Abstract
Production of the human cathelicidin antimicrobial peptide gene (hCAP18/LL-37), is regulated by 1α,25-dihydroxyvitamin D3 (1,25D3) and is critical in the killing of pathogens by innate immune cells. In addition, secreted LL-37 binds extracellular receptors and modulates the recruitment and activity of both innate and adaptive immune cells. Evidence suggests that during infections activated immune cells locally produce increased levels of 1,25D3 thus increasing production of hCAP18/LL-37. The relative expression levels of hCAP18/LL-37 among different immune cell types are not well characterized. The aim of this study was to determine the relative levels of hCAP18/LL-37 in human peripheral blood immune cells and determine to what extent 1,25D3 increased its expression in peripheral blood-derived cells. We show for the first time, a hierarchy of expression of hCAP18 in freshly isolated cells with low levels in lymphocytes, intermediate levels in monocytes and the highest levels found in neutrophils. In peripheral blood-derived cells, the highest levels of hCAP18 following treatment with 1,25D3 were in macrophages, while comparatively lower levels were found in GM-CSF-derived dendritic cells and osteoclasts. We also tested whether treatment with parathyroid hormone in combination with 1,25D3 would enhance hCAP18 induction as has been reported in skin cells, but we did not find enhancement in any immune cells tested. Our results indicate that hCAP18 is expressed at different levels according to cell type and lineage. Furthermore, potent induction of hCAP18 by 1,25D3 in macrophages and dendritic cells may modulate functions of both innate and adaptive immune cells at sites of infection.
Keywords: Dendritic cells, hCAP18, LL-37, Osteoclasts, Parathyroid hormone, Macrophage
1. Introduction
The cathelicidin antimicrobial peptide (CAMP) gene encodes an 18-kDa pro-protein (hCAP18) that is processed to release a peptide, LL-37, that is essential for direct killing of pathogens by professional phagocytes such as neutrophils and macrophages and in barrier defense of epithelial cells that are exposed to the environment [1–3]. Expression of the CAMP gene is strongly induced in macrophages and epithelia by the vitamin D receptor (VDR) and its ligand 1α,25(OH)2D3 (1,25D3) in humans and primates, but not other mammals [4–7]. Unlike other antimicrobial peptide genes that are induced directly during infection and inflammation by NFκB, the CAMP gene is induced by the vitamin D receptor and its ligand 1α,25-dihydroxyvitamin D3 (1,25D3) which are up-regulated via toll-like receptor (TLR)-signaling [8]. Inadequate serum levels of 25-hydroxyvitamin D3 prevent induction of CAMP gene expression by macrophages [8,9] and thus, vitamin D status in humans has direct links to antimicrobial functions in innate immune cells.
The LL-37 peptide is a chemoattractant for T-cells, dendritic cells, neutrophils, and monocytes [10–12], which allows LL-37 to influence cellular traffic at sites of infection or inflammation. LL-37 affects dendritic cell activation and subsequent priming of T-cells when added exogenously [13], demonstrating that LL-37 may also regulate adaptive immune responses, however expression of hCAP18 by dendritic cells has not been well characterized. The presence of hCAP18 has been confirmed in α T cells, B cells, monocytes and NK cells of the peripheral blood by immunohistochemistry and fluorescent microscopy [12], but this approach does not allow for relative quantitative comparisons of levels between different cells types. Identifying which immune cells have high or low baseline levels of hCAP18 and identifying those cells that express higher protein levels in response to 1,25D3 is essential to understanding the cellular sources of hCAP18 that may shape the immune response. This information will provide a better understanding of local effects in specific tissues where 1,25D3 may be produced and induce hCAP18 expression [14]. To determine the relative levels of hCAP18 in different primary immune cell types, we measured it by intracellular staining and flow cytometry in peripheral blood mononuclear cells (PBMC) and neutrophils. Furthermore, we determined the level of induction of CAMP mRNA and hCAP18 in peripheral blood-derived monocytes, macrophages, dendritic cells and osteoclasts in response to treatment with 1,25D3. Parathyroid hormone (PTH) has recently been demonstrated to increase CAMP mRNA expression in human skin cells [15], but the effect of PTH on CAMP expression in immune cells is unknown. We tested whether PTH alone or in combination with 1,25D3 could enhance CAMP expression in macrophages, dendritic cells and osteoclasts.
2. Materials and methods
2.1. Isolation of peripheral blood mononuclear cells
All work with human participants was conducted in accordance with the Declaration of Helsinki. The experiments were conducted with the informed consent of each volunteer participant and approved by the Institutional Review Board of Oregon State University. Human blood was collected into heparinized tubes and diluted with PBS and layered over a density gradient (Lympho-prep, Mediatech Inc., Manassas, VA USA) to separate mononuclear cells. PBMC were washed in PBS and used for either flow cytometry or cell culture. To obtain neutrophils, the red blood cell and granulocyte rich pellet from the density gradient was diluted and incubated with dextran sulfate for 1 h to sediment the red blood cells. The neutrophil rich supernatant was washed with PBS and used for flow cytometry.
2.2. Cell culture
Human PBMC were maintained in RPMI 1640 (Mediatech Inc.) supplemented with 10% (v/v) heat-inactivated FBS, 2 mM L-glutamine, and 1% Pen/Strep (Invitrogen Corporation, Carlsbad, CA, USA). Cell cultures were incubated at 37 °C in a humidified 5% CO2 incubator. To derive macrophages from PBMC, cells were cultured in RPMI as above with human M-CSF (25 ng/ml; Prospec Bio, Rehovet, Israel) for 8 to 9 days. To derive dendritic cells, PBMC were cultured in RPMI containing human GM-CSF (25 ng/ml) and IL-4 (20 ng/ml; Prospec Bio) with 50 μm 2-mercaptoethanol for 8 to 9 days [16]. To derive osteoclasts, we used a modified protocol based on expanding the monocyte/macrophage population first and then inducing osteoclast differentiation. PBMC were cultured in MEMα with human M-CSF (25 ng/ml) for 7 days followed with human RANKL (50 ng/ml) and M-CSF for an additional 14 days [17]. The stock solution of 1,25D3 (1 mM) was diluted in ethanol and subsequently diluted in culture media to a concentration of 10 nM. The human parathyroid hormone 1-34 fragment (Genscript, Piscataway, NJ, USA) was diluted in culture media to concentrations of 10−8 M and 10−11 M for use in cell stimulation experiments.
