Abstract
Background
Diagnosis of adrenal-cortisol insufficiency is often misleading in hospitalized patients as clinical and biochemical features overlap with co-morbidities. We analyzed clinical determinants associated with a biochemical diagnosis of adrenal-cortisol insufficiency in non-ICU hospitalized patients.
Methods
In a retrospective cohort study we reviewed 4668 inpatients with random morning cortisol levels ≤15 μg/dL hospitalized in our center between 2003 and 2010. Using serum cortisol threshold level of 18 μg/dL 30 and/or 60 minutes after cortrosyn (250 μg) injection to define biochemical adrenal-cortisol status, we characterized and compared insufficient (n=108, serum cortisol ≤18 μg/dL) and sufficient ( n=394; serum cortisol >18 μg/dL) non-ICU hospitalized patients.
Results
Commonly reported clinical and routine biochemical adrenal-cortisol insufficiency features were similar between insufficient and sufficient inpatients. Biochemical adrenal-cortisol insufficiency was associated with increased frequency of liver disease, specifically hepatitis C (p=0.01) and prior orthotopic liver transplantation (p<0.001), HIV (p=0.005) and reported preexisting male hypogonadism (p<0.001) as compared to biochemical adrenal-cortisol sufficiency group. Forty percent of insufficient inpatients were not treated with glucocorticoids after diagnosis. Multivariable logistic analysis demonstrated that inpatients with higher cortisol levels (p=0.0001), higher diastolic blood pressure (p=0.05) and females (p=0.009) were more likely not to be treated, while those with previous short-term glucocorticoid treatment (p=0.002), had other co-existing endocrine diseases (p=0.005) or received an inhospital endocrinology consultation (p<0.0001) were more likely to be replaced with glucocorticoids.
Conclusions
Commonly reported adrenal-cortisol insufficiency features do not reliably identify hospitalized patients biochemically confirmed to have this disorder. Co-morbidities including hepatitis C, prior orthotopic liver transplantation, HIV, and reported pre-existing male hypogonadism may help identify hospitalized non-ICU patients for more rigorous adrenal insufficiency assessment.
Keywords: adrenal insufficiency, cortisol, inpatient
INTRODUCTION
As adrenal-cortisol insufficiency, i.e. insufficient cortisol production to support cellular functions and stress responses is associated with increased morbidity and potential mortality (1-3), early diagnosis is crucial. Although endogenous adrenal-cortisol insufficiency is rare, glucocorticoid treatment-induced adrenal insufficiency may be more prevalent (4), as 2.5% of UK elderly and 1.2% of the US population were reported to have received glucocorticoids at some time. (5, 6) In-hospital incidence and prevalence of the disease are unknown.
Adrenal-cortisol insufficiency diagnosis is elusive and challenging, especially in hospitalized patients. Features including anorexia, fatigue, gastrointestinal abnormalities, weight loss and low blood pressure(1), are non-specific and overlap with other co-morbidities. Electrolyte disturbances, azotemia, anemia and eosinophilia (1) may be reflective of other comorbidities or are altered by in-hospital treatments.
Biochemical adrenal-cortisol insufficiency diagnosis relies on measurement of circulating cortisol levels. (1) Random plasma morning cortisol levels >14.5 μg/dL (400 nmol/L) suggest adrenal cortisol sufficiency (1, 7); However, as random morning cortisol levels are not diagnostic, (1, 8) patients suspected of adrenal-cortisol insufficiency should undergo a cortrosyn stimulation test which relies on the acute release of adrenal cortisol upon adrenocorticotropin hormone (ACTH) analog stimulation (β1-24 corticotropin, Cosyntropin, Cortrosyn, Synacthen). This test requires measurement of total cortisol 30 minutes after 250 μg cortrosyn injection (9-11) and rigorously correlates with levels obtained during the gold standard insulin tolerance test (cortisol level following induced hypoglycemia of ≤ 2.2 mmol/L or 40 mg/dL) for adrenal-cortisol insufficiency diagnosis in outpatient. (11) Cortisol measurements 60 minutes after cortrosyn injection (12-15) weakly correlate with insulin tolerance test results. (16) Cortisol level 30 minutes after cortrosyn injection >18 μg/dL (500 nmol/L, in serum) (17) and >20 μg/dL (550 nmol/L, in plasma) are diagnostic for adrenal-cortisol insufficiency(18, 19). Cortrosyn stimulation test (250 μg) can be performed at any time and is not sex or age specific. (1, 20) Low dose (1 μg) cortrosyn (21)elicits similar cortisol responses (20, 22) with inconsistent reliability. (23)Importantly, as this test has not been rigorously validated in hospitalized acutely ill patients often with multiple co-morbidities, the accuracy by which this test validly diagnoses adrenal-cortisol insufficiency is unclear. We therefore chose to use the term biochemical adrenal-cortisol insufficiency or sufficiency in this study.
Relying on a serum cortisol threshold of 18 μg/dL during cortrosyn stimulation test to segregate study subjects, we conducted a retrospective, cohort study to distinguish between biochemically diagnosed adrenal-cortisol insufficient and sufficient hospitalized patients. The results highlight co-morbidities more likely to co-exist with the diagnosis of biochemical adrenal-cortisol insufficiency in non-ICU hospitalized patients with coexisting morbidities and may contribute to facilitating earlier diagnosis of adrenal insufficiency in the setting of an acute illness.
METHODS
Hospitalized patients
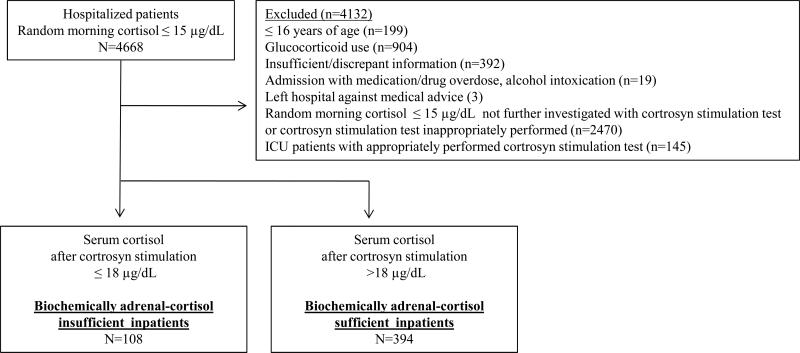
After IRB approval, we identified 4668 hospitalized patients in our electronic clinical data repository with reported serum random morning cortisol levels ≤15 μg/dL measured between 2003 and 2010. Exclusion criteria are detailed in Figure 1. Patients who received glucocorticoid treatment before cortrosyn stimulation test were excluded (n=904), except for inpatients (7.4% in insufficient and 9.1% in sufficient group) who received doses equivalent to ≤30 mg daily hydrocortisone for ≤ 2 weeks up to 24 hours before cortrosyn injection , as these were not expected to have glucocorticoid-induced adrenal insufficiency. (1) Biochemical adrenal-cortisol insufficiency diagnosis in hospitalized patients was based on an abnormal cortrosyn stimulation test. The final cohort included 502 inpatients, 108 biochemically adrenal-cortisol insufficient and 394 biochemically adrenal-cortisol sufficient.
