SUMMARY
Objectives
To investigate the utility of beta-D-glucan (BDG) testing in bronchoalveolar lavage (BAL) fluid for the diagnosis of invasive fungal infection (IFI), as compared to BAL galactomannan (GM).
Methods
We retrospectively reviewed medical records of 132 consecutive patients at the National Institutes of Health (NIH) in whom BAL BDG testing was performed for diagnosis of pneumonia. Using the European Organization for Research and Treatment of Cancer/Mycoses Study Group guidelines, we determined which patients had proven or probable IFI, and assessed the diagnostic performance of BAL BDG testing, relative to BAL GM. We also determined the reproducibility of the BDG assay in BAL via repeat testing of patient samples.
Results
Ten patients had Pneumocystis pneumonia, and 34 patients had proven/probable IFI, including 14 with invasive aspergillosis (IA). BAL BDG was 100% sensitive for Pneumocystis. Although BAL BDG had similar sensitivity to BAL GM for the diagnosis of IA and IFI, it exhibited inferior specificity. Repeat testing demonstrated poor reproducibility of the BDG assay in BAL but not in serum.
Conclusions
BDG testing exhibits poor specificity and reproducibility in BAL. Identification of the BAL-specific factors that may interfere with the performance of the assay could improve the clinical usefulness of BAL BDG testing.
Keywords: invasive fungal infection, aspergillosis, beta-D-glucan, galactomannan, bronchoalveolar lavage
INTRODUCTION
Despite advances in antifungal therapies, invasive fungal infections (IFI), such as invasive aspergillosis (IA) and non-Aspergillus mold infections, continue to cause substantial morbidity and mortality in immunosuppressed patients, in part due to suboptimal diagnosis [1]. The sensitivity of respiratory cultures is unacceptably low, even in histopathology-proven cases, and invasive procedures that have a higher yield are infrequently performed due to patient comorbidities [1,2]. Molecular testing has shown promise but its implementation has been hampered by lack of standardization and non-specific results caused by colonization and/or contamination [1,3].
In recent years, beta-D-glucan (BDG) and galactomannan (GM) tests, which detect fungal cell wall constituents, have emerged as indirect diagnostic markers for IFI. These tests have shown variable sensitivity and specificity depending on several clinical factors. For example, both BDG and GM are more sensitive in patients with neutropenia and/or hematologic malignancies compared to solid organ transplant recipients [4,5]. False-positive results have been reported with concurrent bacterial infections (BDG) or antibiotic administration (GM), and false-negative results for both tests have been associated with antifungal prophylaxis [1,6]. The site of testing also seems to play a role as shown by the higher sensitivity of bronchoalveolar lavage (BAL) over serum GM for diagnosing IA [7]. While serum BDG is an approved test by the Food and Drug Administration and may aid in the diagnosis of IA and non-Aspergillus fungal infections, which are less amenable to detection by GM [1], the role of BDG testing in the BAL for the diagnosis of IFI remains unclear. Thus, our study aimed to examine the performance of BAL BDG (relative to BAL GM) in an unselected population of patients at high-risk for IFI.
PATIENTS AND METHODS
We reviewed medical records of 132 consecutive patients seen at the National Institutes of Health (NIH) between January 2008 and December 2011 for whom BAL BDG was performed for work-up of suspected fungal pneumonia. We obtained exemption from Institutional Review Board review by the NIH Office of Human Subjects Research Protections and collected information on demographics, underlying diseases/predisposing factors, clinical/radiographic features, microbiologic data and clinical outcome. When available, values for BAL GM (n=129), serum BDG (n=76) and serum GM (n=73) were recorded. Samples were tested in real-time after collection of BAL / serum from patients. The Fungitell® assay (Beacon Diagnostics Laboratory, East Falmouth, MA) was used for BDG testing, and the Platelia™ Aspergillus enzyme immunoassay (Bio-Rad, Redmond, WA) was used for GM testing. BDG testing was performed in triplicate from all samples. Per manufacturer recommendations, GM testing was performed in duplicate from all positive samples. For both serum and BAL, BDG values of ≥80 pg/mL and <80 pg/mL were considered positive and negative, respectively, and a GM index of ≥0.5 and <0.5 was considered positive and negative, respectively.
Cases of proven/probable IFI or IA, and their response to treatment, were defined per European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) guidelines [3,8]. Accordingly, PCP cases, defined as positive Pneumocystis polymerase chain reaction and/or direct fluorescent antibody, with compatible clinical picture, were not grouped within IFI, but analyzed separately.