2.3. Flow cytometry
Cells were stained with conjugated antibodies to lineage markers in PBS with 2% FBS at 4 °C. Following staining, the cells were washed and then fixed, permeabilized and blocked using the eBio-science Fixation and Permeabilization Kit as described by the manufacturer (eBioscience, Inc., San Diego, CA, USA). Cells were incubated with an anti-hCAP18 rabbit polyclonal antibody [18] or a rabbit IgG control. A secondary Dylight 649 Fab′2 donkey anti-rabbit antibody (Jackson Immunoresearch, Pike West Grove, PA, USA) was used to detect the rabbit polyclonal. Fluorescence activated cell sorting (FACS) was performed on a BD FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA) and the results were analyzed by BD CellQuest™ Pro software (BD Biosciences). Antibody against CD14 was purchased from Genetex (Irvine, CA, USA) while all other lineage marker antibodies were from eBioscience.
2.4. Quantitative reverse transcriptase PCR (qRT-PCR)
PCR reactions were set up as described previously [19]. PCR was performed on a Bio-Rad CFX-96 QPCR system (Bio-Rad Laboratories, Hercules, CA, USA). Target gene expression was normalized to either 18S rRNA or β-actin. Primers for human CD14 were as follows: forward 5′-agcctagacctcagccacaa-3′ and reverse 5′-cttggctggcagtcctttag-3′. Primers and probe for the calcitonin receptor (CALCR) were obtained from Integrated DNA Technologies (Coralville, Iowa, USA).
2.5. Measurement of serum 25-hydroxyvitamin D
Blood drawn from donors was collected and stored overnight at 4 °C. Serum was removed and stored at −80 °C and was sent for clinical testing at ZRT Laboratories (Beaverton, OR, USA) to determine total serum 25-hydroxyvitamin D levels.
2.6. Statistical analysis
Statistical analysis was performed using GraphPad Prism version 5 software (GraphPad Software, La Jolla, CA, USA). T-test and one-way ANOVA with Tukey’s multiple comparison post-test were used for determination of significance.
3. Results
3.1. Order of hCAP18 expression in freshly isolated human PBMC
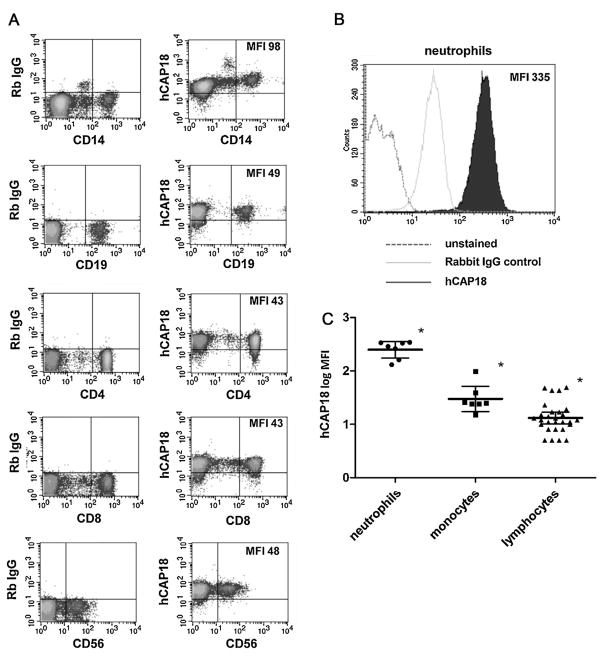
To measure the expression of hCAP18 in PBMC, we prepared cells from freshly drawn blood using standard gradient centrifugation techniques. The PBMC were then stained with primary antibodies to surface lineage markers to identify monocytes (CD14), B-cells (CD19), T-cells (CD4 and CD8), and natural killer (NK) cells (CD56). Intracellular staining for hCAP18 was performed using a polyclonal antibody that recognizes the precursor and the mature LL-37 peptide and cells were analyzed by two-color flow cytometry to measure expression. We refer to the expression of both the precursor and LL-37 peptide as hCAP18 expression in Section 3. Expression of hCAP18 was detected in all cell types examined (Fig. 1A). By comparing the average mean fluorescence intensity (MFI) of hCAP18 staining, a general hierarchy in terms of levels of expression was observed. Within PBMC, monocytes had the highest expression with an MFI of 98, followed by B and T lymphocytes and NK cells with about 50% less expression (MFIs ranging from 49 to 43, Fig. 1A). The expression levels of hCAP18 between the different lymphocyte types were very similar for CD4+ and CD8+ T-cells and CD19+ B-cells and NK cells.
Fig. 1. Expression of hCAP18 in freshly isolated human PBMC and neutrophils.
(A) Freshly isolated human PBMCs were stained for lineage markers and hCAP18 and analyzed by two-color flow cytometry. The left column of the FACS density plots shows staining with a control rabbit IgG and lineage markers, while the right column shows the corresponding hCAP18 expression with the same lineage marker. (B) Human neutrophils stained for hCAP18 compared to a control rabbit IgG and unstained cells in a FACS histogram. In both A and B, the mean fluorescence intensity (MFI) for hCAP18 is noted in the upper right of the FACS plots. The plots are from one donor, representative of seven donors tested. (C) Scatter plot of hCAP18 log MFI for seven donors grouped by cell lineage. In each cell group, the central line represents the mean MFI, while the error bars represent the 95% confidence interval as determined by ANOVA. Asterisks denote that all cell lineage groups were statistically different from one another by ANOVA with Tukey’s Multiple comparison test (p < .05).
Neutrophils from the peripheral blood showed a slightly higher background staining with rabbit IgG as compared to PBMC (Fig. 1B) which was observed before by others [20]. By subtracting the background staining, we could compare the MFIs for hCAP18 in neutrophils and PBMC. Expression of hCAP18 in neutrophils (average MFI of 300) was about three times higher than in monocytes and six times higher than lymphocytes and NK cells (Fig. 1B). This level of expression is consistent with previous studies showing high-level expression of hCAP18 in neutrophils [20,21].
