Abstract
Background
Patients with Anorexia Nervosa (AN) have neuropsychological deficits in set shifting (SS) and central coherence (CC) consistent with an inflexible thinking style and overly detailed processing style, respectively. This study investigates brain activation during SS and CC tasks in patients with AN and tests whether this activation is a biomarker that predicts response to treatment.
Methods
: FMRI data were collected from 21 females with AN while performing a SS task (the Wisconsin Card Sort) and a CC task (embedded figures), and used to predict outcome following 16 weeks of treatment (either 16 weeks of cognitive behavioral therapy or 8 weeks cognitive remediation training followed by 8 weeks of cognitive behavioral therapy).
Results
Significant activation during the SS task included bilateral dorsolateral and ventrolateral prefrontal cortex and left anterior middle frontal gyrus. Higher scores on the neuropsychological test of SS (measured outside the scanner at baseline) were correlated with greater DLPFC and VLPFC activation. Improvements in SS following treatment were significantly predicted by a combination of low VLPFC and high anterior middle frontal activation (R squared = .68, p=.001). For the CC task, the visual and parietal areas were activated, but were not significantly correlated with neuropsychological measures of CC and did not predict outcome.
Conclusion
Cognitive flexibility requires the support of several prefrontal cortex resources. As previous studies suggest that the VLPFC is important for selecting responses, patients who demonstrate that deficit may benefit the most from cognitive therapy with or without cognitive remediation training. The ability to sustain inhibition of an unwanted response, subserved by the anterior middle frontal gyrus, is a cognitive feature that predicts favorable outcome to cognitive treatment. CC deficits may not be an effective predictor of clinical outcome.
INTRODUCTION
Anorexia Nervosa (AN) is a life-threatening psychiatric disorder with an estimated prevalence of 0.48-0.7% in female patients (11). AN is characterized by cognitive distortions about weight and appearance, severe weight loss, minimization of health and emotional problems, and significant co-morbid psychiatric disorders (12-14). Unfortunately, AN is challenging to treat, with few evidenced based interventions, particularly for adults with an enduring form of the disorder (15-17). Currently, cognitive interventions such as cognitive behavioral therapy (CBT) are recommended for adults with AN; however, fewer than 35% achieve remission with this treatment (17-19).
Because of the severe health-related consequences as well as societal costs of persistent AN (20-23) it is important to identify clinical markers or biomarkers to guide treatment selection. Recent studies suggest possible neuroimaging biomarkers for selecting treatment for other psychiatric disorders, including major depressive disorder, wherein subgenual cingulate activation predicts response to CBT(24), visual cortex activation predicts response to scopolamine (25) and insula metabolism differentially predicts response to CBT versus escitalopram (26). For patients suffering from panic disorder with agoraphobia, a negative functional connectivity between the anterior cingulate and amygdala at pre-treatment predicts favorable response to CBT (27). Although prospective studies are needed to determine the utility of these biomarkers for individual patients, the use of neuroimaging biomarkers to predict response to specific treatments is a potential translational use of neuroimaging.
Thus far, no treatment predictor biomarkers have been proposed for AN. However, candidate biomarkers might be identified by examining the brain circuitry underlying the cognitive inefficiencies reported in AN (28-29). AN is associated with a specific neuropsychological profile, including impaired set shifting (e.g. inflexibility and perseveration) and weak central coherence (exaggerated focus on detail to the neglect of the whole) (30-38). This cognitive style appears to be heritable, persists after recovery, and is seen in relatives without AN (39-42). Cognitive inefficiencies are likely to interfere with the ability of a patient to acquire and to use concepts taught in psychotherapy, particularly cognitive therapies (43). Therefore, examining neural correlates of these cognitive processes could identify a biomarker related to treatment response. The purpose of the current study is to describe neural correlates of cognitive inefficiencies in adults with AN, and examine their relationship to treatment outcome after cognitive therapy.
Brain circuitry underlying cognitive function in patients with AN has been investigated in a few studies. Abnormal structure and function has been reported in the anterior cingulate cortex (ACC), prefrontal cortex, insula, and striatum (44-48) regions associated with executive function, interoception, and behavioral inhibition. Zastrow and colleagues reported hypo-activation of the ACC and striatum compared to controls, but exaggerated activation in prefrontal and parietal supervisory regions during a target detection task (49). In another study, blood flow in the superior frontal gyrus was positively correlated with accuracy on a Stroop task requiring response flexibility in patients with AN (50). Taken together this preliminary evidence supports the view that neural correlates of cognitive processes such as SS and CC can be identified in patients with AN and may be useful as a biomarker to predict treatment response.
The current study investigates the neural basis of cognitive inefficiencies in AN, and tests whether neural correlates of these processes are related to treatment outcome. We hypothesized that evidence of greater inefficiencies in CC and SS as evidenced by low activation in key areas of the prefrontal cortex would lead to poorer outcomes from cognitive treatment.
METHODS
Participants
Participants were recruited from among those participating in an NIH-funded treatment study (51). Twenty-three female participants gave informed consent as approved by the Stanford University Institutional Review Board. Participants were between 16-35 years old and met DSM-IV criteria for AN as assessed by the Eating Disorder Examination (EDE) (52). Participants were required to be medically stable for outpatient treatment as determined by their physician (20). All participants reported no history of learning disability or head injury and met safety criteria of the MRI scan environment.
Measures of Symptom severity and comorbid disorders
As part of the clinical trial associated with this project, participants were assessed at baseline and end of treatment (EOT), which was 16 weeks after baseline. At both assessment times, subjects completed the EDE (52-53) to measure severity of ED symptoms and the Beck Depression Inventory (54-55) to measure symptoms of depression. Comorbid diagnoses were determined using the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS, (56)) for participants under 18 years old, and the Structured Clinical Interview for DSM-IV (SCID, (57) for those over 18 years old.
Neuropsychological measures
Standard neuropsychological tests of SS and CC abilities were completed outside the scanner at baseline and EOT. To assess SS, we used the Stroop subtest of the Delis-Kaplan Executive Functioning System (58), as this is a classic measure of response shifting that is different from the task administered in the scanner. The scaled score was used for correlations with activation at baseline, and the difference in raw scores was used to assess change from baseline to EOT. To measure CC ability, we administered the Rey-Osterrieth Complex Figure Test (59), as this is a standard measure of detailed perceptual processing. We correlated both the Style and Coherence scores with brain activation at baseline, and computed outcome as the difference between baseline and EOT.
Treatments
The treatment protocols used in the RCT from which the participants in this study were drawn are described in detail in a previous publication (60). Participants received either 16 weeks of CBT (N=10) or 8 weeks of cognitive remediation training (CRT) followed by 8 weeks of CBT (N=11). CBT is a standard treatment offered to address the behavioral and cognitive distortions in eating disorders and has been used in several randomized clinical studies for adults with AN (18-19). Cognitive Remediation Therapy (CRT) for AN is a novel cognitive therapy that was developed to address the cognitive style specifically associated with AN (61). CRT for AN was derived in part from cognitive re-training therapies developed for patients with traumatic brain injury (62). There were no outcome differences between the randomized groups at week 16 on clinical measures related to eating psychopathology (e.g., BMI, EDE) in the previously published RCT (60).