Figure 1.
Study overview
Our real-time electronic repository system aggregates clinical data for efficient patient centric queries and interactive access for front-end or user-facing applications. The inclusive system contains both electronically inserted data, and scanned documents.
The three abstractors (SMG, AKK, RCS), were similarly trained by the first author, worked separately, according to a pre-designed template, were blinded to results and monitored with random data sampling. Statistical analysis was performed by a blinded statistician (JM).
Cortisol measurements
Utilizing serum random morning cortisol ≤15 μg/dL for screening allowed for inclusion of adrenal-cortisol insufficiency-suspected inpatients and exclusion of critically ill patients with high random morning cortisol level in whom cortrosyn stimulation test is un-interpretable. (24) Baseline cortisol level is defined as total cortisol level taken immediately prior to cortrosyn injection and separately from random morning cortisol measurement. While random morning cortisol levels were measured in all cohort inpatients, baseline cortisol levels were measured in 59 insufficient and in 197 sufficient inpatients. In addition random morning cortisol was measured between 6-10 am while baseline cortisol was taken at any time of the day before cortrosyn injection. Biochemical adrenal-cortisol insufficiency _ENREF_20diagnosis required a serum cortisol value of ≤18 μg/dL and sufficiency- serum cortisol value >18 μg/dL, at 30 and/or 60 minutes after cortrosyn injection. Both time points were included for study as commonly used and extensively published.
Performance of cortrosyn stimulation test (250 μg, IM or IV) was verified by a written test order, evidence of cortrosyn injection followed by serum cortisol measurement within 30 (range 30-40) and 60 (range 50-70) minute cortisol, respectively.
Until 2007 serum cortisol was measured by a solid-phase, competitive chemiluminescent enzyme immunoassay IMMULITE/IMMULITE 1000 cortisol (Siemens), thereafter until 2010 by electrochemiluminescence cortisol immunoassay, Cobas (Roche). The IMMULITE assay range is 0.02-200 mcg/dL (http://www.medical.siemens.com/siemens/en_GLOBAL/gg_diag_FBAs/files/package_inserts/i mmulite/Adrenal_Pituitary_n/pilkco-10_siemens.pdf). Cobas range is 0.036-63.4 μg/dL, within run precision CV% 1-1.4 and total precision CV% 1.4-1.6.
Statistical analysis
Numerical variables were summarized by median, range or interquartile range and compared across groups by t test (normally distributed) or Wilcoxon rank sum test (non-normally distributed). Categorical variables were summarized by percent and frequency and compared across groups by Chi-Square or Fisher exact test. Associations between numerical variables were assessed by Pearson (normally distributed) or Spearman (non-normally distributed) correlation analysis. Linear regression was used to assess whether correlations differed between groups. Logistic regression models were used to assess factors associated with binary outcome variables. A two-sided 1% significance level was used throughout. SAS version 9.2 (SAS Institute, Cary, North Carolina) was used for statistical calculations. All results are presented, however only those with p value ≤ 0.01 and those derived from groups with sufficient data are discussed.
RESULTS
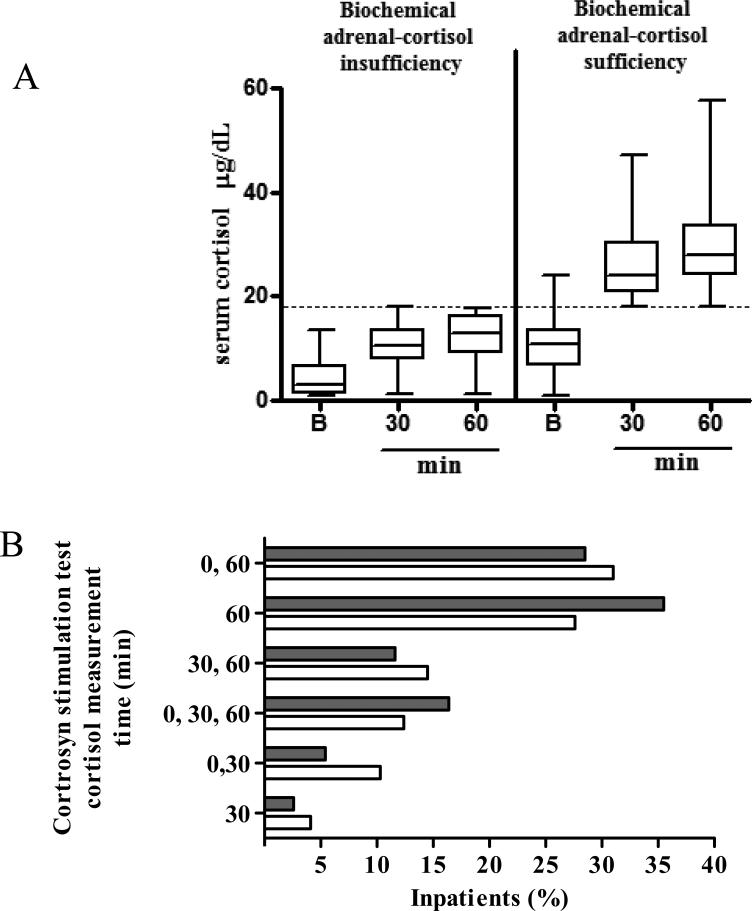
Cortisol levels measured after cortrosyn injection were lower in biochemical adrenal-cortisol insufficient than in sufficient inpatient (p<0.001, Figure 2A) as expected from the study design. Physician preference for cortisol measurement time-point was 60 minutes after cortrosyn injection (Figure 2B). While random morning cortisol level was documented in both groups, baseline cortisol level prior to cortrosyn injection was measured in 59 biochemically adrenal-cortisol insufficient and in 197 sufficient inpatients (Table 1).
Figure 2.