We tested a total of seven donors by flow cytometry for the expression of hCAP18 using lineage markers in PBMC and in neutrophils. We also measured serum levels of 25-hydroxyvitamin D in the donors to ensure that all donors were sufficient for vitamin D. Serum levels of 25-hydroxyvitamin D ranged from 29 to 46 ng/ml, with an average of 33 ng/ml, confirming that all donors were sufficient for vitamin D (data not shown). We observed the same hierarchy of hCAP18 expression in all of the individual donors with neutrophils having the highest hCAP18 expression, monocytes having intermediate expression, and lymphocytes having the lowest expression (Fig. 1C). There was some variability between different donors as to the precise MFI for each of the three major groups of cells; neutrophils, monoctyes, and lymphocytes, yet the groups were significantly different when compared by ANOVA with Tukey’s Multiple comparison test (Fig. 1C). Therefore, we conclude that in healthy adults with sufficient vitamin D, hCAP18 does follow a hierarchy of expression based on cell type.
3.2. 1,25D3 induction of hCAP18 in PBMC
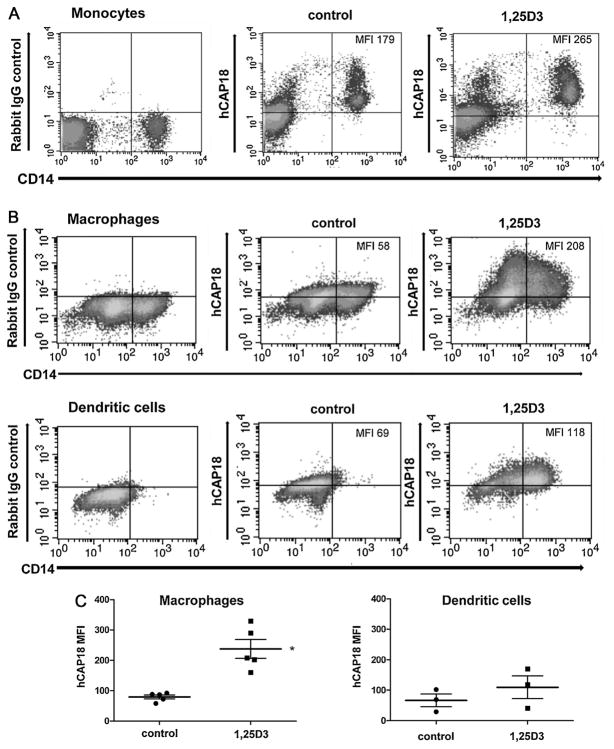
The CAMP gene is induced by 1,25D3 in a number of cell types including monocytes, but has not yet been determined for primary lymphocytes [4–6]. We examined how PBMC in culture responded to treatment with 1,25D3 by measuring hCAP18 induction and CD14 surface expression. PBMC were cultured for 24 h with and without 10 nM 1,25D3 and were then stained for surface lineage markers and hCAP18. We chose 10 nM 1,25D3 as the dose for treatment since this concentration is close to what human macrophages have been reported to synthesize in culture (5 nM) and may reflect the range of concentrations that can be generated endogenously or in localized tissue areas [22]. After 24 h of treatment with 1,25 D3, monocytes showed a clear increase in both CD14 surface expression and in hCAP18 expression (Fig. 2A). Control monocytes had an MFI of 179 for hCAP18 expression and the MFI for monocytes treated with 1,25D3 was 265, a 1.5 fold increase over the control. CD14 expression is also regulated by 1,25D3 [23], thus serving as a second measure for 1,25D3 responsiveness in monocytes. CD14 surface expression for the control had an MFI of 533, which increased 2.5 fold to an MFI of 1350 with 1,25D3 treatment. We did not detect any significant changes in hCAP18 protein in CD3+ T-cells or CD19+ B-cells under these conditions (data not shown).
Fig. 2. Induction of hCAP18 expression by 1,25D3 treatment in monocytes, macrophages and dendritic cells.
(A) Monocytes were cultured for 24 h in control media or media with 10 nM 1,25D3 and then stained for both CD14and hCAP18 or control rabbit IgG. The MFI for hCAP18 is noted in the upper right corner of the FACS density plots. (B) Macrophages and dendritic cells were treated with vehicle or with 10 nM 1,25D3 for 48 h and then stained for both CD14 and hCAP18 expression and shown as density plots. The total MFI for hCAP18 is noted in the upper right corner of the density plots. The plots are representative of 3 donors tested. (C) Comparison of hCAP18 MFI between macrophages and dendritic cells by scatter plot from 5 experiments with macrophages and 3 with dendritic cells treated as in part B. In each group, the central line represents the mean, while the error bars depict the standard error, and the asterisk denotes that induction was statistically significant in macrophages as compared to control and to the dendritic cell 1,25D3 treatment group (p < .05).
3.3. 1,25D3 induction of hCAP18 in monocyte derived macrophages and dendritic cells
1,25D3 induces CAMP gene expression in macrophages derived from primary monocytes in culture using defined growth factors [5], but induction in dendritic cells has not been determined. Monocytes from PBMC fractions were cultured with recombinant M-CSF for 8 to 9 days to allow differentiation to a macrophage phenotype, or GM-CSF + IL-4 for 10 days to allow differentiation to a myeloid dendritic cell phenotype. During the last 48 h of culture, cells were treated with vehicle control or 10 nM 1,25D3 and then harvested for analysis by flow cytometry and parallel isolation of RNA. For two-color flow cytometry, the cells were surface stained for CD14, and then fixed, permeabilized and stained intracellularly for hCAP18 or rabbit IgG control.
Macrophages differentiated for 9 days and treated for 48 h with 1,25D3 showed a 4-fold increase in MFI (58 to 208) for hCAP18 (Fig. 2B). Analysis of the two-color density plots by quadrants showed that an increase in hCAP18 levels occurred in 30% of the cells (Fig. 2B). Macrophages showed two distinct populations of cells (Fig. 2B), one with low CD14 expression (MFI of 50) and the second with high expression (MFI of 423). Although hCAP18 expression increased in both CD14 populations, the percentages of cells expressing low CD14 and high CD14 did not change significantly with 1,25D3 treatment. The slight change in CD14 expression in macrophages upon treatment with 1,25D3 differs from what was observed in monocytes cultured for 24 h in which CD14 surface expression increased 2.5-fold.
