Abstract
Background
Previous data suggest the amount and aerobic intensity of stepping training may improve walking post-stroke. Recent animal and human studies suggest training in challenging and variable contexts can also improve locomotor function. Such practice may elicit substantial stepping errors, although alterations in locomotor strategies to correct these errors could lead to improved walking ability.
Objective
This un-blinded, pretest-posttest pilot study was designed to evaluate the feasibility and preliminary efficacy of providing stepping practice in variable, challenging contexts (tasks and environments) at high aerobic intensities in participants with chronic (< 6 months) and subacute (1–6 months) stroke.
Methods
Twenty-five participants with stroke (gait speeds < 0.9 m/s with no more than moderate assistance) participated in ≤ 40 1-hr training sessions within 10 weeks. Stepping training in variable, challenging contexts was performed at 70–80% heart rate reserve, with feasibility measures of total steps/session, ability to achieve targeted intensities, patient tolerance, dropouts, and adverse events. Measures of daily stepping, gait speed, symmetry, and 6-min walk were performed every 4–5 weeks or 20 sessions with a 3 month follow-up.
Results
Twenty-two participants completed ≥ 4 weeks of training, averaging 2887±780 steps/session over 36±5.8 sessions. Self-selected and fastest speed, paretic single limb stance, and 6-min walk improved significantly at post-training and follow-up.
Conclusions
This preliminary study suggests stepping training at high aerobic intensity in variable contexts was tolerated by participants post-stroke, with significant locomotor improvements. Future trials should delineate the relative contributions of amount, intensity and variability of stepping training to maximize outcomes.
Keywords: locomotion, rehabilitation, gait training
Introduction
Many studies have evaluated the efficacy of specific interventions to improve walking ability in individuals with gait impairments post-stroke, although the critical training parameters which maximize recovery are not clear. Studies from animal models1,2 and humans with neurologic injury3 suggest that the amount of task-specific stepping practice may be an important parameter. Early studies utilizing treadmill training with therapist assistance to approximate speeds and/or kinematics observed in intact individuals4–7 demonstrated significant walking improvements, which may be related to amount of stepping practice. Other studies suggest that the aerobic intensity of stepping training, estimated primarily using heart rate8, may be another important training parameter to improved walking outcomes9,10. Interestingly, in some of these3,10 and other studies11–13 providing large amounts of stepping practice at low or high aerobic intensities, gait speed improvements are often not different compared to changes observed following interventions providing limited walking.
One training parameter that has received less attention is the variability of gait kinematics or tasks. Rather, many training protocols focus on consistent, symmetrical, kinematically correct stepping patterns during training4–7,11,14 and minimize kinematic variability and errors. However, animal15 and human studies16,17 have shown that kinematically constrained treadmill stepping can limit improvements in gait symmetry or joint kinematics, particularly as compared to strategies that allow kinematic variability. Task variability is also limited in many studies, with many protocols providing only forward stepping on a treadmill, with limited practice overground3,10,12,13. Conversely, recent data in animal models of spinal cord injury (SCI) suggest stepping training with variable “challenges” (i.e., multiple directions18 or overground with obstacles or stairs19) improves locomotor performance greater than forward treadmill training. Such variable training protocols required investigators to reduce stepping speeds to ensure task completion, suggesting a tradeoff between the amount and variability of stepping practice within scheduled training sessions.
Few studies have evaluated the feasibility and efficacy of stepping training in variable contexts (tasks or environments) in patients with neurological injury, particularly at high aerobic intensities. Such training may provide less stepping practice as compared to forward treadmill stepping, which may limit walking improvements. Nonetheless, stepping practice in variable contexts may simulate many of the barriers encountered in the community (i.e., stairs/curbs, uneven terrains, altered directions)20 and be more specific to daily stepping activities.
Training challenging variable locomotor tasks at high intensities may however, elicit substantial errors in task performance21, which is often discouraged by traditional physical therapy paradigms22. However, if patients can adjust their locomotor strategies to correct these errors, allowing, inducing, or even augmenting errors during training may improve locomotor performance. The short-term effects of error-augmenting perturbations targeting specific gait deficits (e.g. limb swing23, step-length asymmetry24) have been tested, with recent studies suggesting long-term improvements with sustained training25. Patients post-stroke unfortunately present with many gait impairments, and practice of multiple, challenging stepping tasks targeting these deficits may further improve locomotor function.
The purpose of this study was to evaluate the feasibility and preliminary efficacy of stepping training provided to individuals post-stroke in variable contexts with applied perturbations and at high aerobic intensities. Feasibility was determined by the amount of stepping practice provided, the extent to which high aerobic intensities could be achieved, with estimates of patient tolerance and adverse events. Preliminary efficacy was evaluated in participants with chronic stroke (> 6 months) using repeated baseline assessments to estimate potential effect sizes, with similar assessments performed in participants with subacute stroke (1–6 months) to evaluate feasibility and efficacy early following injury. We hypothesized such training would be tolerated by most subjects with few adverse events, although the efficacy of such training was uncertain.
