Abstract
Cellular membrane affinity chromatography stationary phases have been extensively used to characterize immobilized proteins and provide a direct measurement of multiple binding sites, including orthosteric and allosteric sites. This review will address the utilization of immobilized cellular and tissue fragments to characterize multiple transmembrane proteins co-immobilized onto a stationary phase. This approach will be illustrated by demonstrating that multiple transmembrane proteins were immobilized from cell lines and tissue fragments. In addition, the immobilization of individual compartments/organelles within a cell will be discussed and the changes in the proteins binding/kinetics based on their location.
Keywords: Bioaffinity chromatography, G-Protein Coupled Receptors, Ligand-Gated Ion Channels, ATP-Binding Cassette transporters
1. Introduction
Cell surface transmembrane receptors and transporters are key therapeutic targets in drug discovery and development [1–3] and include large families of proteins such as G-protein coupled receptors (GPCRs), ligand gated ion channels (LGICs) and the ATP Binding Cassette superfamily (ABC transporters). GPCRs make up almost half of the current therapeutic targets receptors and 36% of currently marketed drugs were developed as GPCR agonists or antagonists [4]. LGICs also represent a class of important therapeutic targets such as nicotinic acetylcholine receptors (nAChRs) and N-methyl-D-aspartate receptors (NMDARs) while the ABC transporters such as P-glycoprotein play essential roles in systemic and central bioavailability and are involved in an aspect of multiple drug resistant to therapeutic agents.
The broad range of cellular and pharmacological functions associated with these receptors and transporters has resulted in the development of a large number of in vitro assays for the high-throughput screening of small molecules against a specific receptor. The most commonly used methods for binding affinity determination include classic binding assays, functional assays, monolayer efflux assays and surface plasmon resonance [5, 6]. The classical binding assay approach is based on the concept of competitive interaction of a known analyte and a ligand for the same receptor binding site, while the functional assays allow the determination of a compound’s effect on inhibition of transport, cell proliferation, mobilization of calcium, agonist or antagonistic properties of the ligand, etc. [7, 8]. These approaches allow quantitative determination of the binding affinity of a ligand for its receptor providing valuable information on the potency of the ligand, including effective concentration and selectivity for the targeted receptor.
However, the screening of chemical libraries as well as individual compounds against a single (orthosteric) binding site on the protein target does not always produce an adequate or comprehensive pharmacological profile [9]. For example, the majority of GPCRs possess allosteric binding sites and multiple conformations that can lead to increased or reduced activity or to distinctly different activities via alterations in intracellular signaling cascades. Differences can also arise from slight changes in amino acid residues that produce closely related proteins with widely ranging affinities and tissue expression. This is exemplified by the family of nicotinic acetylcholine receptors (nAChRs) which are composed of a combination of α and β subunits producing a family of structurally related LGICs with a broad range of affinities for the same agonists and antagonists. In addition, transmembrane receptors are expressed in a variety of membrane environments and the composition of the membrane can dramatically affect the function and selectivity of the target protein. This is illustrated by differences in the binding of ligands to the breast cancer resistance protein (BCRP), an ABC transporter, observed between cellular membrane-expressed BCRP and nuclear membrane-expressed BCRP [10].
One approach that provides for the direct measurement of multiple binding sites including orthosteric and allosteric sites, multiple binding configurations as well as subtype ligand interactions is bioaffinity chromatography, where the target biopolymer (protein) is immobilized onto silica based stationary phase. The use of this technology with isolated proteins and enzymes has been extensively reviewed [11–19]. In this review we address the utilization of an immobilized cellular and tissue fragments to characterize multiple proteins co-immobilized onto a stationary phase. The initial studies were carried out by Per Lundahl’s group [20–22], where they immobilized the glucose transporter, GLUT1, through the incorporation of red blood cell membranes in proteo-liposomes [20]. This was quickly expanded by Wainer’s group to include the immobilization of the transmembrane neuronal nicotinic receptor [23], onto the surface of the Immobilized Artificial Membrane (IAM) stationary phase (12 μ, 300Å pore) developed by Pidgeon and Venkataram [28]. The general experimental approach associated with this technology has been reviewed [25] and will not be discussed here, rather this review will concentrate on the study of the immobilization of different families of proteins and the co-immobilization of multiple receptors and differences produced by the cellular membrane environment.
