Abstract
Androgen-independent nuclear localization is required for androgen receptor (AR) transactivation in castration-resistant prostate cancer (CRPC) and should be a key step leading to castration resistance. However, mechanism(s) leading to androgen-independent AR nuclear localization are poorly understood. Since the N-terminal domain (NTD) of AR plays a role in transactivation under androgen-depleted conditions, we investigated the role of NTD in AR nuclear localization in CRPC. Deletion mutagenesis was used to identify amino acid sequences in the NTD essential for its androgen-independent nuclear localization in C4-2, a widely used CRPC cell line. Deletion mutants of AR tagged with green fluorescent protein (GFP) at the 5`-end were generated and their signal distribution was investigated in C4-2 cells by fluorescent microscopy. Our results showed that the region of a.a. 294–556 was required for androgen-independent AR nuclear localization whereas a.a. 1–293 mediates Hsp90 regulation of AR nuclear localization in CRPC cells. Although a.a. 294–556 does not contain a nuclear import signal, it was able to enhance DHT-induced import of the ligand binding domain (LBD). Also, transactivation of the NTD could be uncoupled from its modulation of AR nuclear localization in C4-2 cells. These observations suggest an important role of NTD in AR intracellular trafficking and androgen-independent AR nuclear localization in CRPC cells.
Introduction
Androgens play a vital role in the development and homeostasis of male sex organs (1) as well as the development and progression of benign prostatic hyperplasia (BPH) and prostate cancer (PCa) (2–5). Androgen-deprivation therapy (ADT) is the standard for treating metastatic PCa, however, patients invariably recur with more aggressive castration-resistant prostate cancer (CRPC) (4, 6, 7). During progression to castration resistance, PCa cells utilize a variety of cellular pathways in order to survive and flourish in an androgen-depleted environment [6, 7]. High levels of androgen receptor (AR) expression and renewed expression of androgen-regulated genes indicate that AR transcriptional activity is reactivated in CRPC under castration conditions (8). However, the mechanisms leading to AR activation in CRPC remain incompletely understood.
The human AR is a kD, 919 amino acid protein composed of four domains: 1) the amino terminal activation domain (NTD), 2) the DNA-binding domain (DBD), 3) the hinge region and 4), the carboxyl ligand-binding domain (LBD) (9). The NTD (a.a. 1–556) includes the majority of the AR and is the least conserved allowing AR to differentially recruit co-regulators conferring androgen specific transactivation. Proper activation of the AR requires the first 30 amino acids of the NTD for the amino-carboxyl terminal (N/C) interaction (10–15). AR transactivational activity is primarily mediated through the NTD region containing the activation function 1 (AF1) element, which distinguishes AR from the other steroid receptors that utilize the AF2 region in the LBD (16). In addition to the NTD region, two nuclear localization signals have been reported in the AR. A bipartite nuclear localization signal (NLS1) is present in the DBDH region (17, 18) and the LBD (a.a. 666–919) contains a second nuclear localization signal (NLS2) upon androgen binding. Additionally, the LBD contains a nuclear export signal (NES), which functions in the absence of androgens (19).
A key regulatory step in the action of AR is its translocation to the nucleus. Intracellular trafficking is an important mechanism in the regulation of transcription factors, including AR (20–23). In order for AR to act as a transcription factor it must gain entry to the nucleus. In the prostate, androgens bind to AR in the cytoplasm, causing phosphorylation, dimerization, and subsequent translocation into the nucleus, thereby binding to the androgen-response elements within the DNA, with subsequent activation of genes involved in cell growth and survival. During the progression of prostate cancer to castration-resistance, the tightly regulated androgen signaling pathway is disrupted such that AR can localize to the nucleus and activate its target genes in the absence of androgens. Our recent studies suggest that Hsp90 is required for androgen-independent AR nuclear localization in CRPC, although the mechanism involved remains unclear (24, 25).
Many studies have revealed various types of AR gene mutations that contribute to diseases including spinobulbar muscular atrophy (SBMA) (26), androgen insensitivity syndrome (AIS) (27), and prostate cancer (28, 29). Several reports have revealed that mutant ARs obtained from both AIS and prostate cancer patients may exhibit abnormal intracellular localization and lower capacity for ligand-dependent translocation when compared with wild-type AR. These AR mutants fail to achieve normal nuclear import and exhibit a distinct intracellular aggregation profile (18, 30–33). These findings suggest that abnormal intracellular localization of the AR mutants could be involved in the pathogenesis of these diseases. Elucidation of the nuclear import mechanism of AR will not only contribute to an understanding of androgen influence in AR-related diseases but also provide potential targets to modulate androgen action.
In order to elucidate the mechanism(s) regulating androgen-independent AR nuclear localization in CRPC, the effect of AR deletion mutagenesis on intracellular localization was tested using fluorescent microscopy (18). In this report, C4-2 cells which were generated through multiple stages of co-culture of androgen-sensitive LNCaP prostate cancer cells with human bone fibroblast MS cells in vivo in castrated male athymic mice (34), were used as a model for CRPC. This study demonstrated an important role of the NTD in regulating AR intracellular trafficking and its androgen-independent nuclear localization in CRPC.
Methods
Expression Vector Construction
The expression vector pEGFP-C1 (Clontech) was used to generate fusion protein constructs with GFP at the N terminus of AR and various AR mutants for convenient visualization by fluorescent microscopy. Fusion protein constructs generated were: pEGFP-AR, pEGFP-LBDAR(666–918), pEGFP-DBDH-LBDAR(557–919), GFP-ARΔ(294–556), GFP110 ARΔ(1–293), GFP-NTD, GFP-AR(294–556), GFP-AR(1–293)-LBD, GFP-AR(294–556)-LBD, and GFP-NTD-LBD. Various AR mutants were generated by anchor PCR using the full-length human AR cDNA as the template, which was kindly provided by Dr. Shutsung Liao (University of Chicago). All expression vectors were verified by sequencing analysis, and plasmids were double CsCl gradient purified for transient transfection.
Cell Culture and Transfection
COS-1 and LNCaP cell lines were purchased from American Type Culture Collection (Manassas, VA) and LAPC4 cells were obtained from Dr. Robert Reiter (UCLA). C4-2 cells were obtained from Dr. Leland Chung (Cedars Sinai Medical Center). All cell lines were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 1% glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen) at 37 °C in the presence of 5% CO2 in a humidified incubator. For androgen-free conditions, RPMI 1640 medium was supplemented with FBS stripped 2 times with charcoal to remove all androgens.
AR deletion mutants were transfected into COS-1, LAPC4, LNCaP and C4-2 cells using Lipofectamine™ according to the manufacturer's protocol (Invitrogen). Cells were transfected at >60% confluence in phenol red-free OptiMEM. Localization of GFP fusion proteins was imaged 16 h after transfection or at indicated time with fluorescence microscopy using either a Nikon TE 2000U or Nikon TS100 inverted microscope.
For experiments involving nuclear import and export of GFP-AR fusion proteins, transfected cells were allowed to express the appropriate chimeric protein for 16 hours prior to treatment of indicated amount of DHT for 4 hours in complete medium prior to imaging. For testing the effect of androgen withdrawal, DHT-treated cells were rinsed twice with medium containing charcoal-stripped serum at 0, 30, 60, and 120 min for complete removal of any residual DHT present in the media. Cells were imaged at 12 h after initial androgen withdrawal.
