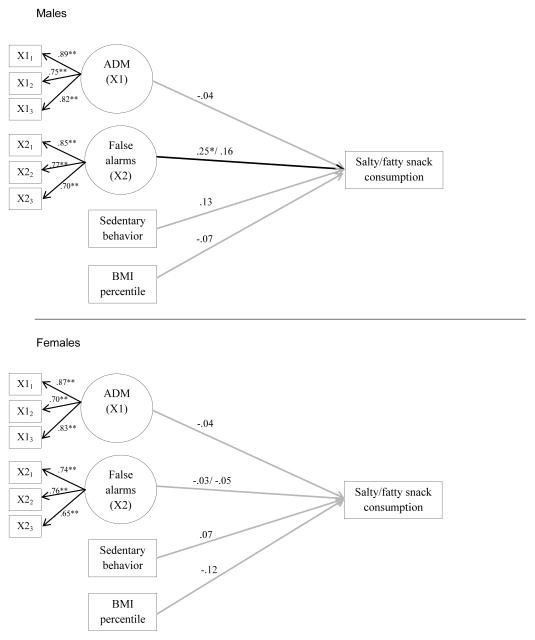
Finally, supplemental structural models for salty/fatty snack food outcomes were evaluated with the same predictor set as in our previous models. These data were analyzed for purposes of comparison with the sugar-sweetened snack food, SSB, and binge models. The fit indices for models focusing on Generic False Alarms was χ2(48) = 70.07 (p=.02), CFI = .95, RMSEA = .07(90% CI: .03, .10), SRMR = .06, and for the Food-Cued False Alarms, χ2(48) = 68.78 (p=.03), CFI = .96, RMSEA = .07(90% CI: .02, .10), SRMR = .06. Standardized path estimates are shown in Figure 3 for the models. The false alarms on the generic Go/No-Go task for males only were associated with more consumption of salty/fatty snacks. No other significant effects were found for males and no significant effects were found for females.
Figure 3.
Standardized path estimates for eating salty/fatty snacks outcome.
Note. Standardized path estimates are shown for the model including generic false alarms or inhibitory failures. IGT and false alarms are latent variables with three parcel indicators each. The path estimates for the food false alarms are given after the slash mark. Predictors were allowed to correlate. P-values are one-tailed at **p<0.01, *p<0.05. Paths that were statistically significant at p < .05 are depicted in bold. N=193 (males=84, females=109). ADM: Affective Decision Making.
Discussion
This study evaluated neurocognitive inhibitory control processes relevant to behavioral regulation and its relationship to snack food consumption in adolescents. Further, this study translated a basic neurocognitive assessment of response inhibition, a Go/No-Go task, to a dietary specific version in order to increase our understanding of the relevance of food cues underlying processes affecting the regulation of eating across a range of healthy weight to obese youth not currently receiving treatment for obesity or eating disorders. Inhibitory processes are relevant primarily when there is a need for inhibition of a pre-potent behavioral tendency and such tendencies do not surface continuously but are activated primarily by antecedent cues associated with the behavior (Wood & Neal, 2007). That is, cues associated with an appetitive behavior can come to trigger a relatively automatic pattern of activation in memory, overwhelming or weakening regulatory control processes. Inhibition or control processes then become most essential when facing relevant cues.
Although we had no a priori hypotheses regarding gender differences, significant differences were revealed in the analyses. Multi-group models revealed that more inhibitory problems, assessed using both false alarms (i.e., inhibitory problems) from the food-cued Go/No-Go and the generic Go/No-Go, to be significantly associated with binge eating behavior in our female adolescent sample, consistent with our hypotheses. In addition, higher BMI percentile and more self-reported sedentary behavior correlated with binge eating in these models. Inhibitory problems on the generic Go/No-Go were as strongly associated with bingeing behavior as BMI percentile. While we expected the magnitude of the effect on the food-specific version of the Go/No-Go task to be stronger, the food-cued version did not perform as well as the generic version. However, this was a first attempt to evaluate the food-specific version of the task in a more diverse adolescent population. It is possible that given the number of Hispanic female youth in the sample that the food cues may not have been culturally sensitive enough to be meaningful, minimizing their effectiveness. The cues were, however, elicited from focus groups held with a similar population. Subsequent tests of the food-cued version might test more personalized tempting food cues assessed as difficult to resist. We would expect individual differences with respect to food-motivated habit and cue relevance, and hence, one’s ability to control impulses in the face of those cues. In the male models, only increased sedentary behavior and BMI percentile were associated with male bingeing behavior.
Affective decision-making was not a significant correlate in the binge eating models or the sweet or salt/fatty snack food models for either males or females and overall did not perform as hypothesized. It is possible that with a more targeted population (e.g., youth with eating disorders, who are overweight or obese), a different pattern of findings may have emerged. Numerous studies with populations demonstrating maladaptive behaviors (e.g., overeating, eating disorders, substance use, externalizing behavior) have shown the IGT detects decreased decision performance in comparison to controls (e.g., Brand et al., 2006; Davis et al., 2004; Davis et al., 2010; Dunn et al., 2006). Further research is needed to evaluate the relationship between affective decision-making and eating behaviors among a general adolescent population, which has been seldom studied.