Neuroimaging Scan Acquisition
All imaging data were acquired within the first 2 weeks of therapy using a 3.0 Tesla General Electric MR750 scanner (gehealthcare.com) housed in the Lucas Imaging Center, using an 8-channel head coil. FMRI scans used a spiral-in/out pulse sequence (63) with the following parameters: 30 axial slices (3 mm thick, 1 mm skip) parallel to the AC-PC line and covering the whole brain; TR = 2000 msec, TE = 30msec, flip angle = 90°, 1 interleave, field of view 22 cm2, 64x64 matrix, in-plane spatial resolution of 3.125mm.
Tasks Presented During FMRI Scans
FMRI tasks were presented by E-Prime software (Psychology Software Tools, Pittsburgh, PA) and responses were recorded using a hand-held 5 button response box. Visual stimuli were projected onto a screen attached to the head coil, which participants viewed by looking directly up into a mirror that reflected the screen. Subjects demonstrated proficiency on all tasks before entering the scan. The two tasks are described below:
Central coherence was assessed using an embedded figures task (64). Two types of trials were presented: embedded and matching. For embedded trials, two complex figures were presented on the upper half of the screen. Centered below the figures was a simple “target” shape. The subject examined the target shape and selected the complex figure that contained the target shape. The matching trials served as the comparison condition that controlled for visual, motor, and task demand characteristics but did not require central coherence skill, as the target shape was identical to one of the two figures. The experiment was self-paced, but all of the subjects finished the task in about 5 minutes, during which 40 embedded and 50 match trials were presented. Accuracy and response times were recorded for each trial.
Set shifting was assessed using the Wisconsin Card Sort Task (7) modeled after the classic neuropsychological test of response shifting and executive function. The experiment was adapted for fMRI and presented in a block design. Two types of blocks were presented: sort blocks and match blocks. The sort blocks were the same as the original Wisconsin Card Sort Task in that subjects used trial and error to deduce which criterion determined a correct response. Three reference cards were presented on the top row of the screen, and the target card was presented below. The subject chose one of the reference cards in response to each target card. The criterion was color, shape, or number of objects. The stimuli on the cards include red, green, and blue stars, circles, and triangles presented in groups of 1, 2, or 3 shapes. The target card was randomly drawn from a 60-card pool and was always different from the 3 reference cards during the sort blocks. The criterion changed if the subject answered 4 consecutive trials correctly. The match blocks served as the comparison condition that controlled for visual, motor, and task demand characteristics, but did not require set-shifting, as the target card was identical to one of the reference cards. For all responses, feedback was given (“correct” or “incorrect”), followed by an intertribal interval of 4 seconds. Each block consisted of 16 trials at 4 seconds per trial. Four blocks of each type were presented. The onset of each block was indicated to the subject by presenting the word “SORT” or “MATCH”.
FMRI data processing
SPM8 software (http://www.fil.ion.ucl.ac.uk/spm) was used for individual and group analyses. First, images were spatially realigned. Then, the ArtRepair toolbox (cibsr.stanford.edu/tools/ArtRepair/ArtRepair.htm) was used to correct motion artifact by replacing affected volumes with a volume interpolated from the nearest unaffected volumes. The threshold for motion correction was a 3 mm translation or rotation, or a spike slope > 1.5 mm/volume. A subject was removed from the analysis if more than 20% of the volumes required motion correction. Data were then normalized to the MNI template and resampled to a 2 mm3 matrix using sinc interpolation. Spatial smoothing was applied using a 7 mm FWHM Gaussian filter, and slow signal drift was corrected using a high pass filter of 120 seconds/cycle.
Individual subject statistical analyses used a fixed effects model. Each task was modeled as a block design and contrasts of interest included ‘SORT minus MATCH’ for the SS task, and ‘Embedded minus Match’ for the CC task.
Overview of FMRI Group Analyses (details of each step are given in the following sections) For each task:
First we identified brain regions significantly activated by the task.
To better understand how these activations were related to cognitive ability, we correlated activation with standard neuropsychological test scores (obtained outside the scanner at baseline).
To determine whether brain activation predicts treatment outcome, we created regression models predicting change in standard neuropsychological test scores, as well as change in BMI, from significant task activation at baseline
Step 1. Brain regions activated by each task
A voxel-wise whole brain analysis of task-related activation was performed using a random effects model and a one-sample t-test implemented in SPM8, including all subjects in a single group. Clusters of activation were determined to be significant if they survived correction for multiple comparisons using the cluster-wise Family Wise Error correction (p<.05).To determine the location of significant clusters, coordinates were converted to Talairach space using the mni2tal function (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach), localized using Talairach daemon software (http://www.talairach.org/client.html), and also visually inspected by an experienced neuroscientist (AG).
Step 2. Correlations between activation and neuropsychological test scores: Mean activation in significant clusters was extracted using the Marsbar toolbox (http://marsbar.sourceforge.net/)) and nonparametric (Spearman's) correlations with neuropsychological test scores were performed in SPSS (v. 21). A nonparametric correlation test was used because we cannot assume that the neuroimaging data conforms to a normal distribution. Activation from the SS task was tested for correlation with the scaled score from the neuropsychological test of SS (Stroop) at baseline, while activation from the CC task was tested for correlations with neuropsychological tests of CC ( the Style and Coherence scores from the Rey Figure test). A threshold of p=.01 corrected for multiple comparisons was used for significance.
Step 3. Regression models predicting treatment outcome:
SPSS software was used to predict treatment outcome from brain activation and patient characteristics at baseline. A hierarchical model was used because we aimed to assess the predictive ability of fMRI data after first accounting for subject variables that were also significant predictors. We tested whether subject variables were relevant to the outcome by first running a linear regression predicting treatment outcome from candidate subject variables, including age, BMI at baseline, type of treatment. Subject variables significantly predicting each outcome were used in the respective hierarchical regression. Step 2 of the model included all the ROIs for that task, in a step-wise procedure, to identify the most relevant brain regions. The SS and CC tasks were modeled separately, using activation from all the ROIs for that task only. The following outcomes were tested: change in BMI (for both the SS and CC tasks), change in Stroop score (for the SS task only), change in Rey Style and Coherence scores (for the CC task only). A corrected threshold of p=.025 (or .05 divided by 2 outcome models) was used for significance for the SS task, and p=.016 (.05 divided by 3 models) was used for the CC task.