Methods
Participants
Individuals with subacute and chronic hemiparesis following unilateral supratentorial stroke were recruited. Eligible participants presented with gait deficits which ranged from requiring moderate assistance to ambulate 10 m overground (i.e., participant can perform 50–74% of the walking task26) up to walking without assistance but at self-selected speeds < 0.9 m/s with devices and an ankle foot orthosis if needed. Additional inclusion criteria consisted of: 18–75 years old; ability to sit unsupported for 30 seconds; Mini-Mental Status Exam score ≥ 23/30; Patient Health Questionnaire < 10; lower extremity passive range of motion of 0–30° ankle plantarflexion, 0–60° knee flexion, and 0–30° hip flexion; and medical clearance to participate. Exclusion criteria included: osteoporosis; cardiovascular instability; inability to ambulate > 150 feet prior to stroke; previous central nervous system injury; and inability to adhere to study requirements including use of pedometers (please see below). Patients could not be concurrently enrolled in physical therapy. The project was approved by the local ethics committee; all participants provided written informed consent.
Experimental Protocol
Participants received ≤ 40 1-hr sessions within 10 weeks, with a goal of 5 days/week. Preliminary baseline assessments (PRE-BSL) were collected on chronic patients 4–5 weeks prior to baseline (BSL) testing to evaluate stability of outcome measures; stability was not expected nor tested in subacute participants. Mid- (MID) and post (POST)-testing were repeated following up to 20 sessions or 5 weeks, with a 3 month follow-up (F/U).
Training sessions were structured and monitored by 1 of 5 therapists with assistance from a research aide if needed. All therapists followed the training protocol for either subacute or chronic participants as described below, with flexibility to address specific gait deficits using strategies outlined. Therapists regularly discussed strategies used with specific participants to ensure protocol adherence. Within the protocol, the primary goals of training were as follows:
Maximizing repetitions of stepping practice: Training consisted of reciprocal stepping in a specific direction (i.e., forwards, backwards or sideways) for ≤ 40 min with rest breaks as needed. Successful stepping was defined as generating positive step lengths (swing limb extending beyond stance limb) without foot drag and absence of limb collapse, while maintaining sagittal/frontal plane stability. Verbal feedback was provided to ensure stepping at targeted intensities (e.g., cues for stepping further, higher, or quicker). Ankle foot orthoses and posterior knee braces were allowed to minimize orthopedic concerns.
Targeting high aerobic intensities: Training was performed with a goal to maintain heart rate (HR) within 70–80% HR reserve27 where maximum predicted HR = 208–0.7*age28, and 70–80% HR reserve = rest HR + [(max HR − rest HR) × (70–80%)].8 The HR reserve was reduced by 10–15 beats/min according to age29 if participants were prescribed medications to reduce HR. Scores of 15–17 (hard to very hard) on the Rating of Perceived Exertion (RPE) were used for 2 subjects unable to achieve 70% HR reserve.
-
Increasing task demands: Specific perturbations were applied to assist or challenge biomechanical subcomponents of upright walking30, including limb swing, weight bearing during stance, forward propulsion, and sagittal-frontal stability. If participants could not demonstrate successful independent stepping as defined above, assistance was provided as needed. With successful stepping, perturbations were applied to increase task difficulty (i.e., error augmentation31), with the types and magnitudes of perturbations varied according to an individual’s gait impairments. If participants could not perform successful stepping after 3–5 consecutive attempts, task difficulty was reduced to allow continuous stepping. A list of perturbation strategies are provided in the Appendix and described below:
Limb swing: Participants unable to generate positive step-lengths without foot drag were provided manual16 or elastic32 assistance only as needed with progression to unassisted practice. With independent limb swing, task difficulty was increased using posterior-directed elastic resistance, leg weights23, or stepping over obstacles33.
Weight bearing during stance: Participants unable to ambulate without limb collapse were provided weight support with a counter-weight harness system only as needed, with support minimized as tolerated6. Task difficulty was increased using a weighted vest34 and reduced upper limb use on handrails6 or assistive devices.
Propulsion: Participants with reduced propulsion (i.e., slow gait speeds) were provided manual or elastic assistance at the pelvis34 to maintain desired walking speeds. Perturbations included reduced upper extremity use, faster stepping speeds5, inclined surfaces35, or elastic resistance at the pelvis34.
Sagittal/frontal plane stability: Participants with reduced frontal/sagittal stability were stabilized at the pelvis with manual or elastic assistance36 or allowed use of handrails6 or assistive devices. Task difficulty was increased by reducing assistance, handrail use or assistive devices, progressing to backward or side-stepping33, stepping over or around obstacles33, over uneven, compliant, or narrow surfaces37, and practicing dual physical tasks (e.g., catching or dribbling a ball during walking).
