Abstract
Purpose
Changes in cancer therapy, in addition to changes in obesity prevalence, suggest the need for a current assessment of weight gain patterns following breast cancer diagnosis. The aim of this study was to evaluate factors associated with weight gain among breast cancer survivors prior to enrolling into a behavioral weight loss intervention.
Methods
Anthropometric measures and data on weight-related factors were collected at baseline on 665 breast cancer survivors. Postdiagnosis weight gain was determined between entry into the trial and previous diagnosis up to 5 years. Multivariate logistic regression analyses were used to evaluate the association between weight gain and influencing factors.
Results
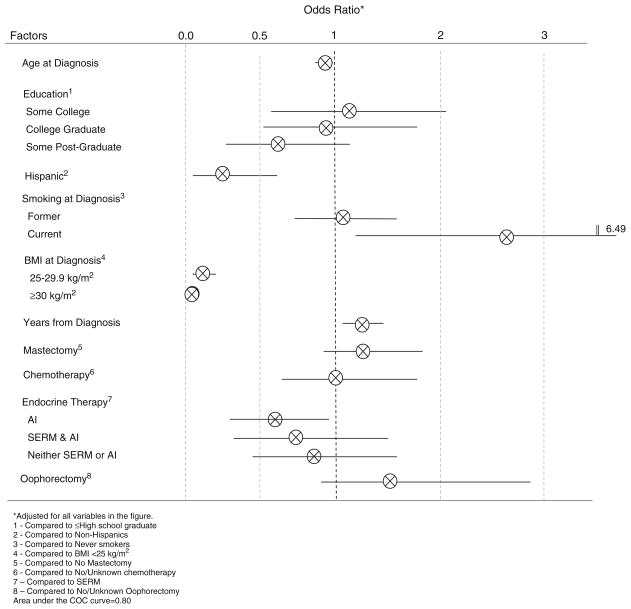
The mean weight gain was 4.5 % body weight (standard deviation=10.6); 44 % of women experienced ≥5 % body weight gain. The risk of weight gain was inversely associated with age (adjusted odds ratio (ORadj)=0.97, 95 % confidence interval (95 % CI) 0.95–0.99), Hispanic ethnicity (ORadj=0.30, 95 % CI 0.13–0.68), and overweight (ORadj= 0.11, 95 % CI 0.05–0.23) or obese (ORadj=0.03, 95 % CI 0.02–0.07) status at diagnosis and positively associated with time elapsed since diagnosis (ORadj=1.19/year, 95 % CI 1.04–1.36). Women prescribed aromatase inhibitors were 46 % less likely to gain weight compared to women prescribed selective estrogen-receptor modulators (ORadj=0.54, 95 % CI 0.31–0.93). The risk of weight gain was positively associated with smoking at diagnosis (ORadj=2.69, 95 % CI 1.12–6.49) although this was attributable to women who subsequently quit smoking.
Conclusions
Postdiagnosis weight gain is common and complex and influenced by age, ethnicity, weight, smoking status, time elapsed since diagnosis, and endocrine-modulating therapy.
Implications for cancer survivors
Weight gain continues to be a concern following a diagnosis of breast cancer. Factors influencing this weight gain include age, ethnicity, weight, smoking status, time elapsed since diagnosis, and endocrine-modulating therapy. Effective weight management strategies are needed for this population of women.
Keywords: Breast cancer survivors, Weight gain, Women
Introduction
Obesity contributes to worse prognosis among women diagnosed with either pre- or postmenopausal breast cancer [1]. A 33 % higher risk for overall mortality, as well as breast cancer specific mortality among women who are obese at the time of diagnosis as compared to normal weight women has been reported in a meta-analysis of 43 studies [1]. Weight gain following breast cancer diagnosis also appears to contribute to worse prognosis, although this finding has not been consistently demonstrated [2–6]. Weight gain after a breast cancer diagnosis has also been shown to be associated with increased hot flashes [7, 8], pain [9], and poorer health-related quality of life [10].
Most breast cancer survivors are overweight or obese at diagnosis in part due to excess adiposity being a risk factor for postmenopausal breast cancer [11], as well as the high prevalence of overweight and obesity in the USA [12]. To compound this problem, breast cancer survivors often experience additional weight gain following diagnosis, partly related to the effects of their cancer treatments [13].
Previous studies have evaluated predictors of weight gain following breast cancer diagnosis [14–16], but ongoing changes to cancer therapy, as well as secular changes in the prevalence of overweight and obesity warrant further evaluation of the weight gain patterns following a diagnosis of breast cancer. The objective of this study was to evaluate the factors associated with weight gain among a self-selected cohort of breast cancer survivors prior to enrolling into the Exercise and Nutrition to Enhance Good Health for You (ENERGY) trial, a multicenter, randomized controlled trial of a behavioral weight loss intervention.
Methods
Design and study population
The ENERGY trial is a multi-center randomized controlled trial designed to evaluate the effect of a group-based cognitive-behavioral weight loss program on intentional weight loss and quality of life measures among early stage breast cancer survivors. Details of the design and recruitment of the trial have been published previously [17]. Briefly, 693 women with stage I (≥1 cm), II, or III breast cancer who had been diagnosed 6 months to 5 years prior to study entry and had completed all their initial breast cancer treatment excluding endocrine-modulating therapy, who were ≥21 years of age, and had a body mass index (BMI) of 25 to 45 kg/m2 were recruited between the fall of 2010 and spring of 2012. Women were recruited from four sites (University of Alabama at Birmingham; University of California, San Diego; University of Colorado Denver; Washington University in St. Louis). Following the baseline study visit, participants were randomized to either a group-based cognitive behavioral weight loss intervention or to a less intensive intervention control group. This trial was approved by the institutional review boards of all collaborating sites, and written informed consent was obtained from all participants.
