Abstract
Introduction
Hydrochlorothiazide, an effective antihypertensive medication commonly prescribed to blacks, decreases urinary calcium excretion. Blacks have significantly higher rates of hypertension and lower levels of 25-hydroxyvitamin D. Thus, they are more likely to be exposed to vitamin D supplementation and thiazide diuretics. The risk for hypercalcemia among blacks using vitamin D and hydrochlorothiazide is undefined.
Methods
We assessed the frequency of hypercalcemia in HCTZ users in a post-hoc analysis of a randomized, double-blind, dose-finding trial of 328 blacks (median age, 51 years) assigned to either placebo, or 1000, 2000, or 4000 international units of cholecalciferol (vitamin D3) daily for 3 months during the winter (2007–2010).
Results
Of the 328 participants, 84 reported hydrochlorothiazide use and had serum calcium levels assessed. Additionally, a comparison convenience group of 44 enrolled participants who were not taking hydrochlorothiazide had serum calcium measurements at 3-months but not at baseline. At 3-months, hydrochlorothiazide participants had higher calcium levels (0.2 mg/dL, p<.001) than non-hydrochlorothiazide participants, but only one participant in the hydrochlorothiazide group had hypercalcemia. In contrast, none of the non-hydrochlorothiazide participants had hypercalcemia. In linear regression model adjusted for age, sex, 25-hydroxyvitamin D at 3-months, and other covariates, only hydrochlorothiazide use [Estimate (SE):0.05(0.01) p=0.01] predicted serum calcium at 3-months.
Conclusion
In summary, vitamin D3 supplementation up to 4000 IU in hydrochlorothiazide users is associated with a rise in serum calcium but a low frequency of hypercalcemia. These findings suggest that participants of this population can use HCTZ with up to 4000 IU of vitamin D3 daily and experience a low frequency of hypercalcemia.
Keywords: black, hypertension, thiazide diuretics, hypercalcemia, vitamin D
INTRODUCTION
Blacks have significantly higher rates of hypertension (1, 2) and lower levels of 25-hydroxyvitamin D [25(OH)D] than whites.(3) Thus, they may be exposed to concurrent thiazide diuretics, commonly prescribed for blacks with hypertension(4–6) and vitamin D supplementation. Thiazide diuretics are inexpensive and regarded as effective therapy for prevention of cardiovascular disease and stroke.(7) They potentiate the blood pressure lowering effects of other classes of antihypertensives such as angiotensin converting enzyme (ACE)-inhibitors, angiotensin receptor blockers (ARBs), and beta blockers.(8, 9) Furthermore, vitamin D supplementation may lower blood pressure.(10)
Patients prescribed HCTZ are routinely monitored for electrolyte abnormalities such as hypokalemia.(11, 12) Thiazide diuretics such as HCTZ also decrease renal excretion of calcium(13, 14), although hypercalcemia and its associated symptoms (muscle aches, fatigue, excessive thirst, and frequent urination) (15) are a less common complication than hypokalemia. When resulting from HCTZ usage, the degree of hypercalcemia is generally mild, with serum calcium values usually less than 11.2 mg/dL, and does not require intervention other than stopping HCTZ. (16, 17) Because vitamin D increases intestinal absorption of calcium (18, 19) (20), we hypothesize that vitamin D supplementation may increase calcium absorption leading to an even higher rate of HCTZ associated hypercalcemia. Therefore, in this post-hoc analysis we assessed the frequency of hypercalcemia among community-based black participants participating in a randomized, double-blind, placebo-controlled dose-finding trial of vitamin D supplementation to examine whether concurrent use of HCTZ and vitamin D increased the risk of thiazide associated hypercalcemia. We monitored serum calcium in a convenience sample of participants taking HCTZ at baseline; moreover, for comparison purposes, we examined serum calcium in a second subset of participants not taking HCTZ.