Cytoplasmic localization of GFP fusion proteins in transfected cells was defined when the cytoplasmic GFP fluorescence was greater than that in the nuclei. Likewise, nuclear localization was defined when nuclear GFP fluorescence was greater than that in the cytoplasm. Even distribution was defined when GFP fluorescence was evenly distributed between the nucleus and cytoplasm as previously described (19). Nuclei were stained with 1 mM/L Hoechst 33342 solution (Sigma-Aldrich).
To determine the effect of Hsp90 inhibition, C4-2 cells were transfected with GFP-tagged AR or AR deletion mutants and then treated with 300 nM 17-AAG (17-AAG was a generous gift of the NCI (Rockville, MD)) 16 hours after transfection. The subcellular localization was determined by fluorescent microscopy 4 hours after treatment with17-AAG.
TIF2 knockdown and western blot analysis
C4-2 cells were transfected with control siRNA or siTIF2 (sc-38882, Santa Cruz Biotechnology) for 72 hours. Cells were lysed in buffer containing 10 mM NaPO4, 1% Triton-X-100, 1 mM EDTA, 150 mM NaCl, 2 mM PMSF, 1 mM Na3VO4, and protease inhibitor cocktail (Sigma). Lysates were subjected to SDS–PAGE and Western blotting was conducted using antibodies against TIF2 (C-20, sc-6976), PSA (A67-B/E3, sc-7316) and GAPDH (FL-335, sc-25778) all from Santa Cruz Biotechnology.
Results
The NTD retards nuclear export of AR in COS-1 cells
Transfected GFP-AR and GFP-LBD were localized in the cytoplasm of COS-1 cells in the absence of DHT, and GFP-DBDH-LBD was evenly distributed between the cytoplasm and nuclei (Fig. 1). Treatment with 1 nM DHT induced nuclear import of GFP-AR and GFP-DBDH-LBD within 4 hours after DHT induction (Fig. 1), whereas 100 nM DHT was required to induce nuclear import of GFP-LBD. A redistribution of GFP-LBD from the nucleus to the cytoplasm was observed 12 hours after DHT withdrawal (Fig. 1), consistent with our previous observation (19). Similarly, hormone withdrawal resulted in transition of GFP-DBDH-LBD from the nucleus to being evenly distributed between the cytoplasm and nucleus in COS-1 cells. In contrast, after hormone withdrawal, GFP-AR remained predominantly in the nucleus, with the cytoplasmic GFP signal being slightly but consistently increased (Fig. 1), in agreement with our previous findings (19). These observations suggest the suppression of AR nuclear export by NTD.
Figure 1. Deletion mutagenesis analysis of AR nuclear import and export in COS1 cells upon androgen manipulation.
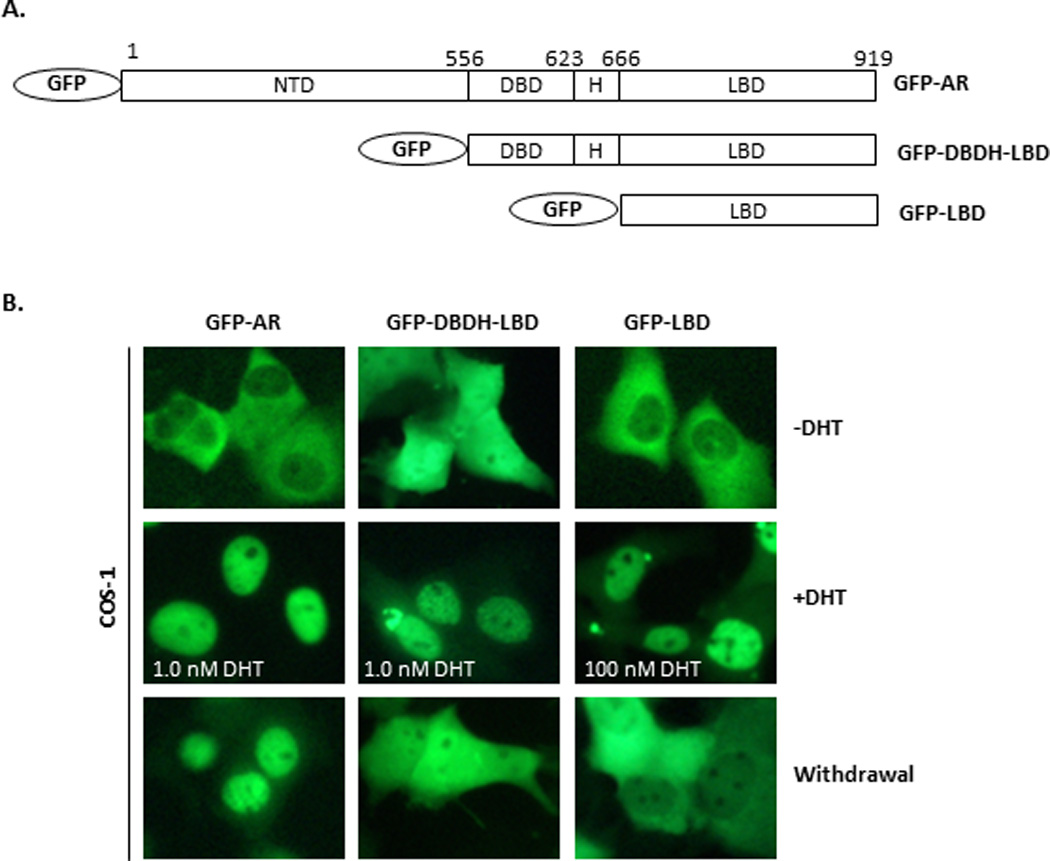
(A) Structures of GFP fusion protein constructs: GFP-AR, GFP-DBDH-LBD, and GFP-LBD. (B) COS1 cells were transiently transfected with GFP-AR, GFP-DBDH-LBD and GFP-LBD cultured in androgen-free conditions. The subcellular signal distribution was visualized by fluorescent microscopy 16 hours post transfection (−DHT), 4 hours after treatment (+DHT) with 1.0 nM DHT for GFP-AR and GFP-DBDH-LBD or 100 nM DHT for GFP-LBD, or 12 hours after androgen withdrawal (Withdrawal). The experiment was reproduced 5 times.
The NTD is required for androgen-independent nuclear localization of AR in C4-2 cells
Transfected GFP-AR is localized to the cytoplasm in AR-negative cell lines such as COS-1 and PC3 in androgen-free medium (Fig. 1) (35, 36). In contrast, GFP-AR is localized to the nucleus in castration resistant C4-2 cells under the same conditions (25). To evaluate the importance of various domains of AR in its androgen-independent nuclear localization in CRPC, we examined the intracellular localization of GFP-AR, GFP-DBDH-LBD and GFP-LBD in C4-2 cells. As expected, GFP-AR was localized to the nuclei of C4-2 cells in androgen-free culture medium (Fig. 2), which is distinctly different from the cytoplasmic localization in COS-1 cells (Fig. 1) and LNCaP cells (37). However, GFP-DBDH-LBD and GFP-LBD exhibited even localization and cytoplasmic localization, respectively, in C4-2 cells, which were the same as their subcellular localizations in COS-1 cells. The above observation suggests that the NTD is responsible for the differential subcellular localization between C4-2 and COS-1 cells in androgen-free medium and plays a key role in androgen-independent nuclear localization of AR in C4-2 cells.
Figure 2. Subcellular distribution of GFP-AR, GFP-DBDH-LBD and GFP-LBD in C4-2 cells.