The current analysis is based on cross-sectional and retrospective data collected by clinic measurements and self-reports at baseline. Subsequent to the baseline publication [17], there was one postrandomization exclusion for a sample of 692 women. Of the 692 women enrolled in the ENERGY trial, 27 women were excluded from this analysis due to missing data on weights at breast cancer diagnosis, mastectomy, endocrine-modulating therapy, Hispanic ethnicity, or smoking status at diagnosis. The analytical sample for this report was therefore 665 breast cancer survivors.
Data collection
At the baseline visit of the ENERGY trial, questionnaires were used to collect self-reported information about education, race, Hispanic ethnicity, smoking status (including the quit date if applicable), menopausal status (including last menstrual period if applicable), and weight at diagnosis. Staff reviewed all questionnaires for completeness. Medical record review was conducted by trained staff to obtain information on breast cancer diagnosis, treatment including surgery and chemotherapy, and endocrine-modulating therapy. Aromatase inhibitors (AIs) included anastrozole, exemestane, and letrozole whereas selective estrogen-receptor modulators (SERMs) included tamoxifen and raloxifene.
According to study protocol, anthropometric measures of height and weight were conducted following a ≥6-hour fast by trained staff at the baseline visit. Height was measured and rounded to the nearest 0.5 in. by having the participant stand fully erect in stocking or bare feet using a wall-mounted stadiometer and a Frankfort horizontal plane. Weight was measured to the nearest 0.5 lb using a calibrated scale by having the participant empty any heavy objects and/or change from their pockets and without shoes.
Analytical methods
The outcome measure for this study is the percentage weight change between breast cancer diagnosis and entry into the ENERGY trial. This measure was calculated by subtracting the participant’s measured weight at ENERGY baseline from the participant’s self-reported weight at diagnosis divided by participant’s self-reported weight at diagnosis and then multiplying by 100 %. The outcome measure of percentage weight change was then dichotomized into <5 % body weight gain versus ≥5 % body weight gain. This cut point was chosen since modest weight loss of ≥5 % body weight has been recommended to improve health outcomes among overweight and obese individuals [18–20] as well as this cut point has been used previously to evaluate the effect of weight change among breast cancer survivors [3, 4, 21, 22]. To further determine the effect of this particular cut point, weight gain was also analyzed and dichotomized as <10 % body weight gain versus ≥10 % body weight gain as well as a continuous weight change variable.
Bivariate analyses were conducted to evaluate the association between ≥5 % weight gain and demographic, breast cancer treatments, and other potential risk factors. Two-sample t tests were used to evaluate continuous variables whereas chi-squared analyses were used for categorical variables. All factors with p values <0.20 by bivariate analyses (except for menopausal status at diagnosis) were included in a multivariate unconditional logistic regression model to evaluate the independent association between the two categories of weight gain and other potential risk factors. Since menopausal status and age at diagnosis were highly correlated (Spearman 0.73, p<0.01), only age was included in those models. Adjusted odds ratios (ORadj) and 95 % confidence intervals (95 % CI) were reported. The final model adjusted for age at diagnosis, education, Hispanic ethnicity, smoking at diagnosis, BMI at diagnosis, years elapsed since diagnosis, mastectomy, chemotherapy, endocrine-modulating therapy, and oophorectomy. The receiver operating characteristic (ROC) curve as a measure of the C-statistic was used to discriminate among participants. Analyses were also conducted with the outcome variable of ≥10 % weight gain, and linear regression analysis was used to evaluate the association between weight change as a continuous variable adjusted for the variables listed above. Quitting smoking is a risk factor for gaining weight [23–26]; however, we only had 14 women who quit smoking within this time period of which 11 gained weight. Given the small number of women, we were unable to adjust for this factor in the analysis; however, a sensitivity analysis was conducted with these 14 women removed. Statistical analyses were based on two-sided statistical tests with an alpha-level of 0.05. Analyses were conducted using Stata/IC version 11 (Stata Statistical Software: Release 11, StataCorp LP, College Station, TX).
Results
Among 665 early stage breast cancer survivors who enrolled in the weight loss trial, the mean age was 53.5 years (standard deviation (SD)=9.5) with the majority being postmenopausal at breast cancer diagnosis (57.1 %), White (84.5 %), non-Hispanic (93.4 %) women with at least a college degree (60.1 %) who never smoked (65.6 %) (Table 1). In this cohort, 166 (25.0 %) women gained ≥10 % of their body weight, 126 (19.0 %) women gained between 5.0 % up to 10.0 % of their body weight, 268 (40.3 %) women maintained their weight with less than 5 % change, and 105 women (15.8 %) lost >5 % body weight up to 5 years following their breast cancer diagnosis. The mean weight gain was 4.5 % body weight (SD=10.8). Fifteen percent of the women were at normal BMI (<25 m/kg2) at breast cancer diagnosis but subsequently gained weight over a mean period of 2.7 years postdiagnosis. Women who experienced ≥5 % weight gain were more likely to be younger, premenopausal, non-Hispanic, smokers, and had a normal BMI upon diagnosis. In the ENERGY trial, there were 30 % diagnosed with stage I (≥1 cm), 52 % with stage II, and 18 % with stage III. The majority of women had received a mastectomy (52.5 %), chemotherapy (77.6 %), and radiation (73.7 %) and also received endocrine-modulating therapy (75.6 %). Women who had ≥5 % gained weight were less likely to have had a mastectomy or use a selective estrogen-receptor modulator (SERM) but more likely to have had an oophorectomy.