We determined the sensitivity and specificity of each test (BAL BDG, BAL GM, serum BDG and serum GM) for all IFI, and for IA and Pneumocystis pneumonia (PCP) separately. We also examined the impact of pre-exposure to antibacterials/antifungals, fungal colonization, and concomitant infections on test performance.
To interrogate the reproducibility of BDG testing in BAL, 17 randomly selected BAL patient samples underwent repeat testing by the same operator at the NIH Microbiology Laboratory or the assay reference laboratory (Beacon Diagnostics) and were evaluated for reproducibility by means of the coefficient of variation (CV). Twenty-two randomly selected serum patient samples also underwent repeat testing as a control to determine the site-specific reproducibility of the BDG assay. For both BAL and serum samples that underwent repeat testing, reproducibility testing was performed on frozen specimens, which were thawed only once. All work was performed in a biological safety cabinet with careful consideration to prevent contamination. Technologists performing the testing were fully trained and had passed proficiency testing for the BDG assay. Per manufacturer guidelines for serum BDG testing, a maximum CV of 20% is expected for replicates of samples with BDG values between 60 and 500 pg/mL. The percent of repeat test values for BAL and serum samples that fell within the expected CV was recorded.
Statistical analysis was performed using SAS, Version 9.3 (SAS Institute Inc., Cary, NC, 2011). The sensitivity and specificity of each test (BAL BDG, BAL GM, serum BDG, serum GM) for the diagnosis of all IFI, IA and PCP was determined. Receiver operating characteristic (ROC) area under the curve (AUC) analysis was used to evaluate whether test values were predictive of IFI. Among those with a positive test, logistic regression was used to assess whether the magnitude of positive test values was predictive of mortality. Fisher's exact test was used to identify associations between clinical factors and false positive or false negative results. For all analyses, P<0.05 was considered statistically significant.
RESULTS
The most common host factors in our cohort were hematologic malignancy (n=58), allogeneic hematopoietic stem cell transplantation (n=40), neutropenia (n=31) and/or corticosteroid use (n=30). The majority of patients (n=78) were on prophylactic or empiric antifungal treatment with fluconazole (n=37), voriconazole (n=33) and/or caspofungin (n=14) prior to BAL; some patients received more than one antifungal agent (Table 1).
Table 1.
Characteristics of patients with a BAL BDG test for evaluation of fungal pneumonia
| Category | Demographic or Clinical Feature | N (%) |
|---|---|---|
| Gender | Male | 72 (54.5) |
| Female | 60 (45.5) | |
| Race | Caucasian | 73 (55.3) |
| Black / African-American | 28 (16.2) | |
| Hispanic | 19 (l4.4) | |
| Other or unknown | 12 (9.l) | |
| aUnderlying Disease or Risk Factor for IFI | Hematologic malignancy | 58 (43.9) |
| Allogeneic stem cell transplantation | 40 (30.3) | |
| bNeutropenia (absolute neutrophil count < 500 / mm3) | 31 (23.5) | |
| Steroids (> 0.3 mg / kg / d prednisone equivalent) Graft versus host disease (acute or chronic) |
30 (22.7) 25 (18.9) |
|
| Severe Aplastic Anemia (SAA) | 17 (l2.9) | |
| Human Immunodeficiency Virus (HIV) | 14 (10.6) | |
| Chronic Granulomatous Disease (CGD) | 6 (4.5) | |
| Concomitant medications | cSystemic antibiotic(s) within one week of BAL | 112 (84.8) |
| dSystemic antifungal(s) at time of diagnosis | 78 (59.1) | |
| Azoles | Fluconazole | 37 (28.0) |
| Voriconazole | 33 (25.0) | |
| Posaconazole | 9 (6.8) | |
| Echinocandins | Caspofungin | 14 (10.6) |
| Micafungin | 12 (9.1) | |
| Anindulafungin | 7 (5.3) | |
| Polyenes | Liposomal amphotericin | 14 (10.6) |
Patients often had more than one underlying disease or risk factor
Average duration of neutropenia prior to diagnosis was 45 days
Antibacterial agent(s); patients often received multiple antibacterial drugs
Antifungal agent(s) within 3 months prior to diagnosis; some patients received more than one antifungal agent
Ten patients had PCP; BAL BDG was positive in 10/10 (median, >500 pg/mL), and serum BDG was positive in 5/6 patients tested (sensitivity, 83%; median among positive tests, >500 pg/mL). Neither BAL nor serum GM was positive in any patient with PCP.