2. Multiple Ligand Gated Ion Channel Columns
The immobilization of solubilized tissue onto the IAM stationary phase was initially demonstrated using solubilized rat brain tissue [11], where the rat brains were homogenized and solubilized and the resulting membrane fragments were immobilized onto an IAM stationary phase. It was clearly demonstrated that the immobilization of solubilized rat forebrain membrane fragments resulted in the co-Immobilization of nicotinic receptors (nAChR), γ-Amino-Butyric Acid Receptors (GABA), and N-Methyl D-Aspartate (NMDA) receptors. Each receptor was characterized by frontal affinity chromatographic studies to determine the binding affinities. The resulting binding affinities correlated with those reported in literature [11]. As each receptor identified had a selective ligand, additional studies were carried out to determine whether the receptors were independent of each other. For example, the nAChR was characterized in the absence and in the presence of a saturating concentration of flunitrazepam, a marker used for the GABAa receptor. The study did not result in any changes in the elution volumes with or without the inhibitor for the other receptor, indicating that the marker ligands were specific for the immobilized receptors and that the immobilized receptors were independent of each other. Specifically, no decrease in the elution volume of [3H]-epibatidine ([3H]-EB) was observed when [3H]-EB was injected on the column with or without saturating concentration of flunitrazepam in the mobile phase. In a subsequent study, human brain tissue was immobilized and the resulting column characterized. In this case, only the nicotinic receptor was probed and a biphasic curve was obtained indicating the presence of multiple sites for epibatidine. The frontal chromatographic studies demonstrated that at least two subtypes of the nicotinic receptor was immobilized, which were believed to be the homomeric α7 nAChRs and heteromeric αxβy nAChRs.
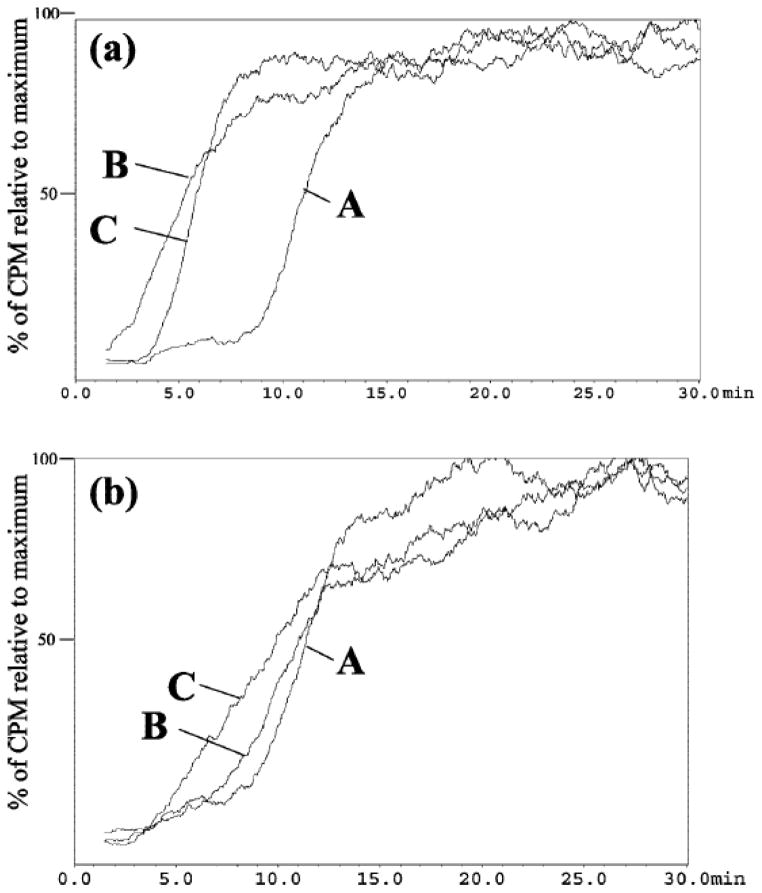
The presence of multiple receptors on the cellular membrane affinity chromatographic columns was not restricted to membrane fragments obtained from tissues. Cellular membranes from the endogenous cell lines, 1321N1 and A172 astrocytoma cell lines, were also immobilized onto the IAM stationary phase [12] and also resulted in a multiple receptor column. Specifically, the presence of multiple ligand gated ion channels, including GABAA and NMDA receptors and multiple subtypes of the nAChR, was demonstrated and characterized by frontal affinity chromatography. Similar to what was observed in the human brain column, the frontal chromatogram of epibatidine resulted in a biphasic curve (Figure 1). This suggested the presence of multiple subtypes of the nicotinic receptor. In order to characterize each subtype of the nAChR independently, a specific ligand for the respective subtypes was added to the mobile phase with epibatidine. To study the homomeric α7 nAChR, a saturating concentration of n-Bungaratoxin, a selective inhibitor of the heteromeric nicotinic receptors, was added to the mobile phase and to study the heteromeric αxβy nAChRs, a saturating concentration of α-Bungarotoxin (α-Bgt), a selective inhibitor of the homomeric nicotinic receptors was added to the mobile phase [12]. The binding affinities obtained for epibatidine for each specific subtype of the nicotinic receptor corresponded to previously reported Kd values. This demonstrates that a ligand that has affinity for multiple subtypes of a receptor can still be used to distinguish between these receptors on a single column, as long as each subtype can be selectively blocked using a subtype specific ligand. While, lacking the advantage of SPR, in terms of throughput, bioaffinity chromatography allows the characterization of multiple proteins in tandem.