The sweet snack and sugar-sweetened beverage (SSB) models revealed a different pattern of findings than the binge eating models. We had expected youth with decision-making deficits and poorer response inhibition on the generic Go/No-Go to report more frequent intake of high sugar snacks and sugar-sweetened beverages (SSBs). Further, we expected more inhibitory problems on the food-specific version in the sugar-sweetened snack food models. In these models, sedentary behavior and inhibitory problems assessed on both the food-specific and generic Go/No-Go tasks were predictive of sweet snack consumption among our male sample. In the male model of sweet snack consumption, both indicators of inhibitory control were stronger correlates of the behavior than sedentary behavior and equal in predictive strength. Interestingly, BMI percentile negatively correlated with sweet snack consumption; that is, those males in the lower BMI percentiles reported more sweet snack consumption than males in the higher percentiles. We speculated that perhaps males were underreporting their intake of sweet snacks since previous research has revealed overweight individuals may underreport food consumption (e.g., Braam, Lavienja, Ocké, Bueno-de-Mesquita, & Seidell, 1998; Heitmann & Lissner, 2005). However, further investigation of these data revealed the negative relationship to be the result of overweight/obese males consuming fewer sweet snacks. The overweight/obese males in our sample had significantly higher restrained eating scores on a measure of restrained eating2 (Van Strien, Frijters, Bergers, & Defares, 1986) than normal/underweight males, indicating an attempt to regulate unhealthy snack consumption. We did not observe a similar negative relationship between BMI percentile in females and sweet snack consumption.
With respect to sugar-sweetened beverage consumption, greater inhibitory problems on the generic Go/No-Go and poorer affective decision-making were associated with SSB consumption in males, but not in females. This was the only model in which affective decision-making was significantly correlated with consumptive behavior. The food-specific Go/No-Go was not predictive in models evaluating SSB consumption, providing some initial discriminant validity for the task, as hypothesized. The food specific version of the task consisted of sweet and fatty snacks as no-go signals, with no sugar-sweetened beverages as cues. Therefore, it would be expected that this task would not be associated with SSB consumption unless some generalization of cue effects had occurred. Among females, only sedentary behavior correlated with sweet snack consumption and none of our predictor variables had an effect on SSB consumption among females. The females in the study were less acculturated than the males. Hence the food cues in the food-cued Go/No-Go may not have been sufficiently meaningful to effectively impact individual differences in self-control (as discussed above); however, this would not explain findings on the generic test of response inhibition.
The central focus of this research was on consumption of sugar-sweetened beverages and snacks since these snacks are commonly consumed by adolescents, are nutrient poor, and have high sugar content (Harrington, 2008; Jahns, Siega-Riz, & Popkin, 2001; Keast, Nicklas, & O’Neil, 2010). However, naturally occurring rewards (like sugar and high fat foods) activate similar reward pathways as non-natural rewards (like drugs), and excessive consumption is associated with change in dopamine as well as opioid signaling (Olsen, 2011). Therefore, supplemental analyses of models with salty/fatty snacks as outcomes were evaluated to compare the specificity of effects. In additional analyses of salty/fatty snack food consumption, greater inhibitory problems in males assessed on the generic Go/No-Go were the only correlate of salty/fatty snack food consumption in our sample. This finding for false alarms on the generic Go/No-Go was similar to that seen in the sweet snack food model for males but the magnitude of the effect for the salty/fatty food models was not as strong. However, the effect was almost identical to that observed in the SSB model for males. No other inhibitory or other effects were observed in the male or female models.
Although speculative, it is possible that our findings may be indicative of gender differences in neurocognitive development. In our sample, the females made significantly fewer inhibitory failures on both Go/No-Go tasks than the males. Gender differences in neurocognitive control function with respect to food-mediated habits have also been observed on a neural level. Relative to males, females have shown greater activity in neural regions implicated in inhibitory processes during food-cue exposure and reactivity tasks (Cornier, Salzberg, Endly, Bessesen, & Tregellas, 2010; Killgore & Yurgelun-Todd, 2010; Killgore & Yurgelun-Todd, 2010; Wang, Volkow, Telang, Thanos, & Fowler, 2011). Further, research in eating behavior has revealed a difference between the daily amount of sugar consumed by boys compared to girls (Ervin, Kit, Carroll, & Ogden, 2012), and studies found that after age 9, males preferred and consumed greater quantities of sweet foods than females of the same age (Conner, Haddon, Pickering, & Booth, 1988; Cooke & Wardle, 2005; Desor, Greene, & Maller, 1975; Desor & Beauchamp, 1987; Greene, Desor, & Maller, 1975). Such gender differences may translate to greater habitual responding by the males to sugary foods and hence more difficulty resisting tempting foods during no-go signals. Further investigation among adolescents is needed to understand gender differences in neurocognitive functioning with respect to inhibitory processes in the face of various types of food cues.