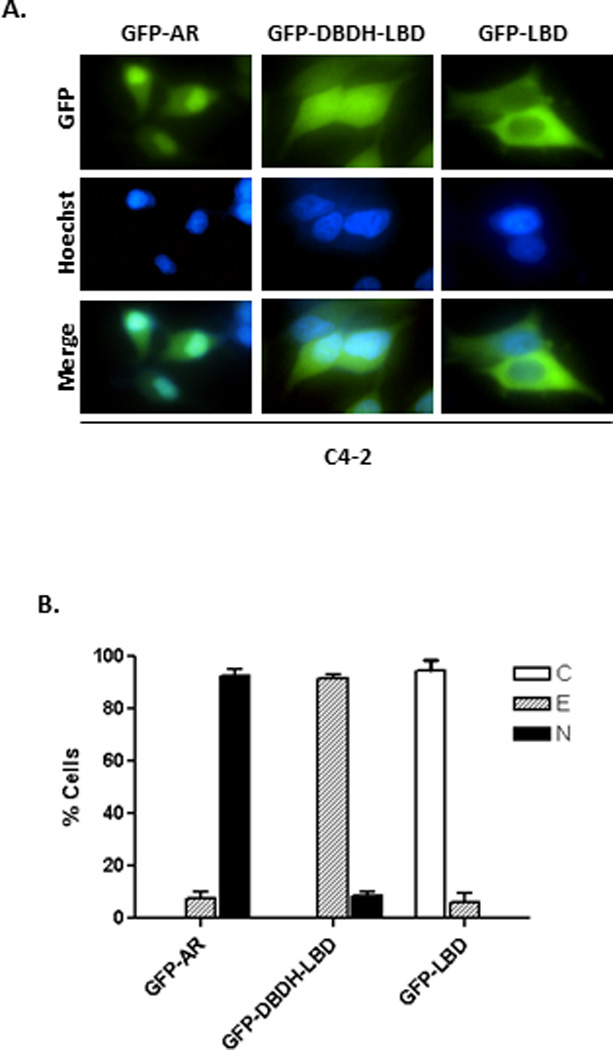
C4-2 cells were transiently transfected with GFP-AR, GFP-DBDH-LBD and GFP-LBD and stained with Hoechst. The subcellular localization (A) and quantification (B) were assessed in androgen-free conditions by fluorescent microscopy 16 hours after transfection. The results are from five transfections for each experimental group. At least 200 cells were counted for each transfection to determine the percentage of cells displaying cytoplasmic (C), even (E), or nuclear (N) localization. The experiment was reproduced 5 times.
Amino acid (a.a.) 294–556 of the NTD is necessary for androgen-independent AR nuclear localization in C4-2 cells
In order to identify the amino acid sequences within the NTD of AR that are essential for androgen-independent nuclear localization in C4-2 cells, we examined the intracellular localization of the deletion mutants GFP-ARΔ(294–556) and GFP-ARΔ(1–293) using fluorescence microscopy (Fig. 3A). GFP-ARΔ(294–556) displayed an even distribution between nucleus and cytoplasm. However, GFP-ARΔ(1–293) exhibited predominant nuclear localization in transfected C4-2 cells, which is comparable to what was observed in the full-length GFP-AR (Fig. 3B–C). These results suggest that a.a. 294–556 of the NTD region is necessary for androgen-independent nuclear localization of AR in C4-2 cells.
Figure 3. Subcellular distribution of GFP-AR, GFP-ARΔ(294–556) and GFP-ARΔ(1–293) in C4-2 cells.
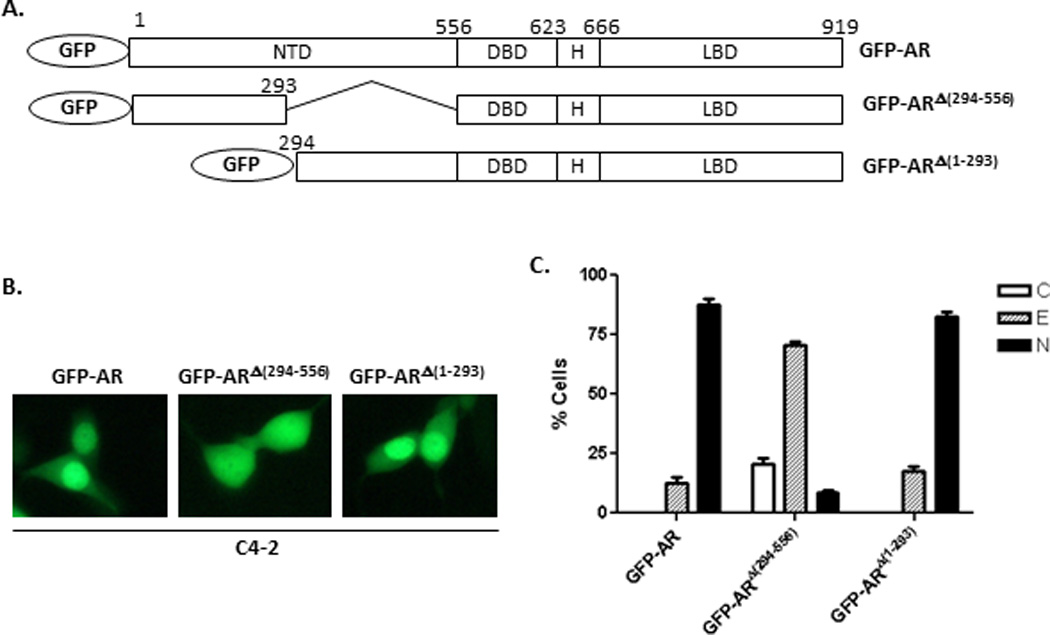
(A) Diagram of different GFP-AR deletion constructs, GFP-AR, GFP-ARΔ(294–556), and GFP-ARΔ(1–293). (B) Representative fluorescent images of C4-2 cells transiently transfected with GFP-AR, GFP-ARΔ(294–556) or GFP-ARΔ(1–293) in in androgen-free conditions 16 hours after transfection. The results were derived from five transfections for each GFP-fusion protein construct. (C) Quantitative analysis of results in (B). At least 200 cells were counted for each transfection to determine the percentage of the cells displaying cytoplasmic (C), even (E), or nuclear (N) localization. The experiment was reproduced 5 times.
The NTD or a.a. 294–556 region alone has no significant effect on tagged-GFP subcellular localization in C4-2 cells
In order to determine whether a.a. 294–556 alone would affect tagged-GFP subcellular localization, GFP-NTD and GFP-AR(294–556) (Fig. 4A) were transfected into LAPC4, C4-2 and LNCaP cells and subcellular localization was assessed. Both GFP-NTD and GFP-AR(294–556) were localized evenly between the nucleus and cytoplasm in LAPC4, C4-2, and LNCaP cells, although GFP-AR(294–556) had a slight propensity towards being more nuclear (Fig. 4B). Similarly, in the androgen-negative PC3 cells, both GFP-NTD and GFP-AR(294–556) exhibited even localization between the nucleus and the cytoplasm (38). These results suggest that a.a. 294–556 together with DBDH-LBD are essential for androgen-independent nuclear localization of AR in LAPC4, C4-2 and LNCaP cells.
Figure 4. Subcellular localization of GFP-AR(294–556) and GFP-NTD in LAPC4, C4-2 and LNCaP cells.