RESULTS
Participants
All participants completed the assessments and scan without difficulty. None of the scans were rejected for head motion. Two participants were removed from the analysis because they reported current alcohol use disorders. The final group included 21 female participants (of whom ten had received CBT and 11 had received CBT+CRT). Three patients did not complete neuropsychological assessments at the end of treatment. Characteristics of the patient sample are shown in Table 1. The most common comorbid diagnosis was Major Depressive Disorder (N=8), followed by anxiety disorders (N=2 Obsessive Compulsive Disorder; N=1 Generalized Anxiety Disorder; N=1 Panic Disorder; N=2 Posttraumatic Stress Disorder). Patients reported a moderate level of AN symptoms based on the EDE assessment. Mean Body Mass Index (BMI) for this sample was 17.43 (standard deviation=1.1). At the time of the scan, five patients were partially weight-restored (using 85% IBW as criteria) and five were taking psychotropic medications (4 taking antidepressant and 1 taking an anxiolytic).
Table 1.
Subject Characteristics
| N | Mean (SD) | |
|---|---|---|
| Age (years) | 21 | 23.43 (5.66) |
| Duration of illness (years) | 21 | 7.05 (5.15) |
| EDE total score | 21 | 2.50 (1.10) |
| Percent of IBW | 21 | 80.88 (5.70) |
| BDI total score | 21 | 18.81 (11.86) |
| N | Percent of sample | |
|---|---|---|
| Purging Subtype (# of participants) | 17 | 80.95 |
| Restricting Subtype (# of participants) | 4 | 19.05 |
| Current psychotropic medication* | 6 | 28.57 |
| Comorbid diagnosis ** | 9 | 42.90 |
| Previous hospitalizations*** | 12 | 57.10 |
| Race | ||
| White | 18 | 85.71 |
| Asian | 3 | 14.28 |
| Education Level: | ||
| High school diploma or less | 6 | 28.6 |
| Attended College | 9 | 42.9 |
| Attended Graduate school | 6 | 28.6 |
| mean | Standard deviation | |
|---|---|---|
| Task Performance in Scanner: | ||
| Set Shifting Task Accuracy (percent correct on sort trials) | 69.24 | 10.17 |
| Set Shifting Task Response Time (milliseconds for sort trials) | 1268.74 | 195.36 |
| Central Coherence Task Accuracy (percent correct for embedded trials) | 82.60 | 14.14 |
| Central Coherence Task Response Time (milliseconds for embedded trials) | 9927.37 | 6415.47 |
| Neuropsychological Test Scores measured outside the scanner | ||
| Stroop scaled score Baseline N=21 | 11.52 | 1.69 |
| Stroop scaled score End of Treatment N=18 | 12.56 | 1.46 |
| Rey Style Baseline N=21 | 1.40 | .45 |
| Rey Style End of Treatment N=18 | 1.65 | .26 |
| Rey Coherence Baseline N=21 | 1.33 | .41 |
| Rey Coherence End of Treatment N=18 | 1.56 | .20 |
| BMI Baseline (N=21) | 17.43 | 1.10 |
| BMI EOT (N=17) | 17.96 | 1.98 |
71.4% none (n=15); 4.7% Cymbalta and Wellbutrin (n=1); 4.7% Cymbalta (n=1), 4.7% Lexapro (n=1); 4.7% Synthroid (n=1); 4.7% Prozac (n=1); 4.7% Luvox and Klonopin (n=1).
4 patients had major depressive disorder; 1 patient had both major depressive disorder and generalized anxiety; 1 patient had major depressive disorder, generalized anxiety, and post-traumatic stress disorder; 1 patient had generalized anxiety and obsessive-compulsive disorder; 1patient had obsessive compulsive disorder; 1 patient had major depressive disorder, generalized anxiety and obsessive-compulsive disorder.
9 patients were hospitalized once in the past, 1 patient was hospitalized twice in the past, and 2 patients were hospitalized 10 times in the past.
^ 3 had some high school, 4 had some college, 3 graduated with a high school diploma, 2 went through some graduate/profession school, 5 graduated with a 4-year college degree, 4 graduated with a graduate/professional degree
*The set-shifting task was the Wisconsin Card Sort Task modified for use in the scanner (see methods for details about the task). The central coherence task was an embedded figures task modified from that previously published {Lee, 2007 #6621} and used with permission.
Imaging Task Performance
Table 2 shows mean scores for the SS and CC tasks performed during fMRI. Scores on these tasks were comparable with those reported previously for similar patients (65). Scores on the neuropsychological tests administered outside the scanner have been reported in a previous publication (51). Task accuracy and response times were not correlated with age or BMI for either task and were not different for participants taking medication compared to those not taking medication (p>.05). Patients having a comorbid anxiety disorder diagnosis responded significantly more slowly during the WCST than those without a comorbid anxiety disorder diagnosis (t(19)=−2.5, p=.023). A comorbid diagnosis of depression did not change performance on either task.
TABLE 2.
Voxel-wise whole brain activation for the Set-Shifting Task. All clusters passed a threshold of p<.05 with cluster-wise Family Wise Error correction for multiple comparisons implemented in SPM8.
| Significant Activation to the Set-Shifting Task | ||||
|---|---|---|---|---|
| Brain Region | BA | k | Peak T | MNI x,y,z |
| R VLPFC/insula | 13/47 | 343 | 18.84 | 34, 22, −6 |
| L VLPFC/insula | 13/47 | 241 | 10.40 | −30, 18, 0 |
| L/R supplementary motor | 32 | 1993 | 13.57 | −2, 20, 46 |
| L/R dorsal anterior cingulate | 32 | 12.49 | 8, 34, 36 | |
| R superior medial frontal | 9 | 8.18 | 8, 38, 34 | |
| R DLPFC | 9/46 | |||
| L DLPFC | 9/6/46 | 1446 | 9.76 | −50, 30, 26 |
| L precentral | 6/8 | 17.84 | −30, −2, 56 | |
| L anterior middle frontal | 10/46 | 111 | 8.30 | −42, 42, 0 |
| R inferior frontal | 10 | 65 | 11.34 | 42, 60, −4 |
| L inferior frontal operculum | 44 | 32 | 8.38 | −50, 10, 14 |
| R caudate | 34 | 8.19 | 12, 12, 6 | |
| L inferior parietal | 40/7 | 2567 | 14.91 | −42, −46, 38 |
| L superior occipital | 19 | 14.00 | −30, −72, 40 | |
| R precuneus | 7 | 2110 | 15.04 | 10, −66, 52 |
| R angular gyrus | 7 | 14.57 | 30, −64, 38 | |
| R inferior parietal | 40 | 10.30 | 52, −38, 50 | |
| R fusiform | 37 | 313 | 12.41 | 42, −62, −14 |
| R inferior occipital | 19 | 10.53 | 50, −62, −10 | |
| R inferior temporal | 20 | 9.74 | 50, −54, −12 | |
| R middle occipital | 19/39 | 126 | 8.54 | 40, −80, 20 |
| L fusiform | 37 | 103 | 10.19 | −40, −52, −14 |
| L inferior occipital | 19/18 | 8.47 | −38, −74, −8 | |
| L Cerebellum | - | 102 | 9.08 | −34, −60, −36 |
| R Cerebellum | - | 71 | 8.94 | 40, −64, −30 |
RESULTS FOR THE SS TASK
Whole brain activation during the SS Task
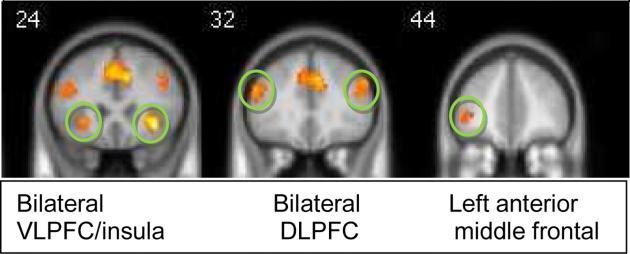
Figure 1 shows significant clusters of activation during the SS task for the contrast of SORT minus MATCH blocks, thus reflecting the executive function skill of response shifting after removing activation associated with motor and visual processes. Activated regions are detailed in Table 2. As expected from previous neuroimaging studies using similar tasks (7, 66) the bilateral DLPFC and VLPFC, inferior parietal cortex, and fusiform gyrus were activated during response switching blocks.
