Abstract
The influence of sex has been neglected in clinical studies on pain and analgesia, with the vast majority of research conducted exclusively in males. However, both preclinical and clinical studies indicate that males and females differ in both the anatomical and physiological composition of central nervous system circuits that are involved in pain processing and analgesia. These differences influence not only the response to noxious stimuli, but also the ability of pharmacological agents to modify this response. Morphine is the most widely prescribed opiate for the alleviation of persistent pain in the clinic; however, it is becoming increasingly clear that morphine is less potent in women compared to men. This review highlights recent research identifying neuroanatomical and physiological dimorphisms underlying sex differences in pain and opioid analgesia, focusing on the endogenous descending pain modulatory circuit.
Sex differences in pain and its control have long been a debated issue for scientists and healthcare providers hoping to optimize pain treatment for the individual. The recent drive towards evidence-based medicine has further highlighted this issue as healthcare providers look to the research literature for making important decisions regarding pain treatment in the clinic. Recently, the Sex, Gender and Pain special interest group of the International Association for the Study of Pain (IASP) issued a consensus paper highlighting the need for inclusion of both males and females in pre-clinical and clinical studies on pain and its management [40]. This multidisciplinary consensus was triggered by the need for application of basic science to clinical problems to continue to advance our understanding of how one’s biological sex influences potential pain mechanisms and therapeutic strategies.
Sex Differences in Pain and Morphine Analgesia
Clinical studies on pain and analgesia are increasingly including sex (or gender) as an independent variable. Indeed, the number of studies examining sex differences in pain and analgesia has increased by 3500% since 1980 [31]. Experimentally induced pain across a wide range of stimuli, including noxious pressure, electrical, ischemic, and thermal stimuli, form the majority of these studies. Measures of pain sensitivity include threshold and tolerance, and self-report ratings of unpleasantness. For the most part, these studies consistently report that females display lower pain thresholds and decreased tolerance to noxious stimuli in comparison to men [12, 77]. However, specific underlying mechanism(s), including sex differences in hormone status, have yet to be identified. Positron emission tomography (PET) scanning studies have reported that experimental pain induces a larger magnitude of activation of the endogenous mu opioid system in males compared to females [95]. Specifically, men demonstrated larger magnitudes of MOR activation than women in the anterior thalamus, ventral basal ganglia, and amygdala. Conversely, women showed reduced activation of mu-opioid system during pain in the nucleus accumbens. These data suggest that the magnitude, direction and site of activation of the endogenous opioid system is sex dependent and likely contributes to the increased pain sensitivity in females reported in pre-clinical experimental pain studies.
While it is clear that females suffer from the majority of chronic pain syndromes, including fibromyalgia, temporomandibular syndrome, and irritable bowel syndrome [15, 43, 44, 58, 72], studies assessing pain levels across sexes for similar ailments are more challenging to interpret [16, 85]. A survey of studies examining sex differences in post-operative and/or procedural pain (including outpatient surgery [18], knee arthroscopic repair [83, 84], and cholecystectomy [26]) reported either no sex difference or greater sensitivity in females [31]. Rarely is it reported that males display increased sensitivity.
Unfortunately, pre-clinical and clinical studies examining sex differences in morphine analgesia are less consistent. Findings of greater analgesia in males versus females, females versus males, and no sex differences following opioid administration have all been reported [16, 33, 38, 85]. One complicating factor is that many of these studies were conducted in an experimental pain setting in which healthy volunteers rated the unpleasantness of a variety of acute noxious stimuli before and after morphine administration. Morphine is typically prescribed for the alleviation of a persistent and/or severe pain state, and it is clear that persistent pain alters the way the central nervous system (CNS) responds to opiates [28, 29]. Future studies using assays that more closely mimic the conditions for which morphine is prescribed may help clarify the impact of sex and/or gender on morphine’s ability to elicit analgesia.
Retrospective studies examining the impact of sex on morphine consumption (including patient controlled analgesia) have reported that males typically consume more morphine than females for post-surgical pain relief [18, 73]. However, given that the side effects associated with morphine consumption, including nausea, headache and dysphoria, are exacerbated in females compared to males, morphine consumption may not be a reliable indicator of morphine analgesia [33]. A limited number of studies have examined the impact of sex on the pain-relieving properties of morphine in a clinically relevant setting. Unfortunately, these results are far from consistent. Cepeda and Carr (2003) reported that females required 30% more morphine to reach the same level of analgesia as males [16]. By contrast, Sarton et al. reported greater morphine analgesia in females [85], while other studies reported no sex difference [33, 38].
Results from preclinical behavioral models examining the impact of sex on opiate analgesia are more consistent, although opiate receptor specificity, route and dose of drug administration, type of analgesiometric test employed, and species and strain of animal tested have been shown to influence the pharmacodynamics of opiate analgesia (see [76] for review). Studies utilizing orofacial, somatosensory or visceral pain assays typically report that morphine produces a significantly greater degree of analgesia in male rodents compared to females [23, 24, 48, 69, 92]. The reported sex differences in morphine analgesia are not trivial; in both persistent inflammatory pain [92] and visceral pain [48] models, the median effective dose (ED50) for systemic morphine is 2-fold higher in females than in males. Importantly, sex differences in morphine analgesia are not due to dimorphisms in the pharmacokinetics of morphine in humans [85] or rodents [20], as no sex differences in morphine elimination rates, or brain or serum levels have been reported [19, 20]. Rather, these studies suggest that there is something inherently different about how morphine acts within the CNS of males and females to alleviate persistent pain.
Neural Correlate of Sexually Dimorphic Pain and Analgesia
The midbrain periaqueductal gray (PAG), and its descending projections to the rostral ventromedial medulla (RVM) and spinal cord, comprises an essential neural circuit for both endogenous and exogenous opioid-mediated analgesia [4–6, 8, 9]. Acute and persistent pain activates the PAG, resulting in the release of endogenous opiates and a reduction in pain sensitivity. PAG stimulation produced analgesia is opioid-mediated, as administration of the opiate antagonist naloxone completely blocks its effects [1]. Indeed, stimulation of the PAG induces a profound analgesic state, such that invasive surgery can be performed in the absence of exogenously administered analgesia [82]. In humans, electrical stimulation of the PAG is used to alleviate intractable pain [39, 59].