A. Serum cortisol levels (μg/dL) in biochemically adrenal-cortisol insufficient and sufficient inpatients before (B, baseline) and 30 and 60 minutes (min) after cortrosyn (250 μg) injection. Presented as Mean ± SD, ***p<.001 (t-test for each time point), B. Physician preference for cortisol measurement timepoints during cortrosyn stimulation tests . Serum cortisol was measured at baseline after which cortrosyn 250 μg was injected and serum cortisol re-measured at times indicated. Black bars, adrenal-cortisol sufficient inpatients, white bars, adrenal-cortisol insufficient inpatients
Table 1.
Characteristics and outcomes: Biochemically adrenal-cortisol insufficient vs. sufficient hospitalized patients
| Biochemically adrenal cortisol insufficient | n | Biochemically adrenal cortisol sufficient | n | p value | ||
|---|---|---|---|---|---|---|
| Age (years), Median [range] | 56.5 [23-93] | 108 | 60.5 [16-95] | 394 | 0.25 | |
| Male/Female (n) | 62/44 | 106 | 196/198 | 394 | 0.11 | |
| Ethnicity % (n) | 108 | 394 | 0.35 | |||
| Caucasian | 44.4 (48) | 50.0 (197) | ||||
| African-American | 13.9 (15) | 14.5 (57) | ||||
| Hispanic | 13.9 (15) | 9.1 (36) | ||||
| Other | 11.1 (12) | 7.1 (28) | ||||
| Unknown | 16.7 (18) | 19.3 (76) | ||||
| Habits % (n) | ||||||
| Smoking | 35.3 (36) | 102 | 37.8 (141) | 373 | 0.64 | |
| Alcohol use | 31.7 (33) | 104 | 24.7 (92) | 372 | 0.15 | |
| Rec. drug use | 21.3 (17) | 80 | 13.3 (37) | 279 | 0.08 | |
| In hospital death % (n) | 8.3 (9) | 108 | 4.1 (16) | 394 | 0.08 | |
| Length of hospital stay (days) | 10 (5-17) | 101 | 9 (5-18) | 369 | 0.34 | |
| Symptoms % (n) | ||||||
| Fatigue | 50.0 (54) | 108 | 37.6 (148) | 394 | 0.02 | |
| Anorexia | 24.1 (26) | 108 | 13.7 (54) | 394 | 0.009 | |
| Gastrointestinal | 33.3 (36) | 108 | 31.7 (125) | 394 | 0.75 | |
| Systolic blood pressure (mm /Hg) | 120 (105-136) | 108 | 115.5 (100-130) | 386 | 0.03 | |
| Diastolic blood pressure (mm/Hg) | 65 (54.5-80) | 108 | 64 (55-71) | 386 | 0.26 | |
| Blood chemistry † | ||||||
| Sodium (mmol/L) | 138 (133-141) | 108 | 139 (134-141) | 390 | 0.31 | |
| Potassium (mmol/L) | 4.2 (3.9-4.6) | 108 | 4.1 (3.7-4.6) | 390 | 0.09 | |
| Calcium (mmol/L) | 2.25 (2.1-2.4) | 108 | 2.3 (2.2-2.4) | 390 | 0.60 | |
| Creatinine (μmol/L) | 79.6 (61.9-128.2) | 108 | 88.4 (70.7-123.8) | 390 | 0.67 | |
| Hemoglobin (g/L) | 123.5 (111.5-132) | 108 | 121 (107-136) | 390 | 0.70 | |
| Eosinophils (1000/UL) | 0.1 (0-0.2) | 103 | 0.1 (0-0.2) | 367 | 0.81 | |
| Total cholesterol (mmol/L) | 4.1 (3.3-4.6) | 23 | 3.9 (3.1-5) | 78 | ||
| HDL cholesterol (mmol/L) | Y 1.0 (0.5-1.3) | 22 | 1.1 (0.8-1.5) | 78 | ||
| LDL cholesterol (mmol/L ) | 2.0 (1.6-2.7) | 22 | 2.2 (1.6-2.8) | 78 | ||
| Triglycerides (mmol/L ) | 1.1 (0.7-2.2) | 22 | 1.3(0.9-1.9) | 78 | ||
| Albumin (g/L) | 34 (28-38) | 93 | 34 (29-38) | 267 | 0.96 | |
| Random morning cortisol level (μg/dL) | 3.25 (1.35-6.75) | 108 | 8.5 (4.5-11.8) | 394 | <0.001 | |
| Cortrosyn stimulation test | baseline | 3.1 (1.1-3.6) | 59 | 10.6 (9.0-14.2) | 197 | <0.001 |
| cortisol level ‡(μg/dL) | 30min | 10.4 (7.8-13.7) | 47 | 21.6 (18.2-28.8) | 134 | <0.001 |
| 60 min | 12.8 (9.1-16.2) | 93 | 27.2 (23.2-33.4) | 362 | <0.001 | |
| Plasma ACTH level (pg/ml) | 6 (2-113) | 51 | 11.5 (2-73) | 102 | 0.002 | |
Normal ranges are as follows: sodium 135-145 mmol/L, potassium 3.5-5 mmol/L, calcium 2.1-2.55 mmol/L (8.4-10.2 mg/dL), creatinine 35.4-106.1 μmol/L (0.4-1.2 mg/dL), hemoglobin Female 116-155 g/L and male 130-170 g/L, eosinophils <0.4 1000/UL, total cholesterol < 5.2 mmol/L (200 mg/dL) , HDL > 1 mmol/L (39 mg/dL), calculated LDL< 3.4 mmol/L (130 mg/dL), triglycerides <4.4 mmol/L (160 mg/dL), albumin 35-55 g/L, ACTH 6-58 pg/ml;
Cortrosyn stimulation test, serum cortisol levels at baseline before and 30 and 60 minutes after cortrosyn (250 μg)injection. Age is presented as median and range while blood pressure values and blood chemistry as median and interquartile range.
Both groups were similar for age, sex, ethnicity and hospitalization unit (90% in each group were in the internal medicine wards), current smoking, alcohol or recreational drug use, and length of hospital stay and mortality (Table 1). Sodium, potassium, calcium, creatinine, hemoglobin and eosinophil count were similar between groups (Table 1). Random morning cortisol and baseline cortisol before cortrosyn injection levels were lower in insufficient compared to sufficient patients (p<0.001). Although plasma ACTH levels were lower in insufficient vs. sufficient patients (p=0.002) the results should be interpreted with caution due to incomplete data.