Figure 1.
The chromatographic traces obtained for 60 pM [3H]-EB on the CMAC(1321N1) column (panel A) and CMAC(A172) column (panel B) where A is the trace obtained using a mobile phase composed of ammonium acetate [10 mM, pH 7.4]; B is the trace obtained after the addition of 5 nM R-BTx to the mobile phase; and C is the trace obtained after the addition of 1 nM κ-BTx to the mobile phase. Reprinted with permission from [12].
3. Multiple G-Protein Coupled Receptor columns
The 1321N1, reported above, was initially homogenized and solubilized using cholate as the detergent. However, the immobilization of membrane fragements of a 1321N1 cell line transfected with the P2Y1 receptor, required the addition of n-octyl-β-d-glucopyranoside to immobilize the G-protein coupled receptor P2Y1 [26]. The importance of the choice of detergent in the solubilization buffer has been previously discussed in detail, c.f. [25]. After the immobilization of n-octyl-β-d-glucopyranoside solubilized membrane fragments from the 1321N1-P2Y1 cells, generating the CMAC (1321N1P2Y1), the full characterization of the P2Y1 was carried out using frontal chromatography. Subsequent studies, demonstrated that the histamine, H1, receptor was also co-immobilized on the CMAC(1321N1P2Y1) column [27]. Both the P2Y1 and H1 receptor were characterized by frontal affinity chromatography and a binding affinity (Kd) of 88.5 nM of 2-MeSADP (2- Methylthioadenosine-5′-O-diphosphate), a P2Y1 agonist, for the P2Y1 receptor and Kd’s of 2.9 nM and 7400 nM for mepyramine and histamine, respectively, for the H1 receptor, were obtained. The independent binding of the ligand to both GPCRs in CMAC (1321N1P2Y1) was demonstrated by showing that no displacement was observed for [3H]mepyramine with 10 mM of 2-MeSADP and for 1 nM of [3H]2-MeSADP with 1 mM of mepyramine.
Another study, reporting on the immobilization of membrane fragments from the KU-812 cell line onto an open tubular (OT) format, also demonstrated the co-immobilization of multiple GPCRs. In this case, however, it was the co-immobilization of two subtypes of the cannabinoid receptors, CB1 and CB2 [28]. The presence of functional CB1/CB2 receptors in the CB1/CB2-OT column was confirmed by the data from frontal affinity chromatography, however, as the subtypes have ligands with cross-selectivity, a new approach was taken. The CB marker ligand [3H]Win 55,212-2 was used in these studies and the compound is 15 time more selective for CB2 than CB1, as a result 0.5 nM (a saturating concentration for CB2) was used to characterize the CB1 receptor and 0.25 nM was used to characterize the CB2 receptor. The calculated Kd value of Win-55,212-2 for the CB1 and CB2 receptors was 8.6 nM and 0.37 nM respectively.[1] In addition, the binding affinities (Ki value) of the selective CB1 agonists R(+)-methanadamide and arachidonyl-2-chloroethylamide were determined and were consistent with previously reported values.