Figure 1.
Clusters of signficant activation during the Set-Shifting (SS) task; threshold=p<.05 Family Wise Error corrected; VLPFC=ventrolateral prefrontal cortex, DLPFC=dorsolateral prefrontal cortex; PFC=prefrontal cortex
Correlations between activation during the SS task and neuropsychological test scores
Spearman's correlation analyses using a corrected threshold of p=.01 found that neuropsychological measure of SS measured outside the scanner at baseline was significantly correlated with right DLPFC activation (rho=.71, p=.0001) and right VLPFC activation (rho=.61, p=.003). Also, BMI at baseline showed a trend for correlation with left anterior prefrontal activation (rho=.50, p=.021).
Hierarchical step-wise regression models predicting clinical change from activation during the SS task
The only subject variable found to significantly predict change in neuropsychological tests of SS ability was type of treatment. Therefore, type of treatment was entered in step 1 of the hierarchical model. Step 2 included activation in all ROIs from the SS taks using a stepwise procedure. The overall model was significant at p=.001 (R squared=.68), as shown in Table 4. Significant factors retained in the model included type of treatment (CBT only vs CRT plus CBT) (p=.021), left anterior prefrontal activation (p=.024) and left VLPFC/insula activation (p=.016). No significant models were found for change in BMI.
Table 4.
Hierarchical Linear Regression Predicting Change in Set Shifting Raw Score* from Baseline to End of Treatment
| Model | R square | Standardized Beta | t | p | |
|---|---|---|---|---|---|
| 1 | (constant) CBT versus CBT+CRT |
.292 | −.54 | 1.29 -2.57 |
.216 .021 |
| 2 | (constant) CBT versus CBT+CRT Anterior middle frontal |
.501 | −1.03 −.67 |
2.91 −3.85 −2.51 |
.011 .002 .024 |
| 3 | (constant) CBT versus CBT+CRT Anterior middle frontal Left VLPFC/Insula |
.676 | −.83 −.81 .53 |
1.94 −3.52 −3.54 2.75 |
.073 .003 .003 .016 |
Raw scores on the SS task are measures of response time, so a lower score indicates better performance
Post-hoc Correlations with Clinical Variables
To elucidate the clinical relevance of activation in regions that were shown to be significantly associated with SS skills, we correlated activation in the regions found to be important correlates or predictors (anterior middle frontal, VLPFC, and DLPFC) with global EDE and total BDI scores at baseline. The correlations were not significant. We tested whether a comorbid anxiety disorder was associated with activation by comparing the five participants with a comorbid anxiety disorder to the 16 without using an independent groups t-test. We found a significant difference in the right DLPFC during the SS task (t=2.4, p=.029), with lower activation in patients with comorbid anxiety disorder, suggesting that symptoms of anxiety may compromise the function of brain regions associated with executive function. However, a comorbid diagnosis of depression was not associated with significant differences in activation in any of the ROIs (p>.05)
RESULTS FOR THE CC TASK
Whole brain activation during the CC Task
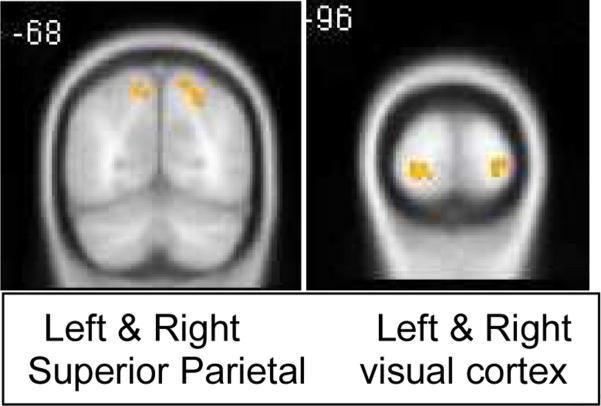
Significant activation during the CC task for the contrast of Embedded minus Match was found in the bilateral posterior occipital lobe, superior parietal lobe, and left superior frontal gyrus. Figure 2 and Table 3 show regions having significant activation during the CC task.
Figure 2.
Clusters of significant activation during the Central Coherence (CC) task (threshold p<.05 Family Wise Error corrected)
Table 3.
Voxel-wise whole brain activation for the Central Coherence Tasks. All clusters passed a threshold of p<.05 with cluster-wise Family Wise Error correction for multiple comparisons implemented in SPM8.
| Significant Activation to the Central Coherence Task | ||||
|---|---|---|---|---|
| Brain Region | BA | k | Peak Z | x,y,z |
| L middle frontal | 6/8 | 112 | 9.54 | −22, 10, 48 |
| L superior frontal | ||||
| L lingual | 18/17 | 211 | 13.99 | −22, −102,−4 |
| L superior occipital | 19 | 58 | 8.86 | −38, −82, 28 |
| R calcarine/lingual | 18/19 | 93 | 8.57 | 24, −100, −6 |
| R superior parietal | 7 | 114 | 8.19 | 18, −70, 56 |
| L superior parietal | 7 | 94 | 8.30 | −10, −70, −54 |
Correlations between CC activation, neuropsychological test scores, and CC task performance
No significant correlations were found between activation during the CC task and neuropsychological test scores of CC ability outside the scanner at baseline.