In dendritic cells, CD14 staining identified a single population of cells with a MFI of 40 in the control that increased almost 5-fold to an MFI of 192 in the 1,25D3 treated cells (Fig. 2B). Expression of hCAP18 increased 1.7-fold in 1,25D3 treated cells (MFI of 118) as compared to the control (MFI of 69; Fig. 2B). Analysis of the two-color density plots by quadrants revealed that 51% of cells increased expression of both CD14 and hCAP18 as shown by the increased density of cells in the upper right quadrant of Fig. 2B. A comparison of the different cell types showed that baseline expression levels of hCAP18 were relatively similar between macrophages and dendritic cells (Fig. 2B and C). Nevertheless, the induction of hCAP18 expression following treatment with 1,25D3 differed among the cell types, with macrophages showing higher levels than the DCs. We confirmed this trend in other replicate experiments using different donors. We compared the hCAP18 MFI from five experiments with macrophages treated with 1,25D3 and three experiments with dendritic cells treated with 1,25D3 (Fig. 2C). The fold change in expression of hCAP18 was significantly higher in macrophages as compared to dendritic cells (Fig. 2C). CD14 expression also differed among the cell types, with macrophages having populations of low and high expression, while dendritic cells had one low expressing population. Following treatment with 1,25D3, dendritic cells showed a 5-fold increase in surface CD14 expression, while macrophages had less than 1.6-fold increase in CD14 (Fig. 2B).
3.4. Induction of CAMP mRNA in macrophages and dendritic cells by 1,25D3
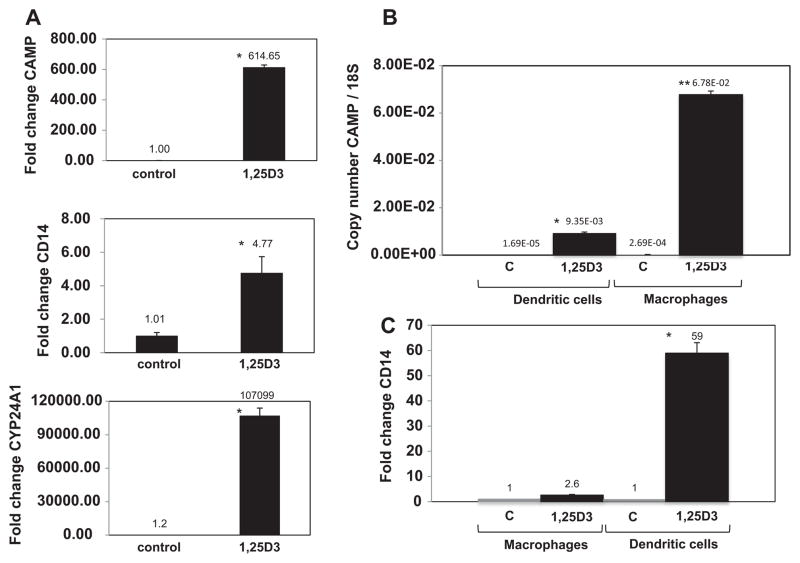
M-CSF-derived macrophages were treated with 1,25D3 for 24 h and compared to untreated cells. We observed a 615-fold increase in CAMP mRNA expression (Fig. 3A) and a 5-fold increase in CD14 mRNA expression. The well-characterized VDR target gene CYP24A1 increased 100,000-fold (Fig. 3A).
Fig. 3. Expression of CAMP, CD14 and CYP24A1 mRNA in response to treatment with 1,25D3.
(A) Macrophages were treated with vehicle (ethanol) or 1,25D3 for 24 h. Total RNA was harvested and cDNAs generated for use in qRT-PCR. The data were normalized to β-actin and expressed as fold change versus the control treatment. (B) Dendritic cells and macrophages were treated with vehicle or 10 nM 1,25D3 for 48 h. CAMP mRNA levels for different cell types were determined by qRT-PCR and normalized to ribosomal 18S RNA using copy number standards. Data shown are from a single experiment and representative of four experimental replicates. (C) CD14 mRNA expression for samples described in panel B. CD14 levels were normalized to β-actin and are expressed as fold-change versus the vehicle control. Asterisks denote significance between 1,25D3 and controls, while two asterisks denote significant difference between the 1,25D3 treatment groups (p < .05).
We next analyzed macrophages and dendritic cells stimulated for 48 h with 1,25D3 for CAMP mRNA induction. In untreated cells, dendritic cells had lower CAMP transcript levels, while macrophages had 16-fold more (Fig. 3B). When the two cell types were treated with 1,25D3 for 48 h, macrophages had 7-fold higher transcript levels than the dendritic cells which was significant by ANOVA. (Fig. 3B). These results are consistent with the hCAP18 protein induction observed by flow cytometry following 1,25D3 treatment. The relative order of the mRNA increase matches the rank of protein expression, with dendritic cells showing lower levels and macrophages showing higher levels.
We further analyzed the mRNA expression of CD14 and CYP24A1 in macrophages and dendritic cells. Macrophages treated with 1,25D3 for 48 h showed a modest increase in CD14 mRNA with 2.6-fold change (Fig. 3C). This result was consistent with the slight induction of CD14 protein detected by flow cytometry in macrophages treated with 1,25D3. Dendritic cells showed a stronger increase in CD14 mRNA with a 59-fold increase following treatment with 1,25D3 for 48 h (Fig. 3C), again matching the protein induction observed for CD14 which was 5-fold for the entire population of cells (Fig. 2B). A wide dynamic range of CYP24A1 mRNA induction was observed in both cell types in response to 1,25D3 (data not shown).