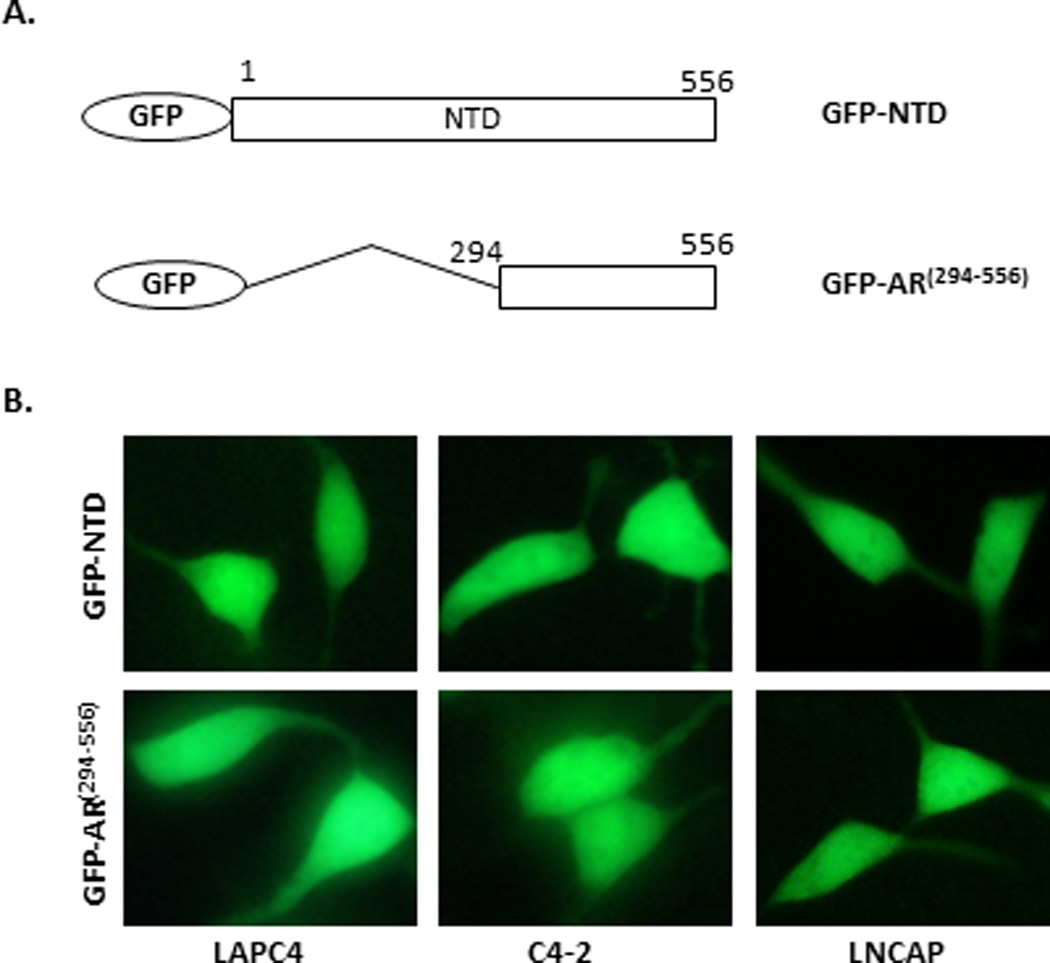
(A) Diagram of GFP fusion constructs GFP-NTD and GFP-AR(294–556). (B) Representative images of GFP-NTD and GFP-AR(294–556) in transiently transfected LAPC4, C4-2, and LNCaP cells. The subcellular localization was assessed in complete medium by fluorescence microscopy after 16 hours of transfection. The experiment was reproduced 5 times.
a.a. 294–556 of NTD enhanced DHT-induced nuclear localization of GFP-LBD in C4-2 cells
Although a.a. 294–556 of the AR does not appear to contain a nuclear localization signal, it may enhance AR nuclear import in C4-2 cells. Since GFP-ARΔ(1–293) is already in the nuclei of C4-2 cells in androgen-free media, it is not feasible to test the effect of a.a. 294–556 on nuclear import of this construct, which contains a nuclear localization signal (NLS1) in the DBDH region (18, 39). Thus, we transfected C4-2 cells with different GFP deletion constructs: GFP-AR(1–293)-LBD, GFP-AR(294–556)-LBD, and GFP-LBD (Fig. 5A). Intracellular localization of various transfected GFP-fusion proteins was examined (Fig. 5B). In the absence of DHT, both GFP-LBD and GFP-AR(1–293)-LBD localized to the cytoplasm of the transfected cells, while GFP-AR(294–556)-LBD displayed even localization between the nucleus and cytoplasm (Fig. 5B). Upon treatment with DHT, specifically at higher doses (10–100nM), GFP-AR(294–556)-LBD was predominantly localized in the nucleus, while at lower doses (0.1–1.0nM) GFP-AR(294–556)-LBD still displayed GFP fluorescent cells that were evenly distributed between the nucleus and cytoplasm of the cell (Fig. 5C). In contrast, GFP-AR(1–293)-LBD localized evenly in the cell at high doses of DHT (10–100nM) (Fig. 5C). GFP-LBD also displayed even localization at 10 nM DHT and it took very high levels of DHT (100nM) for GFP-LBD to begin to enter the nucleus (Fig. 5C). The subcellular localization of GFP-NTD-LBD in response to DHT was similar to GFP-AR(1–293)-LBD, suggesting that a.a.1–293 could counteract the effect of a.a.294–556 in the construct. As a control and as expected, most of the transfected C4-2 cells displayed even or nuclear localization of GFP-AR in the absence of androgens, predominant nuclear localization in the presence of 0.1 nM DHT and complete nuclear localization in the presence of 1–100 nM DHT (Fig. 5C). These results suggest that a.a. 294–556 enhances the androgen stimulated nuclear import of AR in C4-2 cells.
Figure 5. Effect of AR(294–556)on LBD sensitivity to DHT in C4-2 cells.
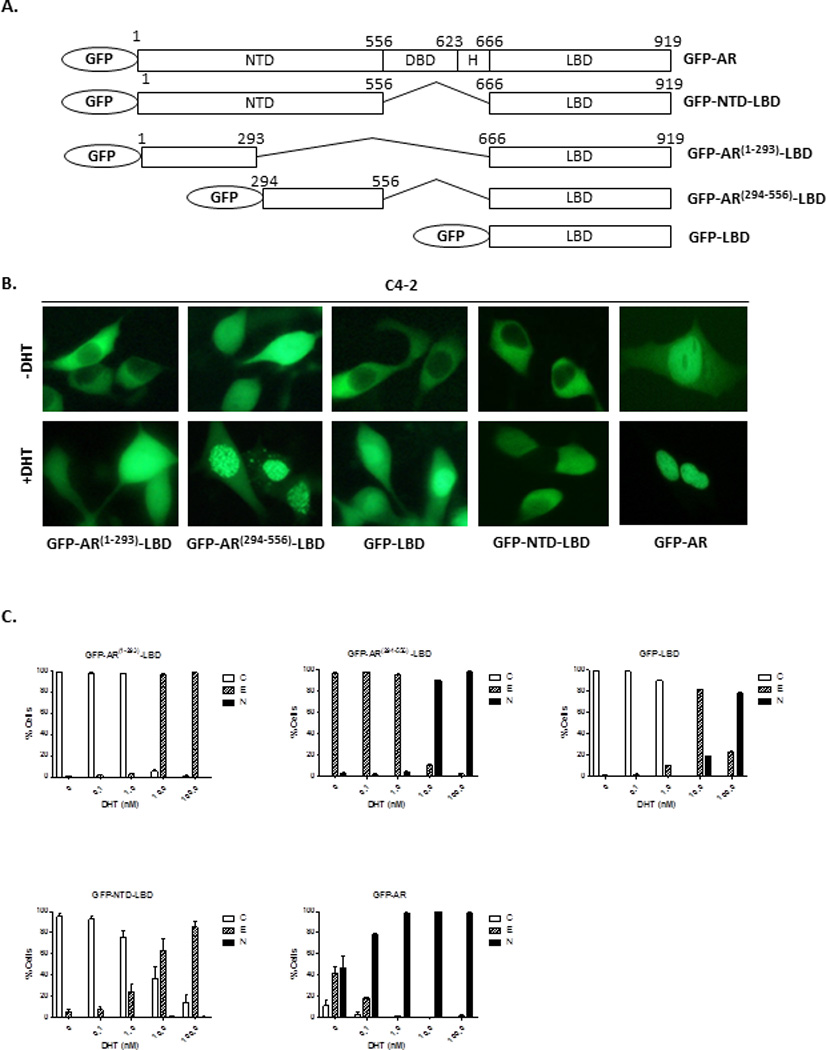
(A) Diagram of GFP fusion constructs GFP-AR, GFP-NTD-LBD, GFP-AR(1–293)-LBD, GFP-AR(294–556)-LBD, and GFP-LBD. (B) Representative images of GFP-tagged protein localization in the absence or presence of 10 nM DHT. (C), Quantification analysis of GFP fusion constructs in C4-2 cells followed by treatment of DHT (0–100 nM) (B). At least 200 cells were counted for each transfection to determine the percentage of the cells displaying cytoplasmic (C), even (E), or nuclear (N) localization. The subcellular localization and quantification of signal were assessed by fluorescent microscopy. The experiment was reproduced 3 times.