Hierarchical step-wise regression models predicting clinical change from activation during the CC task at baseline
Age at baseline predicted change in the Rey Coherence score, but no subject variables predicted change in Rey Style score. For the hierarchical model predicting change in CC ability (Rey Coherence), age was entered in step 1, and activation during the CC task was entered step-wise in step 2. The model was not significant. Similarly, the model predicting change in CC ability (Rey Style score), using no subject variables, was not significant. No significant models were found for change in BMI
DISCUSSION
The current study investigated brain function during set shifting (SS) and central coherence (CC), both of which are commonly seen as inefficient cognitive skills in patients with AN. We also examined correlations between activation and standard neuropsychological test measures of these skills, and explored regression models of brain activation predicting outcome following cognitive treatment for AN. We hypothesized that low activation in key areas of the prefrontal cortex would be a biomarker of poor executive function and predict a poor response to treatment. Our hypothesis was partially supported by our findings. Improvement in SS skill following treatment was predicted by lower VLPFC and higher anterior middle frontal activation, and having received CRT/CBT treatment as opposed to CBT alone. In contrast, although the bilateral visual and superior parietal areas were activated during the CC task, this activation was not associated with standard neuropsychological scores measured outside the scanner at baseline or with changes in CC skill following treatment.
These findings are interesting for several reasons. First, the quest for biomarkers predicting response to treatment is important in psychiatry generally and has been particularly challenging in the field of eating disorders and AN specifically (26). Although executive functioning scores from neuropsychological tests are likely a good outcome predictor, a greater understanding of the underlying brain processes associated with executive function in AN potentially paves the way for a better understanding of the brain basis of the disorder. Second, these findings may shed light on the importance of cognitive inefficiencies in AN as they relate to treatment response. In this study, regional activation associated with cognitive flexibility, but not central coherence predicted treatment response. This finding is consistent with published studies suggesting the clinical importance of cognitive rigidity in chronically ill adults with AN, rather than weak central coherence (28, 67). A rigid cognitive style makes collaboration, goal setting, and thought and behavioral experimentation more challenging—all key treatment targets of cognitive therapies (68). In contrast, although overly detailed processing also interferes with therapy because of preoccupation with and perseveration over details, this may interfere less than cognitive rigidity. Third, the finding that being randomized to receive specific cognitive retraining (CRT) plus CBT predicted greater improvements in executive functioning post treatment is interesting because CRT specifically targets executive function skills such as SS and CC, whereas improvement in executive functioning in CBT would likely be a secondary outcome. In this way, CRT aims to improve general executive functioning rather than directly improving eating related symptoms (69), similar to how it is used for patients with schizophrenia. In turn, improvements in executive function should ultimately support the ability to utilize symptom-targeted treatments.
Our study did not include a healthy comparison group, however, previous neuroimaging studies of healthy controls performing set-shifting tasks have found activation in the dorsolateral prefrontal cortex, ventrolateral prefrontal corex, anterior cingulate cortex, and temporoparietal junction, cerebellum, superior parietal cortex, and retrosplenial cortex (7, 66). By comparison, our sample of patients with AN activated most of the same regions during the SS task, including VLPFC, DLPFC, anterior cingulate, superior parietal cortex, cerebellum, and precuneus. Previous studies of healthy subjects performing a CC (Embedded Figures) task activate the left dorsolateral, medial and dorsal premotor, bilateral parietal and occipital cortex, and bilateral ventral termporal cortex activation (Lee et al., 2007). In comparison, our sample activated parietal, occipital, and ventral temporal regions, and premotor cortex, but not the dorsolateral prefrontal cortex. However, a statistical comparison with a matched group of controls is necessary before determining that these differences are significant.
We speculate that activation in the VLPFC/insula during the SS task reflects a combination of task-related decision making and symptoms of anxiety. Reviews of VLPFC function have hypothesized that activation in this region is associated with context-dependent decision making as well as emotion regulation (1), both of which might be needed to perform the SS task efficiently. In our study, the VLPFC cluster of activation extends prominently into the insula, which is strongly implicated in interoception and state anxiety (70-74). Insula abnormalities have been a focus in imaging studies of eating disorders, for example, recovered AN patients demonstrate hyperactivation of the insula when anticipating food cues (75). Further, anxiety symptoms often predate the onset of AN, and anxiety disorders are commonly comorbid with AN, and appear to have a negative impact on treatment outcome (76-78). Therefore, greater activation in the VLPFC/insula may predict poorer response to cognitive treatment because symptoms of anxiety interfere with treatment processes by undermining learning, motivation, and therapeutic engagement. In support of this hypothesis, a recent study found that state anxiety accounts for executive function deficits in women with AN (79). However, in our study, we did not measure anxiety symptoms so we cannot test this hypothesis, and our post-hoc analyses showed that VLPFC/insula activation did not differ between patients with and without a comorbid anxiety disorder. Neither of the specific forms of CBT or CRT used in the treatment study are known to be effective for anxiety. Future studies are needed to examine such effects, as anxiety is a common feature of AN.
Rather than indicating symptoms of anxiety, activation in the VLPFC may reflect decision making that requires flexible response shifting, as has been suggested by a meta-analysis of fMRI studies using the WCST (8). Similarly, a previous fMRI study of healthy adults showed that the VLPFC activates to error feedback that requires a change in response, but not to error feedback that requires no change in response (2). If so, patients with lower activation in this region may benefit the most from cognitive therapy that directly addresses this skill.
In addition to the VLPFC, better clinical outcome was predicted by greater activation in the anterior middle frontal gyrus. The anterior middle frontal gyrus was activated in studies of healthy adults that use cognitive set shifting tasks, such as reversal learning (6). Also, this region may be particularly important for retaining newly learned responses, as a previous study found that the left anterior middle frontal gyrus inhibits an undesired response in the long term, as opposed to dorsal frontal areas (BA 6) that inhibit an undesired response in immediate trials (9). Therefore, better response to cognitive therapy might be predicted by the ability to retain newly learned cognitive skills over an extended time.
There are important limitations to our findings. Due to the small sample size and the lack of clinical differences in outcome in the 2 treatment arms, both treatment groups were combined for analysis purposes. Additional studies examining treatment differences may better inform clinical care that is tailored for individual patient variables. Additionally, it would be useful to compare this sample to a healthy control group to determine whether activation patterns differ. Future studies with larger numbers of participants and additional clinical and executive function measures are needed to better characterize the neural signature of the cognitive processes associated with executive functioning in AN and their relation to treatment response.
Highlights.
Set-shifting ability is correlated with activation in the prefrontal cortex in patients with AN.
Lower ventral frontal/insula activation predicts improvements in set-shifting after treatment.
Activation during a central coherence task does not predict response to cognitive treatment.
Acknowledgements
The authors thank Dr. Kathleen Kara Fitzpatrick for her contribution as cognitive remediation treatment therapist and neuropsychologist. We also thank Dr. Vaidya and colleagues for generously providing the script for the Central Coherence task, which we modified for use in this study.
This research was supported by the following grants:
NIMH R03 MH096144 (P.I.'s Reiss and Lock)
R01 MH082706 (PI Lock)
K24 MH074467 (PI Lock).