Thirty-four patients had proven or probable pulmonary IFI; 14 had IA, of which 8 were culture-positive. The remaining IFI cases in which a non-Aspergillus mold was isolated were mucormycosis (n=5), fusariosis (n=2), Paecilomyces (n=2), and Scopulariopsis (n=1) (Table 2).
Table 2.
BAL and serum BDG and GM levels and clinical response in the patients with proven/probable pulmonary IFI (n=34)
| aIFI | bRisk factor(s) | BAL BDG (Pg/ml | BAL GM (index) | Serum BDG (pg/ml) | Serum GM (index) | Response at 12 wks |
|---|---|---|---|---|---|---|
| alA | CGD | >500 | 1.02 | 97 | <0.5 | Success |
| alA | ID | 129 | <0.5 | NP | NP | Success |
| IA | AA, HSCT, GVHD, S | >500 | 2.3 | <31 | <0.5 | Success |
| IA | Heme, N | >500 | 5.5 | 296 | 1.8 | Unknown |
| IA | Heme, HSCT, S | >500 | 0.6 | <31 | <0.5 | Death |
| IA | AA, HSCT, GVHD | >500 | 7.33 | NP | NP | Success |
| IA | CGD, HSCT, S | 450 | 4.9 | >500 | 5.6 | Failure |
| IA | Heme, N, S | 117 | 0.7 | <31 | <0.5 | Failure |
| IA | Heme, HSCT, GVHD | 86 | 0.92 | 370 | <0.5 | Failure |
| IA | AA, N | 85 | 4.76 | 183 | 3.213 | Failure |
| IA | ID, HSCT, N | <31 | 11.4 | 92 | 1.2 | Failure |
| IA | CGD | <31 | <0.5 | 204 | NP | Failure |
| IA | AA, N | <31 | <0.5 | 39 | <0.5 | Failure |
| IA | Heme, HSCT, S | <31 | <0.5 | <31 | <0.5 | Failure |
| aIFI | CGD | <31 | 1.02 | <31 | NP | Failure |
| aIFI | ID | <31 | <0.5 | NP | NP | Success |
| IFI | Heme, HSCT, N | 215 | <0.5 | 137 | <0.5 | Success |
| IFI | Heme, HSCT, S | 149 | <0.5 | 93 | <0.5 | Failure |
| IFI | AA, N | 140 | <0.5 | 210 | <0.5 | Success |
| IFI | Heme, HSCT, N, S | 135 | <0.5 | 109 | <0.5 | Death |
| IFI | AA, HSCT, GVHD, N, S | 129 | <0.5 | 189 | <0.5 | Failure |
| IFI | Heme, HSCT, GVHD | <31 | <0.5 | >500 | <0.5 | Death |
| IFI | Heme, HIV, N, S | <31 | <0.5 | >500 | <0.5 | Death |
| IFI | CGD | <31 | NP | 139 | <0.5 | Success |
| aPaecilomyces | ID, HSCT, GVHD, N | >500 | 8.4 | 370 | 3.5 | Death |
| aMucormycosis | Heme, HSCT, GVHD, S | 168 | 0.56 | NP | <0.5 | Death |
| aMucormycosis | Heme, HSCT, GVHD | 86 | 0.92 | 370 | <0.5 | Failure |
| aMucormycosis | AA, N | <31 | <0.5 | <31 | <0.5 | Failure |
| aFusariosis | AA, N | <31 | <0.5 | 74 | <0.5 | Failure |
| aScopulariopsis | AA, N, S | <31 | <0.5 | <31 | <0.5 | Success |
| Fusariosis | Heme, HSCT, GVHD, N, S | 73 | <0.5 | <31 | <0.5 | Death |
| Mucormycosis | Heme, HSCT, N | 54 | <0.5 | NP | NP | Success |
| Paecilomyces | Heme, HSCT, GVHD, N, S | 35 | <0.5 | <31 | <0.5 | Death |
| Mucormycosis | AA, N | <31 | <0.5 | <31 | <0.5 | Death |
NP=not performed
Denotes proven cases; otherwise all other cases are probable IFI per 2008 EORTC/MSG definitions (IA=invasive aspergillosis; IFI=invasive fungal infection not otherwise specified; causative organism listed if known)