The PAG contains a high density of mu opioid receptor (MOR) containing neurons [21, 22, 42, 51, 70, 71, 91] and microinjection of opiate antagonists into the PAG significantly attenuates the analgesic effects of systemic morphine [13, 57, 94]. Similarly, administration of morphine, or other mu opioid receptor agonists, into the PAG produces potent analgesia, which is blocked by central or systemic administration of naloxone [46]. Anatomical studies indicate that approximately 27–50% of PAG neurons projecting to the RVM are MOR+ [21, 91].
The descending PAG-RVM-spinal cord pathway has been characterized anatomically and physiologically in the majority of vertebrate species known to date [2, 3, 7, 8, 10, 11]. Not surprisingly, these studies were conducted exclusively in males with the implicit assumption that CNS neural circuits subserving pain and analgesia were organized in a comparable manner in females. However, recent anatomical and physiological studies in the rat indicate that the PAG-RVM circuit is sexually dimorphic in both its anatomical organization and in its activation during persistent inflammatory pain states [63, 65]. Similarly, the ability of morphine to suppress noxious-stimulus induced excitation of the PAG is also sexually dimorphic.
Using a variety of complementary anatomical techniques, we first examined if there were qualitative and/or quantitative differences in the neural projection from the PAG to the RVM in male and female rats. Consistent with previous anatomical studies [11, 89], we reported dense projections from the dorsomedial, lateral and ventrolateral PAG to the RVM in male and female rats, with no overall qualitative sex differences noted [65]. Interestingly, while the overall distribution pattern of PAG-RVM projection neurons was comparable for both sexes, significant quantitative differences were observed, such that the number of PAG-RVM output neurons was significantly greater in females across the entire rostrocaudal axis of PAG (Figure 2). Indeed, the average number of retrogradely labeled cells across the rostrocaudal extent of the PAG was 33% greater in female compared to male rats. The most prominent sex difference in retrograde labeling was observed within the lateral and ventrolateral regions of the caudal PAG, an area known to contain a dense distribution of mu opioid receptors [51, 90].
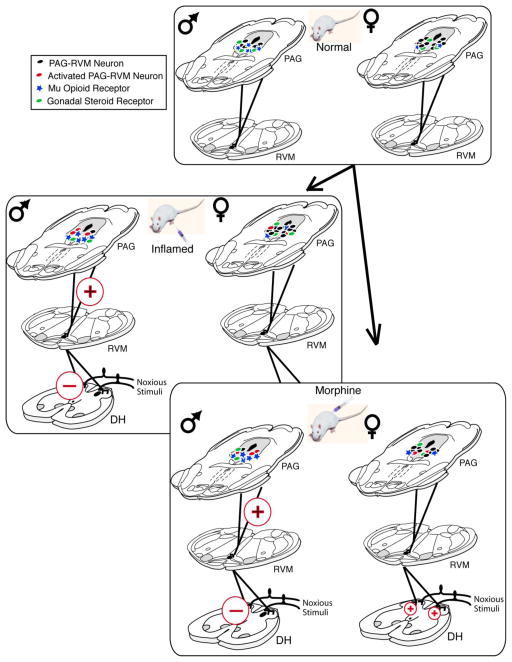
Figure 2.
Proposed model of factors contributing to sex differences in pain and morphine potency. Sex differences in the PAG-RVM projections, mu opioid receptors, and gonadal steroid receptors in the PAG (panel 1) are involved in modulating the activation of the PAG-RVM circuit to a differential degree in males and females to produce sex differences in endogenous pain inhibition under inflammatory conditions (panel 2) and systemic morphine administration (panel 3).
Inflammatory pain results in the activation of descending modulatory circuits [78, 93] and contributes to both hyperalgesia and analgesia. Using Fos expression as a marker for neural activation, we reported that inflammatory hyperalgesia, induced by intraplantar injection of the inflammatory agent Complete Freund’s Adjuvant (CFA), resulted in extensive Fos expression throughout the rostrocaudal axis of PAG in both male and female rats [65]. Importantly, activation of the PAG was comparable (both quantitatively and qualitatively) in male and female rats, and is consistent with our finding of no sex differences in the degree of hyperalgesia observed following intraplantar CFA [65]. However, when the analysis of inflammatory pain-induced Fos was restricted to PAG neurons with direct projections to the RVM, females showed very low levels of activation, despite having almost 2x as many PAG-RVM neurons (Figure 2). This suggests that inflammatory pain preferentially activates the PAG-RVM circuit in males, but not females. Indeed, we found that, overall, persistent inflammatory pain activated approximately 43% of PAG-RVM neurons in the dorsomedial, lateral and ventrolateral PAG of male, but only half as many in females. As activation of the PAG-RVM pathway results in the inhibition of dorsal horn neuronal responses to noxious stimulation and suppresses pain, one would predict that given the greater activation of the PAG-RVM circuit in males in comparison to females, males should have displayed reduced hyperalgesia following induction of hindpaw inflammation. However, this was not the case. Both males and females displayed similar levels of hyperalgesia following intraplantar CFA, suggesting an alternative (non PAG-RVM) circuit for endogenous pain modulation in females.