NIH 1 DP2 OD004445 (P.I. Kesler)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors:
Amy Garrett wrote the majority of the manuscript, analyzed the fMRI data, collected the fMRI data, and created the protocol
James Lock wrote part of the manuscript, designed the study, and was the PI on the treatment protocol that provided the subjects for this study
Nandiini Datta created tables for the manuscript, reviewed the manuscript, helped to collect the fMRI data, and tested the participants
Judy Beenhakker reviewed the manuscript, helped to collect the fMRI data, and tested the participants
Shelli Kesler created the task used in the scanner and reviewed the manuscript
Allan Reiss helped design the study, interpreted the results of the analysis, helped to plan the data analysis, and reviewed the manuscript
Conflicts of Interest: None
REFERENCES
- 1.Mitchell DG. The nexus between decision making and emotion regulation: a review of convergent neurocognitive substrates. Behav Brain Res. 2011;217(1):215–31. doi: 10.1016/j.bbr.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 2.Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J Neurosci. 2002;22(11):4563–7. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pollmann S, Manginelli AA. Anterior prefrontal involvement in implicit contextual change detection. Front Hum Neurosci. 2009;3:28. doi: 10.3389/neuro.09.028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranganath C, Johnson MK, D'Esposito M. Prefrontal activity associated with working memory and episodic long-term memory. Neuropsychologia. 2003;41(3):378–89. doi: 10.1016/s0028-3932(02)00169-0. [DOI] [PubMed] [Google Scholar]
- 5.Huang S, Seidman LJ, Rossi S, Ahveninen J. Distinct cortical networks activated by auditory attention and working memory load. Neuroimage. 2013;83:1098–108. doi: 10.1016/j.neuroimage.2013.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Remijnse PL, Nielen MM, Uylings HB, Veltman DJ. Neural correlates of a reversal learning task with an affectively neutral baseline: an event-related fMRI study. Neuroimage. 2005;26(2):609–18. doi: 10.1016/j.neuroimage.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Lie CH, Specht K, Marshall JC, Fink GR. Using fMRI to decompose the neural processes underlying the Wisconsin Card Sorting Test. Neuroimage. 2006;30(3):1038–49. doi: 10.1016/j.neuroimage.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 8.Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum Brain Mapp. 2005;25(1):35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konishi S, Watanabe T, Jimura K, Chikazoe J, Hirose S, Kimura HM, et al. Role for presupplementary motor area in inhibition of cognitive set interference. J Cogn Neurosci. 2011;23(3):737–45. doi: 10.1162/jocn.2010.21480. [DOI] [PubMed] [Google Scholar]
- 10.Petrides M. The role of the mid-dorsolateral prefrontal cortex in working memory. Exp Brain Res. 2000;133(1):44–54. doi: 10.1007/s002210000399. [DOI] [PubMed] [Google Scholar]
- 11.Hoek H, Hoeken Dv. Review of prevalence and incidence of eating disorders. Int J Eat Disord. 2003;34:383–96. doi: 10.1002/eat.10222. [DOI] [PubMed] [Google Scholar]
- 12.Association AP. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; Arlington, VA: 2013. [Google Scholar]
- 13.Godart N, Flament M, Perdereau F, Jeammet P. Comorbidity between eating disorders and anxiety disorders: A review. Int J Eat Disord. 2002;32:253–70. doi: 10.1002/eat.10096. [DOI] [PubMed] [Google Scholar]
- 14.Holtkamp K, Muller B, Heussen N, Remschmidt H, Herperz-Dahlmann B. Depression, anxiety, and obsessionality in long-term recovered patients with adolescent-onset anorexia nervosa. European Child and Adolescent Psychiatry. 2005;14:106–10. doi: 10.1007/s00787-005-0431-5. [DOI] [PubMed] [Google Scholar]
- 15.Berkman N, Lohr K, Bulik C. Outcomes of eating disorders: A systematic review of the literature. IJED. 2007;40:283–309. doi: 10.1002/eat.20369. [DOI] [PubMed] [Google Scholar]
- 16.Hay P. A systematic review of evidence for psychological treatments in eating disorders: 2005-2012. IJED. 2013;46:462–9. doi: 10.1002/eat.22103. [DOI] [PubMed] [Google Scholar]
- 17.Hay P, Touys W, Sud R. Treatment of severe and enduring anorexia nervosa: a review. Australian and New Zealand Journal of Psychiatry. 2012 doi: 10.1177/0004867412450469. [DOI] [PubMed] [Google Scholar]
- 18.Pike K, Walsh BT, Vitousek K, Wilson GT, Bauer J. Cognitive-Behavioral Therapy in the Posthospitalization Treatment of Anorexia Nervosa. American Journal of Psychiatry. 2004;160:2046–9. doi: 10.1176/appi.ajp.160.11.2046. [DOI] [PubMed] [Google Scholar]
- 19.Fairburn C, Cooper G, Doll H, 0'Connor M, Palmer R, Dalle Grave R. Enhanced cognitive behavioral therapy for adults with anorexia nervosa: a UK-Italy study. Behav Res Ther. doi: 10.1016/j.brat.2012.09.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golden N, Katzman D, Kreipe R, Stevens S, Sawyer S, Rees J, et al. Eating disorders in adolescents: position paper of the Society for Adolescent Medicine:Medical Indications for Hospitalization in an Adolescent with an Eating Disorder. J Adolesc Health. 2003;33:496–503. doi: 10.1016/s1054-139x(03)00326-4. [DOI] [PubMed] [Google Scholar]
- 21.Arcelus J, Mitchell A, Wales J, Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders. Archives of General Psychiatry. 2011;68:724–31. doi: 10.1001/archgenpsychiatry.2011.74. [DOI] [PubMed] [Google Scholar]
- 22.Crow S, Nyman J. The cost-effectiveness of anorexia nervosa treatment. Int J Eat Disord. 2004;35:155–60. doi: 10.1002/eat.10258. [DOI] [PubMed] [Google Scholar]