Limitations and Conclusions
Findings from this study replicate some previous findings for response inhibition and diet-related research in youth with the use of a generic Go/No-Go task (e.g., Pauli-Pott et al., 2010) and a food-specific version (e.g., Batterink et al., 2010). On the other hand, findings from this work did not replicate that of previous studies reporting impaired decision-making and body weight in youth (Verbeken et al., 2013). However, the present study did reveal marked gender differences in inhibitory function relevant to behavioral control and sugary snack food consumption. Further, this work adds to research implicating the relevance of cues in habitual behaviors and their relationship to sweet snack food consumption in a general, understudied adolescent population.
Since cross-sectional data were used in the analyses, only concurrent prediction can be discussed. Longitudinal research is needed to fully verify the observed relationships. Further, several variables were not included in the analyses which could affect observed differences. For example, we did not have good measures of puberty given the nature of the study, and sex hormones are involved in brain maturation during adolescence (Peper, van den Heuvel, Martijn Mandl, Pol, & van Honk, 2011). Also, it should be noted that the no-go signals included both sugary and salty/fatty snacks, but no SSBs, which could have attenuated effects for the food false alarms in the SSB models and to some extent the other models. The food items used on no-go trials were the highest frequency items elicited by focus groups from a similar adolescent population, and inclusion of these items was important for evaluation of the binge eating models. Further, it is important to note that the outcome measures consisted of self-reports and we did not obtain parental verification of youth snacking consumption. Future studies might want to obtain a corroboration of adolescent snacking consumption. Finally, although we did not see strong effects for affective decision-making, it is possible that we could observe a different pattern with a more targeted population (e.g., individuals with bulimia or binge eating disorders) with difficulty resisting snack foods.
Researchers have found that regions implicated in reasoning and other executive control functioning mature during later adolescence and into early adulthood (Giedd et al., 2009; Gogtay et al., 2004). Maturational changes consist of continued cortical sculpting and concomitant function affecting decision-making and behavioral regulation (Crews & Boettiger, 2009; Overman et al., 2004; Sowell, Thompson, & Toga, 2004). Therefore, adolescents are particularly vulnerable with respect to inhibitory control and disadvantageous decision-making with respect to food-mediated behaviors, and perhaps males and females are differentially vulnerable with respect to certain behaviors. Our findings in a general adolescent population revealed gender differences in neurocognitive functions relevant to behavioral control over snack food consumption. Further evaluation of neurocognitive distinctions and gender differences may lead to greater understanding of the multiple processes affecting inhibitory control in dietary behavior and dietary interventions. The neurocognitive functions affecting inhibitory control and behavioral regulation evaluated in this study have direct relevance for engaging in healthy or unhealthy dietary behaviors, yet are distinct enough empirically and conceptually from one another to provide guidance for future interventions.
Table 1.
Demographic Characteristics by Gender (Males N=87; Females N=111).
| Variable | Statistic | Males | Females | Gender Diffa P value |
|---|---|---|---|---|
|
| ||||
| Age | Mean (SD) Years | 15.65 (1.01) | 15.99 (.87) | .014* |
|
| ||||
| Ethnicity | Hispanic | 74.7% (65) | 80.2% (89) | |
| Non-Hispanic | 25.3% (22) | 19.8% (22) | .392 | |
|
| ||||
| Race/Ethnicity | African American/Black | 5.9% (5) | 4.6% (5) | .267 |
| American Indian or Alaskan Native | 1.2% (1) | 8.3% (9) | ||
| Asian | 0% (0) | 0% (0) | ||
| Hispanic or Latino | 56.5% (48) | 55.0% (60) | ||
| Pacific Islander | 0% (0) | 0% (0) | ||
| Non-Hispanic White | 12.9% (11) | 10.1% (11) | ||
| Two or More Races | 23.5% (20) | 22.0% (24) | ||
| Not Reported | (2) | (2) | ||
|
| ||||
| Acculturation - US Orientation | Mean (SD) (range 1 ‘low’ to 8 ‘high’) | 4.17 (2.36) | 3.28 (2.51) | .012* |
|
| ||||
| SES Household Size | Mean (SD) # of people | 4.57 (1.89) | 4.54 (1.71) | .910 |
| Parents’ Education Level | Less than High School | 21.1% (16) | 21.7% (20) | .619 |
| High School Diploma | 25% (19) | 26.1% (24) | ||
| Some College | 31.6% (24) | 29.3% (27) | ||
| College Graduate | 17.1% (13) | 21.7% (20) | ||
| Graduate School | 5.2% (4) | 1.1% (1) | ||
| Not Reported | (11) | (19) | ||
Gender difference p-values correspond to a Chi-square test of dependence for categorical demographic variables and a t-test for the difference between means for continuous demographic variables.
p<.05;
p<.001; Significant difference between males and females on demographic characteristic
Highlights.