Risk factors annotated as follows: AA=aplastic anemia; CGD=chronic granulomatous disease; GVHD=graft versus host disease; Heme=hematologic malignancy; HIV=human immunodeficiency virus; HSCT=hematopoietic stem cell transplantation; ID=other immunodeficiency (e.g. GATA-2, DOCK-8); N=neutropenia; S=steroids (≥0.3 mg/kg/d prednisone equivalent)
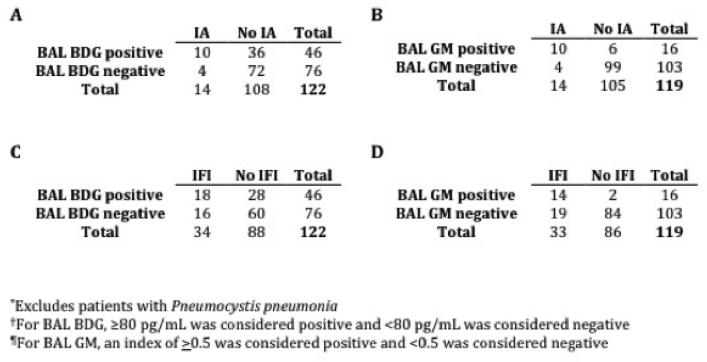
For the diagnosis of IA, BAL BDG was as sensitive as BAL GM (both 71%), but had inferior specificity (67% versus 94%, respectively; Figure 1A-B). Similarly, for all IFI, BAL BDG had comparable sensitivity to BAL GM (53% versus 42%, respectively) but with lower specificity (68% versus 98%, respectively; Figure 1C-D); of note, BAL BDG was positive in 6 patients with IFI who had negative BAL GM, and was negative in 2 patients with IFI who had positive BAL GM (Table 2). Two patients with proven mucormycosis in our study had a positive BDG and/or GM test. Since mucormycosis is not known to produce elevations in BDG or GM, these cases may represent false positive test results or the presence of concomitant, undiagnosed fungal infections causing BDG/GM elevation; therefore, this result should be interpreted with caution. In serum, BDG had similar sensitivity (55%) to BAL BDG for diagnosing IFI, but was more specific (92%). Serum GM was insensitive for both IFI (18%) and IA (36%), but had high specificity (100% and 98%, respectively).
Figure 1.
Performance of BAL BDG† and BAL GM¶ for diagnosis of (A-B) invasive aspergillosis (IA) and (C-D) invasive fungal infection (IFI)
For BAL BDG, ROC analysis revealed that higher test values did not predict the diagnosis of IFI, given an ROC AUC (95% Confidence Interval [CI]) of 0.58 (0.46, 0.70; P>0.05). Similarly, serum GM values were not predictive of the diagnosis of IFI, given an ROC AUC (95% CI) of 0.57 (0.49, 0.65; P>0.05). In contrast, higher BAL GM and serum BDG values were predictive of IFI, with ROC AUC (95% CI) of 0.77 (0.67, 0.86; P<0.001) and 0.76 (0.65, 0.86; P<0.001), respectively. In both BAL and serum, higher test values for neither BDG nor GM were predictive of mortality (P>0.05 for all analyses; data not shown).
The performance of BAL BDG was not affected by underlying disease, concurrent pulmonary bacterial or viral infection, prior antibacterial therapy (including piperacillin/tazobactam or amoxicillin/clavulanate), or respiratory Candida colonization (P>0.05 for all analyses; data not shown). Instead, antifungal therapy was associated with false-negative results for BDG and GM in both BAL (P<0.001), and serum (P<0.05).