The effect of a.a. 294–556 on androgen-independent nuclear localization of AR in C4-2 cells is insensitive to the Hsp90 inhibitor 17-AAG
We have previously reported the regulation of androgen-independent localization of AR by Hsp90 (8, 25). However, the mechanisms of HSP90 regulation of AR androgen-independent nuclear localization remain unclear. Since the NTD plays an important role in androgen-independent AR nuclear localization, we tested whether the HSP90 inhibitor 17-AAG can block the effect of a.a. 294–556 on androgen-independent nuclear localization of AR. As expected, 17-AAG treatment induced cytoplasmic localization of GFP-AR in C4-2 cells (Fig. 6) and had no effect on GFP alone (data not shown) (8). Interestingly, 17-AAG did not affect the subcellular localization of GFP-DBDH-LBD, which remained evenly distributed in the presence of 17-AAG (Fig. 6). The nuclear localization of GFP-ARΔ(1–293), which retains a.a. 294–556, was not disrupted by 17-AAG. However, GFP-ARΔ(294–556) was sensitive to 17-AAG inhibition, which exhibited even distribution between nucleus and cytoplasm in the absence of 17-AAG and cytoplasmic localization in the presence of 17-AAG. This suggests that 17-AAG inhibits androgen-independent nuclear localization of AR and is mediated through a.a. 1–293 of NTD but not a.a. 294–556, although a.a. 294–556 is necessary for androgen-independent nuclear localization of AR.
Figure 6. Localization of GFP-tagged AR and AR deletion mutants in C4-2 cells in presence of Hsp90 inhibitor.
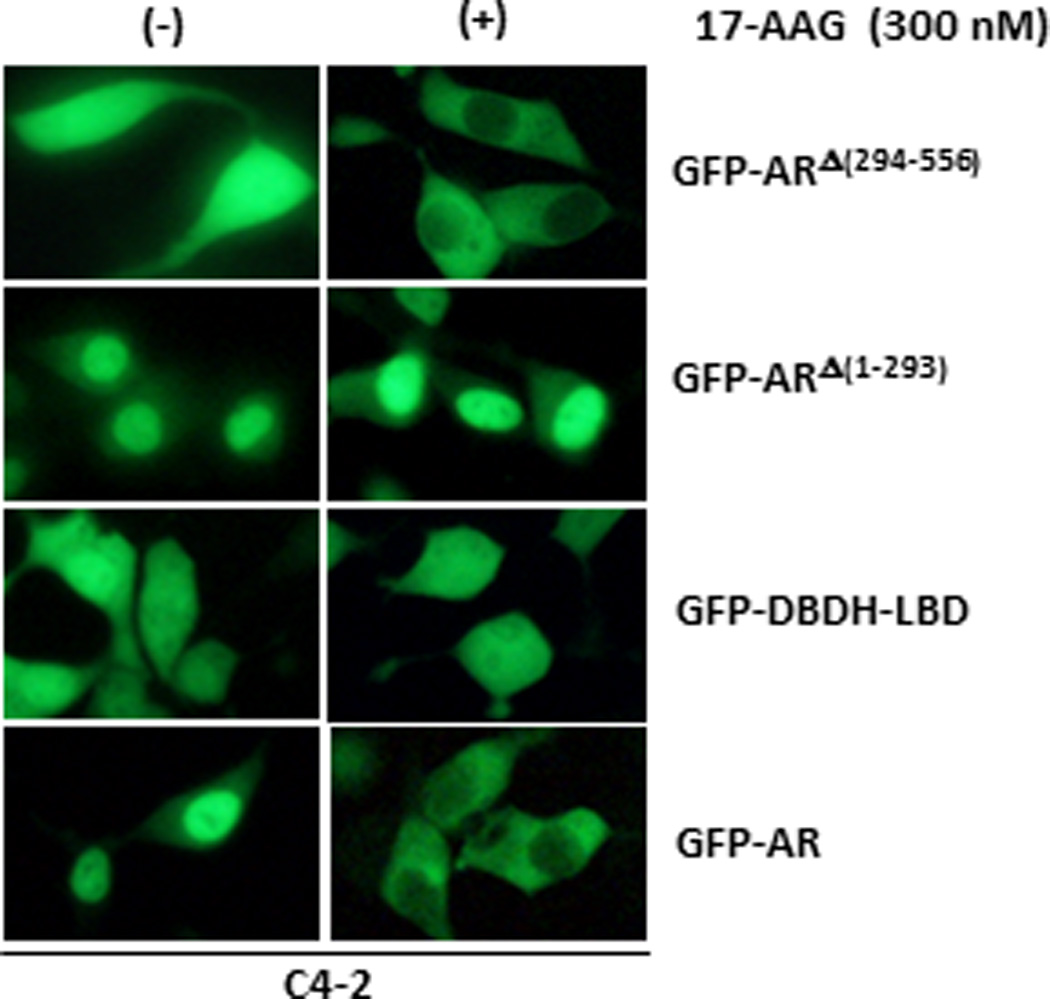
C4-2 cells transfected with GFP-AR, GFP-ARΔ(294–556), GFP-ARΔ(1–293) and GFP-DBDH-LBD were treated with DMSO (control) or HSP90 inhibitor 17-AAG in androgen-free conditions. Localization was assessed by fluorescent microscopy 4 hr after the treatment.
TIF-2 is required for AR activation but not androgen-independent nuclear localization in C4-2 cells
The region of a.a. 294–556 contains the TAU-5 transactivation domain, which is essential for androgen-independent activation of AR (40, 41). Transcription Intermediary Factor 2 (TIF-2), a member of the steroid receptor co-activator family, plays a key role in androgen-independent activation of AR in CRPC cells and has been shown to bind to the TAU-5 motif (39). Knockdown of TIF-2 has been reported to inhibit androgen-independent AR activation in C4-2 cells (40). Since TIF-2 binds to TAU-5 within a.a. 294–556, we decided to test whether knockdown of TIF-2 could also affect androgen-independent nuclear localization of AR in C4-2 cells. As expected, TIF-2 knockdown inhibited the expression of PSA, a gene regulated by AR transcriptionally (Fig. 7A). However, TIF-2 knockdown did not inhibit androgen-independent nuclear localization of GFP-AR in C4-2 cells (Fig. 7B–C).