Latent variable models revealed marked gender differences in inhibitory function.
Inhibitory problems on Go/No-Go tasks correlated with binge eating in females.
Inhibitory problems were strongest correlates of sweet snack consumption in males.
Inhibitory problems and poor decision-making predict sweet drink intake in males.
Results implicate the relevance of cues in sweet snack food intake in adolescents.
Acknowledgments
This research was supported by grants from the National Heart, Lung, & Blood Institute and the National Institute Of Child Health & Human Development (U01HL097839), the National Institute on Drug Abuse (DA023368, DA024659) and the National Cancer Institute (CA152062). We thank James Pike, grant project manager at Claremont Graduate University for his support on this project. Susan L. Ames had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
We conducted supplementary analyses given a reviewer’s concern regarding potential outliers in the data. Upon review of the data, no clear bivariate outliers that would exaggerate the relationships were observed. There was, however, one extreme univariate outlier that could be attenuating, not strengthening, the estimated correlation. The models were reevaluated after removing this extreme outlier, which was due to one female who reported very high consumption of sweet snack foods. These analyses revealed no change in the significance of effects, though the strength of the effect size increased very slightly for false alarms and sweet snack consumption as well as SSB consumption in the female models.
Restrained eating behavior was assessed with the restrained eating subscale from the Dutch Eating Behavior Questionnaire (DEBQ; Van Strien, Frijters, Bergers, Defares, 1986). The Restrained Eating subscale (α = .92) consists of 10 items asking respondents about regulation of their own food intake such as: “If you have put on weight, do you eat less than usual?” All items are scored from 1–5, and the scales were created by summing up the item values and dividing them by the number of completed items. It was used in supplemental analyses only.
Conflict of interest. The authors have no financial or other relationships that might lead to a conflict of interest regarding the material discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abroms L, Jorgensen C, Southwell B, Geller A, Emmons K. Gender differences in young adults’ beliefs about sunscreen use. Health Education & Behavior. 2003;30(1):29–43. doi: 10.1177/1090198102239257. [DOI] [PubMed] [Google Scholar]
- Ames SL, Grenard JL, Thush C, Sussman S, Wiers RW, Stacy AW. Comparison of indirect assessments of association as predictors of marijuana use among at-risk adolescents. Experimental & Clinical Psychopharmacology. 2007;15(2):204–18. doi: 10.1037/1064-1297.15.2.218. 2007-05127-009 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: Relevance for genetic research in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57(11):1285–92. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: An fMRI study. Neuroimage. 2010;52(4):1696–1703. doi: 10.1016/j.neuroimage.2010.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Van der Linden M. Decision-making and impulse control after frontal lobe injuries. Current Opinion in Neurology. 2005;18:734–739. doi: 10.1097/01.wco.0000194141.56429.3c. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: A neurocognitive perspective. Nature Neuroscience. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1–3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Noel X, Crone EA. Loss of willpower: Abnormal neural mechanisms of impulse control and decision-making in addiction. In: Wiers RW, Stacy AW, editors. Handbook of implicit cognition and addiction. Thousand Oaks, CA, US: SAGE Publications; 2006. pp. 215–232. [Google Scholar]
- Bentler P. Comparative fit indexes in structural models. Psychological Bulletin. 1990;107(2):238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Bollen KA, Long JS. Testing structural equation models. Sage; 1993. [Google Scholar]
- Bollen KA. Structural equations with latent variables. New York: Wiley; 1989. [Google Scholar]
- Braam, Lavienja AJLM, Ocké MC, Bueno-de-Mesquita HB, Seidell JC. Determinants of obesity-related underreporting of energy intake. American Journal of Epidemiology. 1998;147(11):1081–1086. doi: 10.1093/oxfordjournals.aje.a009402. [DOI] [PubMed] [Google Scholar]
- Brand M, Labudda K, Markowitsch HJ. Neuropsychological correlates of decision-making in ambiguous and risky situations. Neural Networks. 2006;19(8):1266–1276. doi: 10.1016/j.neunet.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Braver TS, Ruge H. Functional neuroimaging of executive functions. In: Cabeza R, Kingstone A, editors. Handbook of functional neuroimaging of cognition. 2. Cambridge, Massachusetts: Massachusetts Institute of Technology Press; 2006. pp. 307–348. [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54(1–3):241–57. doi: 10.1016/s0301-0511(00)00058-2. S0301051100000582 [pii] [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) CDC growth chart. 2010 Retrieved, 2014, from http://www.cdc.gov/growthcharts/)
- Centers for Disease Control and Prevention (CDC) Childhood obesity facts. 2013 Retrieved May 9 from http://www.cdc.gov/healthyyouth/obesity/facts.htm.