Table 1.
Prevalence of ≥5 % weight gain by demographic and risk factor characteristics among ENERGY trial participants
| Characteristic | Overall | <5 % weight gain | ≥5 % weight gain | p value |
|---|---|---|---|---|
| N | 665 | 373 | 292 | |
| Age at diagnosis, mean (SD) | 53.5 (9.5) | 55.3 (9.4) | 51.1 (9.5) | <0.01 |
| Site, n (%) | ||||
| San Diego | 215 (32.3) | 124 (57.7) | 91 (42.3) | 0.81 |
| Denver | 177 (26.6) | 101 (57.1) | 76 (42.9) | |
| Birmingham | 107 (16.1) | 60 (56.1) | 47 (43.9) | |
| St Louis | 166 (25.0) | 88 (53.0) | 78 (47.0) | |
| Education | ||||
| ≥High school graduate | 94 (14.4) | 56 (59.6) | 38 (40.4) | 0.14 |
| Some college | 171 (25.7) | 90 (52.6) | 81 (47.4) | |
| College graduate | 185 (27.8) | 95 (51.4) | 90 (48.7) | |
| Some postgraduate | 219 (32.3) | 132 (61.4) | 83 (38.6) | |
| Hispanic | ||||
| No | 621 (93.4) | 342 (55.1) | 279 (44.9) | 0.05 |
| Yes | 44 (6.6) | 31 (70.5) | 13 (29.6) | |
| Race | ||||
| White | 562 (84.5) | 311 (55.3) | 251 (44.7) | 0.61 |
| Black | 68 (10.2) | 40 (58.8) | 28 (41.2) | |
| Other | 35 (5.3) | 22 (5.9) | 13 (37.1) | |
| Smoking status at diagnosis | ||||
| Never | 436 (65.6) | 244 (56.0) | 192 (44.0) | <0.01 |
| Former | 194 (29.2) | 118 (60.8) | 76 (39.2) | |
| Smoker | 35 (5.3) | 11 (31.4) | 24 (68.6) | |
| Menopausal status at diagnosis | ||||
| Premenopausal | 199 (29.9) | 89 (44.7) | 110 (55.3) | <0.01 |
| Perimenopausal | 86 (12.9) | 47 (54.7) | 39 (45.4) | |
| Postmenopausal | 380 (57.1) | 237 (62.4) | 143 (37.6) | |
| Years since diagnosis, mean (SD) | 2.65 (1.4) | 2.5 (1.4) | 2.9 (1.4) | <0.01 |
| BMI at diagnosis, kg/m2 | ||||
| <25 | 99 (14.9) | 10 (10.1) | 89 (89.9) | <0.01 |
| 25<30 | 276 (41.5) | 141 (51.1) | 135 (48.9) | |
| ≥30 | 290 (43.6) | 222 (76.6) | 68 (23.5) | |
| Height, inches, mean (SD) | 64.6 (2.5) | 64.5 (2.6) | 64.7 (2.3) | 0.29 |
| Stage | ||||
| I (≥1 cm) | 200 (30.1) | 119 (59.5) | 81 (40.5) | 0.46 |
| II | 344 (51.7) | 190 (55.2) | 154 (44.8) | |
| III | 121 (18.2) | 64 (52.9) | 57 (47.1) | |
| Mastectomy | ||||
| Yes | 349 (52.5) | 210 (60.2) | 139 (39.8) | 0.03 |
| No | 316 (47.5) | 163 (51.6) | 153 (48.4) | |
| Chemotherapy | ||||
| No/unknown | 149 (22.4) | 91 (61.1) | 58 (38.9) | 0.16 |
| Yes | 516 (77.6) | 282 (54.7) | 234 (45.4) | |
| Radiation | ||||
| No/unknown | 175 (26.3) | 93 (53.1) | 82 (46.9) | 0.36 |
| Yes | 490 (73.7) | 280 (57.1) | 210 (42.9) | |
| Endocrine-modulating therapy | ||||
| SERM | 144 (21.7) | 63 (45.8) | 81 (56.3) | <0.01 |
| AI | 269 (40.5) | 178 (66.2) | 91 (33.8) | |
| Both SERM and AI | 89 (13.4) | 42 (47.2) | 47 (52.8) | |
| Neither SERM or AI | 163 (24.5) | 90 (55.2) | 73 (44.8) | |
| Ooporectomy | ||||
| No/unknown | 602 (90.5) | 349 (58.0) | 253 (42.0) | <0.01 |
| Yes | 63 (9.5) | 24 (38.1) | 39 (61.9) | |
The factors that were independently associated with ≥5 % gained weight included younger age at diagnosis (ORadj= 0.97/year, 95 % CI 0.95–s0.99), non-Hispanic ethnicity (ORadj=0.30, 95 % CI 0.13–0.68), being a smoker at the time of diagnosis (ORadj=2.69, 95 % CI 1.12–6.49), and time elapsed since diagnosis (ORadj=1.19/year, 95 % CI 1.04–1.36) (Fig. 1). When the women who quit smoking were removed from the analysis, the association between being a smoker at diagnosis and weight gain was no longer significant (ORadj=1.92, 95 % CI 0.64–5.75). After adjustment, women with a higher BMI at diagnosis were less likely to gain weight as compared to normal weight women; overweight (ORadj= 0.11, 95 % CI 0.05–0.23) and obese (ORadj=0.03, 95 % CI 0.02–0.07). Women on AI alone were 46 % less likely to gain ≥ 5 % as compared to those who were prescribed a SERM after adjustment for covariates (ORadj=0.54, 95 % CI 0.31–0.93). In addition, the greater the amount of time elapsed since diagnosis, the greater the gain in weight such that for each year postdiagnosis the odds of weight gain increased by 19 %. When the analysis was conducted with ≥10 % weight gain versus <10 % gain, comparable estimates were observed although only age, years since diagnosis, and BMI at diagnosis remained significant while adjusting for the same covariates (data not shown). Furthermore, age, Hispanic ethnicity, current smoking, years since diagnosis, and BMI at diagnosis remained significant in the linear regression analysis with change in body weight as the outcome variable (data not shown).