More recently, we have co-immobilized CB1 and β2 adrenergic GPCRs onto the surface of open tubular capillary from HepG2 cell line. The resulting HepG2-OT column was characterized using frontal affinity chromatography to determine the binding affinity of selective agonists of the CB1 receptor and β2 adrenergic receptor, Win 55,2122 and CGP-12177, respectively. In addition, the selective antagonist S-propanolol of β2 adrenergic receptor was also determined. The binding affinity of Win 55,212-2 for the immobilized CB1 receptor was 23 nM, similar to previously reported values and the binding affinity of CGP-12177 and propranolol was determined to be 108.1 nM and 70.2 nM, respectively, also in agreement with previously reported values [29].
4. Multiple ATP Binding Cassette transporter columns
In drug discovery and drug development, a significant interest has been placed in the ATP Binding Cassette (ABC) superfamily. This family consists of 48 members, of which P-glycoprotein, MRP1, MRP2 and BCRP are the most well-known members. These transporters are targeted due to their role in drug resistance [30]. The immobilization of the Pgp transporter has been previously carried out on both IAM stationary phase and open tubular format. The Pgp(+)-OT column was used to obtain the binding affinity of known substrates for the Pgp transporter, the resulting affinities correlated with literature results, indicating that the Pgp(+)-OT can be used to quantitatively estimate binding affinities [31]. Subsequently, a cohort of known Pgp substrates was used for ranking the compounds based on the differential retention times on the Pgp (+)-OT and Pgp(−)-OT columns (Δt), into high affinity, moderate affinity and no affinity groups. A comparison between the results obtained on the Pgp-OT columns and the monolayer efflux assay (Caco-2 cell monolayer model) [32], resulted in a statistically significant correlation between the Δt values and the permeability ratios, r2 = 0.7749 (p = 0.0063), indicating that the differential chromatography approach could be used to quantitatively assess permeability ratios. 11 of the 13 compounds tested on both the systems were correctly predicted by the rapid frontal screening using Pgp-OT, with the exception of ketoconazole and imipramine, which both had disagreement in literature as well.
A multiple ABC transporter column was subsequently developed using immobilized cellular membranes from the LN-229 cell line [10]. The resulting column was demonstrated to contain immobilized Pgp, BCRP and MRP1 transporters. The presence of these transporters was demonstrated using etoposide as the marker ligand. As etoposide is a substrate for all three transporters, selective inhibition of the multiple transporters (2 of the 3 transporters) was carried out in order to study a single targeted transporter. For example, the addition of a saturating concentration of verapamil, a Pgp and MRP1 substrate, allowed for the specific characterization of the BCRP transporter. In the same manner, the addition of estrone-3-sulfate, a MRP1 and BCRP substrate, allowed the characterization of the Pgp transporter; and the addition of prazosin, a Pgp and BCRP substrate allowed the characterization of the MRP1 transporter. The binding affinity obtained for etoposide for LN-229-IAM column was reported to be 3.75 ± 0.97μM, a combination of the binding affinity for all three immobilized transporters. The binding affinity for etoposide was determined for each transpoter by the addition of the aforementioned substrates and the resulting binding affinities were 5.83 ± 0.68 μM, 4.39 ± 1.93 μM and 1.41 ± 0.54 μM for Pgp, MRP1 and BCRP, respectively. A multiple ABC transporter column could be beneficial for drug development studies.