Figure 7. Effect of TIF2 knockdown on AR subcellular localization and PSA expression in C4-2 cells.
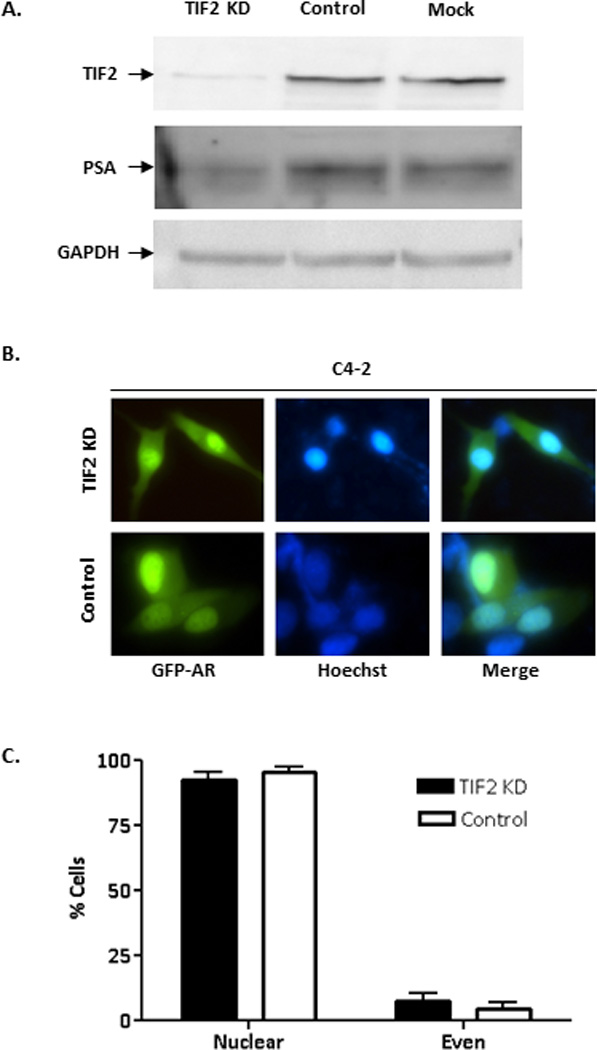
(A) Western blot analysis of C4-2 cells transfected with control siRNA or siTIF2 for 72 hours. The Western blots were probed with anti-TIF2, anti-PSA, or anti-GAPDH (loading control) antibodies. (B) Representative images of GFP-AR in C4-2 cells co-transfected with GFP-AR and either control siRNA or siTIF2 for 72 hours. Localization of GFP-AR was determined using fluorescence microscopy. (C) Percentage of cells displaying nuclear or even localization was determined for GFP-AR in cells co-transfected with either control siRNA or siTIF2. The experiment was reproduced 2 times.
Discussion
Multiple AR domains/motifs and their interactions within AR and with AR co-factors are likely involved in determining the ultimate subcellular localization of AR. Identification of the domains/motifs important in regulating AR intracellular trafficking and/or subcellular localization provides a foundation for further characterization of their interactions essential for androgen-independent AR nuclear localization in CPRC. We have explored the mechanism of androgen-independent nuclear localization of AR by characterizing the subcellular localization of different AR deletion mutants in castration-resistant C4-2 cells. Our findings suggest an important role of the NTD in castration-resistance of prostate cancer, with a.a. 294–556 in the NTD being required for androgen-independent AR nuclear localization.
Previous studies have shown the important roles of the DBDH and LBD regions in nucleocytoplasmic trafficking of AR, with NLS1 present in the DBDH region and NLS2 and NES in the LBD (17–19). Numerous studies have demonstrated the importance of the NTD in AR transactivation (11, 36, 42–45). However, the role of the NTD in AR intracellular trafficking is poorly understood, particularly with regards to the androgen-independent nuclear localization of AR. NTD modulation of AR nuclear localization has previously been reported to involve tubulin, which can be targeted by microtubule-targeting chemotherapy drugs such as docetaxel (37). Our findings of the NTD inhibiting nuclear export of AR in COS-1 cells and its requirement in androgen-independent nuclear localization of AR in C4-2 cells further argue for an important role of the NTD in modulating AR subcellular localization.
Regulation of AR intracellular localization and/or trafficking by the NTD appears to be complex. The presence of the NTD led to the cytoplasmic localization of full-length AR in COS-1 cells, whereas GFP-DBDH-LBD, which lacks NTD, was evenly distributed in COS-1 cells. This finding is consistent with the presence of a region, AR50–250, within the NTD capable of promoting cytoplasmic localization of the AR in PC3 cells (38). Furthermore, GFP-AR and GFP-ARΔ (294–556) were localized in the cytoplasm in virtually all of the transfected cells whereas GFP-ARΔ (1–293) was evenly distributed in PC3 cells (46). These observations suggest that a.a.294–556 of NTD is not sufficient to drive complete nuclear localization of AR in PC3 cells. It is possible that a.a.294–556 of NTD can only drive AR nuclear localization in AR-positive castration-resistant prostate cancer cells such as C4-2. The presence of the NTD did not inhibit androgen-induced nuclear import of AR because DHT induced efficient nuclear import of GFP-AR when compared to the nuclear import of GFP-DBDH-LBD in COS-1 cells (Fig. 1). In contrast, the NTD significantly retarded export of AR from the nuclei of COS-1 cells after androgen withdrawal (Fig. 1). This suggests that the effect of the NTD on AR nuclear export is likely more dramatic than its influence on AR nuclear import. The mechanisms responsible for differential effects of the NTD on nuclear import versus export are unclear and will require further investigation.
Our studies suggest that the NTD is responsible for the differential localization of AR in C4-2 and COS-1 cells and plays a key role in determining androgen-independent nuclear localization of AR in C4-2 cells. The sensitivity of AR to androgens in different cell lines can be very different, with CRPC cells being hypersensitized to low levels of androgens (47). Since the NTD is involved in differential localization of AR in COS-1 and C4-2 cells and is required for androgen-independent nuclear localization of AR, it may play an important role in regulating androgen hypersensitivity of AR in C4-2 cells. Understanding the mechanisms by which the NTD regulates AR subcellular localization may provide new insights into AR hypersensitization that can lead to castration-resistance.
Androgen-independent activation of AR requires its androgen-independent nuclear localization in CRPC cells. However, it is not clear whether AR nuclear localization is sufficient for AR transactivation in CRPC cells. The region of a.a. 294–556 contains the TAU-5 transactivation domain essential for AR transactivation and recognized by TIF2 (48). In our study, TIF-2 knockdown inhibited AR transactivation but not nuclear localization in C4-2 cells, suggesting that transactivation activity of the NTD can be uncoupled from its function in modulating AR subcellular localization.