Fig. 1.
Factors independently associated with 5 % weight gain
Discussion
This study evaluated weight change patterns among breast cancer survivors who self-selected to enter a weight loss trial and thereby more likely to have a higher starting BMI on average. This study found that among the 665 breast cancer survivors, those who gained more than 5 % of their body weight over an average 2.5 years postdiagnosis, were significantly more likely to be younger, non-Hispanic White, current smokers (attributable to those who quit following diagnosis), less obese, and had a longer time since diagnosis. Additionally, breast cancer survivors who were initially prescribed AIs were less likely to gain ≥5 % body weight as compared to those prescribed SERMs.
In this cohort, the mean weight gain was 4.5 % body weight, and 44 % of women gained ≥5 % body weight postdiagnosis between 6 months to 5 years after diagnosis. Previous studies have reported that 10–48 % of breast cancer survivors gain ≥5 % over an average of 2 to 7 years postdiagnosis [3, 4, 6, 15, 21, 22, 27–32] with rates ranging from 36–46 % among US and European cohorts [3, 4, 15, 21, 22, 31, 32]. In a pooling project of 12,915 breast cancer patients with prospectively collected data from the USA and China, the mean weight gain was 34.7 and 33.7 % in the USA and 36.6 % in China [6]. In the Women’s Healthy Eating and Living (WHEL) study, a dietary intervention trial without a weight loss component, 45 % of the 3,088 early-stage breast cancer survivors gained weight from 1 year before breast cancer diagnosis up to 6 years postdiagnosis [21] with similar weight gain observed in an early subset of this cohort [15]. In the Life After Cancer Epidemiology (LACE) cohort study, 36 % of 1,692 breast cancer survivors gained weight from 1 year pre-diagnosis to on average 2 years postdiagnosis [4]. Among smaller cohort studies, weight gain was noted among 46 % of French breast cancer survivors over 15 months [31] and 36 % for US breast cancer survivors over 18 months [32].
In the general population, weight gain is also commonly seen among women as they age [33, 34]. Among healthy women age 30–55 years enrolled in the Nurses’ Health Study, a mean weight gain of 1.9 kg (SD=4.6) was observed over the first 4 years of follow-up and 1.6 kg (SD=4.6) over the second 4-year period [34]. In the Australian Longitudinal Study on Women’s Health, an average weight gain of 2.42 kg (95 % CI 2.29–2.54) was reported among 8,071 women, age 45 to 55 years old, over 5 years [35]. In the current investigation, breast cancer survivors gained an average of 3 kg (6.5 lbs) which is similar to the 2.4 kg weight gain from the US sites of the pooling project among breast cancer survivors [6].
Even though the national obesity rates are higher in Hispanics as compared with non-Hispanic Whites, Hispanic women had a lower odds of gaining ≥5 % of body weight in the current investigation. Forty-five percent of non-Hispanic Whites gained ≥5 % of body weight as compared with only 30 % of Hispanic breast cancer survivors in our study, though these statistics should be interpreted cautiously given the small number of Hispanic women (n=44). In the Healthy Eating, Activity, and Lifestyle (HEAL) study of 514 women with stage 0–IIIA breast cancer, the adjusted mean weight gained experienced by 78 Hispanic women also was lower compared to 436 non-Hispanic Whites women (1.1 vs. 1.9 kg) 3 years following diagnosis [36]. In the WHEL study, no significant difference in weight gain was observed between Hispanic (n=154) as compared to non-Hispanic Whites (n= 2,625) breast cancer survivors [21]. Within the prospective SUNSHINE study of 214 non-Hispanic Whites and 91 Hispanic breast cancer survivors, 40 % of Hispanic women and 35 % of non-Hispanic Whites women gained ≥5 % of body weight after a mean follow-up of 6 years [22].