Training sessions were divided between treadmill, overground, and stair climbing activities, with continued focus on stepping amount and aerobic intensity during practice of multiple challenging tasks. In the initial 2 weeks (8–10 sessions), all training was performed on the treadmill at the highest speeds tolerated within the targeted intensities (i.e., speed-dependent treadmill training). Weight support was applied as necessary with treadmill speeds between 0.5–2.0 kmph; with minimal or no weight support (minimal tension on safety support system) speeds were increased > 2.0 kmph. Participants could hold onto the handrail but were encouraged to minimize weightbearing.
Training over the remaining sessions was divided in 10 min increments between speed-dependent treadmill training, skill-dependent treadmill training, overground training, and stair climbing. Skill-dependent treadmill training was performed by applying perturbations as described above, including walking in multiple directions or over obstacles with limited handrail use. Perturbations were applied such that 2–5 different tasks were randomly alternated and repeated within 10 min to ensure substantial task repetition. Overground training focused on high speeds or applied perturbations described above, with 2–5 tasks alternated within the 10 min period. Participants were guarded by therapists with use of a gait belt, or an overhead mobile or rail suspension system for safety as available. Stair climbing was performed over static or rotating stairs (i.e., similar to walking up a down-going escalator; Stairmaster, Vancouver, WA), using reciprocal gait patterns leading with the paretic limb, with progression to higher speeds and reduced hand rail use.
Outcome Measures
Primary outcomes included changes in daily stepping (steps/day), self-selected (SSS) and fastest possible gait speeds (FS) over short distances, and walking distance over 6 minutes (6 min walk). Outcomes were performed by un-blinded assessors. Daily stepping was recorded using Step Activity Monitors (Orthocare Innovations, Seattle, WA). Participants were requested to wear monitors for all waking hours throughout the study, with stepping activity recorded per minute. Changes in daily stepping without concurrent training were obtained at BSL, POST and F/U by averaging data over 5–14 days as available (MID stepping activity was not available without training). Additional analyses included weekly averaged steps/day throughout training, and averaged steps/session (1 week encompassed 4–5 sessions).
For overground SSS and FS, data were recorded using the Gait Mat II (Equitest Inc, Chalfont, PA), with assistive devices and braces. If participants required physical assistance, the amount of assistance and a SSS of 0 m/s were recorded. The 6 min walk was recorded at SSS; the test was terminated after 6 min or when participants required assistance.
Secondary measures of spatiotemporal symmetry were utilized to estimate gait quality; such measures have been linked to impaired paretic limb power generation38 and may reflect altered lower limb impairments and gait function post-stroke. Temporal symmetry was evaluated using paretic single limb stance time (expressed as % gait cycle), with typical values ~ 40%. Step length asymmetry was assessed using a ratio of step length used previously:16
This calculation results in a maximum value of 100% irrespective of which limb demonstrates longer step lengths, with improvements observed as positive values.
Measures of feasibility included number of sessions attended, average steps/sessions, and the sessions reaching the targeted aerobic intensities. Additional measures included number of dropouts and adverse events, including orthopedic injuries, cardiovascular events (e.g., incidence of hypertension or angina), and falls during and outside of training.
Data and Statistical Analysis
Outcome measures are presented as mean ± standard deviation in the text and tables, with mean ± standard errors in figures. Paired t-tests revealed no differences between PRE-BSL to BSL assessments in chronic participants; further analyses utilized only BSL data. Repeated-measures ANOVAs were utilized with subacute and chronic participants considered separately. Bonferroni corrections were applied for the 16 measures (α=0.0031), and post-hoc Tukey-Kramer tests used to identify specific differences. Daily stepping (steps/day) without concurrent training was evaluated at BSL, POST, and F/U. Separate ANOVAs determined differences in weekly steps/day and steps/session during training. Associations between changes in primary outcomes and steps/session or steps/day throughout the study were determined using Pearson’s correlation analyses.
Results
Twenty five (13 chronic, 12 subacute) of 36 individuals consecutively referred to the study fulfilled the inclusion criteria and were enrolled. Data from 3 chronic participants were excluded secondary to inability to complete at least 4 weeks of training. One participant reported exercise intolerance, a second participant relocated to another city, and a third was dismissed secondary to an unstable vascular condition (pulsatile mass in neck). Only the first participant was unable to sustain the targeted aerobic intensity prior to withdrawal.
Of those who completed ≥ 4 weeks of training, 3 subacute participants required moderate assistance to ambulate at BSL, with all other participant’s SSS < 0.9 m/s (Table 2). Two participants completed only 4–5 weeks of training secondary to exercise intolerance (1 chronic) or family obligations (1 subacute), with MID training outcomes imputed to POST. Four participants sustained a single fall outside of training (one with humeral fracture) but all completed the study. One reported angina during training which was controlled with prescribed medications. Incidence of hypertension limited training in 4 participants until consultation with their physician. Five reported transient lower extremity discomfort during training which did not interfere with study completion, with no additional orthopedic injuries. Demographic and clinical characteristics are presented in Tables 1–2.