5. Membrane-Enclosed Organelle Columns
The lipid environment of the transmembrane protein (boundary lipids) plays a pivotal role in the immobilization protocol, and are required for the generation of a stable column [33]. For example, the immobilization of α4β2 nAChR using membrane fragments from a SH-EP1-pCEP4-hα4β2 cell line and a HEK-293 cells, required the addition of l-α-phosphatidylserine and l-α-phosphatidylethanolamine in the former case to generate a stable and functional column. Epibatidine had had a pronounced difference in its binding affinity for the α4β2, 0.5 vs 0.01 nM, respectively, which is believed to result from the change in the lipid environment, clearly indicating the importance of the lipid environment for the binding affinity. Thus, the presence of a transporter/protein on a different membrane environment, for example, nuclear versus cellular may result in differences in affinity for the ligands.
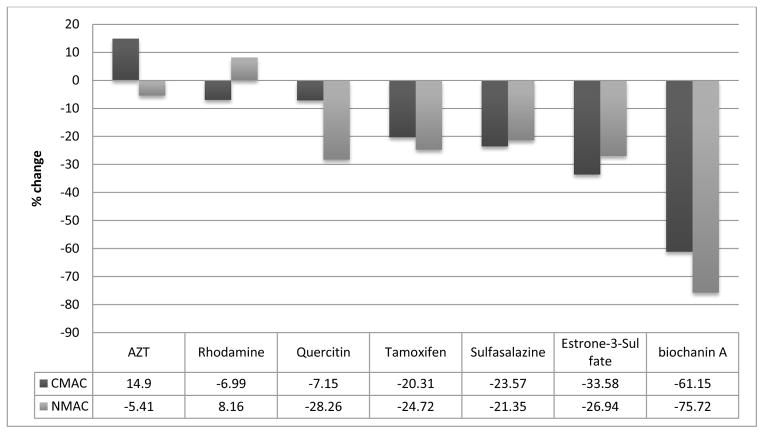
To this end, it was recently demonstrated that the BCRP was expressed in nuclear and cellular membranes of human-derived glioblastoma and astrocytoma cell lines [34]. As a result, the characterization of a transporter/protein in its native environment was carried out to determine if there were differences in the binding characteristics and inhibitory properties between the nuclear and cytoplasmic BCRP. The role of the nuclear BCRP for MDR was demonstrated by its increased sensitivity to the anti-cancer agent mitoxantrone after knockdown or chemical inhibition of nuclear BCRP. The nuclear membranes were immobilized in a similar fashion to the cellular membranes with the exception that the nuclear membranes were initially isolated with a Nuclear Protein Extraction Kit [35]. The nuclear BCRP was characterized using known substrates/inhibitors, including etoposide, biochanin A and the selective inhibitor FTC. The resulting binding affinities correlated with literature values. In addition to BCRP, the presence of Pgp and MRP1 were confirmed on the nuclear membranes by western blot analysis and frontal chromatography. Similarly, the cellular membranes from LN-229 cells were also shown to contain these three transporters. The selective characterization of each transporter was carried out by saturation of the binding sites of the non-targeted transporters. While there was little difference in the binding affinities obtained for verapamil, etoposide and prazosin between the nuclear and cellular membrane columns, the screening of the BCRP transporter between cellular membranes versus nuclear membrane was of interest. The selective screening of BCRP was carried out by the addition of verapamil (a Pgp and MRP1 substrate) to the mobile phase. This allowed the comparative screening of 7 compounds, not including etoposide, for the nuclear and cellular BCRP (Figure 2). Of the 7 compounds tested, 4 compounds had no major differences in the binding to the BCRP in both columns. However, for the three other compounds tested, AZT, quercetin and biochanin A had more significant differences. All three compounds had stronger affinity for the cellular BCRP, with an increase of 20%, 21% and 15%, respectively, in displacement of etoposide. The data suggest that these compounds display a higher affinity for the BCRP transporter found within the cellular membrane that of the BCRP found within the nuclear membrane. AZT was the strongest of the tested compounds for the cellular BCRP, while rhodamine123 was the strongest for nuclear BCRP; and biochanin A was the weakest substrate for both nuclear and cellular BCRP columns. The results of the study indicated that differences between the nuclear and cellular BCRP could be determined using bioaffinity chromatography. While, the differences could result from changes in post-translational modifications in different membranes or from conformational differences between the two proteins produced by the lipid membrane environment.
Figure 2.