Smoking at the time of breast cancer diagnosis was associated with a 2.7-fold increased risk for weight gain as compared to never smokers. Of the 35 women who were smokers at their breast cancer diagnosis, 40 % (n=14) quit smoking following their breast cancer diagnosis. The high rate of quitting may be due in part to smoking being contraindicated for reconstructive surgery [37], thereby these women would likely have been counseled to quit prior to surgery. When these women were removed from the model, the association was no longer significant. Weight gain is common following smoking cessation, with weight gains ranging from 1.5 to 3.8 kg [23–26]. The mechanism for this weight gain is not fully elucidated, but possible factors include increase in energy intake, decrease in resting metabolic rate and physical activity, and changes in lipoprotein lipase activity [38]. In the current investigation, smoking at diagnosis was significantly associated with modest weight gain of ≥5 % of body weight but not with weight gains of 10 % or more.
Breast cancer treatments and treatment-related factors have also been associated with weight gain. Chemotherapy has been associated with weight gain among breast cancer survivors in some [14–16, 21, 39, 40] but not in all studies [22, 28, 32, 41]. A significant association was not observed between chemotherapy and weight gain following a breast cancer diagnosis after adjustment for other factors. As reported previously [3, 4, 6, 15, 21, 22, 32, 39], we observed an inverse association between BMI at diagnosis and subsequent weight gain such that normal weight women were more likely to gain weight as compared to heavier women. This finding remained significant when we used either a 5 %- or 10 %-body weight cut point, or weight change as a continuous variable. The average BMI in this study is considerably higher than in other cohorts, which is expected; given the eligibility criteria included only women who were overweight or obese. In the current investigation, the average BMI at diagnosis was 30.3 kg/m2 (SD=5.3); 27.7 kg/m2 (SD=4.1) for those women who gained ≥5 % body weight as compared to 32.4 kg/m2 (SD=5.3) in those who had <5 % body weight gain. Within the US sites of the pooling project, the average pre-diagnosis BMI was reported at 26.5 kg/m2 (SD=5.4) [6]. Additionally at the time of diagnosis, 19 % of ENERGY participants reported having a BMI less than 25 kg/m2 as compared with 30 to 60 % from other investigations [3, 4, 21, 22, 31].
In the current study, women on AI therapy had a 44 % reduced risk of gaining ≥5 % body weight as compared to those on tamoxifen therapy after adjustment for other covariates. A finding of a lower weight gain among women prescribed aromatase inhibitors as compared to tamoxifen has not been previously reported. There is considerable epidemiologic literature, recently reviewed by Vance and colleagues [13], reporting no difference in weight gain associated with tamoxifen among breast cancer treatment, but fewer data are available investigating the association between AIs (anastrozole, exemestane, and letrozole) and weight gain. Within the Arimidex (anastrozole), Tamoxifen Alone, or in Combination (ATAC) trial conducted among 9,366 postmenopausal breast cancer survivors, weight gain was similar in the three study arms with an average weight gain of 1.65 kg or 2.5 % body weight over 2 years [42]. Similarly, no significant difference in weight change was observed in a small study (n= 55) conducted among postmenopausal breast cancer survivors who received exemestane following 2 years of tamoxifen as compared to those who remained on tamoxifen, although a significant reduction in fat mass and the ratio of fat mass to fat-free mass was observed among patients who also took exemestane as compared to those who remained on tamoxifen [43]. Our findings of an association between lower weight gain and AIs was limited to analyses of ≥5 % body weight. When weight change was evaluated with a cut point of ≥10 % or as a continuous variable the association was attenuated and no longer significant. The current standard of care for estrogen-receptor-positive postmenopausal breast cancers recommends adjuvant endocrine-modulating therapy with an AI whereas endocrine-modulating therapy with tamoxifen is recommended for premenopausal women [37]. However, this relationship is complex because many cancer therapies can cause premenopausal women to undergo early menopause [16, 44, 45], and both age and menopausal status are associated with weight gain [3, 4, 15, 27, 32, 36, 39]. The median age of our study cohort at diagnosis was younger than US breast cancer patients that included localized stage; 53 versus 62 years [46]. In our analysis, we adjusted for age and not menopausal status because they were highly correlated. When we used either menopausal status with age or menopausal status alone in the analysis, similar results were observed (data not shown). Given that differences may exist by the type of AI and possible differences in fat free mass, our findings of reduced ≥5 % body weight gain associated with AIs as compared to those prescribed SERMs warrants further investigation.
Strengths and limitation of this epidemiologic study should be acknowledged. This is a large study of well-characterized breast cancer survivors in which treatment variables have been determined through medical chart abstraction. Given the ENERGY trial recruited overweight and obese breast cancer survivors for a weight loss intervention, this study cannot be generalizable to all early stage breast cancer survivors; however, it may be generalizable to breast cancer survivors who are younger and overweight or obese. It also should be noted that our observation of weight gain of ≥5 % body weight among 44 % of participants is similar to what has been observed in other investigations [3, 4, 15, 21, 22, 31, 32]. Due to weight at diagnosis being measured by self-report, there is the potential for misclassification especially among obese women who may underreport their weight [47]. To minimize this potential bias, we adjusted our models for BMI at diagnosis. The difference in mean weight was 0.77 kg (1.7 lb). Since dietary or physical activity measures at diagnosis as well as time on endocrine-modulating therapy or adherence measures were not collected, we were unable to address these factors. Also, given the small number of women who quit smoking following their breast cancer diagnosis, we could not fully explore this relationship.