Sex Differences in Response to Systemic Morphine: Role of the PAG-RVM Circuit
In the majority of pre-clinical pain studies, morphine consistently produces a greater degree of analgesia in male compared with female rats, with similar, although not unequivocal effects observed in humans. As reviewed above, several lines of evidence indicate that the PAG is an essential locus for exogenous opioid-mediated analgesia. In our previous studies, we reported that systemic morphine administration attenuated persistent inflammatory pain-induced Fos in the PAG of male, but not female, rats [65], a finding consistent with studies reporting that the ED50 value for systemic morphine is approximately 2-fold higher in females compared to males [18]. Interestingly, morphine administration, in the absence of pain, resulted in a 2-fold greater activation of PAG neurons in both males and females compared to saline administration [63]. No sex difference was observed in the activation of PAG neurons by morphine, suggesting that in the absence of pain, morphine is equipotent in its ability to depolarize PAG neurons. However, when analysis is limited to PAG-RVM neurons, the number of neurons activated by morphine is consistently and significantly higher in males compared to females. Indeed, approximately half of the PAG-RVM neurons in males were activated by morphine, whereas only 20% were activated in females.
In subsequent studies, we examined the role of the PAG-RVM circuit in the development of morphine tolerance [64]. Repeated administration of an ED50 dose of morphine induced tolerance in males to a significantly greater extent than in females. In parallel, morphine activation of PAG-RVM neurons was significantly attenuated following repeated morphine administration in males. While no sex difference in the overall activation of the PAG was observed following 3 or 6 doses of morphine over 3 days (5 mg/kg; i.p.), the specific activation of the PAG-RVM circuit by morphine steadily declined in males only. Morphine activation of this pathway in female rats was minimal, and therefore did not decline significantly following repeated administration of morphine. Together, these studies suggest that sex differences in morphine’s ability to engage the PAG-RVM pathway contributes its dimorphic pain-relieving properties.
Direct administration of morphine or MOR selective agonists into the PAG also results in sex-dependent analgesia. Krzanowska and Bodnar [55] reported intra-PAG morphine ED50 values of 1.2 μg for male rats in comparison to >50 μg in estrus female rats. In a model of persistent inflammatory pain, we reported intra-PAG morphine ED50 values for males of 7.5 μg versus 15 μg for females [68]. The antinociceptive effects of morphine are mediated primarily by mu opioid receptors; therefore, our subsequent experiments tested the hypothesis that sex differences in MOR expression within the PAG contributed to our observed sex differences in morphine analgesia. Using both immunohistochemistry and autoradiography, we report that males have significantly higher levels of MOR expression and binding along the rostrocaudal axis of PAG (Figure 2). Furthermore, we found that mu opioid receptor-expressing PAG neurons appear to be necessary for eliciting the sexually dimorphic response to morphine as site-directed lesions of mu opioid receptor-expressing PAG neurons dose-dependently reduced morphine analgesia in males only [68], making them similar to females in their response to morphine.
In addition to MOR, sex differences in the initiation of second messenger signaling cascades by morphine have also been reported [14, 25, 75, 86]. Morphine post-synaptically inhibits G protein-coupled inwardly rectifying potassium channels (GIRKs) and sex differences in signal transduction of morphine by GIRK have been reported [75]. While wild-type male mice exhibit higher pain thresholds and greater morphine analgesia than female mice, male mice lacking the GIRK2 channel subunit exhibit reduced pain thresholds and morphine analgesia levels similar to wild-type females [75]. Altered signal transduction following activation of membrane estrogen receptors may also be involved in modulating analgesia in females.
Role of Gonadal Hormones in Sex Differences in Morphine Analgesia
Sex differences in gonadal hormone concentrations appear to play a contributing role in sex differences in pain and analgesia [88]. In women, there is evidence that pain fluctuates across the ovarian cycle, as well as during pregnancy and menopause [12]. Circulating levels of estradiol across the rat estrous cycle reportedly influence pain and morphine analgesia as well, with greater potency reported during diestrus, when circulating estradiol is lowest [24]. An organizational effect of gonadal steroids is also likely. For example, male rats feminized at birth demonstrate reduced morphine potency in adulthood, while masculinized female rats demonstrate greater morphine potency [54].
The PAG-RVM circuit is an essential pathway by which morphine produces an analgesic response; therefore, we hypothesized that sex differences in the steroid regulation of the PAG-RVM pathway may contribute to sex-dependent pain thresholds or opioid analgesia. The PAG contains a large population of both estrogen (ERα) and androgen (AR) receptor containing neurons [79, 80]. Indeed, this region contains the largest population of steroid receptors outside of the hypothalamus. Both ERα and AR immunoreactive neurons are localized primarily within the dorsomedial, lateral and ventrolateral regions of PAG [66]. While the expression of ERα in the PAG is comparable between the sexes, males have a significantly greater number of AR immunoreactive neurons localized within the dorsomedial, lateral and ventrolateral PAG compared to females (Figure 2) [66]. AR binds 5,7-DHT, the 5α-reduced metabolite of testosterone. Future studies manipulating 5,7-DHT levels are warranted to determine the role of increased PAG AR expression in morphine analgesia.
Approximately 30–37% of PAG neurons projecting to the RVM express AR or ERα, with the highest density of colocalization noted in the lateral/ventrolateral region of the caudal PAG. This PAG region also contains the highest density of MOR, and suggests a direct mechanism whereby changes in endogenous gonadal steroid levels could modulate morphine analgesia. Consistent with previous studies, we found that the antinociceptive properties of intra-PAG morphine were significantly reduced in female rats during both proestrus and estrus in comparison to diestrus (when estrogen and progesterone are lowest) [45, 53, 55, 56]. In fact, analgesia resulting from intra-PAG morphine to diestrus females was not significantly different from males [68]. These results parallel our findings of reduced MOR protein levels and binding during proestrus compared with diestrus, and provide further support that the amount of available MOR is positivity related to the degree of analgesia produced by morphine.
Estradiol has been shown to uncouple the mu opioid receptor from G protein-gated inwardly rectifying potassium channels [52], resulting in an attenuation of morphine-induced hyperpolarization. Estradiol has also been shown to induce MOR internalization [27], thereby reducing available opioid binding sites on the cell membrane. Interestingly, ERα is required for estradiol-induced MOR internalization [74] supporting the hypothesis that colocalization of MOR and ERα in the PAG-RVM output neurons provides a unique mechanism through which estrogens may differentially affect morphine potency in male and female rats (see [37] for review).