Single frontal displacement studies of 8 compounds (4.25 μM), including etoposide, for the BCRP carried out on both CMAC(LN-229)-OT and NMAC(LN-229)-OT using ammonium acetate [10 mM, pH 7.4] in the presence of 2 μM verapamil and 0.75 μM etoposide as the mobile phase. The data was normalized to the change in breakthrough volume observed with etoposide and the relative changes from etoposide (0%) are reported. Reprinted with permission from [10].
6. Conclusions
The results discussed in this review, suggests that bioaffinity chromatography has a new role to play in the characterization of simultaneous characterizations of multiple proteins/multiple subtypes of a receptor and in the characterization of proteins in different membrane environments, for example, membrane-enclosed organelles. The immobilization of individual compartments/organelles within a cell could open up possibilities of studying differences in receptor binding/kinetics based on their location. For example, a cellular, nuclear and mitochondrial column can be made from the same cell line.
Highlights.
Tissue based columns
Multiple transmbembrane protein stationary phases
Membrane Enclosed Organelle columns
Acknowledgments
This work was supported in part by the Intramural Research Program of the National Institute on Aging, NIH. K.L.H. and R.S. were supported by the European Union through the European Regional Development Fund (Centre of Excellence “Mesosystems: Theory and Applications”, TK114) and by the Ministry of Education and Research of Estonia under targeted financing No. SF0130010s12.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hutchings CJ, Koglin M, Marshall FH. MAbs. 2010;6:594–606. doi: 10.4161/mabs.2.6.13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bianchi MT, Botzolakis EJ. BMC Pharmacology. 2010;10:1–8. doi: 10.1186/1471-2210-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pierce KL, Premont RT, Lefkowitz RJ. Cell Biol. 2002;9:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 4.Tang XL, Wang Y, Li DL, Luo J, Liu MY. Acta Pharmacol Sin. 2012;33:363–371. doi: 10.1038/aps.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper MA. J Mol Recognit. 2004;17:286–315. doi: 10.1002/jmr.675. [DOI] [PubMed] [Google Scholar]
- 6.de Jong LA, Uges DR, Franke JP, Bischoff R. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;829:1–25. doi: 10.1016/j.jchromb.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Major JS. Journal of Recptor Signal Transduction Reasearch. 1995;15:595–607. doi: 10.3109/10799899509045242. [DOI] [PubMed] [Google Scholar]
- 8.Sittampalam GS, Kahls SD, Janzen WP. Curr Opin Chem Biol. 1997;1:384–91. doi: 10.1016/s1367-5931(97)80078-6. [DOI] [PubMed] [Google Scholar]
- 9.Lane JR, Sexton PM, Christopoulos A. Trends Pharmacol Sci. 2013;34:59–66. doi: 10.1016/j.tips.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Habicht K-L, Singh NS, Khadeer MA, Shimmo R, Wainer IW, Moaddel R. Characterization of a multiple endogenously expressed ATP-Binding Cassette transporters using nuclear and cellular membrane affinity chromatography columns. doi: 10.1016/j.chroma.2014.02.076. (Accepted for publication in J Chromatogr A. 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moaddel R, Cloix JF, Ertem G, Wainer IW. Pharmaceutical Research. 2002;19:104–107. doi: 10.1023/a:1013619802766. [DOI] [PubMed] [Google Scholar]
- 12.Kitabatake T, Moaddel R, Cole R, Gandhari M, Frazier C, Hartenstein J. Anal Chem. 2008;80:8673–8680. doi: 10.1021/ac8016407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loun B, Hage DS. J Chromatogr. 1992;579:225–235. [PubMed] [Google Scholar]