3.5. Vitamin D induction of hCAP18 in monocyte derived osteoclasts and the effects of PTH on hCAP18 induction
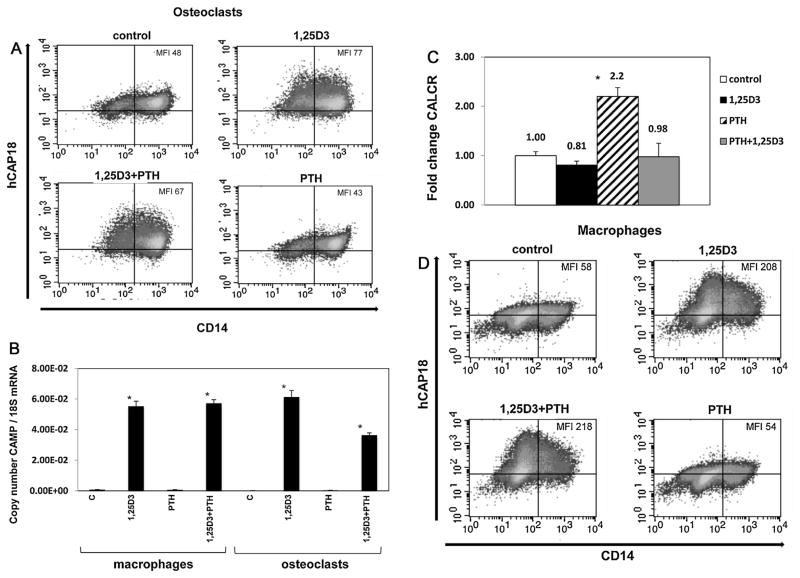
Osteoclasts are bone-remodeling cells of immune origin. Currently it is unknown whether human osteoclasts express CAMP or if 1,25D3 treatment would induce expression of CAMP. Osteoclasts are targets of vitamin D action in calcium metabolism, making these cells a logical target to examine for CAMP expression. Osteoclasts were derived from monocytes cultured with M-CSF and RANKL for 21 days. To test for induction, they were treated with vehicle control or 10 nm 1,25D3 for 48 h. The majority of cells expressed high levels of CD14 and both low and high CD14+ populations expressed hCAP18 (Fig. 4A). Following 1,25D3 treatment, the low CD14+ population showed a 2.3-fold increase of hCAP18, while the high CD14+ population showed a 1.4-fold increase in hCAP18 levels (Fig. 4A). CD14 expression did not change significantly in either population, which was similar to that seen in more mature macrophages (Fig. 2B). The amount of hCAP18 induced in osteoclasts was similar in magnitude to that observed in GM-CSF-derived dendritic cells, but lower than that observed in macrophages.
Fig. 4. Lack of cooperative induction of CAMP gene expression by 1,25D3 and PTH in osteoclasts and macrophages.
(A) Osteoclasts were treated 48 h with combinations of 10 nM 1,25D3 and 10 nM parathyroid hormone (PTH) and then stained for hCAP18 and CD14. FACS density plots show hCAP18 expression on the y-axis and CD14 expression on the x-axis, with the MFI for hCAP18 noted in the upper right hand corner. (B) qRT-PCR of CAMP mRNA expression from osteoclasts and macrophages treated with 1,25D3 and PTH normalized to ribosomal 18S RNA using copy number standards, with the asterisks denoting significance from controls by ANOVA (p < .05). (C) Quantitative PCR measuring CALCR mRNA expression in osteoclasts treated as in panel A. The data are expressed as fold-change normalized to ribosomal 18S RNA relative to the control vehicle treatment, and the asterisk denotes significance by ANOVA versus other treatments (p < .05). (D) Macrophages were treated with combinations of 1,25D3 and PTH for 48 h and then stained for CD14 and hCAP18 expression. FACS density plots for each treatment have the MFI for hCAP18 noted in the upper right corner. Macrophages were also treated with a lower dose of 10−11 M PTH in combination with 1,25D3 as in part D (Supplemental Fig. 1). GM-CSF derived dendritic cells were also tested for responses to PTH and 1,25D3 (Supplemental Fig. 2).
Parathyroid hormone (PTH) is involved in regulating calcium metabolism and influences bone remodeling. It was recently reported that PTH in combination with 1,25D3 increased CAMP gene transcription in human skin cells [15]. To determine if PTH and 1,25D3 increased hCAP18 expression in osteoclasts and macrophages, we treated monocyte-derived osteoclasts with or without PTH in combination with 1,25D3 to test for cooperative induction. Measuring protein levels by flow cytometry, we observed a 1.6-fold (MFI 48 to 77) increase in hCAP18 protein after 1,25D3 treatment. Treatment with 10 nM PTH alone for 48 h had no effect on hCAP18 protein levels. When osteoclasts were treated with both PTH and 1,25D3 for 48 h, the MFI increased from 48 to 67, less than the induction observed for 1,25D3 alone (Fig. 4A). Treatment with 1,25D3 alone induced CAMP at the transcript level, while treatment with only PTH had no effect on CAMP gene expression (Fig. 4B). Combining PTH and 1,25D3 resulted in lower levels of transcript as compared with 1,25D3 alone (Fig. 4B). Taken together, we conclude that in osteoclasts the combination of PTH and 1,25D3 does not result in enhanced expression of CAMP.
As a positive control for PTH activity, we examined expression of a known PTH target gene in osteoclasts, the calcitonin receptor (CALCR). CALCR is part of a feedback circuit induced by PTH that limits the bone resorbing activity of activated osteoclasts once calcium levels have increased [24]. We detected CALCR in resting osteoclasts, and treatment with PTH alone led to a significant 2.2-fold increase in CALCR mRNA. Treatment with 1,25D3 led to a slight decrease in CALCR mRNA, while the combination of PTH with 1,25D3 was similar to the baseline mRNA level (Fig. 4C). Taken together, the data demonstrate that osteoclasts responded to PTH at the dose used in these experiments.
In similar experiments with macrophages, we observed a 3.6-fold increase in hCAP18 levels (MFI from 58 to 208) with 1,25D3 treatment. Treatment with PTH alone had no effect on hCAP18 protein, while treatment with both PTH and 1,25D3 resulted in a 3.7-fold increase in hCAP18 levels, comparable to that observed with 1,25D3 alone (Fig. 4D). At the transcript level, PTH had no effect on CAMP mRNA expression with or without 1,25D3 (Fig. 4B). A lower dose of PTH, 10−11 M, in combination with 1,25D3 did not affect CAMP induction in macrophages (Supplemental Fig. 1). We do not believe that 10 nM 1,25D3 was masking the potential of PTH to increase hCAP18, since in skin cells synergy was observed at 100 nM 1,25D3 in combination with 10−11 M PTH [15]. We have also tested 1,25D3 at several concentrations for induction of hCAP18 in macrophages and have found that 10 nM 1,25D3 is not a maximal dose for these cells (data not shown). In addition, PTH had no effect on hCAP18 expression in DCs when given alone or in combination with 1,25D3 (Supplemental Fig. 2). Taken together, these data indicate that PTH does not affect expression of the CAMP gene in cells of myeloid origin as it does in skin cells.