Co-morbidities more commonly observed in biochemically adrenal-cortisol insufficient than sufficient inpatients (Table 2) included liver disease (p<0.001), specifically hepatitis C (p=0.002) and prior orthotopic liver transplantation (p<0.001). Odds Ratio (OR) for observing hepatitis C in insufficient inpatients was 3.0 (95% confidence interval:1.5-6.1), p=0.004 as compared to sufficient inpatients. Of the 7 liver transplantation patients, 6 had active hepatitis C, 4 had hepatocellular carcinoma, 1 hepatitis B and 1 alcoholic cirrhosis and 1 primary biliary cirrhosis. All patients were transplanted and followed at our institution with ages ranging from 47 to 58 years, median time from liver transplantation 96 months (range 24-192) and median time from last recorded glucocorticoid treatment administration 60 months (range 12-90).
Table 2.
Co-existing diseases: Biochemical adrenal-cortisol insufficient vs. sufficient inpatients *
| Biochemical adrenal- cortisol insufficient (n=108) | Biochemical adrenal-cortisol sufficient (n=394) | p value | |
|---|---|---|---|
| Cardiovascular | 53.7 (58) | 63.5 (250) | 0.07 |
| Neurological | 38.0 (41) | 27.7 (109) | 0.04 |
| Pulmonary | 25.9 (28) | 25.4 (100) | 0.91 |
| Renal | 25.9 (28) | 19.8 (78) | 0.17 |
| Gastrointestinal | 32.4 (35) | 32.0 (126) | >0.99 |
| Liver | 20.4 (22) | 9.4 (37) | 0.004 |
| Pancreas | 5.6 (6) | 5.3 (21) | 0.99 |
| Skeletal | 37.0 (40) | 31.5 (124) | 0.27 |
| Hematological | 35.2 (38) | 35.0 (138) | >0.99 |
| Immunological | 25.9 (28) | 18.5 (73) | 0.09 |
| Cancer | 19.4 (21) | 18.3 (72) | 0.78 |
| Urogenital | 17.6 (19) | 13.5 (53) | 0.28 |
| Infections | 54.6 (59) | 40.9 (161) | 0.01 |
| Sepsis | 13.9 (15) | 7.6 (30) | 0.04 |
| Hepatitis (any) | 13.0 (14) | 5.3 (21) | 0.01 |
| Hepatitis C | 10.2 (11) | 3.6 (14) | 0.01 |
| Hepatitis B | 0.9 (2) | 1.5 (6) | n/a |
| Both | 0 | 0.3 (1) | n/a |
| HIV | 22.2 (24) | 11.7 (46) | 0.005 |
| Urinary tract | 8.3 (9) | 11.2 (44) | 0.48 |
| Pneumonia | 9.3 (10) | 10.2 (40) | 0.86 |
| Cellulitis | 9.3 (10) | 3.3 (13) | 0.02 |
| Osteomyelitis | 2.8 (3) | 2.3 (9) | 0.73 |
| CNS | 0 | 1.0 (4) | 0.58 |
| Colitis | 7.4 (8) | 5.8 (23) | 0.51 |
| other | 8.3 (9) | 7.6 (30) | 0.84 |
| Endocrine | 56.5 (61) | 42.4 (167) | 0.009 |
| Diabetes mellitus | 22.2 (24) | 20.8 (82) | 0.75 |
| Adrenal† | 4.6 (5) | 0.8 (3) | n/a |
| Gonads‡ | 22.6 (14) | 3.1 (6) | <0.001 |
| Thyroid | 14.8 (16) | 21.8 (86) | 0.11 |
| Other | 7.4 (8) | 5.3 (21) | 0.48 |
| Transplantation | 9.3 (10) | 2.8 (11) | 0.006 |
| Liver | 6.5 (7) | 0 | <0.001 |
| Kidney | 1.9 (2) | 0.5 (2) | n/a |
| Liver & kidney | 0.9 (1) | 0 | n/a |
| Bone marrow | 1.9 (2) | 1.5 (6) | n/a |
| Heart | 0 | 0.5 (2) | n/a |
| pancreas | 0 | 0.8 (3) | n/a |
| Psychiatric | 21.3 (23) | 20.8 (82) | 0.91 |
presented as % (n)
Including unilateral adrenalectomy for pheochomocytoma and lung metastases and adrenal incidentaloma
Reported pre-existing male hypogonadism reported by the patient or a physician upon hospitalization; n/a, not applicable due to small sample size
Biochemically adrenal-cortisol insufficient inpatients had more HIV [OR=2.2 (1.2-3.7), p=0.007] than sufficient inpatients.
Other endocrine diseases were more frequent in biochemically adrenal-cortisol insufficient than sufficient inpatients (p=0.009) (Table 2). Specifically male inpatients with biochemical adrenal-cortisol insufficiency were more likely to have reported hypogonadism as compared to those with biochemical adrenal-cortisol sufficiency [OR=6.2 (2.67-14.2), p<0.0001]. Using a multivariable model, both HIV [OR=5.7 (2.1-15.9), p<0.001] and biochemical adrenal-cortisol insufficiency [OR=7.4, (2.6-21.0), p<0.001] increased the risk of reported hypogonadism. Diabetes mellitus and thyroid abnormalities were similarly frequent in both study groups.
As compared to biochemically adrenal-cortisol sufficient, insufficient inpatients exhibited higher rates of infectious disease (p=0.01) (Table 2) and accordingly also received more anti-infectious medications (p=0.005) (Table 3), and received more pulmonary medications (including glucocorticoid inhalers) (p=0.01) (Table 3). Inpatients in both study groups were similarly administered drugs known to inhibit endogenous cortisol axis and prior short-term, low-dose glucocorticoid treatment (7.4% and 9.1%, respectively) (Table 3).
Table 3.