Spinal Antinociception is Sexually Dimorphic and Dependent on Gonadal Hormones
In addition to the PAG, numerous studies suggest that sex differences in the anatomical, physiological and biochemical organization of the spinal cord also contribute to the dimorphic effects of opiates. The dorsal horn of the spinal cord is densely populated with MOR, and sex differences in analgesia can be elicited following intrathecal administration of either endogenous or exogenous opioid ligands. For instance, endomorphin, the predominant endogenous opioid ligand in the spinal cord, is more effective at producing spinal antinociception in male rats [60]. This effect is hormone dependent. During diestrus, when circulating estrogens are low, spinal antinociception to endomorphin was minimal. In contrast, during proestrus, when circulating estrogens are high, spinal endomorphin antinociception was robust and comparable in magnitude to that noted in males.
Sex differences in the neuroendocrine organization of the spinal cord likely contribute to the dimorphic effects of morphine [87]. The spinal cord dorsal horn contains high levels of estrogen receptors (both ERα and ERβ; [62, 81]), and there is evidence that these receptors interact with both MOR and KOR at the level of the spinal cord to alter antinociception [41, 61]. Kappa opioid receptors form heterodimers with MOR (KOR/MOR) in the spinal cord [17], and the levels of KOR/MOR are approximately 4-fold greater in the spinal cord of proestrus female versus male rats. Sex differences in KOR/MOR heterodimers contributes to the sexually dimorphic effects of intrathecal morphine such that in females, but not males, activation of spinal κ-opioid receptors is a prerequisite for spinal morphine antinociception. Interestingly, activation of spinal kappa receptors alone does not induce antinociception, indicating the requirement for KOR/MOR dimer activation in morphine analgesia [62].
Changes in hormonal status have also been reported to influence peripheral pain processing [32, 34–36, 47, 49, 50]. For example, using a recently-developed in vitro superfusion method to measure proinflammatory peptide release from human dental pulp from extracted teeth [30], Loyd et al. [67] reported sex differences in inflammation-induced proinflammatory peptide release that was dependent on stage of menstrual cycle. Specifically, inflammatory mediator-evoked proinflammatory peptide release was highest in amenstrual females and females in the last week of menses [67]. Changes in hormonal status have also been reported to contribute to a variety of pain disorders that are more common in women, including migraine, fibromyalgia and irritable bowel syndrome. Together, these data should be considered when assessing pain and providing pain therapy to women, especially in persistent pain disorders that involve an inflammatory component.
Implications on Future Research and Pain Management
Research spanning four decades has shown that the PAG, and its descending projections to the RVM and spinal cord dorsal horn, constitute an essential neural circuit for opioid-based analgesia. During the last half of that period, numerous rodent and human studies have established sex differences in pain and the analgesic effects of morphine at each level of this circuit. Sex differences in pain and morphine analgesia are likely due to the inherent differences in how the central nervous system responds to pain and opioid-based analgesia. The anatomical, physiological and biochemical properties by which morphine produces analgesia are sexually dimorphic in the PAG and spinal cord, with clear biological consequences in terms of pain modulation and morphine action. Current research suggests that morphine may not be the drug of choice for pain management in women; thus, research efforts need to be devoted toward the identification of more effective pain therapeutics for the management of persistent pain in women.
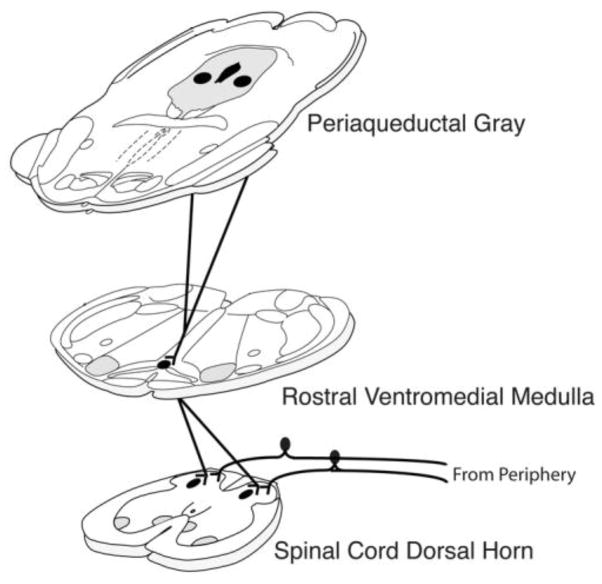
Figure 1.