4. Discussion
4.1. Expression of hCAP18 in immune system cells
Expression of the CAMP gene is essential for innate immune defense against many pathogens including bacteria, viruses, and fungi [1]. In humans, CAMP expression was initially described in bone marrow and neutrophils where the protein is stored in specific granules [25,26]. Neutrophils constitutively express high levels of hCAP18 (630 μg per 109 cells) [18]. CAMP also is expressed by other immune cells and by epithelial cells of the skin, gut, urinary tract and mucous membranes where it helps provide a barrier to infection at these sites [27–30]. Immunofluorescent microscopy showed that hCAP18 is present in peripheral blood monocytes, B-lymphocytes, NK cells, tonsillar dendritic cells [12] and γδ T-lymphocytes express hCAP18 as well as macrophages and lymphocytes from bronchiolar lavage samples [12,31]. Nevertheless, studies quantitating hCAP18 levels are lacking and knowledge of differences in hCAP18 expression levels between these cells types is still unknown. This is the first study using intracellular staining and flow cytometry to more quantitatively compare hCAP18 expression in freshly isolated PBMC and neutrophils. We found a hierarchy of relative protein expression, with neutrophils showing the highest levels, monocytes intermediate levels, and lymphocytes the lowest levels. In the lymphocyte population, B-cells, NK cells, CD4+ T-cells and CD8+ T-cells had similar levels of hCAP18 expression.
Monocytes, macrophages, dendritic cells and osteoclasts all have roles in the innate immune response, while dendritic cells also shape the adaptive immune response. In vivo, all of these cell types locally produce 1,25D3 through the actions of the CYP27B1 enzyme expressed in these cell types [32,33]. Toll-like receptor (TLR) signal pathways in immune cells are activated when they encounter pathogens leading to an increase in CYP27B1 and 1,25D3 production when sufficient 25(OH)D3 levels are present [8]. Thus, the effect of 1,25D3 on these cells may be due to systemic sources or locally from autocrine or paracrine sources, both of which may influence CAMP expression. Identifying which immune cells efficiently induce CAMP in response to 1,25D3 is an important first step in understanding local tissue responses influenced by vitamin D and hCAP18/LL37. 1,25D3 induction of CAMP gene expression in dendritic cells and osteoclasts has not yet been studied. We compared induction of hCAP18 in monocytes, macrophages, dendritic cells, and osteoclasts after 1,25D3 treatment. The highest levels of hCAP18 following treatment with 1,25D3 were seen in macrophages and monocytes, while lower levels were observed in osteoclasts and dendritic cells.
4.2. Potential roles for hCAP18 in dendritic cells
CAMP modulation of the immune response and its influence on the adaptive immune response has been described for both human cells and in mouse models. LL-37 is a chemotactic factor for T-cells, monocytes, dendritic cells, neutrophils, and mast cells [11,12,34]. In vitro, dendritic cell development is altered with treatment of exogenous LL-37 resulting in increased T-cell co-stimulation and an enhanced Th-1 response [13]. The Th-1 promoting effect of LL-37 was confirmed in a knockout model for mouse cathelicidin (CRAMP) as these mice displayed increased Th2 T-cell responses [35]. Upon maturation, human dendritic cells increase expression of CYP27B1 and interaction of T-cells with dendritic cells through CD40 and CD40L strongly induces CYP27B1 in dendritic cells [36]. Taken in context with our finding that GM-CSF-derived dendritic cells induce hCAP18 expression in response to 1,25D3, it is likely that endogenously produced LL-37 by dendritic cells may also act on T-cells or other immune cells and modulate local immune responses. Possible outcomes include increased chemotaxis of T-cells toward LL-37 producing dendritic cells and influences on T-helper cell development toward a Th-1 profile. Several studies have shown that 1,25D3 treatment of human dendritic cells generally leads to a more anti-inflammatory tolerogenic condition in which co-stimulatory ligands for T-cell activation and inflammatory cytokines such as IFN-γ, and IL-17 are reduced [36–38]. Dendritic cell maturation is a complex series of events with inflammatory cytokines, TLR signals and vitamin D status all shaping the outcome. A study using global gene pro-filing in human monocyte-derived dendritic cells suggests that, independently of the inflammatory environment, 1,25D3 regulates approximately 200 genes, including CAMP [39]. Whether CAMP acts as a pro-inflammatory factor or tolerogenic factor during adaptive immunity may depend on the context of other immune signals.
The levels of CAMP expression in specific tissues may alter adaptive immune responses as suggested by the observation that low levels of CAMP are seen in atopic dermatitis patients [40], whereas CAMP is present at abnormally high levels in patients with psoriasis [27,41]. During wound healing in the skin plasmacytoid dendritic cells are recruited and produce Type1 interferons that are needed for the initial inflammatory reaction and to promote subsequent healing [42]. At this time the expression of CAMP increases locally, coinciding with the recruitment of plasmacytoid dendritic cells to the wound site. In mouse models, injection of CRAMP into skin causes increased expression of Type 1 interferons [42]. Thus CAMP has a normal role in skin healing that may involve recruitment of plasmacytoid dendritic cells and the subsequent activation of these cells responding to cellular damage [43]. When CAMP expression is dysregulated, as in psoriasis, increased Type 1 interferon production appears to lead to the development of autoimmune T-cells [42]. Precisely how hCAP18 or LL-37 influences T-cell activation and dendritic cell function is not well understood, but it may alter cytokine profiles or the type of dendritic cells that are recruited to the local tissue.
4.3. Lack of cooperative induction of CAMP by PTH and 1,25D3 in myeloid origin cells
Increased CAMP expression has been seen in human skin cells when both 1,25D3 and PTH, a hormone involved in calcium metabolism, are combined in vitro [15]; therefore, we determined if PTH could cooperatively induce CAMP expression with 1,25D3 in osteoclasts and macrophages. This synergy is proposed to offer an alternative pathway for CAMP expression in the skin during vitamin D deficiency, when PTH may be increased. Osteoclasts are cells of myeloid origin and express PTH type 1 receptors [44], making them a logical choice to test this synergy since osteoclasts are also responsive to 1,25D3. Although osteoclasts do increase CAMP transcription and hCAP18 protein following treatment with 1,25D3, they do not show enhanced expression when both PTH and 1,25D3 are present. We were able to show an increase in CALCR mRNA with PTH treatment, confirming that the cells could indeed respond to PTH. We extended this observation further by measuring CAMP expression at both transcript and protein level in macrophages and DCs treated with PTH and again did not see any cooperative effects of PTH with 1,25D3. Thus, it is likely that in cells of myeloid origin, PTH does not act in synergy with 1,25D3 as it does in keratinocytes [15].