- 23.Streigel-Moore R, Leslie D, Petrill SA, Garvin V, Rosenheck RA. One-year use and cost of inpatient and outpatient services among female and male patients with an eating disorder: Evidence from a national database of health insurance claims. International Journal of Eating Disorders. 2000;27:381–9. doi: 10.1002/(sici)1098-108x(200005)27:4<381::aid-eat2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 24.Siegle GJ, Thompson WK, Collier A, Berman SR, Feldmiller J, Thase ME, et al. Toward clinically useful neuroimaging in depression treatment: prognostic utility of subgenual cingulate activity for determining depression outcome in cognitive therapy across studies, scanners, and patient characteristics. Arch Gen Psychiatry. 2012;69(9):913–24. doi: 10.1001/archgenpsychiatry.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furey ML, Drevets WC, Hoffman EM, Frankel E, Speer AM, Zarate CA., Jr Potential of pretreatment neural activity in the visual cortex during emotional processing to predict treatment response to scopolamine in major depressive disorder. JAMA Psychiatry. 2013;70(3):280–90. doi: 10.1001/2013.jamapsychiatry.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGrath CL, Kelley ME, Holtzheimer PE, Dunlop BW, Craighead WE, Franco AR, et al. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry. 2013;70(8):821–9. doi: 10.1001/jamapsychiatry.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lueken U, Straube B, Konrad C, Wittchen HU, Strohle A, Wittmann A, et al. Neural substrates of treatment response to cognitive-behavioral therapy in panic disorder with agoraphobia. Am J Psychiatry. 2013;170(11):1345–55. doi: 10.1176/appi.ajp.2013.12111484. [DOI] [PubMed] [Google Scholar]
- 28.Tchanturia K, Davies H, Harrison A, Roberts M, Nakazato M, Schmidt U, et al. Poor cognitive flexibility in eating disorders: Examining the evidence. PloS ONE. 2012;7:e28331. doi: 10.1371/journal.pone.0028331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fonville L, Lao-Kaim N, Giampietro V, Van den Eynde F, Davies H, Lounes N, et al. Evaluation of enhanced attention to local detail in anorexia nervosa using the Embedded Figures Test: an fMRI study. PloS ONE. 2013;8(5):e63964. doi: 10.1371/journal.pone.0063964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tchanturia K, Davies H, Harrison A, Fox JR, Treasure J, Schmidt U. Altered social hedonic processing in eating disorders. Int J Eat Disord. 2012;45(8):962–9. doi: 10.1002/eat.22032. [DOI] [PubMed] [Google Scholar]
- 31.Tchanturia K, Serpell L, Troop N, Treasure J. Perceptual illusions in eating disorders: rigid and fluctuating styles. J Behav Ther Exp Psychiatry. 2001;32(3):107–15. doi: 10.1016/s0005-7916(01)00025-8. [DOI] [PubMed] [Google Scholar]
- 32.Tchanturia K, Morris RG, Surguladze S, Treasure J. An examination of perceptual and cognitive set shifting tasks in acute anorexia nervosa and following recovery. Eat Weight Disord. 2002;7(4):312–5. doi: 10.1007/BF03324978. [DOI] [PubMed] [Google Scholar]
- 33.Tchanturia K, Anderluh MB, Morris RG, Rabe-Hesketh S, Collier DA, Sanchez P, et al. Cognitive flexibility in anorexia nervosa and bulimia nervosa. J Int Neuropsychol Soc. 2004;10(4):513–20. doi: 10.1017/S1355617704104086. [DOI] [PubMed] [Google Scholar]
- 34.Holliday J, Landau S, Collier D, Treasure J. Do illness characteristics and familial risk differ between women with anorexia nervosa grouped on the basis of personality pathology? Psychol Med. 2006;36(4):529–38. doi: 10.1017/S0033291705006641. [DOI] [PubMed] [Google Scholar]
- 35.Lock J, Garrett A, Beenhakker J, Reiss AL. Aberrant brain activation during a response inhibition task in adolescent eating disorder subtypes. Am J Psychiatry. 2011;168(1):55–64. doi: 10.1176/appi.ajp.2010.10010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts ME, Tchanturia K, Stahl D, Southgate L, Treasure J. A systematic review and meta-analysis of set-shifting ability in eating disorders. Psychological Medicine. 2007;37(8):1075–84. doi: 10.1017/S0033291707009877. [DOI] [PubMed] [Google Scholar]
- 37.Benson RT, Tavares SP, Robertson SC, Sharp R, Marshall RW. Conservatively treated massive prolapsed discs: a 7-year follow-up. Ann R Coll Surg Engl. 2010;92(2):147–53. doi: 10.1308/003588410X12518836438840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harrison A, O'Brien N, Lopez C, Treasure J. Sensitivity to reward and punishment in eating disorders. Psychiatry Res. 2010;177(1-2):1–11. doi: 10.1016/j.psychres.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 39.Fossella JA, Sommer T, Fan J, Pfaff D, Posner MI. Synaptogenesis and heritable aspects of executive attention. Ment Retard Dev Disabil Res Rev. 2003;9(3):178–83. doi: 10.1002/mrdd.10078. [DOI] [PubMed] [Google Scholar]
- 40.Treasure JL. Getting beneath the phenotype of anorexia nervosa: the search for viable endophenotypes and genotypes. Can J Psychiatry. 2007;52(4):212–9. doi: 10.1177/070674370705200402. [DOI] [PubMed] [Google Scholar]
- 41.Wade TD, Bulik CM. Shared genetic and environmental risk factors between undue influence of body shape and weight on self-evaluation and dimensions of perfectionism. Psychol Med. 2007;37(5):635–44. doi: 10.1017/S0033291706009603. [DOI] [PubMed] [Google Scholar]
- 42.Grice DE, Halmi KA, Fichter MM, Strober M, Woodside DB, Treasure JT, et al. Evidence for a susceptibility gene for anorexia nervosa on chromosome 1. Am J Hum Genet. 2002;70(3):787–92. doi: 10.1086/339250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baldock E, Tchanturia K. Translating laboratory research into practice: foundations, functions, and future of cognitive remediation therapy for anorexia nervosa. Therapy. 2007;4:1–8. [Google Scholar]
- 44.Wagner A, Aizenstein H, Venkatraman VK, Fudge J, May JC, Mazurkewicz L, et al. Altered reward processing in women recovered from anorexia nervosa. Am J Psychiatry. 2007;164(12):1842–9. doi: 10.1176/appi.ajp.2007.07040575. [DOI] [PubMed] [Google Scholar]
- 45.Muhlau M, Gaser C, Ilg R, Conrad B, Leibl C, Cebulla MH, et al. Gray matter decrease of the anterior cingulate cortex in anorexia nervosa. Am J Psychiatry. 2007;164(12):1850–7. doi: 10.1176/appi.ajp.2007.06111861. [DOI] [PubMed] [Google Scholar]
- 46.Uher R, Murphy T, Friederich HC, Dalgleish T, Brammer MJ, Giampietro V, et al. Functional neuroanatomy of body shape perception in healthy and eating-disordered women. Biol Psychiatry. 2005;58(12):990–7. doi: 10.1016/j.biopsych.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Uher R, Murphy T, Brammer MJ, Dalgleish T, Phillips ML, Ng VW, et al. Medial prefrontal cortex activity associated with symptom provocation in eating disorders. Am J Psychiatry. 2004;161(7):1238–46. doi: 10.1176/appi.ajp.161.7.1238. [DOI] [PubMed] [Google Scholar]
- 48.Uher F. [Impact of DNA chips on haematological oncology]. Magy Onkol. 2001;45(1):59–66. [PubMed] [Google Scholar]
- 49.Zastrow A, Kaiser S, Stippich C, Walther S, Herzog W, Tchanturia K, et al. Neural correlates of impaired cognitive-behavioral flexibility in anorexia nervosa. Am J Psychiatry. 2009;166(5):608–16. doi: 10.1176/appi.ajp.2008.08050775. [DOI] [PubMed] [Google Scholar]