Table 2.
Outcomes for chronic and subacute subjects.
| Chronic participants | BSL | POST | F/U | p-value | effect size |
|---|---|---|---|---|---|
| SSS (m/s) | 0.44±0.26 | 0.67±0.32 | 0.69±0.36 | <0.001* | 1.34 |
| single limb stance (%) | 20±6.5 | 24±6.4 | 23±7.9 | <0.001* | 1.46 |
| step length symm (%) | 70±24 | 79±18 | 74±25 | 0.370 | 0.36 |
| FS (m/s) | 0.56±0.40 | 0.94±0.48 | 0.83±0.44 | <0.001* | 1.62 |
| single limb stance (%) | 21±8.7 | 27±7.1 | 25±7.4 | <0.001* | 1.13 |
| step length symm (%) | 63±35 | 77±16 | 72±23 | 0.102 | 0.55 |
| 6 min walk (m) | 167±103 | 256±119 | 285±136 | <0.001* | 1.49 |
| daily stepping | 3357±2224 | 4021±1872 | 4564±1719 | 0.030 | 0.50 |
| Subacute participants | BSL | POST | F/U | p-value | effect size |
|---|---|---|---|---|---|
| SSS (m/s) | 0.33±0.27 | 0.66±0.35 | 0.65±0.36 | <0.001* | 1.64 |
| single limb stance (%) | 20±5.6 | 27±7.6 | 27±7.8 | <0.001* | 1.57 |
| step length symm (%) | 67±24 | 82±15 | 78±18 | 0.160 | 0.56 |
| FS (m/s) | 0.47±0.41 | 1.0±0.67 | 0.95±0.62 | <0.001* | 1.53 |
| single limb stance (%) | 23±5.9 | 30±7.4 | 30±7.9 | <0.001* | 2.11 |
| step length symm (%) | 66±20 | 82±10 | 80±13 | 0.048 | 0.70 |
| 6 min walk (m) | 119±94 | 263±170 | 260±169 | <0.001* | 1.47 |
| daily stepping | 2482±2110 | 3677±2796 | 3855±2289 | 0.022 | 0.84 |
BSL = baseline, POST = post training, F/U = follow-up,
Table 1.
Demographic and baseline characteristics of participants who completed the study; significant differences for duration post-stroke. Cardiac history denotes those with diagnosed coronary artery disease or congestive heart failure.
| Patient demographics | chronic (n=10) | subacute (n=12) |
|---|---|---|
| age (years) | 55±8.8 | 52±13 |
| gender (male/female) | 6/4 | 8/4 |
| hemiparesis (right/left) | 4/6 | 8/4 |
| duration (months) | 42±58 | 3.2±1.8 |
| ankle foot orthosis (n) | 6 | 4 |
| assistive device (n) | 9 | 9 |
| comorbidities (n) | ||
| cardiac history | 3 | 4 |
| hypertension | 9 | 11 |
| peripheral vascular disease | 3 | 1 |
| diabetes mellitus | 4 | 6 |
| renal disease | 0 | 2 |
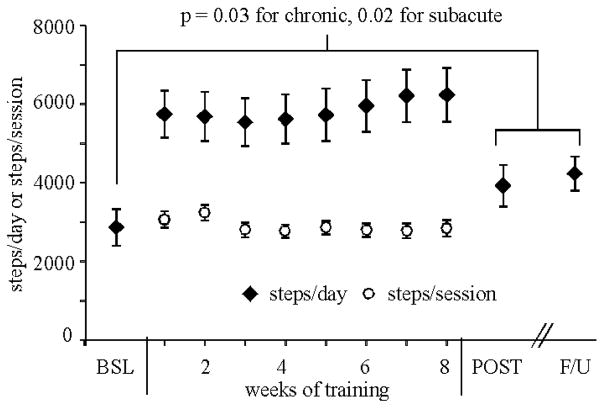
Participants performed an average of 36±5.8 training sessions (range 17–40). Nineteen of 22 participants achieved either 70% HR reserve or RPE ≥ 15 at the 1st session; all others reached the targeted aerobic intensity by the 6th session and were able to reach this intensity at every session thereafter. Figure 1 depicts mean steps/day of all participants at BSL, POST, and F/U assessments, with weekly average steps/day during training and steps/sessions. An average of 2887±780 steps/session (subacute: 2845±869; chronic: 2967±722) was performed with no differences across the weeks of training. An average of 5824±2772 steps/day was achieved during the weeks of training (training sessions included). Non-significant improvements were observed from BSL to POST (subacute: 1216±454 steps/day; chronic: 658±1367 steps/day) or F/U (subacute: 1395±1368 steps/day; chronic: 1206±1294 steps/day; Table 2).
Figure 1.