- Chiara G, Tanda G, Bassareo V, Pontieri F, Acquas E, Fenu S, Carboni E. Drug addiction as a disorder of associative learning: Role of nucleus accumbens shell/extended amygdala dopamine. Annals of the New York Academy of Sciences. 1999;877(1):461–485. doi: 10.1111/j.1749-6632.1999.tb09283.x. [DOI] [PubMed] [Google Scholar]
- Conner MT, Haddon AH, Pickering ES, Booth DA. Sweet tooth demonstrated: Individual differences in preference for both sweet foods and foods highly sweetened. Journal of Applied Psychology. 1988;73(2):275. doi: 10.1037/0021-9010.73.2.275. [DOI] [PubMed] [Google Scholar]
- Cooke LJ, Wardle J. Age and gender differences in children’s food preferences. British Journal of Nutrition. 2005;93(5):741–746. doi: 10.1079/bjn20051389. [DOI] [PubMed] [Google Scholar]
- Cornier M, Salzberg AK, Endly DC, Bessesen DH, Tregellas JR. Sex-based differences in the behavioral and neuronal responses to food. Physiology & Behavior. 2010;99(4):538–543. doi: 10.1016/j.physbeh.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacology, Biochemistry and Behavior. 2009;93(3):237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Levitan RD, Muglia P, Bewell C, Kennedy JL. Decision-making deficits and overeating: A risk model for obesity. Obesity Research. 2004;12(6):929–935. doi: 10.1038/oby.2004.113. [DOI] [PubMed] [Google Scholar]
- Davis C, Patte K, Curtis C, Reid C. Immediate pleasures and future consequences. A neuropsychological study of binge eating and obesity. Appetite. 2010;54(1):208–213. doi: 10.1016/j.appet.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Desor J, Beauchamp GK. Longitudinal changes in sweet preferences in humans. Physiology & Behavior. 1987;39(5):639–641. doi: 10.1016/0031-9384(87)90166-1. [DOI] [PubMed] [Google Scholar]
- Desor J, Greene LS, Maller O. Preferences for sweet and salty in 9-to 15-year-old and adult humans. Science. 1975;190(4215):686–687. doi: 10.1126/science.1188365. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Dalgleish T, Lawrence AD. The somatic marker hypothesis: A critical evaluation. Neuroscience and Biobehavioral Reviews. 2006;30(2):239. doi: 10.1016/j.neubiorev.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Durston S, Casey BJ. What have we learned about cognitive development from neuroimaging? Neuropsychologia. 2006;44(11):2149–57. doi: 10.1016/j.neuropsychologia.2005.10.010. S0028-3932(05)00339-8 [pii] [DOI] [PubMed] [Google Scholar]
- Eagle DM, Bari A, Robbins TW. The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology. 2008;199:439–456. doi: 10.1007/s00213-008-1127-6. [DOI] [PubMed] [Google Scholar]
- Ervin R, Kit B, Carroll M, Ogden C. Consumption of added sugar among US children and adolescents, 2005–2008. 87. Hyattsville, MD: National Center for Health Statistics; 2012. [Google Scholar]
- Everitt B, Robbins T. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nature Neuroscience. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fillmore MT. Increased sensitivity to the disinhibiting effects of alcohol in adults with ADHD. Experimental and Clinical Psychopharmacology. 2009;17(2):113. doi: 10.1037/a0015418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Marczinski CA, Bowman AM. Acute tolerance to alcohol effects on inhibitory and activational mechanisms of behavioral control. Journal of Studies on Alcohol and Drugs. 2005;66(5):663. doi: 10.15288/jsa.2005.66.663. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Polydrug abusers display impaired discrimination-reversal learning in a model of behavioural control. Journal of Psychopharmacology. 2006;20(1):24–32. doi: 10.1177/0269881105057000. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Ostling EW, Martin CA, Kelly TH. Acute effects of alcohol on inhibitory control and information processing in high and low sensation-seekers. Drug and Alcohol Dependence. 2009;100(1–2):91–99. doi: 10.1016/j.drugalcdep.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Kelly TH, Martin CA. Effects of d-amphetamine in human models of information processing and inhibitory control. Drug and Alcohol Dependence. 2005;77(2):151–159. doi: 10.1016/j.drugalcdep.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: The bogalusa heart study. The Journal of Pediatrics. 2007;150(1):12–17. e2. doi: 10.1016/j.jpeds.2006.08.042. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Lalonde FM, Celano MJ, White SL, Wallace GL, Lee NR, Lenroot RK. Anatomical brain magnetic resonance imaging of typically developing children and adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48(5):465–470. doi: 10.1097/CHI.0b013e31819f2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LS, Desor JA, Maller O. Heredity and experience: Their relative importance in the development of taste preference in man. Journal of Comparative and Physiological Psychology. 1975;89(3):279–284. doi: 10.1037/h0076802. [DOI] [PubMed] [Google Scholar]