- 14.Fitos I, Visy J, Simonyi M, Hermansson J. J Chromatogr. 1992;609:163–171. doi: 10.1016/0021-9673(92)80159-r. [DOI] [PubMed] [Google Scholar]
- 15.Noctor TAG, Wainer IW, Hage DS. J Chromatogr. 1992;577:305–315. doi: 10.1016/0378-4347(92)80252-l. [DOI] [PubMed] [Google Scholar]
- 16.Noctor TAG, Pham CD, Kaliszan R, Wainer IW. Mol Pharm. 1992;42:506–511. [PubMed] [Google Scholar]
- 17.Domenici E, Bertucci C, Salvadori P, Wainer IW. J Pharm Sci. 1991;80:164–166. doi: 10.1002/jps.2600800216. [DOI] [PubMed] [Google Scholar]
- 18.Dalgaard L, Hansen JJ, Pedersen JL. J Pharm Biomed Anal. 1989;7:361–368. doi: 10.1016/0731-7085(89)80103-7. [DOI] [PubMed] [Google Scholar]
- 19.Kaliszan R, Noctor TAG, Wainer IW. Mol Pharmacol. 1992;42:512–517. [PubMed] [Google Scholar]
- 20.Brekkan E, Lundqvist A, Lundahl P. Biochemistry. 1996;35:12141–12145. doi: 10.1021/bi9603231. [DOI] [PubMed] [Google Scholar]
- 21.Gottschalk L, Li YM, Lundahl P. J Chromatogr B. 2000;739:55–62. doi: 10.1016/s0378-4347(99)00383-7. [DOI] [PubMed] [Google Scholar]
- 22.Gottschalk I, Lagerquist C, Zuo SS, Lundqvist A, Lundahl P. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;768:31–40. doi: 10.1016/s0378-4347(01)00483-2. [DOI] [PubMed] [Google Scholar]
- 23.Wainer IW, Zhang Y, Xiao Y, Kellar KJ. J Chromatogr B Biomed Sci Appl. 1999;724:65–72. doi: 10.1016/s0378-4347(98)00579-9. [DOI] [PubMed] [Google Scholar]
- 24.Pidgeon C, Venkataram UV. Anal Biochem. 1989;176:36–47. doi: 10.1016/0003-2697(89)90269-8. [DOI] [PubMed] [Google Scholar]
- 25.Moaddel R, Wainer IW. Nat Protoc. 2009;4:197–205. doi: 10.1038/nprot.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moaddel R, Calleri E, Massolini G, Frazier C, Wainer IW. Anal Biochem. 2007;364:216–218. doi: 10.1016/j.ab.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moaddel R, Musyimi HK, Sanghvi M, Bashore C, Frazier C, Khadeer M, Bhatia P, Wainer IW. J Pharm Biomed Anal. 2010;52:416–419. doi: 10.1016/j.jpba.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moaddel R, Rosenberg A, Spelman K, Frazier J, Frazier C, Nocerino S, Brizzi A, Mugnaini C, Wainer IW. Anal Biochem. 2011;412:85–91. doi: 10.1016/j.ab.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beigi F, Chakir K, Xiao RP, Wainer IW. Anal Chem. 2004;76:7187–7193. doi: 10.1021/ac048910c. [DOI] [PubMed] [Google Scholar]
- 30.Mogg AJ, Whiteaker P, McIntosh JM, Marks M, Collins AC, Wonnacott S. Journal of Pharmacology and Experimental Therapeutics. 2002;302:197–204. doi: 10.1124/jpet.302.1.197. [DOI] [PubMed] [Google Scholar]
- 31.Moaddel R, Bullock PI, Wainer IW. J Chromatogr B. 2004;799:255–63. doi: 10.1016/j.jchromb.2003.10.054. [DOI] [PubMed] [Google Scholar]
- 32.Moaddel R, Hamid R, Patel S, Wainer IW, Bullock P. Anal Chimica Acta. 2006;578:25–30. doi: 10.1016/j.aca.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Moaddel R, Oliveira RV, Kimura T, Hyppolite P, Juhaszova M, Xiao Y, Kellar KJ, Bernier M, Wainer IW. Anal Chem. 2008;80:48–54. doi: 10.1021/ac701943b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhatia P, Bernier M, Sanghvi M, Moaddel R, Schwarting R, Ramamoorthy A, Wainer IW. Xenobiotica. 2012;42:748–755. doi: 10.3109/00498254.2012.662726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Habicht KL, Frazier C, Singh N, Shimmo R, Wainer IW, Moaddel R. J Pharm Biomed Anal. 2013;72:159–62. doi: 10.1016/j.jpba.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]