Medications received: Biochemically adrenal-cortisol insufficient vs. sufficient inpatients *
| Biochemically adrenal- cortisol insufficient n=108 | Biochemically adrenal- cortisol sufficient n=394 | p value | ||
|---|---|---|---|---|
| Anti-infectious | 62.0 (67) | 46.8 (184) | 0.005 | |
| Pulmonary | 29.6 (32) | 18.5 (73) | 0.01 | |
| Cardiovascular | 53.7 (58) | 60.7 (239) | 0.19 | |
| Diuretics | 12.0 (13) | 18 (71) | 0.14 | |
| Neuro- modulators | 66.7 (72) | 58.4 (230) | 0.12 | |
| Hematologic | 48.1 (52) | 44.4 (175) | 0.49 | |
| Endocrine | 40.7 (44) | 43.7 (172) | 0.59 | |
| Chemotherapy & radiation | 13.0 (14) | 12.9 (51) | >0.99 | |
| Gastrointestinal | 65.7 (71) | 65.2 (257) | 0.92 | |
| Skeletal | 7.4 (8) | 10.9 (43) | 0.37 | |
| Megestrol | 15.7 (17) | 8.4 (33) | 0.02 | |
| Azole antifungals † | 11.1 (12) | 6.3 (25) | 0.10 | |
| Mitotane/metyrapone | 0 | 0.3 (1) | ||
| Etomidate | 0.9 (1) | 0 | ||
| Prior glucocorticoids ‡ | 7.4 (8) | 9.1 (36) | 0.70 | |
| Prior fludrocortisone | 2.8 (3) | 1.8 (7) | 0.45 |
presented as % (n)
Anti-fungal azole drugs including ketoconazole, fludroconazole and voriconazole
Patients who received glucocorticoids at doses equivalent to ≥ 30 mg daily hydrocortisone for ≥ 2 weeks up to 24 hours prior to cortrosyn injection.
Sixty percent of biochemically adrenal-cortisol insufficient inpatients received glucocorticoid treatment after diagnosis (Table 4). We analyzed factors associated with lack of glucocorticoid replacement after biochemical diagnosis by multivariable logistic regression analysis of factors which may have determined physician judgments. Factors associated with higher odds of no treatment were higher random morning cortisol level [OR=1.2, (1.1-1.3), for 1-unit increase, p<0.0001] and higher diastolic blood pressure [OR=1.1 (1.0-1.2), for 5-unit increase p=0.05] as well as female sex [OR=2.0 (1.2-3.6), p=0.009) and not receiving an inhospital endocrinology consultation [OR=9.2 (5.1-16.7), p<0.0001]. Factors associated with lower odds of no replacement were co-existing endocrine diseases [OR=0.3 (0.1-0.7), p=0.005] and brief and low-dose glucocorticoid treatment up to 24 hours before cortrosyn stimulation test [OR=0.3 (0.2-0.7), p=0.002]. Mortality was similar whether or not biochemically adrenal-cortisol insufficient inpatients received glucocorticoid treatment post diagnosis [2 deaths among 49 untreated (4.1%) and 6 deaths among 59 treated inpatients (10.2%), p=0.3].
Table 4.
Patient management: Biochemically adrenal-cortisol insufficient vs. sufficient inpatients *
| Biochemically adrenal- cortisol insufficient N=108 | Biochemically adrenal- cortisol sufficient N=394 | p value | |
|---|---|---|---|
| Inhospital endocrine consult | 49.1 (53) | 14.5 (57) | <0.001 |
| Glucocorticoid treatment after diagnosis | 54.6 (59) | 6.3 (25) | <0.001 |
| Hydrocortisone | 36.1 (39) | 2.8 (11) | |
| Prednisone | 11.1 (12) | 1.8 (7) | |
| Dexamethasone | 0 | 0 | |
| Other | 7.4 (8) | 1.8 (7) |
Presented as % (n)
Albumin levels negatively and weakly correlated with both random morning cortisol (r= -0.26, p<0.001) and baseline cortisol levels before cortrosyn injection (r=-0.26, p<0.001), but not with cortisol levels obtained 30 and 60 minutes after cortrosyn injection (Table 5). The negative correlation of albumin with cortisol was similar in both study groups (insufficient group: r = -0.36, p < 0.001; sufficient group: r = -0.26, p < 0.001). Frequencies of hypoalbuminemia (≤ 25 g/L) were not different between patients with or without hepatitis C (p=0.2), prior liver transplantation (p=0.18), HIV (p=0.51) or reported male hypogonadism (p>0.99).
Table 5.
Biochemical correlations
| Albumin | Random morning Cortisol | Cortrosyn stimulation test: cortisol level | Cholesterol | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 30 min | 60 min | Total | HDL | LDLcal | ||||
| Albumin | 1 | −0.26† | −0.26 | 0.06 | 0.06 | 0.45 | 0.46 | 0.39 | |
| <0.001 | <0.001 | 0.40 | 0.20 | <0.001 | <0.001 | <0.001 | |||
| 303 | 264 | 204 | 450 | 114 | 111 | 107 | |||
| Random morning Cortisol a | 1 | 0.68 | 0.40 | 0.40 | −0.22 | −0.22 | −0.18 | ||
| <0.001 | <0.001 | <0.001 | 0.04 | 0.04 | 0.11 | ||||
| 200 | 151 | 305 | 90 | 89 | 86 | ||||
| Conrtrosyn stimulation test cortisol level | Baseline | 1 | 0.68 | 0.52 | −0.22 | −0.11 | −0.20 | ||
| <0.001 | <0.001 | 0.04 | 0.31 | 0.07 | |||||
| 150 | 305 | 85 | 83 | 82 | |||||
| 30 min | 1 | 0.9 | 0.07 | −0.03 | 0.14 | ||||
| <0.001 | 0.55 | 0.83 | 0.27 | ||||||
| 189 | 65 | 64 | 62 | ||||||
| 60 min | 1 | 0.06 | 0.07 | 0.09 | |||||
| 0.45 | 0.44 | 0.29 | |||||||
| 139 | 137 | 133 | |||||||
Data analyzed with Pearson (Albumin and random morning Cortisol) or Spearman correlation coefficients (other correlations), results presented in 3 values: upper, coefficient; middle, p value; bottom, number of inpatients.
In a regression model, adrenal-cortisol insufficiency and adrenal-cortisol sufficiency groups were similar,interaction p=0.17
b, Cortrosyn stimulation test, serum cortisol levels were taken at baseline, 30 and 60 minutes after cortrosyn (250 μg) injection; HDL, high density lipoprotein; LDLcal, calculated low density lipoprotein
Random morning cortisol levels correlated moderately with baseline cortisol levels taken before cortrosyn injection (r=0.68, p<0.001) and both positively correlated with cortisol levels measured 30 (r=0.40 and r=0.68, respectively, p<0.001) and 60 minutes (r=0.40 and r=0.52, respectively, p<0.001) after cortrosyn injection. Serum cortisol levels obtained 30 minutes after cortrosyn injection strongly correlated with levels observed at 60 minutes (r=0.90, p<0.001). Albumin, but not cortisol levels correlated moderately with cholesterol levels.