The N-terminal a.a. 1–293 also appears to play an important role in modulating AR subcellular localization in C4-2 cells. The deletion mutant GFP-ARΔ(294–556), which retains a.a. 1–293, displayed even distribution in transfected C4-2 cells and became localized to the cytoplasm in the presence of 300 nM 17-AAG, an HSP90 inhibitor. This suggests that a.a. 1–293 mediates the 17-AAG inhibition of nuclear localization of AR in C4-2 cells, which is further substantiated by the observation that GFP-ARΔ(1–293) is insensitive to 17-AAG inhibition. The region a.a. 1–293 contains a motif essential for N/C interaction within AR. Hsp90 inhibition by 17-AAG may influence the N/C interaction via the LBD, because Hsp90 can bind to the LBD (8). Alternatively, 17-AAG may affect some signaling molecules that could modulate the function of a.a.1–293 and subsequently inhibit androgen-independent nuclear localization of AR.
In summary, this study presents evidence for an important role of the NTD in AR androgen-independent nuclear localization in CRPC cells. The region of a.a. 294–556 is required for androgen-independent AR nuclear localization whereas a.a. 1–293 mediates Hsp90 regulation of AR nuclear localization in CRPC cells. Although a.a. 294–556 does not contain a nuclear import signal, it was able to enhance DHT-induced import of the LBD. This finding is consistent with the findings of Jenster et al. (11), and Zhou et al (49), which showed that NTD is present in both the nucleus and cytoplasm. However, according to a recent report by Kaku et al., the NTD of AR was localized to the nucleus, suggestion the presence of an NLS in NTD (18). Future studies will be required to determine the reasons for this discrepancy in observations. Although we found that the NTD could modulate AR localization when present in the full-length AR, region a.a. 294–556 did not confer nuclear localization alone. Our study also showed that transactivation of the NTD can be uncoupled from its modulation of AR subcellular localization. Future identification and characterization of cellular factors involved in NTD modulation of AR subcellular localization will be essential for elucidating the mechanisms of androgen-independent nuclear localization and may lead to new therapeutic targets for the treatment of CRPC.
HIGHLIGHTS.
Region a.a. 294–556 was required for AR androgen-independent nuclear localization.
Region a.a. 1–293 mediates Hsp90 regulation of AR nuclear localization in CRPC cells.
Region a.a. 294–556 was able to enhance DHT-induced import of LBD.
Transactivation of NTD is uncoupled from its modulation of AR nuclear localization.
Acknowledgements
This investigation was supported in part by National Institutes of Health Grants R01 CA108675 1 P50 CA90386, 5 R37 DK51193. K.E. is a Post-doctoral Scholar supported by T32 DK007774. K.Z.M. is a Mellam Scholar and L.E.P. is a Tippins Scholar. Z.W. holds UPMC Chair in Urological Research and J.B.N. is the Frederic N. Schwentker Professor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Welsh M, et al. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. The Journal of clinical investigation. 2008 Apr;118:1479. doi: 10.1172/JCI34241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isaacs JT, Coffey DS. Etiology and disease process of benign prostatic hyperplasia. The Prostate. Supplement. 1989;2:33. doi: 10.1002/pros.2990150506. [DOI] [PubMed] [Google Scholar]
- 3.O'Malley KJ, et al. The expression of androgen-responsive genes is up-regulated in the epithelia of benign prostatic hyperplasia. The Prostate. 2009 Dec 1;69:1716. doi: 10.1002/pros.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kozlowski JM, Ellis WJ, Grayhack JT. Advanced prostatic carcinoma. Early versus late endocrine therapy. The Urologic clinics of North America. 1991 Feb;18:15. [PubMed] [Google Scholar]
- 5.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: a cancer journal for clinicians. 2010 Sep-Oct;60:277. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 6.Chen CD, et al. Molecular determinants of resistance to antiandrogen therapy. Nature medicine. 2004 Jan;10:33. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 7.Zegarra-Moro OL, Schmidt LJ, Huang H, Tindall DJ. Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer research. 2002 Feb 15;62:1008. [PubMed] [Google Scholar]
- 8.Saporita AJ, Ai J, Wang Z. The Hsp90 inhibitor, 17-AAG, prevents the ligand-independent nuclear localization of androgen receptor in refractory prostate cancer cells. The Prostate. 2007 Apr 1;67:509. doi: 10.1002/pros.20541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yong EL, Ghadessy F, Wang Q, Mifsud A, Ng SC. Androgen receptor transactivation domain and control of spermatogenesis. Reviews of reproduction. 1998 Sep;3:141. doi: 10.1530/ror.0.0030141. [DOI] [PubMed] [Google Scholar]
- 10.Simental JA, Sar M, Lane MV, French FS, Wilson EM. Transcriptional activation and nuclear targeting signals of the human androgen receptor. The Journal of biological chemistry. 1991 Jan 5;266:510. [PubMed] [Google Scholar]
- 11.Jenster G, et al. Domains of the human androgen receptor involved in steroid binding, transcriptional activation, and subcellular localization. Mol Endocrinol. 1991 Oct;5:1396. doi: 10.1210/mend-5-10-1396. [DOI] [PubMed] [Google Scholar]
- 12.Jenster G, et al. Changes in the abundance of androgen receptor isotypes: effects of ligand treatment, glutamine-stretch variation, and mutation of putative phosphorylation sites. Biochemistry. 1994 Nov 29;33:14064. doi: 10.1021/bi00251a015. [DOI] [PubMed] [Google Scholar]
- 13.Jenster G, van der Korput HA, Trapman J, Brinkmann AO. Identification of two transcription activation units in the N-terminal domain of the human androgen receptor. The Journal of biological chemistry. 1995 Mar 31;270:7341. doi: 10.1074/jbc.270.13.7341. [DOI] [PubMed] [Google Scholar]
- 14.Chamberlain NL, Whitacre DC, Miesfeld RL. Delineation of two distinct type 1 activation functions in the androgen receptor amino-terminal domain. The Journal of biological chemistry. 1996 Oct 25;271:26772. doi: 10.1074/jbc.271.43.26772. [DOI] [PubMed] [Google Scholar]
- 15.Gao T, Marcelli M, McPhaul MJ. Transcriptional activation and transient expression of the human androgen receptor. The Journal of steroid biochemistry and molecular biology. 1996 Sep;59:9. doi: 10.1016/s0960-0760(96)00097-0. [DOI] [PubMed] [Google Scholar]
- 16.Reid J, Kelly SM, Watt K, Price NC, McEwan IJ. Conformational analysis of the androgen receptor amino-terminal domain involved in transactivation. Influence of structure-stabilizing solutes and protein-protein interactions. The Journal of biological chemistry. 2002 May 31;277:20079. doi: 10.1074/jbc.M201003200. [DOI] [PubMed] [Google Scholar]
- 17.Ylikomi T, Bocquel MT, Berry M, Gronemeyer H, Chambon P. Cooperation of proto-signals for nuclear accumulation of estrogen and progesterone receptors. The EMBO journal. 1992 Oct;11:3681. doi: 10.1002/j.1460-2075.1992.tb05453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaku N, Matsuda K, Tsujimura A, Kawata M. Characterization of nuclear import of the domain-specific androgen receptor in association with the importin alpha/beta and Ran-guanosine 5'-triphosphate systems. Endocrinology. 2008 Aug;149:3960. doi: 10.1210/en.2008-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saporita AJ, et al. Identification and characterization of a ligand-regulated nuclear export signal in androgen receptor. The Journal of biological chemistry. 2003 Oct 24;278:41998. doi: 10.1074/jbc.M302460200. [DOI] [PubMed] [Google Scholar]