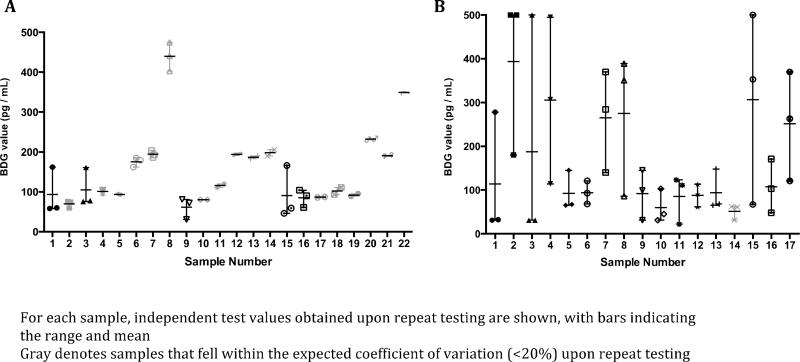
To investigate the poor specificity of BAL BDG relative to serum BDG, 17 BAL and 22 serum samples were randomly selected for repeat testing and the CV was determined per manufacturer’s guidelines. For serum samples, 17 of 22 (77.3%) yielded reproducible BDG values within the expected 20% CV, a percentage within the acceptable manufacturer range (71.1-99.1%) (Figure 2A). Conversely, for BAL samples, only 1 of 17 (5.9%) yielded reproducible BDG values within the 20% CV (Figure 2B). In fact, in 11 BAL samples, the repeat result would have led to a different clinical interpretation; that is to say a negative result was positive on repeat testing (4 samples), or a positive result was negative on repeat testing (7 samples).
Figuere 2.
Reproducibility of serum (A) and BAL (B) BDG values upon repeat testing of patient samples
DISCUSSION
To our knowledge, we present the largest study evaluating the utility of BAL BDG for the diagnosis of IFI in an unselected population of high-risk patients, and the first study to directly compare the performance of BDG and GM in BAL.
Several observations arise from our analysis. First, BAL (and serum) BDG are highly sensitive for PCP, consistent with previous reports [9,10]. As 60% of patients with PCP in our cohort were HIV-negative, our data suggest that BDG testing may be a useful adjunct test to rule out PCP in the non-HIV population, in which microscopy is insensitive [11].
The performance of BAL GM for the diagnosis of IA in our cohort is in keeping with previous reports [7,12], with sensitivity and specificity of 71% and 94%, respectively. BAL BDG appeared to be as sensitive as BAL GM for the diagnosis of IA (71%).
For the diagnosis of all IFI, including infections caused by non-Aspergillus molds that may be amenable to BDG but not GM detection, BAL BDG had comparable sensitivity to BAL GM (53% vs. 42%). As shown previously [1,6], the high proportion of patients on antifungal therapy at the time of bronchoscopy in our study, which is a common occurrence in immunosuppressed patients at risk for IFIs, may have affected the sensitivity of both BDG and GM testing.
Although the sensitivity of BAL BDG was similar to that of BAL GM, BAL BDG demonstrated inferior specificity for the diagnosis of IA and IFI. We were unable to identify clinical factors such as prior antibacterial therapy, concomitant infections and respiratory Candida colonization that accounted for the false-positive BAL BDG results in our study.
Notably, the poor specificity of BDG testing appeared specific to the BAL (68% in BAL versus 92% in serum). Strikingly, upon repeat testing, only 5.9% of BAL BDG samples yielded consistent results, compared to 77.3% of serum BDG samples. Same-patient BAL BDG repeat testing was discrepant independent of whether repeat testing was performed at NIH or Beacon Diagnostics, indicating that the poor reproducibility results in BAL were not accounted for by inter-laboratory variability, but by yet-unknown BAL-intrinsic factors that may adversely affect the reliability of the BDG assay. Additional studies are warranted to validate these initial findings in larger number of samples from different hospitals. However, our study suggests that the BDG assay may yield unreliable results when performed specifically on BAL fluid, thus potentially compromising the interpretability of BAL BDG in its current assay format in clinical practice.
Limitations of the study include its retrospective, single-center design, and the relatively low number of proven/probable IFI among the study population. In addition, due to lack of sample availability, repeat testing of BAL and serum samples was not performed on all patient samples but on a subset of samples, which were randomly selected for analysis. Therefore, future studies that will test a larger number of samples at a fixed time-point after sample acquisition will be required to test the reproducibility of BDG testing in BAL versus serum samples. Furthermore, future studies should incorporate methods to adjust for BAL sample dilution (such as urea measurement [13]) in an effort to minimize the inter-patient sample variability of BAL results.
In summary, our study provides the first comparative analysis of the performance of BAL BDG relative to BAL GM in a sizeable, unselected population of high-risk patients. While BAL BDG is highly sensitive for PCP and has comparable sensitivity with BAL GM for IFI and IA, its poor specificity and reproducibility limit its clinical usefulness. Future work should aim to identify the BAL-intrinsic factors that appear to adversely affect the BDG assay reproducibility in BAL, as this could result in devising strategies to enhance the reproducibility and diagnostic utility of BAL BDG testing.