A schematic of the descending inhibitory pathway for pain modulation illustrating the projections from the midbrain periaqueductal gray to the brainstem rostral ventromedial medulla and the spinal cord dorsal horn at the level of incoming stimulation from the periphery.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akil H, Mayer DJ, Liebeskind JC. Antagonism of stimulation-produced analgesia by naloxone, a narcotic antagonist. Science. 1976;191(4230):961–2. doi: 10.1126/science.1251210. [DOI] [PubMed] [Google Scholar]
- 2.Bandler R, Carrive P, Zhang SP. Integration of somatic and autonomic reactions within the midbrain periaqueductal grey: Vicerotopic, somatotopic and functional organization. Prog Brain Res. 1991;87:269–305. doi: 10.1016/s0079-6123(08)63056-3. [DOI] [PubMed] [Google Scholar]
- 3.Bandler R, Tork I. Midbrain periaqueductal grey region in the cat has afferent and efferent connections with solitary tract nuclei. Neurosci Letters. 1987;74:1–6. doi: 10.1016/0304-3940(87)90041-3. [DOI] [PubMed] [Google Scholar]
- 4.Basbaum AI, Clanton CH, Fields HL. Three bulbospinal pathways from the rostral medulla of the cat: an autoradiographic study of pain modulating systems. J Comp Neurol. 1978;178(2):209–24. doi: 10.1002/cne.901780203. [DOI] [PubMed] [Google Scholar]
- 5.Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol. 1978;4(5):451–62. doi: 10.1002/ana.410040511. [DOI] [PubMed] [Google Scholar]
- 6.Basbaum AI, Fields HL. Endogenous pain control systems: Brainstem spinal pathways and endorphin circuitry. Ann Rev Neurosci. 1984;7:309–38. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 7.Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- 8.Behbehani MM, Fields HL. Evidence that an excitatory connection between the periaqueductal gray and nucleus raphe magnus mediates stimulation produced analgesia. Brain Res. 1979;170(1):85–93. doi: 10.1016/0006-8993(79)90942-9. [DOI] [PubMed] [Google Scholar]
- 9.Behbehani MM, Pomeroy SL. Effect of morphine injected in periadueductal gray on the activity of single units in nucleus raphe magnus of the rat. Brain Res. 1978;149(1):266–9. doi: 10.1016/0006-8993(78)90609-1. [DOI] [PubMed] [Google Scholar]
- 10.Beitz AJ. The organization of afferent projections to the midbrain periaqueductal gray of the rat. Neuroscience. 1982;7:133–59. doi: 10.1016/0306-4522(82)90157-9. [DOI] [PubMed] [Google Scholar]
- 11.Beitz AJ, Shepard RD, Wells WE. The periaqueductal gray-raphe magnus projection contains somatostatin, neurotensin and serotonin but not cholecystokinin. Brain Res. 1983;261:132–7. doi: 10.1016/0006-8993(83)91292-1. [DOI] [PubMed] [Google Scholar]
- 12.Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20(3):371–80. doi: 10.1017/s0140525x97221485. discussion 435–513. [DOI] [PubMed] [Google Scholar]
- 13.Bernal SA, Morgan MM, Craft RM. PAG mu opioid receptor activation underlies sex differences in morphine antinociception. Behavioural brain research. 2007;177(1):126–33. doi: 10.1016/j.bbr.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burstein SR, Williams TJ, Lane DA, Knudsen MG, Pickel VM, McEwen BS, Waters EM, Milner TA. The influences of reproductive status and acute stress on the levels of phosphorylated delta opioid receptor immunoreactivity in rat hippocampus. Brain Res. 2013;1518:71–81. doi: 10.1016/j.brainres.2013.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cairns BE. The influence of gender and sex steroids on craniofacial nociception. Headache. 2007;47(2):319–24. doi: 10.1111/j.1526-4610.2006.00708.x. [DOI] [PubMed] [Google Scholar]
- 16.Cepeda MS, Carr DB. Women experience more pain and require more morphine than men to achieve a similar degree of analgesia. Anesthesia and analgesia. 2003;97(5):1464–8. doi: 10.1213/01.ANE.0000080153.36643.83. [DOI] [PubMed] [Google Scholar]
- 17.Chakrabarti S, Liu NJ, Gintzler AR. Formation of mu-/kappa-opioid receptor heterodimer is sex-dependent and mediates female-specific opioid analgesia. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(46):20115–9. doi: 10.1073/pnas.1009923107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chia YY, Chow LH, Hung CC, Liu K, Ger LP, Wang PN. Gender and pain upon movement are associated with the requirements for postoperative patient-controlled iv analgesia: a prospective survey of 2,298 Chinese patients. Can J Anaesth. 2002;49(3):249–55. doi: 10.1007/BF03020523. [DOI] [PubMed] [Google Scholar]
- 19.Cicero TJ, Nock B, Meyer ER. Gender-related differences in the antinociceptive properties of morphine. J Pharmacol Exp Ther. 1996;279(2):767–73. [PubMed] [Google Scholar]
- 20.Cicero TJ, Nock B, Meyer ER. Sex-related differences in morphine’s antinociceptive activity: relationship to serum and brain morphine concentrations. J Pharmacol Exp Ther. 1997;282(2):939–44. [PubMed] [Google Scholar]
- 21.Commons KG, Aicher SA, Kow LM, Pfaff DW. Presynaptic and postsynaptic relations of mu-opioid receptors to gamma-aminobutyric acid-immunoreactive and medullary-projecting periaqueductal gray neurons. J Comp Neurol. 2000;419(4):532–42. doi: 10.1002/(sici)1096-9861(20000417)419:4<532::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 22.Commons KG, van Bockstaele EJ, Pfaff DW. Frequent colocalization of mu opioid and NMDA-type glutamate receptors at postsynaptic sites in periaqueductal gray neurons. J Comp Neurol. 1999;408(4):549–59. [PubMed] [Google Scholar]
- 23.Craft RM. Sex differences in drug- and non-drug-induced analgesia. Life sciences. 2003;72(24):2675–88. doi: 10.1016/s0024-3205(03)00178-4. [DOI] [PubMed] [Google Scholar]