Average daily stepping across all participants at BSL (baseline), POST (post-training), and F/U (follow-up) at 3 months, with differences not significantly different between BSL compared to POST and F/U. Weekly averages of steps/session and steps/day throughout training were not different throughout training.
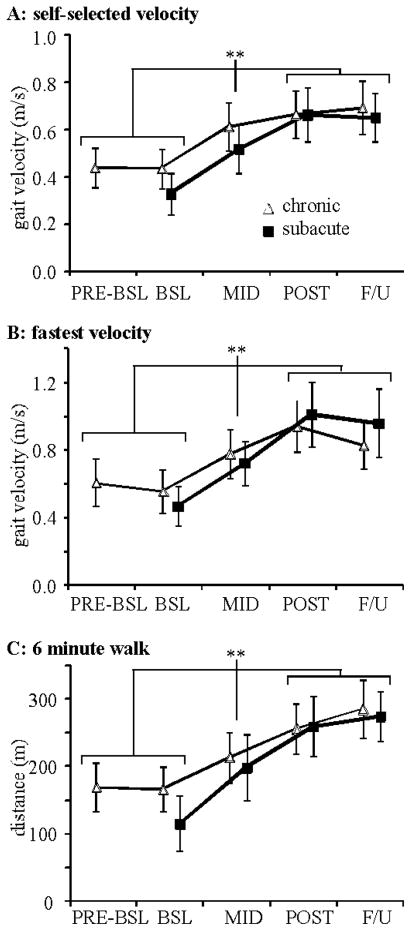
Figure 2 and Table 2 depict changes in SSS, FS, and 6 min walk. For chronic participants, there were no differences from pre-BSL to BSL assessments (Fig 2). Post-hoc analyses revealed improvements from BSL to MID, and MID to POST and F/U, with no differences between POST and F/U. Specific differences from BSL to POST included improvements in SSS (subacute: 0.33±0.20; chronic: 0.23±0.17 m/s), FS (subacute: 0.54±0.35 m/s; chronic: 0.39±0.23 m/s) and 6 min walk (subacute: 144±98 m; chronic: 89±60 m).
Figure 2.
Average changes in: (a) SSS, b) FS, and c) 6-minute walk at PRE-BSL (chronic only), BSL, MID, POST, and F/U (asterisks indicate Tukey-Kramer differences from BSL to MID to POST and F/U, with no differences at F/U).
Significant changes in gait symmetry were limited to paretic single limb stance time at SSS and FS in both participant populations, with ~6–7% improvements at POST training (normal ~40%; Table 2). Step length symmetry demonstrated positive but non-significant changes.
Correlation analyses revealed a moderate association between mean steps/session across all training and changes in 6 min walk from BSL to POST (r = 0.50; p = 0.02; Table 3). However, non-significant correlations were observed with changes in other primary outcomes (SSS: r = 0.00, p = 0.99; FS; r = 0.41, p = 0.06; daily stepping: r = 0.23, p = 0.34). Changes in primary outcomes were not correlated to mean steps/day during training (all r < 0.32). Further, changes in outcome measures were not associated to initial walking impairments, as demonstrated for initial vs change in 6 min walk distance (Fig 3B; r = 0.02; p = 0.92), with non-significant correlations for all other initial vs change scores (all r < 0.10).
Figure 3.
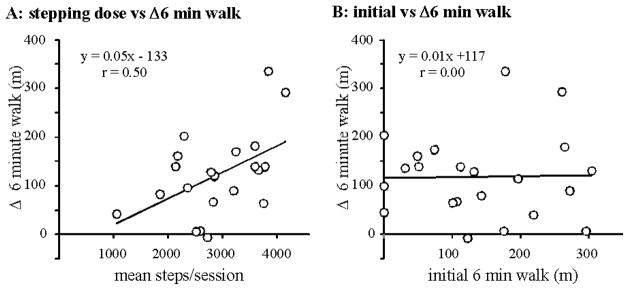
Correlations between: a) average stepping per session throughout session and changes in 6-minute walk, and b) initial and changes in 6-minute walk
Discussion
The present study assessed the feasibility and preliminary efficacy of providing stepping practice in variable challenging contexts at high aerobic intensities. Of those enrolled, 12% (3 chronic participants) dropped out or were dismissed prior to completing 4 weeks of training, similar to other stepping training protocols.11,14. Two additional participants did not complete 8 weeks with data imputed to POST training. Adverse events regarding fall incidence and cardiovascular events were noted, with only one event requiring study dismissal. Significant changes were observed for SSS, FS, and 6 min walk at MID training, with continued improvements at POST training. There was no significant improvement observed for daily stepping. Despite the primary focus on task completion (i.e., successful stepping as described), significant changes in paretic single limb stance but not step length symmetry were observed.
The average steps/session provided in the present study was nearly 3–10 times greater than previously observed in clinical physical therapy sessions of individuals post-stroke (average range of 200–900 steps/session)3,39. However, stepping activity in this study was approximately ~1000 steps/session less than forward treadmill training at high aerobic intensities3 in individuals with similar diagnoses and locomotor function (average SSS: 0.51±0.21m/s). The moderate, significant correlation with only 6 min walk suggests that amount of stepping practice may contribute to walking outcomes, although this relationship is uncertain.