4.4. Potential roles of hCAP18 expression in osteoclasts
Increased expression of CAMP in response to 1,25D3 in osteoclasts is an intriguing finding because bone is an important source of 1,25D3 production. Osteoclasts express CYP27B1 and thus are likely to regulate CAMP expression in both autocrine and paracrine manners since other bone resident cells such as osteoblasts also produce 1,25D3 [45]. LL-37 has been shown to increase the chemotactic response of hematopoietic stem cells to SDF-1 during bone marrow transplantation [46]. The bone remodeling function of osteoclasts is necessary to preserve and create available niches in the marrow compartment for hematopoietic stem cells [47]. It is possible that cycles of osteoclast remodeling activity followed by 1,25D3 production may enable osteoclasts to couple the creation of new niches with chemotactic signals mediated by LL-37 and other bone marrow derived factors. Other roles for LL-37 in bone are not well defined in vivo, but in vitro differentiation of osteoclasts appears to be reduced by the addition of exogenous LL-37, suggesting additional functions for CAMP expression [48]. A recent study using an induced model of arthritis in rats found increased expression of rCRAMP, the rat homolog to hCAP18, in macrophages and osteoclasts in inflamed joints [49]. However, unlike the human gene, the rCRAMP gene is not directly regulated by 1,25D3 [5,7] so the mechanism of increased expression likely differs.
In summary, our data comparing the baseline expression of CAMP and the induced response to 1,25D3 provides a basis to understand the cell type specific role of CAMP expression in local tissue sites. Quantitative data showing which cell types increase CAMP expression in response to vitamin D improves our understanding of how CAMP shapes both the innate and adaptive immune responses.
Supplementary Material
Acknowledgments
This work was supported by the US National Institutes of Health grant (5R01AI065604) to A.F.G. We thank Mary L. Fantacone (Linus Pauling Institute, Oregon State University, Corvallis, OR USA) for critically reading the manuscript and providing insightful comments.
Abbreviations
- 1,25D3
1α,25-dihydroxyvitamin D3
- hCAP18
human 18-kDa cationic antimicrobial protein
- LL-37
37 amino acid cathelicidin antimicrobial peptide
- GM-CSF
granulocyte macrophage colony stimulating factor
- CAMP
cathelicidin antimicrobial peptide
- VDR
vitamin D receptor
- NFκB
nuclear factor kappa-B
- TLR
toll-like receptor
- PBMC
peripheral blood mononuclear cell
- PTH
parathyroid hormone
- PBS
phosphate buffered saline
- M-CSF
macrophage CSF
- RANKL
receptor activator of NFκB ligand
- qRT-PCR
quantitative reverse transcriptase polymerase chain reaction
- CALCR
calcitonin receptor
- NK
natural killer
- MFI
mean fluorescence intensity
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jsbmb.2014.02.004.
Footnotes
Authorship
M.B.L. and A.F.G. were responsible for experimental design and data analysis. M.B.L. and C.G. were responsible for performing experiments and data collection. M.B.L., N.B. and A.F.G. were involved in intellectual input and manuscript preparation.
Conflict of interest
The authors have no financial conflicts of interest.
References
- 1.Lehrer RI, Ganz T. Cathelicidins: a family of endogenous antimicrobial peptides. Curr Opin Hematol. 2002;9:18–22. doi: 10.1097/00062752-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Gombart AF. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4:1151–1165. doi: 10.2217/fmb.09.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White JH. Vitamin D as an inducer of cathelicidin antimicrobial peptide expression: past, present and future. J Steroid Biochem Mol Biol. 2010;121:234–238. doi: 10.1016/j.jsbmb.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 4.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 5.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 6.Weber G, Heilborn JD, Chamorro Jimenez CI, Hammarsjo A, Torma H, et al. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J Invest Dermatol. 2005;124:1080–1082. doi: 10.1111/j.0022-202X.2005.23687.x. [DOI] [PubMed] [Google Scholar]
- 7.Gombart AF, Saito T, Koeffler HP. Exaptation of an ancient Alu short interspersed element provides a highly conserved vitamin D-mediated innate immune response in humans and primates. BMC Genomics. 2009;10:321. doi: 10.1186/1471-2164-10-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 9.Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, et al. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009;182:4289–4295. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chertov O, Michiel DF, Xu L, Wang JM, Tani K, et al. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J Biol Chem. 1996;271:2935–2940. doi: 10.1074/jbc.271.6.2935. [DOI] [PubMed] [Google Scholar]
- 11.De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agerberth B, Charo J, Werr J, Olsson B, Idali F, et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–3093. [PubMed] [Google Scholar]
- 13.Davidson DJ, Currie AJ, Reid GS, Bowdish DM, MacDonald KL, et al. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J Immunol. 2004;172:1146–1156. doi: 10.4049/jimmunol.172.2.1146. [DOI] [PubMed] [Google Scholar]
- 14.Hewison M. Vitamin D and the immune system: new perspectives on an old theme. Endocrinol Metab Clin North Am. 2010;39:365–379. doi: 10.1016/j.ecl.2010.02.010. (table of contents) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muehleisen B, Bikle DD, Aguilera C, Burton DW, Sen GL, et al. PTH/PTHrP and vitamin D control antimicrobial peptide expression and susceptibility to bacterial skin infection. Sci Transl Med. 2012;4:135ra166. doi: 10.1126/scitranslmed.3003759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romani N, Gruner S, Brang D, Kampgen E, Lenz A, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorensen MG, Henriksen K, Schaller S, Henriksen DB, Nielsen FC, et al. Characterization of osteoclasts derived from CD14+ monocytes isolated from peripheral blood. J Bone Miner Metab. 2007;25:36–45. doi: 10.1007/s00774-006-0725-9. [DOI] [PubMed] [Google Scholar]
- 18.Sorensen O, Cowland JB, Askaa J, Borregaard N. An ELISA for hCAP-18, the cathelicidin present in human neutrophils and plasma. J Immunol Methods. 1997;206:53–59. doi: 10.1016/s0022-1759(97)00084-7. [DOI] [PubMed] [Google Scholar]
- 19.Guo C, Rosoha E, Lowry MB, Borregaard N, Gombart AF. Curcumin induces human cathelicidin antimicrobial peptide gene expression through a vitamin D receptor-independent pathway. J Nutr Biochem. 2013;24:754–759. doi: 10.1016/j.jnutbio.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misawa Y, Baba A, Ito S, Tanaka M, Shiohara M. Vitamin D(3) induces expression of human cathelicidin antimicrobial peptide 18 in newborns. Int J Hematol. 2009;90:561–570. doi: 10.1007/s12185-009-0452-9. [DOI] [PubMed] [Google Scholar]