- 24.Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. European J Pain. 2004;8(5):397–411. doi: 10.1016/j.ejpain.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Craft RM, Tseng AH, McNiel DM, Furness MS, Rice KC. Receptor-selective antagonism of opioid antinociception in female versus male rats. Behavioural Pharm. 2001;12(8):591–602. doi: 10.1097/00008877-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 26.De Cosmo G, Congedo E, Lai C, Primieri P, Dottarelli A, Aceto P. Preoperative psychologic and demographic predictors of pain perception and tramadol consumption using intravenous patient-controlled analgesia. Clin J Pain. 2008;24(5):399–405. doi: 10.1097/AJP.0b013e3181671a08. [DOI] [PubMed] [Google Scholar]
- 27.Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of mu-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J Neurosci. 1998;18(10):3967–76. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eidson LN, Murphy AZ. Blockade of Toll-like receptor 4 attenuates morphine tolerance and facilitates the pain relieving properties of morphine. J Neurosci. 2013;33(40):15952–63. doi: 10.1523/JNEUROSCI.1609-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eidson LN, Murphy AZ. Persistent peripheral inflammation attenuates morphine-induced periaqueductal gray glial cell activation and analgesic tolerance in the male rat. J Pain. 2013;14(4):393–404. doi: 10.1016/j.jpain.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fehrenbacher JC, Sun XX, Locke EE, Henry MA, Hargreaves KM. Capsaicin-evoked iCGRP release from human dental pulp: a model system for the study of peripheral neuropeptide secretion in normal healthy tissue. Pain. 2009;144(3):253–61. doi: 10.1016/j.pain.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10(5):447–85. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fillingim RB, Ness TJ. Sex-related hormonal influences on pain and analgesic responses. Neurosci Biobehav Rev. 2000;24(4):485–501. doi: 10.1016/s0149-7634(00)00017-8. [DOI] [PubMed] [Google Scholar]
- 33.Fillingim RB, Ness TJ, Glover TL, Campbell CM, Hastie BA, Price DD, Staud R. Morphine responses and experimental pain: sex differences in side effects and cardiovascular responses but not analgesia. J Pain. 2005;6(2):116–24. doi: 10.1016/j.jpain.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Flake NM, Hermanstyne TO, Gold MS. Testosterone and estrogen have opposing actions on inflammation-induced plasma extravasation in the rat temporomandibular joint. Am J Physiol Regul Integr Comp Physiol. 2006;291(2):R343–8. doi: 10.1152/ajpregu.00835.2005. [DOI] [PubMed] [Google Scholar]
- 35.Gintzler AR. Endorphin-mediated increases in pain threshold during pregnancy. Science. 1980;210(4466):193–5. doi: 10.1126/science.7414330. [DOI] [PubMed] [Google Scholar]
- 36.Gintzler AR, Bohan MC. Pain thresholds are elevated during pseudopregnancy. Brain Res. 1990;507(2):312–6. doi: 10.1016/0006-8993(90)90288-m. [DOI] [PubMed] [Google Scholar]
- 37.Gintzler AR, Liu NJ. Importance of sex to pain and its amelioration; relevance of spinal estrogens and its membrane receptors. Frontiers Neuroendo. 2012;33(4):412–24. doi: 10.1016/j.yfrne.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon NC, Gear RW, Heller PH, Paul S, Miaskowski C, Levine JD. Enhancement of morphine analgesia by the GABAB agonist baclofen. Neuroscience. 1995;69(2):345–9. doi: 10.1016/0306-4522(95)00335-g. [DOI] [PubMed] [Google Scholar]
- 39.Green AL, Hyam JA, Williams C, Wang S, Shlugman D, Stein JF, Paterson DJ, Aziz TZ. Intra-operative deep brain stimulation of the periaqueductal grey matter modulates blood pressure and heart rate variability in humans. Neuromodulation. 2010;13(3):174–81. doi: 10.1111/j.1525-1403.2010.00274.x. [DOI] [PubMed] [Google Scholar]
- 40.Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ, Pain SIGotI Consensus Working Group of the Sex G. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132 (Suppl 1):S26–45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta DS, von Gizycki H, Gintzler AR. Sex-/ovarian steroid-dependent release of endomorphin 2 from spinal cord. JPET. 2007;321(2):635–41. doi: 10.1124/jpet.106.118505. [DOI] [PubMed] [Google Scholar]
- 42.Gutstein HB, Mansour A, Watson SJ, Akil H, Fields HL. Mu and kappa opioid receptors in periaqueductal gray and rostral ventromedial medulla. Neuroreport. 1998;9(8):1777–81. doi: 10.1097/00001756-199806010-00019. [DOI] [PubMed] [Google Scholar]
- 43.Heitkemper M, Jarrett M. Irritable bowel syndrome: does gender matter? J Psychosomatic Res. 2008;64(6):583–7. doi: 10.1016/j.jpsychores.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 44.Heitkemper MM, Jarrett ME. Update on irritable bowel syndrome and gender differences. Nutrition in Clinical Practice. 2008;23(3):275–83. doi: 10.1177/0884533608318672. [DOI] [PubMed] [Google Scholar]
- 45.Islam AK, Cooper ML, Bodnar RJ. Interactions among aging, gender, and gonadectomy effects upon morphine antinociception in rats. Physiol Behav. 1993;54(1):45–53. doi: 10.1016/0031-9384(93)90042-e. [DOI] [PubMed] [Google Scholar]
- 46.Jensen TS, Yaksh TL., III Comparison of the antinociceptive action of mu and delta opioid receptor ligands in the periaqueductal gray matter, medial and paramedial ventral medulla in the rat as studied by microinjection technique. Brain Res. 1986;372:301–12. doi: 10.1016/0006-8993(86)91138-8. [DOI] [PubMed] [Google Scholar]
- 47.Ji Y, Murphy AZ, Traub RJ. Estrogen modulates the visceromotor reflex and responses of spinal dorsal horn neurons to colorectal stimulation in the rat. J Neurosci. 2003;23(9):3908–15. doi: 10.1523/JNEUROSCI.23-09-03908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ji Y, Murphy AZ, Traub RJ. Sex differences in morphine-induced analgesia of visceral pain are supraspinally and peripherally mediated. American journal of physiology Regulatory, integrative and comparative physiology. 2006;291(2):R307–14. doi: 10.1152/ajpregu.00824.2005. [DOI] [PubMed] [Google Scholar]