MATERIALS AND METHODS
Study Design and Participants
The parent study was a prospective, randomized, double-blind, placebo-controlled trial of oral vitamin D supplementation in a healthy black population (Clinical Trials.gov: NCT00585637). Protocol has been previously described (10). Participants were recruited through 12 low-income housing sites in the metropolitan Boston area (21) as well as community and faith-based organizations, and a refer-a-friend incentive program, resulting in 328 enrolled participants. Participants of Open Doors to Health (ODH) were invited to participate in the study if they were aged 30–80 years, able to understand written and spoken English, and self-identified as Black or African-American, and had permission from their primary care doctors. Participants were enrolled during winter to minimize the influence of sun exposure on vitamin D levels. The project was approved by the Institutional Review Board of Harvard School of Public Health (HSPH) and also annually monitored by a Data Safety Monitoring Board. The procedures followed were in accordance with the ethical standards of the institution. All participants gave written informed consent.
Exclusion Criteria
The exclusion criteria were: pre-existing disorders of calcium metabolism and parathyroid function (including prevalent hypercalcemia at baseline); type I diabetes; renal disease; sarcoidosis; concurrent active malignancies (other than non-melanoma skin cancer); cognitive impairment; active thyroid disease (e.g., Graves, Hashimoto’s or thyroiditis); plans for vacation or extended travel to a sunny region during the supplementation phase of the study; and supplementation with vitamin D. Persons already taking calcium supplements were permitted to continue calcium supplements.
Randomization, Treatment, and Calcium Safety Monitoring
Participants were randomly assigned to one of four treatment arms: placebo, 1000 IU (25µg), 2000 IU (50µg), or 4000 IU (100µg) of vitamin D (as cholecalciferol). Treatment consisted of tablets that in addition to their vitamin D, also contained 200 mg of calcium carbonate. The tablets were formulated and manufactured by Pharmavite LLC (Mission Hill, CA). Study medications were started during early winter (November or December) and were taken orally once a day for 3 months (completed in February or March). Study statisticians generated the random allocation sequence, and subjects were enrolled by research assistants. All participants, providers, and study staff were blinded. Dietary intake of calcium and vitamin D was estimated by using a modified Food Frequency Questionnaire (FFQ) at baseline, 3 months, and 6 months to evaluate intake of calcium and vitamin D-rich foods.
All participants were assessed for adverse events by study staff over the phone at week 2 of each month and in-person at the beginning of each month when the next month’s supply of vitamins was provided. To assess signs of elevated calcium, participants were informed of the potential symptoms of hypercalcemia and advised to contact study coordinators if symptoms occurred. At each adverse event assessment, study staff ascertained absence of symptoms (such as muscle aches, fatigue, excessive thirst, frequent urination, loss or change in appetite, changes to the skin-e.g. pruritus, and nausea). Baseline serum calcium was not measured; nonetheless, to be eligible for the trial, the primary care physicians of all enrolled patients were required to provide documentation that the participant had no prior history of hypercalcemia. As part of the clinical trial, serum calcium measurements were mandated in all enrolled participants using HCTZ at 4 to 6 weeks following study initiation and again at 12 weeks. In addition, as part of the regular toxicity assessments, participants who reported any symptoms possibly associated with hypercalcemia were required to undergo measurement of serum calcium at the time of the adverse event report. Finally, to assess any potential change in serum calcium level attributable primarily to vitamin D supplementation (and not to HCTZ), a subset of 44 participants who were not taking HCTZ underwent protocol serum calcium tests at 12 weeks. Serum venous total calcium was analyzed using standard auto analyzer methodology. Any participant with serum calcium greater than 10.5 mg/dL (unadjusted for serum albumin) was immediately withdrawn from the study and the participant’s primary care physician was notified. During the 3 months of the trial, no participant stopped or started HCTZ. We did not quantify how long HCTZ users had been taking the medication prior to the beginning of the supplementation trial.