DISCUSSION
Adrenal-cortisol insufficiency in hospitalized patient is difficult to diagnose and requires high level of alertness to suspect the disease. (1-3) Biochemical diagnosis relies on cortisol level after cortrosyn injection, however as this test was validated in outpatients its accuracy in acutely ill hospitalized patients, whether in the ICU or not, is unclear. As no other biochemical test is currently available for diagnosis of adrenal insufficiency we relied on the cortrosyn stimulation test to determine the presence of biochemical adrenal-cortisol insufficiency in non-ICU hospitalized patients, a diagnostically challenging population.
Interestingly, although uncontrolled adrenal-cortisol insufficiency is considered fatal our results did not demonstrate increased mortality in biochemically adrenal-cortisol insufficient inpatients. In addition, no difference in mortality was observed within the insufficient group even when comparing glucocorticoid treated vs. non-treated inpatients. Small sample size, cortrosyn stimulation not being an optimal test for this patient population, inadequate glucocorticoid treatment and the retrospective nature of this study are among the explanations for this surprising observation. Similar lack of increased mortality has been reported in a prospective study in ICU inpatients with septic shock. Mortality was similar whether or not patients were diagnosed with biochemical adrenal-cortisol insufficiency and whether or not insufficient inpatients were treated with hydrocortisone.(25)
We demonstrate that commonly described features (1, 24) were similar for biochemically adrenal-cortisol insufficient and sufficient inpatients and therefore not reliable in the hospital setting. Whether this similarity is due to overlap with co-existing comorbidities and/or treatments received in-hospital or that the biochemical adrenal-cortisol abnormality has little, if any, clinical consequences in this inpatient population, is unclear. In-contrast, we retrospectively identified that non-ICU biochemically adrenal-cortisol insufficient inpatients were more likely to have certain co-morbidities including liver disease, specifically hepatitis C and prior liver transplantation, HIV and to report male hypogonadism. These comorbidities could potentially alert the hospital physician of inpatients who may require further assessment for possible cortisol deficiency
We show that biochemical adrenal-cortisol insufficiency is associated with liver disease, specifically active hepatitis C. (26) Moreover, inpatients with biochemical adrenal-cortisol insufficiency were more likely to have prior orthotopic liver transplantation associated with recurrent viral hepatitis years after transplantation and last glucocorticoid treatment, while none of the sufficient inpatients had prior liver transplantation. This observation is in line with reports documenting increased frequency of biochemical adrenal-cortisol insufficiency in cirrhotic patients and after liver transplantation.(27, 28) Low circulating total cortisol levels in the presence of advanced liver failure can also be the result of low levels of circulating cortisol binding proteins i.e. cortisol-binding globulin and albumin (produced solely by the liver and carrying 80% and 10% of circulating cortisol, respectively). (1) Although significantly reduced binding protein level in the failing liver was shown to reduce unstimulated serum total cortisol, (27, 28) the effect on post cortrosyn stimulation cortisol levels is unclear. Hypoalbuminemia (albumin ≤25 g/L) was shown to interfere with the correlation between total serum cortisol and salivary free cortisol 60 minutes after cortrosyn injection, (29) however, biochemically adrenal-cortisol insufficient inpatients with co-morbidities highlighted here did not exhibit increased frequency of hypoalbuminemia. Moreover, we demonstrate no correlation between albumin and post-stimulation cortisol levels. Cortisol-binding globulin levels were previously shown to negatively correlate with disease (29) and stress (30) severity and may have had a more significant impact on cortrosyn stimulation test results than albumin, however to which extent and how different from albumin is unclear, as acute stress is also associated with increased cortisol per se. Cortisol-binding globulin is not measured routinely in our center.
Interestingly, hypogonadism was more frequently reported in biochemically adrenal-cortisol insufficient than sufficient male inpatients, especially if interviewed by an endocrinologist, who would be more likely to inquire about the disorder. The observed coexistence if proven correct in prospective studies is interesting and may indicate a pathophysiological link between the two major steroid producing organs. Lower levels of HDL, a major distributer of cholesterol for tissue steroidogenesis, were reported in adrenal-cortisol insufficient patients (31, 32), liver failure (33, 34) and HIV infected patients (35) all of whom also exhibit high frequency of hypogonadism..
Receiving pulmonary medications was associated with increased adrenal-cortisol insufficiency frequency. As biochemically adrenal-cortisol insufficient and sufficient inpatients had similar frequency of lung diseases, increased biochemical adrenal-cortisol insufficiency frequency may have been associated with inhaled glucocorticoids as previously reported. (36, 37)
Physician uncertainty in adrenal-cortisol insufficiency diagnosis is also reflected by their likelihood to treat with glucocorticoid after biochemical confirmation. Only 60% of biochemically adrenal-cortisol insufficient inpatients were treated with glucocorticoids. We show that factors which may influence physician judgment independently contributed to physician treatment-decision. Relatively higher cortisol levels and higher diastolic blood pressure may have encouraged avoiding glucocorticoid use and their serious side effects. Surprisingly, female sex was an independent variable determining the choice not to treat. Although females have higher free cortisol levels than males (40), and estrogen increases cortisol-binding globulin production thereby increasing total cortisol levels, (41) our study demonstrates that even after adjustment to random morning cortisol levels, females were less likely to be replaced with glucocorticoids. In contrast, may be in anticipation of higher probability for adrenal-cortisol insufficiency, physicians were more likely to treat inpatients with co-existing endocrine diseases and those with previous short-term low-dose glucocorticoid treatment history. Whether rigorously adhering to clinical suspicion and cortrosyn stimulation test results in determining glucocorticoid treatment and how physician interpretation of cortrosyn stimulation test results determines inpatient outcome requires prospective study. The finding that females are less likely to receive glucocorticoids also raises the question of medical sex bias, a topic of high current interest. (42)
The clinical benefit of an inhospital endocrinology consultation remains unclear. Inpatients were more likely to receive glucocorticoids if they had an endocrinology consultation, likely reflecting the level of specialist certainty with, and bias towards, the diagnosis. It is generally accepted that patients with proven adrenal insufficiency should be replaced with glucocorticoids, however, effects of such replacement on patient outcomes could not be addressed by our study.