- 49.Ji Y, Murphy AZ, Traub RJ. Estrogen modulation of morphine analgesia of visceral pain in female rats is supraspinally and peripherally mediated. J Pain. 2007;8(6):494–502. doi: 10.1016/j.jpain.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 50.Ji Y, Tang B, Traub RJ. Modulatory effects of estrogen and progesterone on colorectal hyperalgesia in the rat. Pain. 2005;117(3):433–42. doi: 10.1016/j.pain.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Kalyuzhny AE, Arvidsson U, Wu W, Wessendorf MW. mu-Opioid and delta-opioid receptors are expressed in brainstem antinociceptive circuits: studies using immunocytochemistry and retrograde tract-tracing. J Neurosci. 1996;16(20):6490–503. doi: 10.1523/JNEUROSCI.16-20-06490.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kelly MJ, Qiu J, Ronnekleiv OK. Estrogen modulation of G-protein-coupled receptor activation of potassium channels in the central nervous system. Ann N Y Acad Sci. 2003;1007:6–16. doi: 10.1196/annals.1286.001. [DOI] [PubMed] [Google Scholar]
- 53.Kepler KL, Kest B, Kiefel JM, Cooper ML, Bodnar RJ. Roles of gender, gonadectomy and estrous phase in the analgesic effcts of intracerebroventricular morphine in rats. Pharmacol Biochem Behav. 1989;34:119–27. doi: 10.1016/0091-3057(89)90363-8. [DOI] [PubMed] [Google Scholar]
- 54.Krzanowska EK, Bodnar RJ. Morphine antinociception elicited from the ventrolateral periaqueductal gray is sensitive to sex and gonadectomy differences in rats. Brain Res. 1999;821(1):224–30. doi: 10.1016/s0006-8993(98)01364-x. [DOI] [PubMed] [Google Scholar]
- 55.Krzanowska EK, Bodnar RJ. Morphine antinociception elicted from the ventrolateral periaqueductal gray is sensitive to sex and gonadectomy differences in rats. Brain Res. 1999;821:224–30. doi: 10.1016/s0006-8993(98)01364-x. [DOI] [PubMed] [Google Scholar]
- 56.Krzanowska EK, Ogawa S, Pfaff DW, Bodnar RJ. Reversal of sex differences in morphine analgesia elicited from the ventrolateral periaqueductal gray in rats by neonatal hormone manipulations. Brain Res. 2002;929(1):1–9. doi: 10.1016/s0006-8993(01)03350-9. [DOI] [PubMed] [Google Scholar]
- 57.Lane DA, Patel PA, Morgan MM. Evidence for an intrinsic mechanism of antinociceptive tolerance within the ventrolateral periaqueductal gray of rats. Neuroscience. 2005;135(1):227–34. doi: 10.1016/j.neuroscience.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 58.Leresche L. Defining gender disparities in pain management. Clinical orthopaedics and related research. 2011;469(7):1871–7. doi: 10.1007/s11999-010-1759-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levy R, Deer TR, Henderson J. Intracranial neurostimulation for pain control: a review. Pain Physician. 2010;13(2):157–65. [PubMed] [Google Scholar]
- 60.Liu NJ, Gintzler AR. Spinal endomorphin 2 antinociception and the mechanisms that produce it are both sex- and stage of estrus cycle-dependent in rats. J Pain. 2013;14(11):1522–30. doi: 10.1016/j.jpain.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu NJ, Schnell S, Wessendorf MW, Gintzler AR. Sex, pain, and opioids: interdependent influences of sex and pain modality on dynorphin-mediated antinociception in rats. JPET. 2013;344(2):522–30. doi: 10.1124/jpet.112.199851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu NJ, von Gizycki H, Gintzler AR. Sexually dimorphic recruitment of spinal opioid analgesic pathways by the spinal application of morphine. JPET. 2007;322(2):654–60. doi: 10.1124/jpet.107.123620. [DOI] [PubMed] [Google Scholar]
- 63.Loyd DR, Morgan MM, Murphy AZ. Morphine preferentially activates the periaqueductal gray-rostral ventromedial medullary pathway in the male rat: a potential mechanism for sex differences in antinociception. Neuroscience. 2007;147(2):456–68. doi: 10.1016/j.neuroscience.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Loyd DR, Morgan MM, Murphy AZ. Sexually dimorphic activation of the periaqueductal gray-rostral ventromedial medullary circuit during the development of tolerance to morphine in the rat. Eur J Neurosci. 2008;27(6):1517–24. doi: 10.1111/j.1460-9568.2008.06100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Loyd DR, Murphy AZ. Sex differences in the anatomical and functional organization of the periaqueductal gray-rostral ventromedial medullary pathway in the rat: a potential circuit mediating the sexually dimorphic actions of morphine. J Comp Neurol. 2006;496(5):723–38. doi: 10.1002/cne.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Loyd DR, Murphy AZ. Androgen and estrogen (alpha) receptor localization on periaqueductal gray neurons projecting to the rostral ventromedial medulla in the male and female rat. J Chem Neuroanatomy. 2008;36(3–4):216–26. doi: 10.1016/j.jchemneu.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Loyd DR, Sun XX, Locke EE, Salas MM, Hargreaves KM. Sex differences in serotonin enhancement of capsaicin-evoked calcitonin gene-related peptide release from human dental pulp. Pain. 2012;153(10):2061–7. doi: 10.1016/j.pain.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Loyd DR, Wang X, Murphy AZ. Sex differences in micro-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. J Neurosci. 2008;28(52):14007–17. doi: 10.1523/JNEUROSCI.4123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Loyd DR, Wang X, Murphy AZ. Sex differences in micro-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. J Neurosci. 2008;28(52):14007–17. doi: 10.1523/JNEUROSCI.4123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci. 1987;7(8):2445–64. [PMC free article] [PubMed] [Google Scholar]
- 71.Mansour A, Lewis ME, Khachaturian H, Akil H, Watson SJ. Pharmacological and anatomical evidence of selective mu, delta, and kappa opioid receptor binding in rat brain. Brain Res. 1986;399(1):69–79. doi: 10.1016/0006-8993(86)90601-3. [DOI] [PubMed] [Google Scholar]
- 72.Mayer EA, Berman S, Chang L, Naliboff BD. Sex-based differences in gastrointestinal pain. European J Pain. 2004;8(5):451–63. doi: 10.1016/j.ejpain.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 73.Miaskowski C, Gear RW, Levine JD. Sex related differences in analgesic responses. In: Fillingim RB, editor. Sex, Gender, and Pain. Seattle: IASP Press; 2000. pp. 209–32. [Google Scholar]