- 21.Sorensen O, Arnljots K, Cowland JB, Bainton DF, Borregaard N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood. 1997;90:2796–2803. [PubMed] [Google Scholar]
- 22.Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, et al. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103:316–321. doi: 10.1016/j.jsbmb.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 23.Bhalla AK, Williams MM, Lal S, Lydyard PM. 1,25-Dihydroxyvitamin D3, but not retinoic acid, induces the differentiation of U937 cells. Clin Exp Immunol. 1989;76:274–277. [PMC free article] [PubMed] [Google Scholar]
- 24.Hoff AO, Catala-Lehnen P, Thomas PM, Priemel M, Rueger JM, et al. Increased bone mass is an unexpected phenotype associated with deletion of the calcitonin gene. J Clin Invest. 2002;110:1849–1857. doi: 10.1172/JCI200214218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larrick JW, Hirata M, Balint RF, Lee J, Zhong J, et al. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995;63:1291–1297. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cowland JB, Johnsen AH, Borregaard N. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett. 1995;368:173–176. doi: 10.1016/0014-5793(95)00634-l. [DOI] [PubMed] [Google Scholar]
- 27.Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M, Liden S, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–15263. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 28.Bals R, Wang X, Zasloff M, Wilson JM. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. PNAS. 1998;95:9541–9546. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 30.Chromek M, Slamova Z, Bergman P, Kovacs L, Podracka L, et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med. 2006;12:636–641. doi: 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- 31.Wah J, Wellek A, Frankenberger M, Unterberger P, Welsch U, et al. Antimicrobial peptides are present in immune and host defense cells of the human respiratory and gastrointestinal tracts. Cell Tissue Res. 2006;324:449–456. doi: 10.1007/s00441-005-0127-7. [DOI] [PubMed] [Google Scholar]
- 32.Hewison M, Gacad MA, Lemire J, Adams JS. Vitamin D as a cytokine and hematopoetic factor. Rev Endocr Metab Disord. 2001;2:217–227. doi: 10.1023/a:1010015013211. [DOI] [PubMed] [Google Scholar]
- 33.Fritsche J, Mondal K, Ehrnsperger A, Andreesen R, Kreutz M. Regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase and production of 1 alpha,25-dihydroxyvitamin D3 by human dendritic cells. Blood. 2003;102:3314–3316. doi: 10.1182/blood-2002-11-3521. [DOI] [PubMed] [Google Scholar]
- 34.Niyonsaba F, Iwabuchi K, Someya A, Hirata M, Matsuda H, et al. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology. 2002;106:20–26. doi: 10.1046/j.1365-2567.2002.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kin NW, Chen Y, Stefanov EK, Gallo RL, Kearney JF. Cathelin-related antimicrobial peptide differentially regulates T- and B-cell function. Eur J Immunol. 2011;41:3006–3016. doi: 10.1002/eji.201141606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeffery LE, Wood AM, Qureshi OS, Hou TZ, Gardner D, et al. Availability of 25-hydroxyvitamin D(3) to APCs controls the balance between regulatory and inflammatory T cell responses. J Immunol. 2012;189:5155–5164. doi: 10.4049/jimmunol.1200786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berer A, Stockl J, Majdic O, Wagner T, Kollars M, et al. 1,25-Dihydroxyvitamin D(3) inhibits dendritic cell differentiation and maturation in vitro. Exp Hematol. 2000;28:575–583. doi: 10.1016/s0301-472x(00)00143-0. [DOI] [PubMed] [Google Scholar]
- 38.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 39.Szeles L, Keresztes G, Torocsik D, Balajthy Z, Krenacs L, et al. 1,25-Dihydroxyvitamin D3 is an autonomous regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotype. J Immunol. 2009;182:2074–2083. doi: 10.4049/jimmunol.0803345. [DOI] [PubMed] [Google Scholar]
- 40.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 41.Yamasaki K, Kanada K, Macleod DT, Borkowski AW, Morizane S, et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol. 2011;131:688–697. doi: 10.1038/jid.2010.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gregorio J, Meller S, Conrad C, Di Nardo A, Homey B, et al. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med. 2010;207:2921–2930. doi: 10.1084/jem.20101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dorschner RA, Pestonjamasp VK, Tamakuwala S, Ohtake T, Rudisill J, et al. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J Invest Dermatol. 2001;117:91–97. doi: 10.1046/j.1523-1747.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- 44.Dempster DW, Hughes-Begos CE, Plavetic-Chee K, Brandao-Burch A, Cosman F, et al. Normal human osteoclasts formed from peripheral blood monocytes express PTH type 1 receptors and are stimulated by PTH in the absence of osteoblasts. J Cell Biochem. 2005;95:139–148. doi: 10.1002/jcb.20388. [DOI] [PubMed] [Google Scholar]
- 45.van Driel M, Koedam M, Buurman CJ, Hewison M, Chiba H, et al. Evidence for auto/paracrine actions of vitamin D in bone: 1alpha-hydroxylase expression and activity in human bone cells. FASEB J. 2006;20:2417–2419. doi: 10.1096/fj.06-6374fje. [DOI] [PubMed] [Google Scholar]
- 46.Wu W, Kim CH, Liu R, Kucia M, Marlicz W, et al. The bone marrow-expressed antimicrobial cationic peptide LL-37 enhances the responsiveness of hematopoietic stem progenitor cells to an SDF-1 gradient and accelerates their engraftment after transplantation. Leukemia. 2012;26:736–745. doi: 10.1038/leu.2011.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mansour A, Abou-Ezzi G, Sitnicka E, Jacobsen SE, Wakkach A, et al. Osteoclasts promote the formation of hematopoietic stem cell niches in the bone marrow. J Exp Med. 2012;209:537–549. doi: 10.1084/jem.20110994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Supanchart C, Thawanaphong S, Makeudom A, Bolscher JG, Nazmi K, et al. The antimicrobial peptide, LL-37, inhibits in vitro osteoclastogenesis. J Dent Res. 2012;91:1071–1077. doi: 10.1177/0022034512460402. [DOI] [PubMed] [Google Scholar]
- 49.Hoffmann MH, Bruns H, Backdahl L, Neregard P, Niederreiter B, et al. The cathelicidins LL-37 and rCRAMP are associated with pathogenic events of arthritis in humans and rats. Ann Rheum Dis. 2013;72:1239–1248. doi: 10.1136/annrheumdis-2012-202218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.