For 25(OH)D assays, blood samples collected at baseline, three months, and six months were separated, and plasma was stored in liquid nitrogen in the Dana-Farber Cancer Institute Clinical Research Laboratory (Boston, MA). After completion of the study, all plasma samples were sent as a single batch to the laboratory of Dr. Bruce Hollis (Medical University of South Carolina, Charleston, SC), where 25(OH)D concentrations were measured using the Diasorin radioimmunoassay.(22) All laboratory personnel were blinded to treatment assignment. Masked quality control samples were also assayed; the mean coefficient of variation of 25(OH)D measurements was 9%.
Statistical Analysis
The primary purpose of this investigation was to quantify the potential risk for hypercalcemia among participants taking HCTZ and those not taking HCTZ who underwent protocol calcium measurements. Those individuals with signs or symptoms of hypercalcemia and measured serum calcium ≥ 10.5 mg/dL at 1-month were withdrawn from the study at 1-month. Withdrawal from the study was based on the assumption that the participants’ hypercalcemia existed prior to starting vitamin D and would continue or recur if they were retained in the study. The difference in serum calcium levels between the HCTZ and non-HCTZ groups was determined with a Wilcoxon rank sum analysis of variance. Additionally, independent predictors of serum calcium levels at 3-months were identified by step-wise regression, with a model that included the following covariates: HCTZ status, sex, age, BMI, vitamin D dose, adherence to medication, exercise frequency, number of cigarettes smoked daily, dietary calcium, and plasma vitamin D level and at 3-months; vitamin D dose and baseline plasma vitamin D level were forced into the model. Statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). Statistical power for the vitamin D supplementation trial was based on the intent-to-treat population of 80 subjects per arm. During the study, the sample size was expanded to allow up to 100 participants per arm to account for the time gap between subject consent and PCP approval, and a 10% withdrawal and lost-to-follow-up rate. Using a two-sided t-test at the 0.05 significance level, the minimum detectable difference in 25(OH)D between treatment arms was 13.2 and 15.5 nmol/L (5.3 and 6.2 ng/mL) with 80% and 90% power, respectively.
RESULTS
At baseline, the HCTZ and non-HCTZ participants were similar in terms of BMI, dietary intake of vitamin D and calcium, and serum vitamin D, but those not taking HCTZ were approximately 10 years younger and predominantly male. (Table 1).
Table 1.
Baseline Characteristicsa
| Characteristic | Overall | HCTZ | Non-HCTZ |
|---|---|---|---|
| Total No. | 128 | 84 | 44 |
| Mean Age (SD) | 54.8 (10.7) | 58.1 (9.8) | 48.7 (10.4)* |
| Sex, No. (%) | |||
| Female | 73 (57) | 63 (75) | 10 (23)* |
| Male | 55 (43) | 21 (25) | 34 (77)* |
| BMI, median(IQR) | 30.5 (26.8–35.6) | 32.2 (26.8–35.9) | 29.5 (26.9–34.8) |
| Serum 25(OH)D, ng/ml, median (IQR) | 15.6 (10.6–24.0) | 16.3 (11.5–24.5) | 13.0 (9.7–22.6) |
| Dietary calcium, mg/d, median (IQR) | 314.4 (177.9–646.2) | 333.5 (186.5–611.0) | 260.2 (177.6–721.6) |
| Dietary vitamin D, IU/d, median(IQR) | 119.8 (61.1–254.6) | 113.9 (78.8–256.6) | 180.1 (78.8–256.6) |
| Calcium supplement use, No. | 0 | 0 | 0 |
Data are reported as No.(%) unless otherwise indicated.
P<.0001
The Kruskal-Wallis test was used to calculate P values for continuous variables. All statistical tests were two-sided. P values were calculated by Fisher’s exact test for dichotomous variables.