- 74.Micevych PE, Rissman EF, Gustafsson JA, Sinchak K. Estrogen receptor-alpha is required for estrogen-induced mu-opioid receptor internalization. J Neurosci Res. 2003;71(6):802–10. doi: 10.1002/jnr.10526. [DOI] [PubMed] [Google Scholar]
- 75.Mitrovic I, Margeta-Mitrovic M, Bader S, Stoffel M, Jan LY, Basbaum AI. Contribution of GIRK2-mediated postsynaptic signaling to opiate and alpha 2-adrenergic analgesia and analgesic sex differences. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):271–6. doi: 10.1073/pnas.0136822100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nature Reviews Neuroscience. 2012;13(12):859–66. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- 77.Mogil JS, Bailey AL. Sex and gender differences in pain and analgesia. Prog Brain Res. 2010;186:141–57. doi: 10.1016/B978-0-444-53630-3.00009-9. [DOI] [PubMed] [Google Scholar]
- 78.Morgan MM, Gold MS, Liebeskind JC, Stein C. Periaqueductal gray stimulation produces a spinally mediated, opioid antinociception for the inflamed hindpaw of the rat. Brain Res. 1991;545:17–23. doi: 10.1016/0006-8993(91)91264-2. [DOI] [PubMed] [Google Scholar]
- 79.Murphy AZ, Hoffman GE. Distribution of androgen and estrogen receptor containing neurons in the male rat periaqueductal gray. Horm Beh. 1999;36:98–108. doi: 10.1006/hbeh.1999.1528. [DOI] [PubMed] [Google Scholar]
- 80.Murphy AZ, Hoffman GE. Distribution of gonadal steroid receptor-containing neurons in the preoptic-periaqueductal gray-brainstem pathway: A potential circuit for the initiation of male sexual behavior. J Comp Neurol. 2001;438(2):191–212. doi: 10.1002/cne.1309. [DOI] [PubMed] [Google Scholar]
- 81.Papka RE, Storey-Workley M, Shughrue PJ, Merchenthaler I, Collins JJ, Usip S, Saunders PT, Shupnik M. Estrogen receptor-alpha and beta- immunoreactivity and mRNA in neurons of sensory and autonomic ganglia and spinal cord. Cell and tissue research. 2001;304(2):193–214. doi: 10.1007/s004410100363. [DOI] [PubMed] [Google Scholar]
- 82.Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164(3878):444–5. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- 83.Rosseland LA, Solheim N, Stubhaug A. Pain and disability 1 year after knee arthroscopic procedures. Acta Anaesthesiol Scand. 2008;52(3):332–7. doi: 10.1111/j.1399-6576.2007.01541.x. [DOI] [PubMed] [Google Scholar]
- 84.Rosseland LA, Stubhaug A. Gender is a confounding factor in pain trials: women report more pain than men after arthroscopic surgery. Pain. 2004;112(3):248–53. doi: 10.1016/j.pain.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 85.Sarton E, Olofsen E, Romberg R, den Hartigh J, Kest B, Nieuwenhuijs D, Burm A, Teppema L, Dahan A. Sex differences in morphine analgesia: an experimental study in healthy volunteers. Anesthesiology. 2000;93(5):1245–54. doi: 10.1097/00000542-200011000-00018. discussion 6A. [DOI] [PubMed] [Google Scholar]
- 86.Schwindinger WF, Borrell BM, Waldman LC, Robishaw JD. Mice lacking the G protein gamma3-subunit show resistance to opioids and diet induced obesity. American journal of physiology Regulatory, integrative and comparative physiology. 2009;297(5):R1494–502. doi: 10.1152/ajpregu.00308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Small KM, Nag S, Mokha SS. Activation of membrane estrogen receptors attenuates opioid receptor-like1 receptor-mediated antinociception via an ERK-dependent non-genomic mechanism. Neuroscience. 2013;255:177–90. doi: 10.1016/j.neuroscience.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stoffel EC, Ulibarri CM, Craft RM. Gonadal steroid hormone modulation of nociception, morphine antinociception and reproductive indices in male and female rats. Pain. 2003;103(3):285–302. doi: 10.1016/s0304-3959(02)00457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van Bockstaele EJ, Aston-Jones G, Pieribone VA, Ennis M, Shipley MT. Subregions of the periaqueductal gray topographically innervate the rostral ventral medulla in the rat. J Comp Neurol. 1991;309:305–27. doi: 10.1002/cne.903090303. [DOI] [PubMed] [Google Scholar]
- 90.Wang H, Wessendorf MW. Mu- and delta-opioid receptor mRNAs are expressed in spinally projecting serotonergic and nonserotonergic neurons of the rostral ventromedial medulla. J Comp Neurol. 1999;404(2):183–96. doi: 10.1002/(sici)1096-9861(19990208)404:2<183::aid-cne4>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 91.Wang H, Wessendorf MW. Mu- and delta-opioid receptor mRNAs are expressed in periaqueductal gray neurons projecting to the rostral ventromedial medulla. Neuroscience. 2002;109(3):619–34. doi: 10.1016/s0306-4522(01)00328-1. [DOI] [PubMed] [Google Scholar]
- 92.Wang X, Traub RJ, Murphy AZ. Persistent pain model reveals sex difference in morphine potency. American journal of physiology Regulatory, integrative and comparative physiology. 2006;291(2):R300–6. doi: 10.1152/ajpregu.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Williams FG, Mullet MA, Beitz AJ. Basal release of Met-enkephalin and neurotensin in the ventrolateral periaqueductal gray matter of the rat: a microdialysis study of antinociceptive circuits. Brain Res. 1995;690:207–16. doi: 10.1016/0006-8993(95)00554-4. [DOI] [PubMed] [Google Scholar]
- 94.Zambotti F, Zonta N, Parenti M, Tommasi R, Vicentini L, Conci F, Mantegazza P. Periaqueductal gray matter involvement in the muscimol-induced decrease of morphine antinociception. Naunyn-Schmiedeberg’s archives of pharmacology. 1982;318(4):368–9. doi: 10.1007/BF00501180. [DOI] [PubMed] [Google Scholar]
- 95.Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. mu-opioid receptor-mediated antinociceptive responses differ in men and women. J Neurosci. 2002;22(12):5100–7. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]