- 50.Ferro AM, Brugnolo A, De Leo C, Dessi B, Girtler N, Morbelli S, et al. Stroop interference task and single-photon emission tomography in anorexia: a preliminary report. Int J Eat Disord. 2005;38(4):323–9. doi: 10.1002/eat.20203. [DOI] [PubMed] [Google Scholar]
- 51.Lock J, Agras WS, Fitzpatrick KK, Bryson SW, Jo B, Tchanturia K. Is outpatient cognitive remediation therapy feasible to use in randomized clinical trials for anorexia nervosa? Int J Eat Disord. 2013;46(6):567–75. doi: 10.1002/eat.22134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cooper Z, Fairburn CG. The Eating Disorder Examination: A semi-structured interview for the assessment of the specific psychopathology of eating disorders. International Journal of Eating Disorders. 1987;6:1–8. [Google Scholar]
- 53.Fairburn CG, Cooper I. The eating disorder examination (12th edition. In: Fairburn CG, Wilson GT, editors. Binge eating: Nature, Assessment, and Treatment. Guilford Press; New York: 1993. [Google Scholar]
- 54.Beck AT. Beck Depression Inventory. San Antonio, TX: p. 1987. [Google Scholar]
- 55.Beck AT, Steer RA, Garbin M. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psycho Rev. 1988;8:77–100. [Google Scholar]
- 56.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 57.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P, version 2.0) New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- 58.Delis D, E. K, Kramer J. Examiner's Manual: Delis-Kaplan Executive Functioning Systems (D-KEFS) The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- 59.Osterrieth P. Test of copying complex figures: Contribution to the study of perception and memory. Archives de Psychologie. 1944;20:206–356. [Google Scholar]
- 60.Lock J, Agras WS, Fitzpatrick K, Jo B, Bryson S, Tchanturia K. Addressing treatment dropout in anorexia nervosa using cognitive remediation therapy. IJED. doi: 10.1002/eat.22134. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tchanturia K, Lock J. Cognitive remediation therapy (CRT) for eating disorders: Development, refinement, and future directions. Current Topics in Behavioral Neuroscience. 2011;6:269–97. doi: 10.1007/7854_2010_90. [DOI] [PubMed] [Google Scholar]
- 62.Malia K, G., Powell G, Torode S. Personality and psychosocial function after brain injury. Brain Injury. 1995;(9):697–712. doi: 10.3109/02699059509008226. [DOI] [PubMed] [Google Scholar]
- 63.Glover GH, Lai S. Self-navigated spiral fMRI: interleaved versus single-shot. Magn Reson Med. 1998;39(3):361–8. doi: 10.1002/mrm.1910390305. [DOI] [PubMed] [Google Scholar]
- 64.Lee PS, Foss-Feig J, Henderson JG, Kenworthy LE, Gilotty L, Gaillard WD, et al. Atypical neural substrates of Embedded Figures Task performance in children with Autism Spectrum Disorder. Neuroimage. 2007;38(1):184–93. doi: 10.1016/j.neuroimage.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tchanturia K, Davies H, Roberts M, Harrison A, Nakazato M, Schmidt U, et al. Poor cognitive flexibility in eating disorders: examining the evidence using the Wisconsin Card Sorting Task. PLoS One. 2012;7(1):e28331. doi: 10.1371/journal.pone.0028331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith AB, Taylor E, Brammer M, Rubia K. Neural correlates of switching set as measured in fast, event-related functional magnetic resonance imaging. Hum Brain Mapp. 2004;21(4):247–56. doi: 10.1002/hbm.20007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tchanturia K, Davies H, Roberts M, Harrison A, Nakazato M, Schmidt U, et al. Poor cognitive flexibility in eating disorders: Examining the evidence using the Wisconsin Card Sorting Task. PloS ONE. 2012;7:e28331. doi: 10.1371/journal.pone.0028331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Block A, Dhanhi H, Thompson-Tardif S, Floresco S. Thalamic-prefrontal cortical-ventral striatal circuity mediates dissociable components of strategy set shifting. Cerbr Corex. 2007;17:1625–36. doi: 10.1093/cercor/bhl073. [DOI] [PubMed] [Google Scholar]
- 69.Wykes T, Huddy V, McGurk S, Czobor p. A meta-analysis of cognitive remediation for schizophrenia: Methodology and effect sizes. AJP. 2011;168:472–85. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- 70.Klumpp H, Angstadt M, Phan KL. Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biol Psychol. 2012;89(1):273–6. doi: 10.1016/j.biopsycho.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Simmons AN, Stein MB, Strigo IA, Arce E, Hitchcock C, Paulus MP. Anxiety positive subjects show altered processing in the anterior insula during anticipation of negative stimuli. Hum Brain Mapp. 2011;32(11):1836–46. doi: 10.1002/hbm.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simmons A, Matthews SC, Paulus MP, Stein MB. Intolerance of uncertainty correlates with insula activation during affective ambiguity. Neurosci Lett. 2008;430(2):92–7. doi: 10.1016/j.neulet.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164(2):318–27. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- 74.Paulus MP, Feinstein JS, Castillo G, Simmons AN, Stein MB. Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Arch Gen Psychiatry. 2005;62(3):282–8. doi: 10.1001/archpsyc.62.3.282. [DOI] [PubMed] [Google Scholar]
- 75.Oberndorfer T, Simmons A, McCurdy D, Strigo I, Matthews S, Yang T, et al. Greater anterior insula activation during anticipation of food images in women recovered from anorexia nervosa versus controls. Psychiatry Res. 2013;214(2):132–41. doi: 10.1016/j.pscychresns.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bulik C, Sullivan P, Tozzi F, Furberg H, Lichtenstein P, Pedersen N. Prevalence, heritability and prospective risk factors for anorexia nervosa. Archives of General Psychiatry. 2006;63:305–12. doi: 10.1001/archpsyc.63.3.305. [DOI] [PubMed] [Google Scholar]
- 77.Bulik CM, Sullivan PF, Fear J, Joyce PR. Eating disorders and antecedent anxiety disorders: a controlled study. Acta Psychiatrica Scandinavica. 1997;96:101–7. doi: 10.1111/j.1600-0447.1997.tb09913.x. [DOI] [PubMed] [Google Scholar]
- 78.Kaye W, Bulik CM, Thonton L, Barbarich B, Masters K, Fichter M, et al. Anxiety disorders comorbid with bulimia and anorexia nervosa. Am J Psychiatry. 2004;161:2215–21. doi: 10.1176/appi.ajp.161.12.2215. [DOI] [PubMed] [Google Scholar]
- 79.Billingsley-Marshall RL, Basso MR, Lund BC, Hernandez ER, Johnson CL, Drevets WC, et al. Executive function in eating disorders: the role of state anxiety. Int J Eat Disord. 2013;46(4):316–21. doi: 10.1002/eat.22086. [DOI] [PubMed] [Google Scholar]