- 20.Zhou ZX, Wong CI, Sar M, Wilson EM. The androgen receptor: an overview. Recent progress in hormone research. 1994;49:249. doi: 10.1016/b978-0-12-571149-4.50017-9. [DOI] [PubMed] [Google Scholar]
- 21.Gelmann EP. Molecular biology of the androgen receptor. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002 Jul 1;20:3001. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Chang CS, Kokontis J, Liao ST. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science. 1988 Apr 15;240:324. doi: 10.1126/science.3353726. [DOI] [PubMed] [Google Scholar]
- 23.Lubahn DB, et al. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science. 1988 Apr 15;240:327. doi: 10.1126/science.3353727. [DOI] [PubMed] [Google Scholar]
- 24.O'Malley KJ, et al. Hsp90 inhibitor 17-AAG inhibits progression of LuCaP35 xenograft prostate tumors to castration resistance. The Prostate. 2012 Jul 1;72:1117. doi: 10.1002/pros.22458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ai J, et al. HDAC6 regulates androgen receptor hypersensitivity and nuclear localization via modulating Hsp90 acetylation in castration-resistant prostate cancer. Mol Endocrinol. 2009 Dec;23:1963. doi: 10.1210/me.2009-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monks DA, et al. Androgen receptor and Kennedy disease/spinal bulbar muscular atrophy. Hormones and behavior. 2008 May;53:729. doi: 10.1016/j.yhbeh.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazen I, Lumbroso S, Abdel Ghaffar S, Salah N, Sultan C. Mutation of the androgen receptor (R840S) in an Egyptian patient with partial androgen insensitivity syndrome: review of the literature on the clinical expression of different R840 substitutions. Journal of endocrinological investigation. 2004 Jan;27:57. doi: 10.1007/BF03350912. [DOI] [PubMed] [Google Scholar]
- 28.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocrine reviews. 2004 Apr;25:276. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 29.Tilley WD, Buchanan G, Hickey TE, Bentel JM. Mutations in the androgen receptor gene are associated with progression of human prostate cancer to androgen independence. Clinical cancer research : an official journal of the American Association for Cancer Research. 1996 Feb;2:277. [PubMed] [Google Scholar]
- 30.Kawate H, et al. Impaired nuclear translocation, nuclear matrix targeting, and intranuclear mobility of mutant androgen receptors carrying amino acid substitutions in the deoxyribonucleic acid-binding domain derived from androgen insensitivity syndrome patients. The Journal of clinical endocrinology and metabolism. 2005 Nov;90:6162. doi: 10.1210/jc.2005-0179. [DOI] [PubMed] [Google Scholar]
- 31.Cutress ML, Whitaker HC, Mills IG, Stewart M, Neal DE. Structural basis for the nuclear import of the human androgen receptor. Journal of cell science. 2008 Apr 1;121:957. doi: 10.1242/jcs.022103. [DOI] [PubMed] [Google Scholar]
- 32.Zoppi S, et al. Amino acid substitutions in the DNA-binding domain of the human androgen receptor are a frequent cause of receptor-binding positive androgen resistance. Mol Endocrinol. 1992 Mar;6:409. doi: 10.1210/mend.6.3.1316540. [DOI] [PubMed] [Google Scholar]
- 33.Nazareth LV, et al. A C619Y mutation in the human androgen receptor causes inactivation and mislocalization of the receptor with concomitant sequestration of SRC-1 (steroid receptor coactivator 1) Mol Endocrinol. 1999 Dec;13:2065. doi: 10.1210/mend.13.12.0382. [DOI] [PubMed] [Google Scholar]
- 34.Wu HC, et al. Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells. International journal of cancer. Journal international du cancer. 1994 May 1;57:406. doi: 10.1002/ijc.2910570319. [DOI] [PubMed] [Google Scholar]
- 35.Tyagi RK, et al. Dynamics of intracellular movement and nucleocytoplasmic recycling of the ligand-activated androgen receptor in living cells. Mol Endocrinol. 2000 Aug;14:1162. doi: 10.1210/mend.14.8.0497. [DOI] [PubMed] [Google Scholar]
- 36.Roy AK, et al. Androgen receptor: structural domains and functional dynamics after ligand-receptor interaction. Annals of the New York Academy of Sciences. 2001 Dec;949:44. doi: 10.1111/j.1749-6632.2001.tb04001.x. [DOI] [PubMed] [Google Scholar]
- 37.Zhu ML, et al. Tubulin-targeting chemotherapy impairs androgen receptor activity in prostate cancer. Cancer research. 2010 Oct 15;70:7992. doi: 10.1158/0008-5472.CAN-10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dar JA, et al. N-terminal domain of the androgen receptor contains a region that can promote cytoplasmic localization. The Journal of steroid biochemistry and molecular biology. 2013 Oct 4; doi: 10.1016/j.jsbmb.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chmelar R, Buchanan G, Need EF, Tilley W, Greenberg NM. Androgen receptor coregulators and their involvement in the development and progression of prostate cancer. International Journal of Cancer. 2007;120:719. doi: 10.1002/ijc.22365. [DOI] [PubMed] [Google Scholar]
- 40.Callewaert L, Van Tilborgh N, Claessens F. Interplay between two hormone-independent activation domains in the androgen receptor. Cancer research. 2006 Jan 1;66:543. doi: 10.1158/0008-5472.CAN-05-2389. [DOI] [PubMed] [Google Scholar]
- 41.Dehm SM, Regan KM, Schmidt LJ, Tindall DJ. Selective role of an NH2-terminal WxxLF motif for aberrant androgen receptor activation in androgen depletion independent prostate cancer cells. Cancer research. 2007 Oct 15;67:10067. doi: 10.1158/0008-5472.CAN-07-1267. [DOI] [PubMed] [Google Scholar]
- 42.Han G, et al. Hormone status selects for spontaneous somatic androgen receptor variants that demonstrate specific ligand and cofactor dependent activities in autochthonous prostate cancer. The Journal of biological chemistry. 2001 Apr 6;276:11204. doi: 10.1074/jbc.M008207200. [DOI] [PubMed] [Google Scholar]
- 43.Han G, et al. Mutation of the androgen receptor causes oncogenic transformation of the prostate. Proceedings of the National Academy of Sciences of the United States of America. 2005 Jan 25;102:1151. doi: 10.1073/pnas.0408925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andersen RJ, et al. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer cell. 2010 Jun 15;17:535. doi: 10.1016/j.ccr.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 45.Rundlett SE, Wu XP, Miesfeld RL. Functional characterizations of the androgen receptor confirm that the molecular basis of androgen action is transcriptional regulation. Mol Endocrinol. 1990 May;4:708. doi: 10.1210/mend-4-5-708. [DOI] [PubMed] [Google Scholar]
- 46.Dar JA, et al. N-terminal domain of the androgen receptor contains a region that can promote cytoplasmic localization. The Journal of steroid biochemistry and molecular biology. 2014 Jan;139:16. doi: 10.1016/j.jsbmb.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonit M, et al. Hormone depletion-insensitivity of prostate cancer cells is supported by the AR without binding to classical response elements. Mol Endocrinol. 2011 Apr;25:621. doi: 10.1210/me.2010-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Metzger E, Muller JM, Ferrari S, Buettner R, Schule R. A novel inducible transactivation domain in the androgen receptor: implications for PRK in prostate cancer. The EMBO journal. 2003 Jan 15;22:270. doi: 10.1093/emboj/cdg023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou ZX, Sar M, Simental JA, Lane MV, Wilson EM. A ligand-dependent bipartite nuclear targeting signal in the human androgen receptor. Requirement for the DNA-binding domain and modulation by NH2-terminal and carboxyl-terminal sequences. The Journal of biological chemistry. 1994 May 6;269:13115. [PubMed] [Google Scholar]