In conclusion, within this study of breast cancer survivors who enrolled in a weight loss trial, weight gain of ≥5 % was reported by 44 % approximately 2.5 years after diagnosis. Gaining weight following a breast cancer diagnosis may be associated with a higher risk of recurrence and mortality [2–6] as well as cancer-related symptoms and poorer health-related quality of life [7–10]. Furthermore, being overweight or obese is associated with comorbid conditions such as diabetes, cardiovascular disease as well as other cancers [48–50]. Weight and weight gain continues to be an issue for breast cancer survivors that has implications for the patients as well as the healthcare system. Public health efforts need to focus on developing effective approaches of weight management that can be disseminated within this growing population of women.
Acknowledgments
Funding for the ENERGY trial was provided by the National Cancer Institute (CA148791). This research was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under award number UL1TR00165 at the University of Alabama at Birmingham and by the NIH/NCATS Colorado CTSI Grant Number UL1 TR001082 at the University of Colorado Denver. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views. The authors would also like to recognize the subjects for their participation and to acknowledge the ENERGY Trial Group.
Footnotes
The ENERGY trial group University of California, San Diego: Cheryl Rock, PhD, RD, Bilge Pakiz, EdD, Barbara Parker, MD, Chris Zoumas, MS, RD, Shirley Flatt, MS, Hava Shoshana Barkai, MS, RD, Dennis Heath, MS, Lea Jacinto, Mila Pruitt.
University of California, Los Angeles: Patricia A. Ganz, MD.
University of Colorado Denver: Tim Byers, MD, MPH, Rebecca Sedjo, PhD, Holly Wyatt, MD, Anthony Elias, MD, Anna Van Pelt, MPH, Kim Gorman, MS, RD.
Washington University in St. Louis: Graham Colditz, MD, Kathleen Wolin, ScD, Esther Liu, PhD, Michael Naughton, MD, Casey Fagin, MA, Jennifer Tappenden, Sonya Izadi.
University of Alabama at Birmingham: Wendy Demark-Wahnefried, PhD, RD, Helen Krontiras, MD, Maria Azrad, PhD, RD, Cindy Blair, PhD, Sonthe Burge, MS, RD.
Conflict of interest The authors (RLS, TB, PG, GC, WDW, KYW, MA, CLR) declare no disclosures of any financial or personal relationships that could pose a conflict of interest with the subject matter of this article.
Contributor Information
Rebecca L. Sedjo, Email: rebecca.sedjo@ucdenver.edu, Department of Community and Behavioral Health, Colorado School of Public Health, University of Colorado Denver, 13001 East 17th Place, MS F519, Aurora, CO 80045, USA
Tim Byers, Department of Epidemiology, Colorado School of Public Health, University of Colorado Denver, 13001 E. 17th Place, Aurora, CO 80045, USA.
Patricia A. Ganz, Jonsson Comprehensive Cancer Center, University of California, 650 Charles Young Drive South, Room A2-125 CHS, Los Angeles, CA 90095-6900, USA
Graham A. Colditz, Washington University School of Medicine, 660 South Euclid Avenue, Campus Box 8100, Saint Louis, MO 63110, USA
Wendy Demark-Wahnefried, University of Alabama Comprehensive Cancer Center, University of Alabama at Birmingham, 1824 6th Avenue, Birmingham, AL 35294, USA.
Kathleen Y. Wolin, Department of Public Health Sciences, Loyola University Chicago Stritch School of Medicine, 2160 S. First Ave, Maywood, IL 60153, USA
Maria Azrad, Department of Nutrition Sciences, University of Alabama at Birmingham, 1675 University Blvd, 348 Webb, Birmingham, AL 35294, USA.
Cheryl L. Rock, Department of Family and Preventive Medicine, School of Medicine, University of California San Diego, 3855 Health Sciences Drive, La Jolla, CA 92093, USA
References
- 1.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123:627–35. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 2.Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23:1370–8. doi: 10.1200/JCO.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 3.Caan BJ, Emond JA, Natarajan L, Castillo A, Gunderson EP, Habel L, et al. Post-diagnosis weight gain and breast cancer recurrence in women with early stage breast cancer. Breast Cancer Res Treat. 2006;99:47–57. doi: 10.1007/s10549-006-9179-y. [DOI] [PubMed] [Google Scholar]
- 4.Caan BJ, Kwan ML, Hartzell G, Castillo A, Slattery ML, Sternfeld B, et al. Pre-diagnosis body mass index, post-diagnosis weight change, and prognosis among women with early stage breast cancer. Cancer Causes Control. 2008;19:1319–28. doi: 10.1007/s10552-008-9203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nichols HB, Trentham-Dietz A, Egan KM, Titus-Ernstoff L, Holmes MD, Bersch AJ, et al. Body mass index before and after breast cancer diagnosis: associations with all-cause, breast cancer, and cardiovascular disease mortality. Cancer Epidemiol Biomarkers Prev. 2009;18:1403–9. doi: 10.1158/1055-9965.EPI-08-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caan BJ, Kwan ML, Shu XO, Pierce JP, Patterson RE, Nechuta SJ, et al. Weight change and survival after breast cancer in the After Breast Cancer Pooling Project. Cancer Epidemiol Biomarkers Prev. 2012;21:1260–71. doi: 10.1158/1055-9965.EPI-12-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su HI, Sammel MD, Springer E, Freeman EW, DeMichele A, Mao JJ. Weight gain is associated with increased risk of hot flashes in breast cancer survivors on aromatase inhibitors. Breast Cancer Res Treat. 