- Grenard JL, Stacy AW, Shiffman S, Baraldi AN, MacKinnon DP, Lockhart G, Reynolds KD. Sweetened drink and snacking cues in adolescents: A study using ecological momentary assessment. Appetite. 2013;67(1):61–73. doi: 10.1016/j.appet.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenard JL, Ames SL, Wiers RW, Thush C, Sussman S, Stacy AW. Working memory capacity moderates the predictive effects of drug-related associations on substance use. Psychol Addict Behav. 2008;22(3):426–32. doi: 10.1037/0893-164X.22.3.426. 2008-11981-012 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigson PS. Like drugs for chocolate: Separate rewards modulated by common mechanisms ? Physiol Behav. 2002;76(3):389–95. doi: 10.1016/s0031-9384(02)00758-8. S0031938402007588 [pii] [DOI] [PubMed] [Google Scholar]
- Guerrieri R, Nederkoorn C, Jansen A. How impulsiveness and variety influence food intake in a sample of healthy women. Appetite. 2007;45:119–122. doi: 10.1016/j.appet.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Guerrieri R, Nederkoorn C, Stankiewicz K, Alberts H, Geschwind N, Martijn C, Jansen A. The influence of trait and induced state impulsivity on food intake in normal-weight healthy women. Appetite. 2007;49(1):66–73. doi: 10.1016/j.appet.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Guerrieri R, Nederkoorn C, Schrooten M, Martijn C, Jansen A. Inducing impulsivity leads high and low restrained eaters into overeating, whereas current dieters stick to their diet. Appetite. 2009;53(1):93–100. doi: 10.1016/j.appet.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Harrington S. The role of sugar-sweetened beverage consumption in adolescent obesity: A review of the literature. The Journal of School Nursing. 2008;24(1):3–12. doi: 10.1177/10598405080240010201. [DOI] [PubMed] [Google Scholar]
- Heitmann BL, Lissner L. Can adverse effects of dietary fat intake be overestimated as a consequence of dietary fat underreporting? Public Health Nutrition. 2005;8(08):1322–1327. doi: 10.1079/phn2005750. [DOI] [PubMed] [Google Scholar]
- Houben K, Nederkoorn C, Jansen A. Eating on impulse: the relationship between overweight and food-specific inhibitory control. Obesity. 2014;22:E6–E8. doi: 10.1002/oby.20670. [DOI] [PubMed] [Google Scholar]
- Hoyle RH. Structural equation modeling: Concepts, issues, and applications. Sage; 1995. [Google Scholar]
- Hu L, Bentler PM. Fit indices in covariance structural equation modeling: Sensitivity to underparameterized model misspecification. Psychological Methods. 1998;3:424–453. [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6:1–55. [Google Scholar]
- Jahns L, Siega-Riz AM, Popkin BM. The increasing prevalence of snacking among US children from 1977 to 1996. The Journal of Pediatrics. 2001;138(4):493–498. doi: 10.1067/mpd.2001.112162. [DOI] [PubMed] [Google Scholar]
- Jasik CB, Lustig RH. Adolescent obesity and puberty: The “perfect storm”. Annals of the New York Academy of Sciences. 2008;1135(1):265–279. doi: 10.1196/annals.1429.009. [DOI] [PubMed] [Google Scholar]
- Keast DR, Nicklas TA, O’Neil CE. Snacking is associated with reduced risk of overweight and reduced abdominal obesity in adolescents: National health and nutrition examination survey (NHANES) 1999–2004. The American Journal of Clinical Nutrition. 2010;92(2):428–435. doi: 10.3945/ajcn.2009.28421. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Schiltz CA, Landry CF. Neural systems recruited by drug- and food-related cues: Studies of gene activation in corticolimbic regions. Physiol Behav. 2005;86(1–2):11–4. doi: 10.1016/j.physbeh.2005.06.018. S0031-9384(05)00234-9 [pii] [DOI] [PubMed] [Google Scholar]
- Kenny PJ. Reward mechanisms in obesity: New insights and future directions. Neuron. 2011;69(4):664–679. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Sex differences in cerebral responses to images of high vs low calorie food. Neuroreport. 2010;21(5):354. doi: 10.1097/WNR.0b013e32833774f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273(5280):1399–402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behavioral Neuroscience. 2001;115(2):493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Jollant F, O’Daly O, Zelaya F, Phillips ML. Distinct roles of prefrontal cortical subregions in the iowa gambling task. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn154. bhn154 [pii] [DOI] [PubMed] [Google Scholar]
- Li CR, Zhang S, Duann J, Yan P, Sinha R, Mazure CM. Gender differences in cognitive control: An extended investigation of the stop signal task. Brain Imaging and Behavior. 2009;3(3):262–276. doi: 10.1007/s11682-009-9068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little TD, Rhemtulla M, Gibson K, Schoemann A. Why the items versus parcels controversy needn’t be one. Psychological Methods. 2013;18(3):285–300. doi: 10.1037/a0033266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little T, Cunningham W, Shahar G, Widaman K. To parcel or not to parcel: Exploring the question, weighing the merits. Structural Equation Modeling: A Multidisciplinary Journal. 2002;9(2):151–173. [Google Scholar]
- Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychological Science. 1997;8(1):60–64. doi: 10.1111/j.1467-9280.1997.tb00545. [DOI] [Google Scholar]
- MacCallum RC, Browne MW, Sugawara HM. Power analysis and determination of sample size for covariance structure modeling. Psychological Methods. 1996;1(2):130–149. [Google Scholar]
- Marczinski CA, Fillmore MT. Dissociative antagonistic effects of caffeine on alcohol-induced impairment of behavioral control. Experimental and Clinical Psychopharmacology. 2003;11(3):228. doi: 10.1037/1064-1297.11.3.228. [DOI] [PubMed] [Google Scholar]
- Meule A, Lukito S, Vögele C, Kübler A. Enhanced behavioral inhibition in restrained eaters. Eating Behaviors. 2011;12(2):152–155. doi: 10.1016/j.eatbeh.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Mobbs O, Van der Linden M, d’Acremont M, Perroud A. Cognitive deficits and biases for food and body in bulimia: Investigation using an affective shifting task. Eat Behav. 2008;9(4):455–61. doi: 10.1016/j.eatbeh.2008.07.002. S1471-0153(08)00061-5 [pii] [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 6. Los Angeles, CA: Muthén & Muthén; 1998–2011. [Google Scholar]
- Nederkoorn C, Guerrieri R, Havermans R, Roefs A, Jansen A. The interactive effect of hunger and impulsivity on food intake and purchase in a virtual supermarket. International Journal of Obesity. 2009;33(8):905–912. doi: 10.1038/ijo.2009.98. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Braet C, Van Eijs Y, Tanghe A, Jansen A. Why obese children cannot resist food: The role of impulsivity. Eating Behaviors. 2006;7(4):315–322. doi: 10.1016/j.eatbeh.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Coelho JS, Guerrieri R, Houben K, Jansen A. Specificity of the failure to inhibit responses in overweight children. Appetite. 2012;59(2):409–413. doi: 10.1016/j.appet.2012.05.028. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Houben K, Hofmann W, Roefs A, Jansen A. Control yourself or just eat what you like? weight gain over a year is predicted by an interactive effect of response inhibition and implicit preference for snack foods. Health Psychology. 2010;29(4):389. doi: 10.1037/a0019921. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Jansen E, Mulkens S, Jansen A. Impulsivity predicts treatment outcome in obese children. Behaviour Research and Therapy. 2007;45(5):1071–1075. doi: 10.1016/j.brat.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Nelson DL, Goodmon LB. Disrupting attention: The need for retrieval cues in working memory theories. Memory & Cognition. 2003;31(1):65–76. doi: 10.3758/bf03196083. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA: The Journal of the American Medical Association. 2012;307(5):483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CM. Natural rewards, neuroplasticity, and non-drug addictions. Neuropharmacology. 2011;61(7):1109–1122. doi: 10.1016/j.neuropharm.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overman WH, Frassrand K, Ansel S, Trawalter S, Bies B, Redmond A. Performance on the IOWA card task by adolescents and adults. Neuropsychologia. 2004;42(13):1838–1851. doi: 10.1016/j.neuropsychologia.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Patterson CM, Newman JP. Reflectivity and learning from aversive events: Toward a psychological mechanism for the syndromes of disinhibition. Psychol Rev. 1993;100(4):716–36. doi: 10.1037/0033-295x.100.4.716. [DOI] [PubMed] [Google Scholar]
- Pauli-Pott U, Albayrak Ö, Hebebrand J, Pott W. Association between inhibitory control capacity and body weight in overweight and obese children and adolescents: Dependence on age and inhibitory control component. Child Neuropsychology. 2010;16(6):592–603. doi: 10.1080/09297049.2010.485980. [DOI] [PubMed] [Google Scholar]
- Peper JS, van den Heuvel Martijn P, Mandl RC, Pol HEH, van Honk J. Sex steroids and connectivity in the human brain: A review of neuroimaging studies. Psychoneuroendocrinology. 2011;36(8):1101–1113. doi: 10.1016/j.psyneuen.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Conditioning of stimuli with nonzero initial value. Journal of Experimental Psychology: Animal Behavior Processes. 2008;34(3):315–323. doi: 10.1037/0097-7403.34.3.315. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Drug addiction: Bad habits add up. Nature. 1999;398(6728):567–570. doi: 10.1038/19208. [DOI] [PubMed] [Google Scholar]
- Rockett HRH, Breitenbach M, Frazier AL, Witschi J, Wolf AM, Field AE, Colditz GA. Validation of a Youth/Adolescent food frequency questionnaire. Preventive Medicine. 1997;26(6):808. doi: 10.1006/pmed.1997.0200. [DOI] [PubMed] [Google Scholar]