The aerobic intensity of training may have also contributed to improved walking function. Training protocols that provide treadmill stepping at high aerobic intensities nearly always elicit improvements in cardiovascular function, but demonstrate variable changes in locomotor outcomes (6 min10,12 vs daily stepping3). However, improvements in standard measures such as SSS are often not significantly different from control interventions3,10,12.
Practice of multiple, variable tasks is not a novel rehabilitation strategy for individuals post-stroke. Multicenter evaluation of clinical physical therapy sessions with patients post-stroke in different settings indicate practice of many tasks occurs within single sessions (e.g., transfers, strengthening, balance, and gait)39. However, sufficient practice of any one task may be limited with this approach. Conversely, training of variable tasks while focusing entirely on stepping activities at high intensities has received very limited investigation in individuals post-stroke. In a higher functioning population (initial SSS: 0.62±0.24 m/s), Ada and colleagues33 employed a treadmill and overground walking program in multiple environments and directions with additional cognitive tasks, but did not focus on application of perturbations to elicit errors, nor on training intensity (see also 40 for similar protocols in SCI). Studies focusing on inducing or augmenting gait errors have revealed transient changes in specific biomechanical walking tasks (limb swing23, step symmetry24) following short-term training perturbations with recent promising data detailing long term improvements with sustained training25. However, focus on single gait deficits may not address the multiple impairments that contribute to locomotor dysfunction post-stroke.
An additional rationale for providing stepping training in variable challenging contexts was to simulate tasks and environments encountered in the community to improve daily stepping. The changes in daily stepping observed here were similar to other studies in patients post-stroke (subacute11, chronic3), although not statistically significant. Lack of significant improvements may be due in part to the strict alpha level utilized with multiple comparisons, but also the inherent variability of daily stepping observed in patients post-stroke (see Table 2). Nonetheless, the observed changes were not sufficient to increase average daily stepping above the threshold of sedentary activity (i.e., 5000 steps/day)41. Greater focus towards developing training paradigms or community programs directed towards enhancing daily stepping may be warranted.
Improvement in other primary outcomes were significant, with average changes well above minimally clinically important differences in gait speed (0.10 m/s) or 6 min walk (50 m)42. Improvements in paretic single limb stance time at SSS and FS were also observed, although changes may partly reflect increases in walking speed. In general, the kinematic patterns approached normal gait symmetry without substantial focus on gait patterns during training other than task completion.
Primary limitations of this preliminary study included a lack of a control group and un-blinded assessors; such limitations cannot exclude the possibility of history, testing, or selection effects, or potential bias by the assessors evaluating the reported outcomes. Further, the small sample size may limit the generalizability of the findings. The present data and derived effect sizes now permit sample sizes calculations for future randomized trials in similar patient populations. Given the variability of daily stepping, SSS, FS, and 6 min walk would be likely choices for primary outcomes, all of which demonstrated very large effect sizes. Defining comparative training strategies remains a primary question, however, as stepping amount, intensity and variability/challenge may all represent important independent training parameters. For example, high intensity treadmill training provides substantial amounts of stepping practice but limited variability, with improvements in chronic stroke ranging from 0.05–0.13 m/s for SSS3,5,10,43 and 27–58 m for 6 min walk3,10,12. For subacute stroke, changes in SSS range from 0.23–0.26 m/s4,11, with 6 min walk improvements from 60–82 m. Such changes are also substantial and above specific MCIDs, and evaluation of the present vs previous protocols requires further investigation.
In summary, substantial amounts of walking practice provided during high intensity stepping training in variable contexts was tolerated by nearly all participants throughout 4–8 weeks, and elicited significant improvements in selected outcome measures. Future investigations should focus on identifying the contribution of each training variable to locomotor outcomes.