2010;124:205–11. doi: 10.1007/s10549-010-0802-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caan BJ, Emond JA, Su HI, Patterson RE, Flatt SW, Gold EB, et al. Effect of postdiagnosis weight change on hot flash status among early-stage breast cancer survivors. J Clin Oncol. 2012;30:1492–7. doi: 10.1200/JCO.2011.36.8597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forsythe LP, Alfano CM, George SM, McTiernan A, Baumgartner KB, Bernstein L, et al. Pain in long-term breast cancer survivors: the role of body mass index, physical activity, and sedentary behavior. Breast Cancer Res Treat. 2013;137:617–30. doi: 10.1007/s10549-012-2335-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imayama I, Alfano CM, Neuhouser ML, George SM, Wilder Smith A, Baumgartner RN, et al. Weight, inflammation, cancer-related symptoms and health-related quality of life among breast cancer survivors. Breast Cancer Res Treat. 2013;140:159–76. doi: 10.1007/s10549-013-2594-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 12.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2010. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 13.Vance V, Mourtzakis M, McCargar L, Hanning R. Weight gain in breast cancer survivors: prevalence, pattern and health consequences. Obes Rev. 2011;12:282–94. doi: 10.1111/j.1467-789X.2010.00805.x. [DOI] [PubMed] [Google Scholar]
- 14.Demark-Wahnefried W, Rimer BK, Winer EP. Weight gain in women diagnosis with breast cancer. J Am Diet Assoc. 1997;97:519–29. doi: 10.1016/s0002-8223(97)00133-8. [DOI] [PubMed] [Google Scholar]
- 15.Rock CL, Flatt SW, Newman V, Caan BJ, Haan MN, Stefanick ML, et al. Factors associated with weight gain in women after diagnosis of breast cancer. J Am Diet Assoc. 1999;99:1212–21. doi: 10.1016/s0002-8223(99)00298-9. [DOI] [PubMed] [Google Scholar]
- 16.Goodwin P, Ennis M, Pritchard K, McCready D, Koo J, Sidlofsky S, et al. Adjuvant treatment and onset of menopause predict weight gain after breast cancer diagnosis. J Clin Oncol. 1999;17:120–9. doi: 10.1200/JCO.1999.17.1.120. [DOI] [PubMed] [Google Scholar]
- 17.Rock CL, Byers TE, Colditz GA, Demark-Wahnefried W, Ganz P, Wolin KY, et al. Reducing breast cancer recurrence with weight loss, a vanguard trial: the Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) Trial. Contemp Clin Trials. 2013;34:282–95. doi: 10.1016/j.cct.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi-Sunyer X, et al. Clinical implications of obesity with specific focus on cardiovascular disease. A statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2004;110:2952–67. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- 19.Doyle C, Kushi LH, Byers T, Courneya KS, Demark-Wahnefried W, Grant B, et al. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA Cancer J Clin. 2006;56:323–53. doi: 10.3322/canjclin.56.6.323. [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes Association Task Force for Writing Nutrition Principles and Recommendations for the management of diabetes: American Diabetes Association position statement: evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. J Am Diet Assoc. 2002;102:109–18. doi: 10.1016/s0002-8223(02)90031-3. [DOI] [PubMed] [Google Scholar]
- 21.Saquib N, Flatt SW, Natarajan L, Thomson CA, Bardwell WA, Caan B, et al. Weight gain and recovery of pre-cancer weight after breast cancer treatments: evidence from the women’s healthy eating and living (WHEL) study. Breast Cancer Res Treat. 2007;105:177–86. doi: 10.1007/s10549-006-9442-2. [DOI] [PubMed] [Google Scholar]
- 22.Sedjo RL, Hines LM, Byers T, Giuliano AR, Marcus A, Vadaparampil S, et al. Long-term weight gain among Hispanic and non-Hispanic White women with and without breast cancer. Nutr Cancer. 2013;65:34–42. doi: 10.1080/01635581.2013.741750. [DOI] [PubMed] [Google Scholar]
- 23.Travier N, Agudo A, May AM, Gonzalez C, Luan J, Wareham NJ, et al. Longitudinal changes in weight in relation to smoking cessation in participants of the EPIC-PANACEA study. Prev Med. 2012;54:183–92. doi: 10.1016/j.ypmed.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Williamson DF, Madans J, Anda RF, Kleinman JC, Giovino GA, Byers T. Smoking cessation and severity of weight gain in a national cohort. NEJM. 1991;324:739–45. doi: 10.1056/NEJM199103143241106. [DOI] [PubMed] [Google Scholar]
- 25.Basterra-Cortari FJ, Forga L, Bes-Rastrollo M, Toledo E, Martinez JA, Martinex-Gonzalez MA. Effects of smoking on body weight: longitudinal analysis of the SUN cohort. Rev Esp Cardiol. 2010;63:20–7. doi: 10.1016/s1885-5857(10)70005-0. [DOI] [PubMed] [Google Scholar]