Supplementary Material
Highlights.
We review bronchoalveolar-lavage (BAL) beta-D-glucan (BDG) testing in 132 patients.
We compare the performance of BDG with galactomannan (GM) in BAL.
BAL BDG is highly sensitive for Pneumocystis pneumonia.
BAL BDG has similar sensitivity but inferior specificity to GM for aspergillosis.
BDG results are poorly reproducible upon retesting in BAL but not in serum.
ACKOWLEDGMENTS
This work was supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA. Results were presented in part at the 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy in Denver, Colorado, 2013.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT:
The authors state that no conflict of interest exists.
REFERENCES
- 1.Ostrosky-Zeichner L. Invasive mycoses: diagnostic challenges. Am J Med. 2012;125(1 Suppl):S14–24. doi: 10.1016/j.amjmed.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Tarrand J, Lichterfeld M, Warraich I, Luna M, Han XY, May GS, et al. Diagnosis of invasive septate mold infections. A correlation of microbiological culture and histologic or cytologic examination. Am J Clin Pathol. 2003;119(6):854–58. doi: 10.1309/EXBV-YAUP-ENBM-285Y. [DOI] [PubMed] [Google Scholar]
- 3.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer / Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–21. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfeiffer CD, Fine JP, Safdar N. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin Infect Dis. 2006;42(10):1417–27. doi: 10.1086/503427. [DOI] [PubMed] [Google Scholar]
- 5.Karageorgopoulos DE, Vouloumanou EK, Ntziora F, Michalopoulos A, Rafailidis PI, Falagas ME. β-D-glucan assay for the diagnosis of invasive fungal infections: a meta-analysis. Clin Infect Dis. 2011;52(6):750–70. doi: 10.1093/cid/ciq206. [DOI] [PubMed] [Google Scholar]
- 6.Ostrosky-Zeichner L, Alexander BD, Kett DH, Vazquez J, Pappas PG, Saeki F, et al. Multicenter clinical evaluation of the (1→3) beta-D-glucan assay as an aid to diagnosis of fungal infections in humans. Clin Infect Dis. 2005;41(5):654–9. doi: 10.1086/432470. [DOI] [PubMed] [Google Scholar]
- 7.Zou M, Tang L, Zhao S, et al. Systematic review and meta-analysis of detecting galactomanan in bronchoalveolar lavage fluid for diagnosing invasive aspergillosis. PLos One. 2012;7(8):e43347. doi: 10.1371/journal.pone.0043347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Segal BH, Herbrecht R, Stevens DA, Ostrosky-Zeichner L, Sobel J, Viscoli C, et al. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria. Clin Infect Dis. 2008;47(5):674–83. doi: 10.1086/590566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karageorgopoulos DE, Qu JM, Korbila IP, Zhu YG, Vasileiou VA, Falagas ME. Accuracy of β-D-glucan for the diagnosis of Pneumocystis jirovecii pneumonia: a meta-analysis. Clin Microbiol Infect. 2013;19(1):39–49. doi: 10.1111/j.1469-0691.2011.03760.x. [DOI] [PubMed] [Google Scholar]
- 10.Theel ES, Jespersen DJ, Iqbal S, Bestrom JE, Rollins LO, Misner LJ, et al. Detection of (1,3)- β –D-glucan in bronchoalveolar lavage and serum samples collected from immunocompromised hosts. Mycopathologia. 2013;175(1-2):33–41. doi: 10.1007/s11046-012-9579-y. [DOI] [PubMed] [Google Scholar]
- 11.Tasaka S, Tokuda H. Pneumocystis jirovecii pneumonia in non-HIV infected patients in the era of novel immunosuppressive therapies. J Infect Chemother. 2012;18(6):793–806. doi: 10.1007/s10156-012-0453-0. [DOI] [PubMed] [Google Scholar]
- 12.Fisher CE, Stevens AM, Leisenring W, Pergam SA, Boeckh M, Hohl TM. The serum galactomannan index predicts mortality in hematopoietic stem cell transplant recipients with invasive aspergillosis. Clin Infect Dis. 2013;57(7):1001–4. doi: 10.1093/cid/cit393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones KP, Edwards JH, Reynolds SP, Peters TJ, Davies BH. A comparison of albumin and urea as reference markers in bronchoalveolar lavage fluid from patients with interstitial lung disease. Eur Respir J. 1990;3(2):152–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.