Acknowledgments
Funding provided by NIDRR grant #H133B031127
Footnotes
Disclosures: none
References
- 1.Cha J, Heng C, Reinkensmeyer DJ, Roy RR, Edgerton VR, De Leon RD. Locomotor ability in spinal rats is dependent on the amount of activity imposed on the hindlimbs during treadmill training. J Neurotrauma. 2007;24:1000–12. doi: 10.1089/neu.2006.0233. [DOI] [PubMed] [Google Scholar]
- 2.De Leon RD, Hodgson JA, Roy RR, Edgerton VR. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J Neurophysiol. 1998;79:1329–40. doi: 10.1152/jn.1998.79.3.1329. [DOI] [PubMed] [Google Scholar]
- 3.Moore JL, Roth EJ, Killian C, Hornby TG. Locomotor training improves daily stepping activity and gait efficiency in individuals poststroke who have reached a “plateau” in recovery. Stroke. 2010;41:129–35. doi: 10.1161/STROKEAHA.109.563247. [DOI] [PubMed] [Google Scholar]
- 4.Plummer P, Behrman AL, Duncan PW, et al. Effects of stroke severity and training duration on locomotor recovery after stroke: a pilot study. Neurorehabil Neural Repair. 2007;21:137–51. doi: 10.1177/1545968306295559. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan K, Knowlton BJ, Dobkin BH. Step training with body weight support: effect of treadmill speed and practice paradigms on poststroke locomotor recovery. Arch Phys Med Rehabil. 2002;83:683–91. doi: 10.1053/apmr.2002.32488. [DOI] [PubMed] [Google Scholar]
- 6.Visintin M, Barbeau H, Korner-Bitensky N, Mayo NE. A new approach to retrain gait in stroke patients through body weight support and treadmill stimulation. Stroke. 1998;29:1122–8. doi: 10.1161/01.str.29.6.1122. [DOI] [PubMed] [Google Scholar]
- 7.Hesse S, Bertelt C, Jahnke MT, et al. Treadmill training with partial body weight support compared with physiotherapy in nonambulatory hemiparetic patients. Stroke. 1995;26:976–81. doi: 10.1161/01.str.26.6.976. [DOI] [PubMed] [Google Scholar]
- 8.Katch VL, McArdle WD, Katch FI. Essentials of Exericse Physiology. Baltimore, MD: Lippincott Williams & Williams; 2011. [Google Scholar]
- 9.Macko RF, DeSouza CA, Tretter LD, et al. Treadmill aerobic exercise training reduces the energy expenditure and cardiovascular demands of hemiparetic gait in chronic stroke patients. A preliminary report. Stroke. 1997;28:326–30. doi: 10.1161/01.str.28.2.326. [DOI] [PubMed] [Google Scholar]
- 10.Macko RF, Ivey FM, Forrester LW, et al. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke: a randomized, controlled trial. Stroke. 2005;36:2206–11. doi: 10.1161/01.STR.0000181076.91805.89. [DOI] [PubMed] [Google Scholar]
- 11.Duncan PW, Sullivan KJ, Behrman AL, et al. Body-weight-supported treadmill rehabilitation after stroke. N Engl J Med. 2011;364:2026–36. doi: 10.1056/NEJMoa1010790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Globas C, Becker C, Cerny J, et al. Chronic stroke survivors benefit from high-intensity aerobic treadmill exercise: a randomized control trial. Neurorehabil Neural Repair. 2012;26:85–95. doi: 10.1177/1545968311418675. [DOI] [PubMed] [Google Scholar]
- 13.Luft AR, Macko RF, Forrester LW, et al. Treadmill exercise activates subcortical neural networks and improves walking after stroke: a randomized controlled trial. Stroke. 2008;39:3341–50. doi: 10.1161/STROKEAHA.108.527531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hidler J, Nichols D, Pelliccio M, et al. Multicenter randomized clinical trial evaluating the effectiveness of the Lokomat in subacute stroke. Neurorehabil Neural Repair. 2009;23:5–13. doi: 10.1177/1545968308326632. [DOI] [PubMed] [Google Scholar]
- 15.Cai LL, Fong AJ, Otoshi CK, et al. Implications of assist-as-needed robotic step training after a complete spinal cord injury on intrinsic strategies of motor learning. J Neurosci. 2006;26:10564–8. doi: 10.1523/JNEUROSCI.2266-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hornby TG, Campbell DD, Kahn JH, Demott T, Moore JL, Roth HR. Enhanced gait-related improvements after therapist- versus robotic-assisted locomotor training in subjects with chronic stroke: a randomized controlled study. Stroke. 2008;39:1786–92. doi: 10.1161/STROKEAHA.107.504779. [DOI] [PubMed] [Google Scholar]
- 17.Lewek MD, Cruz TH, Moore JL, Roth HR, Dhaher YY, Hornby TG. Allowing intralimb kinematic variability during locomotor training poststroke improves kinematic consistency: a subgroup analysis from a randomized clinical trial. Phys Ther. 2009;89:829–39. doi: 10.2522/ptj.20080180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah PK, Gerasimenko Y, Shyu A, et al. Variability in step training enhances locomotor recovery after a spinal cord injury. Eur J Neurosci. 2012;36:2054–62. doi: 10.1111/j.1460-9568.2012.08106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Brand R, Heutschi J, Barraud Q, et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science. 2012;336:1182–5. doi: 10.1126/science.1217416. [DOI] [PubMed] [Google Scholar]
- 20.Shumway-Cook A, Patla AE, Stewart A, Ferrucci L, Ciol MA, Guralnik JM. Environmental demands associated with community mobility in older adults with and without mobility disabilities. Phys Ther. 2002;82:670–81. [PubMed] [Google Scholar]