We recognize the inherent limitations of a retrospective study, evaluating patients from a single, although large and quaternary medical center. Several potential biases are evident in this study. Physician intent to assess cortisol levels in an inpatient setting, as these are not routinely measured and using random morning cortisol threshold of ≤15 μg/dL for screening. Post-cortrosyn serum cortisol threshold up to 20 rather than 18 μg/dL is used by some for the diagnosis of adrenal-cortisol insufficiency. To maximally reduce false-positives we used the lowest threshold indicated for serum cortisol in the literature (18) ,which is lower than plasma cortisol. (19) The cortrosyn stimulation test has been validated against the gold-standard insulin tolerance test only in outpatients and not in acutely ill hospitalized patients. Cortisol collection time after cortrosyn injection was not rigorously adherent but rather ranged up to 10 minute beyond the 30 minute time-point and 10 minutes around the 60 minute time point. The limited laxity permitted in the time range for cortisol collections was dictated by nurse availability and report. The use of 2 different cortisol assays increases variability in addition to intra-assay variability, and limitations of relying on total cortisol levels dependent on cortisol-binding globulin levels that had not been measured. The definition of male hypogonadism is based on the patient's or his physician report rather than on pre-hospitalization documentation of low testosterone levels. The extent to which endocrinologist consultation altered inpatient care is unclear. Importantly, although the association between liver disease or HIV with adrenal-cortisol insufficiency had been previously shown, our study approach differs. While prior studies assessed adrenal insufficiency in pre-selected patients with either HIV or liver disease, we study liver disease or HIV frequency in patients with biochemical adrenal-cortisol insufficiency as compared to sufficient patients.
We identify co-morbidities including hepatitis C, prior liver transplantation, HIV infection and reported male hypogonadism to be more commonly associated with biochemical adrenal-cortisol insufficiency in non-ICU hospitalized patients. As the clinical and routine biochemical features of this disorder are unhelpful for diagnosis in the hospital setting, these comorbidities may alert for higher risk of adrenal insufficiency, and help identify inpatients for more rigorous pursuit of the diagnosis.
CLINICAL SIGNIFICANCE BULLET POINTS.
Biochemical adrenal-cortisol insufficiency in hospitalized patients is associated with co-morbidities including hepatitis C, prior liver transplantation, HIV and reported male hypogonadism.
Almost half of inpatients with diagnosed biochemical adrenal-cortisol insufficiency were not replaced with corticosteroids. Random morning cortisol level, elevated blood pressure, sex, endocrinology consultation, co-existing endocrine diseases and prior brief or low-dose glucocorticoid treatment were independent determinants of treatment decision.
ACKNOWLEDGEMENTS
The authors thank Mr. Billy Gellepis for administrative assistance.
Funding/Support: Supported by the National Center for Advancing Translational Sciences through UCLA CTSI Grant UL1TR000124 and NIH grant CA75979. The content is solely the responsibility of the authors and does not represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest disclosure: The authors have nothing to disclose.
All authors had access to the primary data. AB, NL, SM participated in study design, follow up, interpretation and manuscript writing, JM performed all statistical analysis, SMG, AKK, RCS participated in data gathering, AB and SM wrote the paper.
REFERENCES
- 1.Stewart PM, Krone NP. The Adrenal Cortex. In: Melmed SP, K.S., Larsen PR, Kronenberg HM, editors. Williams Textbook of Endocrinology. 12th ed. Saunders, Elsevier; Philadelphia: 2011. pp. 479–544. [Google Scholar]
- 2.Bergthorsdottir R, Leonsson-Zachrisson M, Oden A, Johannsson G. Premature mortality in patients with Addison's disease: a population-based study. J Clin Endocrinol Metab. 2006;91(12):4849–53. doi: 10.1210/jc.2006-0076. [DOI] [PubMed] [Google Scholar]
- 3.Bensing S, Brandt L, Tabaroj F, et al. Increased death risk and altered cancer incidence pattern in patients with isolated or combined autoimmune primary adrenocortical insufficiency. Clin Endocrinol (Oxf) 2008;69(5):697–704. doi: 10.1111/j.1365-2265.2008.03340.x. [DOI] [PubMed] [Google Scholar]
- 4.Sacre K, Dehoux M, Chauveheid MP, et al. Pituitary-adrenal function after prolonged glucocorticoid therapy for systemic inflammatory disorders: an observational study. J Clin Endocrinol Metab. 2013;98(8):3199–205. doi: 10.1210/jc.2013-1394. [DOI] [PubMed] [Google Scholar]
- 5.van Staa TP, Leufkens HG, Abenhaim L, et al. Use of oral corticosteroids in the United Kingdom. QJM. 2000;93(2):105–11. doi: 10.1093/qjmed/93.2.105. [DOI] [PubMed] [Google Scholar]
- 6.Overman RA, Yeh JY, Deal CL. Prevalence of oral glucocorticoid usage in the United States: A general population perspective. Arthritis Care Res (Hoboken) 2012 doi: 10.1002/acr.21796. [DOI] [PubMed] [Google Scholar]
- 7.Hagg E, Asplund K, Lithner F. Value of basal plasma cortisol assays in the assessment of pituitary-adrenal insufficiency. Clin Endocrinol (Oxf) 1987;26(2):221–6. doi: 10.1111/j.1365-2265.1987.tb00780.x. [DOI] [PubMed] [Google Scholar]
- 8.Grinspoon SK, Biller BM. Clinical review 62: Laboratory assessment of adrenal insufficiency. J Clin Endocrinol Metab. 1994;79(4):923–31. doi: 10.1210/jcem.79.4.7962298. [DOI] [PubMed] [Google Scholar]
- 9.Landon J, James VH, Cryer RJ, et al. Adrenocorticotropic Effects of a Synthetic Polypeptide--Beta 1-24-Corticotropin--in Man. J Clin Endocrinol Metab. 1964;24:1206–13. doi: 10.1210/jcem-24-11-1206. [DOI] [PubMed] [Google Scholar]