- 26.Luo J, Rossouw J, Margolis KL. Smoking cessation, weight change, and coronary heart disease among postmenopausal women with and without diabetes. JAMA. 2013;310(1):94–6. doi: 10.1001/jama.2013.6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu K, Chen X, Zhen Y, Chen Z, Zheng W, Lu W, et al. Weight change patterns among breast cancer survivors: results from the Shanghai breast cancer survival study. Cancer Causes Control. 2010;21(4):621–9. doi: 10.1007/s10552-009-9491-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han HS, Lee KW, Kim JH, Kim SW, Kim IA, Oh DY, et al. Weight changes after adjuvant treatment in Korean women with early breast cancer. Breast Cancer Res Treat. 2009;114:147–53. doi: 10.1007/s10549-008-9984-6. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Lu W, Gu K, Chen Z, Zheng Y, Zheng W, et al. Weight change and its correlates among breast cancer survivors. Nutr Cancer. 2011;63:538–48. doi: 10.1080/01635581.2011.539316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yaw WH, Shariff ZM, Kaniah M, Mun CY, Yusof RM, Othman Z, et al. Weight changes and lifestyle behaviors in women after breast cancer diagnosis: a cross-sectional study. BMC Public Health. 2011;11:309–18. doi: 10.1186/1471-2458-11-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trédan O, Bajard A, Meunier A, Roux P, Fiorletta I, Gargi T, et al. Body weight change in women receiving adjuvant chemotherapy for breast cancer: a French prospective study. Clin Nutr. 2010;29:187–91. doi: 10.1016/j.clnu.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Reddy S, Sadim M, Li J, Yi N, Agarwal S, Mantzoroas CS, et al. Clinical and genetic predictors of weight gain in patients diagnosed with breast cancer. Br J Cancer. 2013;109:872–81. doi: 10.1038/bjc.2013.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wing RR. Obesity and weight gain during adulthood: a health problem for United States women. WHI. 1992;2:114–22. doi: 10.1016/s1049-3867(05)80279-8. [DOI] [PubMed] [Google Scholar]
- 34.Colditz GA, Willett WC, Stampfer MJ, London SJ, Segal MR, Speizer FE. Patterns of weight change and their relations to diet in a cohort of healthy women. Am J Clin Nutr. 1990;51:1100–5. doi: 10.1093/ajcn/51.6.1100. [DOI] [PubMed] [Google Scholar]
- 35.Brown WJ, Williams L, Ford JH, Ball K, Dobson AJ. Identifying the energy gap: magnitude and determinants of 5-year weigh gain in midage women. Obes Res. 2005;13:1431–44. doi: 10.1038/oby.2005.173. [DOI] [PubMed] [Google Scholar]
- 36.Irwin ML, McTiernan A, Baumgartener R, Bumgartener KB, Bernstein L, Gilliland FD, et al. Changes in body fat and weight after a breast cancer diagnosis: influence of demographic, prognastic, and lifestyle factors. J Clin Oncol. 2005;23:774–82. doi: 10.1200/JCO.2005.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theriault RL, Carlson RW, Anderson BO, Burstein HJ, Edge SB, Farrar WB, et al. Breast cancer. [Accessed: 10/23/2013];National Comprehensive Cancer Network Guidelines, version 3.2013. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 38.Filozof C, Pinilla CF, Fernández-Cruz A. Smoking cessation and weight gain. Obes Rev. 2004;5:95–103. doi: 10.1111/j.1467-789X.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- 39.Makari-Judson G, Judson CH, Mertens WC. Longitudinal patterns of weight gain after breast cancer diagnosis: observations beyond the first year. Breast J. 2007;13(3):258–65. doi: 10.1111/j.1524-4741.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- 40.Heideman WH, Russell NS, Gundy C, Rookus MA, Voskuil DW. The frequency, magnitude and timing of post-diagnosis body weight gain in Dutch breast cancer survivors. Eur J Cancer. 2009;45:119–26. doi: 10.1016/j.ejca.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Freedman RJ, Aziz N, Albanes D, Hartman T, Danforth D, Hill S, et al. Weight and body composition changes during and after adjuvant chemotherapy in women with breast cancer. J Clin Endocrinol Metab. 2004;89:2248–53. doi: 10.1210/jc.2003-031874. [DOI] [PubMed] [Google Scholar]
- 42.The ATAC (Arimidex, Tamoxifen Alone or in Combination) Trialists’ Group. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359:2131–9. doi: 10.1016/s0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- 43.Francini G, Petrioli R, Montagnani A, Cadirni A, Campagna S, Francini E, et al. Exemestane after tamoxifen as adjuvant hormonal therapy in postmenopausal women with breast cancer: effects on body composition and lipids. Br J Cancer. 2006;95:153–8. doi: 10.1038/sj.bjc.6603258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petrek JA, Naughton MJ, Case LD, Paskett ED, Naftalis EZ, Singletary SE, et al. Incidence, time course, and determinates of menstrual bleeding after breast cancer treatment: a prospective study. J Clin Oncol. 2006;24:1045–51. doi: 10.1200/JCO.2005.03.3969. [DOI] [PubMed] [Google Scholar]
- 45.Partridge A, Gelber S, Gelber RD, Castiglione-Gertsch M, Goldhirsch A, Winer E. Age of menopause among women who remain premenopausal following treatment for early breast cancer: long-term results from International Breast Cancer Study Group Trials V and VI. Eur J Cancer. 2007;43:1646–53. doi: 10.1016/j.ejca.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 46.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivor-ship statistics, 2012. CA Cancer J Clin. 2012;62:220–41. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 47.Lin CJ, Deroo LA, Jacobs SR, Sandler DP. Accuracy and reliability of self-reported weight and height in the Sister Study. Public Health Nutr. 2011;9:1–11. doi: 10.1017/S1368980011003193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88–108. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pi-Sunyer X. The medical risks of obesity. Postgrad Med. 2009;121:21–33. doi: 10.3810/pgm.2009.11.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington DC: AICR; 2007. [Google Scholar]