- 21.Schmidt R, Lee T. Motor Control and Learning: A Behavioral Emphasis. 3. Champaign, IL: Human Kinetics Inc; 1999. [Google Scholar]
- 22.Bobath B. Adult Hemiplegia: Evaluation and Treatment. 3. Oxford: Butterworth-Heinemann; 1990. [Google Scholar]
- 23.Lam T, Wirz M, Lunenburger L, Dietz V. Swing phase resistance enhances flexor muscle activity during treadmill locomotion in incomplete spinal cord injury. Neurorehabil Neural Repair. 2008;22:438–46. doi: 10.1177/1545968308315595. [DOI] [PubMed] [Google Scholar]
- 24.Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain. 2007;130:1861–72. doi: 10.1093/brain/awm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reisman DS, McLean H, Keller J, Danks KA, Bastian AJ. Repeated split-belt treadmill training improves poststroke step length asymmetry. Neurorehabil Neural Repair. 2013;27:460–8. doi: 10.1177/1545968312474118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Functional Independence Measure: Guide for the Uniform Data Set for Medical Rehabilitation (Adult FIM). Version 4.0. Buffalo, NY: State University of New York at Buffalo; 1993. [Google Scholar]
- 27.Pang MY, Eng JJ, Dawson AS, McKay HA, Harris JE. A community-based fitness and mobility exercise program for older adults with chronic stroke: a randomized, controlled trial. J Am Geriatr Soc. 2005;53:1667–74. doi: 10.1111/j.1532-5415.2005.53521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37:153–6. doi: 10.1016/s0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- 29.Cohen-Solal A, Baleynaud S, Laperche T, Sebag C, Gourgon R. Cardiopulmonary response during exercise of a beta 1-selective beta-blocker (atenolol) and a calcium-channel blocker (diltiazem) in untrained subjects with hypertension. Journal of cardiovascular pharmacology. 1993;22:33–8. doi: 10.1097/00005344-199307000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Gottschall J, Kram R. Energy cost and muscular activity required for leg swing during walking. J Appl Physiol. 2005;99:23–0. doi: 10.1152/japplphysiol.01190.2004. [DOI] [PubMed] [Google Scholar]
- 31.Reisman DS, Bastian AJ, Morton SM. Neurophysiologic and rehabilitation insights from the split-belt and other locomotor adaptation paradigms. Phys Ther. 2010;90:187–95. doi: 10.2522/ptj.20090073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gottschall J, Kram R. Energy cost and muscular activity required for propulsion during walking. J Appl Physiol. 2003;94:1766–72. doi: 10.1152/japplphysiol.00670.2002. [DOI] [PubMed] [Google Scholar]
- 33.Ada L, Dean CM, Hall JM, Bampton J, Crompton S. A treadmill and overground walking program improves walking in persons residing in the community after stroke: a placebo-controlled, randomized trial. Arch Phys Med Rehabil. 2003;84:1486–91. doi: 10.1016/s0003-9993(03)00349-6. [DOI] [PubMed] [Google Scholar]
- 34.Grabowski A, Farley CT, Kram R. Independent metabolic costs of supporting body weight and accelerating body mass during walking. J Appl Physiol. 2005;98:579–83. doi: 10.1152/japplphysiol.00734.2004. [DOI] [PubMed] [Google Scholar]
- 35.Leroux A, Fung J, Barbeau H. Adaptation of the walking pattern to uphill walking in normal and spinal-cord injured subjects. Exp Brain Res. 1999;126:359–68. doi: 10.1007/s002210050743. [DOI] [PubMed] [Google Scholar]
- 36.Donelan JM, Shipman DW, Kram R, Kuo AD. Mechanical and metabolic requirements for active lateral stabilization in human walking. J Biomech. 2004;37:827–35. doi: 10.1016/j.jbiomech.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Domingo A, Ferris DP. The effects of error augmentation on learning to walk on a narrow balance beam. Exp Brain Res. 2010 doi: 10.1007/s00221-010-2409-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch Phys Med Rehabil. 2007;88:43–9. doi: 10.1016/j.apmr.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Lang CE, Macdonald JR, Reisman DS, et al. Observation of amounts of movement practice provided during stroke rehabilitation. Arch Phys Med Rehabil. 2009;90:1692–8. doi: 10.1016/j.apmr.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Musselman KE, Fouad K, Misiaszek JE, Yang JF. Training of walking skills overground and on the treadmill: case series on individuals with incomplete spinal cord injury. Phys Ther. 2009;89:601–11. doi: 10.2522/ptj.20080257. [DOI] [PubMed] [Google Scholar]
- 41.Tudor-Locke C, Bassett DR., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34:1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 42.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–9. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 43.Sullivan KJ, Brown DA, Klassen T, et al. Effects of task-specific locomotor and strength training in adults who were ambulatory after stroke: results of the STEPS randomized clinical trial. Phys Ther. 2007;87:1580–602. doi: 10.2522/ptj.20060310. discussion 603–7. [DOI] [PubMed] [Google Scholar]