- 10.Lindholm J, Kehlet H. Re-evaluation of the clinical value of the 30 min ACTH test in assessing the hypothalamic-pituitary-adrenocortical function. Clin Endocrinol (Oxf) 1987;26(1):53–9. doi: 10.1111/j.1365-2265.1987.tb03638.x. [DOI] [PubMed] [Google Scholar]
- 11.Lindholm J, Kehlet H, Blichert-Toft M, et al. Reliability of the 30-minute ACTH test in assessing hypothalamic-pituitary-adrenal function. J Clin Endocrinol Metab. 1978;47(2):272–4. doi: 10.1210/jcem-47-2-272. [DOI] [PubMed] [Google Scholar]
- 12.Streeten DH, Anderson GH, Jr., Dalakos TG, et al. Normal and abnormal function of the hypothalamic-pituitary-adrenocortical system in man. Endocr Rev. 1984;5(3):371–94. doi: 10.1210/edrv-5-3-371. [DOI] [PubMed] [Google Scholar]
- 13.Alesci S, Ilias I, Souvatzoglou E, et al. Intramuscular administration of ACTH1-24 vs. 24-hour blood sampling in the assessment of adrenocortical function. Hormones (Athens) 2005;4(2):96–100. [PubMed] [Google Scholar]
- 14.Cooper MS, Stewart PM. Adrenal insufficiency in critical illness. J Intensive Care Med. 2007;22(6):348–62. doi: 10.1177/0885066607307832. [DOI] [PubMed] [Google Scholar]
- 15.Lipiner-Friedman D, Sprung CL, Laterre PF, et al. Adrenal function in sepsis: the retrospective Corticus cohort study. Crit Care Med. 2007;35(4):1012–8. doi: 10.1097/01.CCM.0000259465.92018.6E. [DOI] [PubMed] [Google Scholar]
- 16.Hurel SJ, Thompson CJ, Watson MJ, et al. The short Synacthen and insulin stress tests in the assessment of the hypothalamic-pituitary-adrenal axis. Clin Endocrinol (Oxf) 1996;44(2):141–6. doi: 10.1046/j.1365-2265.1996.555381.x. [DOI] [PubMed] [Google Scholar]
- 17.Greig WR, Maxwell JD, Boyle JA, et al. Criteria for distinguishing normal from subnormal adrenocortical function using the Synacthen test. Postgrad Med J. 1969;45(523):307–13. doi: 10.1136/pgmj.45.523.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart PMaKNP. The adrenal cortex. In: Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, editors. Williams Textbook of Endocrinology. 12th ed. Elsevier; Philadelphia: 2011. pp. 479–544. [Google Scholar]
- 19.Tuchelt H, Dekker K, Bahr V, Oelkers W. Dose-response relationship between plasma ACTH and serum cortisol in the insulin-hypoglycaemia test in 25 healthy subjects and 109 patients with pituitary disease. Clin Endocrinol (Oxf) 2000;53(3):301–7. doi: 10.1046/j.1365-2265.2000.01089.x. [DOI] [PubMed] [Google Scholar]
- 20.Dorin RI, Qualls CR, Crapo LM. Diagnosis of adrenal insufficiency. Ann Intern Med. 2003;139(3):194–204. doi: 10.7326/0003-4819-139-3-200308050-00009. [DOI] [PubMed] [Google Scholar]
- 21.Crowley S, Hindmarsh PC, Holownia P, et al. The use of low doses of ACTH in the investigation of adrenal function in man. J Endocrinol. 1991;130(3):475–9. doi: 10.1677/joe.0.1300475. [DOI] [PubMed] [Google Scholar]
- 22.Stewart PM, Clark PM. The low-dose corticotropin-stimulation test revisited: the less, the better? Nat Clin Pract Endocrinol Metab. 2009;5(2):68–9. doi: 10.1038/ncpendmet1038. [DOI] [PubMed] [Google Scholar]
- 23.Fleseriu M, Gassner M, Yedinak C, et al. Normal hypothalamic-pituitary-adrenal axis by high-dose cosyntropin testing in patients with abnormal response to low-dose cosyntropin stimulation: a retrospective review. Endocr Pract. 2010;16(1):64–70. doi: 10.4158/EP09153.OR. [DOI] [PubMed] [Google Scholar]
- 24.Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med. 2003;348(8):727–34. doi: 10.1056/NEJMra020529. [DOI] [PubMed] [Google Scholar]
- 25.Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358(2):111–24. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 26.Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705–14. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 27.Fede G, Spadaro L, Tomaselli T, et al. Adrenocortical dysfunction in liver disease: a systematic review. Hepatology. 2012;55(4):1282–91. doi: 10.1002/hep.25573. [DOI] [PubMed] [Google Scholar]
- 28.Trifan A, Chiriac S, Stanciu C. Update on adrenal insufficiency in patients with liver cirrhosis. World J Gastroenterol. 2013;19(4):445–56. doi: 10.3748/wjg.v19.i4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galbois A, Rudler M, Massard J, et al. Assessment of adrenal function in cirrhotic patients: salivary cortisol should be preferred. J Hepatol. 2010;52(6):839–45. doi: 10.1016/j.jhep.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 30.Dhillo WS, Kong WM, Le Roux CW, et al. Cortisol-binding globulin is important in the interpretation of dynamic tests of the hypothalamic--pituitary--adrenal axis. Eur J Endocrinol. 2002;146(2):231–5. doi: 10.1530/eje.0.1460231. [DOI] [PubMed] [Google Scholar]
- 31.Bochem AE, Holleboom AG, Romijn JA, et al. High-density lipoprotein as a source of cholesterol for adrenal steroidogenesis; a study in individuals with low plasma HDL-C. J Lipid Res. 2013 doi: 10.1194/jlr.P033449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ooi LS, Panesar NS, Masarei JR. Urinary excretion of testosterone and estradiol in Chinese men and relationships with serum lipoprotein concentrations. Metabolism. 1996;45(3):279–84. doi: 10.1016/s0026-0495(96)90279-6. [DOI] [PubMed] [Google Scholar]
- 33.Marik PE. Adrenal-exhaustion syndrome in patients with liver disease. Intensive Care Med. 2006;32(2):275–80. doi: 10.1007/s00134-005-0005-5. [DOI] [PubMed] [Google Scholar]
- 34.Etogo-Asse FE, Vincent RP, Hughes SA, et al. High density lipoprotein in patients with liver failure; relation to sepsis, adrenal function and outcome of illness. Liver Int. 2012;32(1):128–36. doi: 10.1111/j.1478-3231.2011.02657.x. [DOI] [PubMed] [Google Scholar]
- 35.Giannarelli C, Klein RS, Badimon JJ. Cardiovascular implications of HIV-induced dyslipidemia. Atherosclerosis. 2011;219(2):384–9. doi: 10.1016/j.atherosclerosis.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Wass JA, Arlt W. How to avoid precipitating an acute adrenal crisis. BMJ. 2012;345:e6333. doi: 10.1136/bmj.e6333. [DOI] [PubMed] [Google Scholar]
- 37.Mak VH. Inhaled corticosteroids: first do no harm. BMJ. 2012;345:e8204. doi: 10.1136/bmj.e8204. [DOI] [PubMed] [Google Scholar]