- Rockett H, Wolf A, Colditz G. Development and reproducibility of a food frequency questionnaire to assess diets of older children and adolescents. Journal of the American Dietetic Association. 1995;95(3):336–40. doi: 10.1016/S0002-8223(95)00086-0. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46(1):224–32. doi: 10.1016/j.neuropsychologia.2007.07.015. S0028-3932(07)00268-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10(4):372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Stacy AW, Wiers RW. Implicit cognition and addiction: A tool for explaining paradoxical behaviors. Annual Review of Clinical Psychology. 2010;6:551–575. doi: 10.1146/annurev.clinpsy.121208.131444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy AW. Memory activation and expectancy as prospective predictors of alcohol and marijuana use. J Abnorm Psychol. 1997;106(1):61–73. doi: 10.1037//0021-843x.106.1.61. [DOI] [PubMed] [Google Scholar]
- Stacy AW, Ames SL, Knowlton BJ. Neurologically plausible distinctions in cognition relevant to drug use etiology and prevention. Substance use & Misuse. 2004;39(10–12):1571–1623. doi: 10.1081/ja-200033204. [DOI] [PubMed] [Google Scholar]
- Stice E, Fisher M, Martinez E. Eating disorder diagnostic scale: Additional evidence of reliability and validity. Psychological Assessment. 2004;16(1):60–70. doi: 10.1037/1040-3590.16.1.60. [DOI] [PubMed] [Google Scholar]
- Stice E, Telch C, Rizvi S. Development and validation of the eating disorder diagnostic scale: A brief self-report measure of anorexia, bulimia, and binge-eating disorder. Psychological Assessment. 2000;12(2):123–131. doi: 10.1037//1040-3590.12.2.123. [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of tow response inhibition tasks. NeuroImage. 2011;(56):1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Tchanturia K, Liao P, Uher R, Lawrence N, Treasure J, Campbell IC. An investigation of decision making in anorexia nervosa using the iowa gambling task and skin conductance measurements. Journal of the International Neuropsychological Society. 2007;13(04):635–641. doi: 10.1017/S1355617707070798. [DOI] [PubMed] [Google Scholar]
- Unger JB, Gallaher P, Shakib S, Ritt-Olson A, Palmer PH, Johnson CA. The AHIMSA acculturation scale: A new measure of acculturation for adolescents in a multicultural society. The Journal of Early Adolescence. 2002;22(3):225–251. doi: 10.1177/02731602022003001. [DOI] [Google Scholar]
- Van Strien T, Frijters JE, Bergers G, Defares PB. The dutch eating behavior questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. International Journal of Eating Disorders. 1986;5(2):295–315. [Google Scholar]
- Verbeken S, Braet C, Bosmans G, Goossens L. Comparing decision-making in average and overweight children and adolescents. International Journal of Obesity. 2013 doi: 10.1038/ijo.2013.235. [DOI] [PubMed] [Google Scholar]
- Verbeken S, Braet C, Claus L, Nederkoorn C, Oosterlaan J. Childhood obesity and impulsivity: An investigation with performance-based measures. Behaviour Change. 2009;26(03):153–167. [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nature Neuroscience. 2005;8(5):555–60. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Wang G, Volkow ND, Telang F, Thanos PK, Fowler JS. Handbook of behavior, food and nutrition. Springer; 2011. Gender differences in brain activation by food stimulation; pp. 505–514. [Google Scholar]
- White NM. Addictive drugs as reinforcers: Multiple partial actions on memory systems. Addiction. 1996;91(7):921–49. [PubMed] [Google Scholar]
- Wiers RW, Bartholow BD, van den Wildenberg E, Thush C, Engels RC, Sher KJ, Stacy AW. Automatic and controlled processes and the development of addictive behaviors in adolescents: A review and a model. Pharmacol Biochem Behav. 2007;86(2):263–83. doi: 10.1016/j.pbb.2006.09.021. S0091-3057(06)00323-6 [pii] [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: Translation between clinical and preclinical studies. Clinical Psychology Review. 2006;26(4):379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Rompré P. Brain dopamine and reward. Annual Review of Psychology. 1989;40(1):191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Wood W, Neal DT. A new look at habits and the habit-goal interface. Psychol Rev. 2007;114(4):843–63. doi: 10.1037/0033-295X.114.4.843. 2007-13558-001 [pii] [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. Addiction and learning in the brain. In: Wiers RW, Stacy AW, editors. Handbook of implicit cognition and addiction. Thousand Oaks, CA: SAGE Publications; 2006a. pp. 167–184. [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nature Reviews Neuroscience. 2006b;7(6):464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]