Occurrence of Elevated Calcium
Based on the 1-month and 3-month assessments, 5 of the 84 participants reporting HCTZ use (6.0%) had a serum calcium level above the upper limit of normal (Table 2). All 4 of the 84 participants that had hypercalcemia during month 1 were asked to stop taking the study medication and were withdrawn from the study. The study medication was not resumed. More participants had hypercalcemia at 1-month than at 3-months. After 1 month of the 12-week vitamin D supplementation period, 4 participants taking HCTZ experienced modestly elevated serum calcium levels, ranging from 10.7 to 11.0 mg/dL (Table 3). Among the HCTZ participants at 1-month, 3 of the 1000 IU group and 1 of the 2000 IU group had hypercalcemia, but no participants in the 4000 IU HCTZ group had hypercalcemia (Table 3). At 3-months, only one HCTZ participant had elevated calcium (calcium= 11.2 mg/dL; Table 3). None of the participants in the non-HCTZ group experienced elevated serum calcium during the 12 weeks of supplementation (Table 2). Baseline serum calcium was not measured.
Table 2.
Calcium (Ca) levels in HCTZ and non-HCTZ participants by vitamin D3 supplementation arm
| TIME POINT | VITAMIN D3 DOSE ASSIGNMENT (IU/DAY FOR THREE MONTHS) | TOTAL |
P Value Within HCTZ or non-HCTZ group* |
P Value Between HCTZ and non-HCTZ group# |
|||
|---|---|---|---|---|---|---|---|
| PLACEBO | 1000 | 2000 | 4000 | ||||
| One month# | |||||||
| HCTZ, n | 22 | 23 | 16 | 18 | 79 | ||
| Ca, Median(IQR), mg/dL | 9.5 (9.3 –9.8) | 9.8 (9.4–10.2) | 9.8 (9.5–9.9) | 9.5 (9.3–9.8) | 9.6 (9.4–9.9) | 0.139 | |
| Ca >10.5 mg/dL,n(%) | 0 | 3 (13) | 1 (6) | 0 | 4 (5) | ||
| Three months | |||||||
| Total, n | 29 | 28 | 31 | 31 | 119 | ||
| HCTZ, n | 22 | 20 | 15 | 18 | 75 | ||
| Ca, Median(IQR), mg/dL | 9.7 (9.4–10.0) | 9.6 (9.2–9.8) | 9.8 (9.7–10.1) | 9.7 (9.3–10.0) | 9.7(9.4–10.0) | 0.28 | 0.01 |
| Ca >10.5 mg/dL,n(%) | 0 | 0 | 0 | 1 (5) | 1 (1) | ||
| Non-HCTZ, n | 7 | 9 | 16 | 12 | 44 | 0.78 | |
| Ca, Median(IQR), mg/dL | 9.7(9.3–9.8) | 9.4 (9.3–9.9) | 9.5(9.2–9.7) | 9.4(9.3–9.5) | 9.5(9.3–9.7) | ||
| Ca >10.5 mg/dL, n | 0 | 0 | 0 | 0 | 0 | ||
Table 3.
Description of cases with elevated serum calciuma
| Case | Time point |
Calcium level (mg/dL) |
Vitamin D Dose (IU/day) |
Gender | Age | Description |
|---|---|---|---|---|---|---|
| 1 | 1 month | 10.7 | 1000 | Female | 63 | HCTZ subject, polydipsia and polyuria at calcium safety check and blood draw |
| 2 | 1 month | 10.9 | 1000 | Female | 65 | HCTZ subject, no symptoms at calcium safety check and blood draw |
| 3 | 1 month | 10.9 | 1000 | Female | 57 | HCTZ subject, no symptoms at calcium safety check and blood draw |
| 4 | 1 month | 11.0 | 2000 | Female | 53 | HCTZ subject, no symptoms at calcium safety check and blood draw |
| 5 | 3 months | 11.2 | 4000 | Female | 73 | HCTZ subject, no symptoms at calcium safety check and blood drawb |
normal serum calcium range: 8.8–10.5 mg/dL (not adjusted for albumin)
month 1 calcium=10 mg/dL
Differences in Calcium Levels Among HCTZ and non-HCTZ Participants
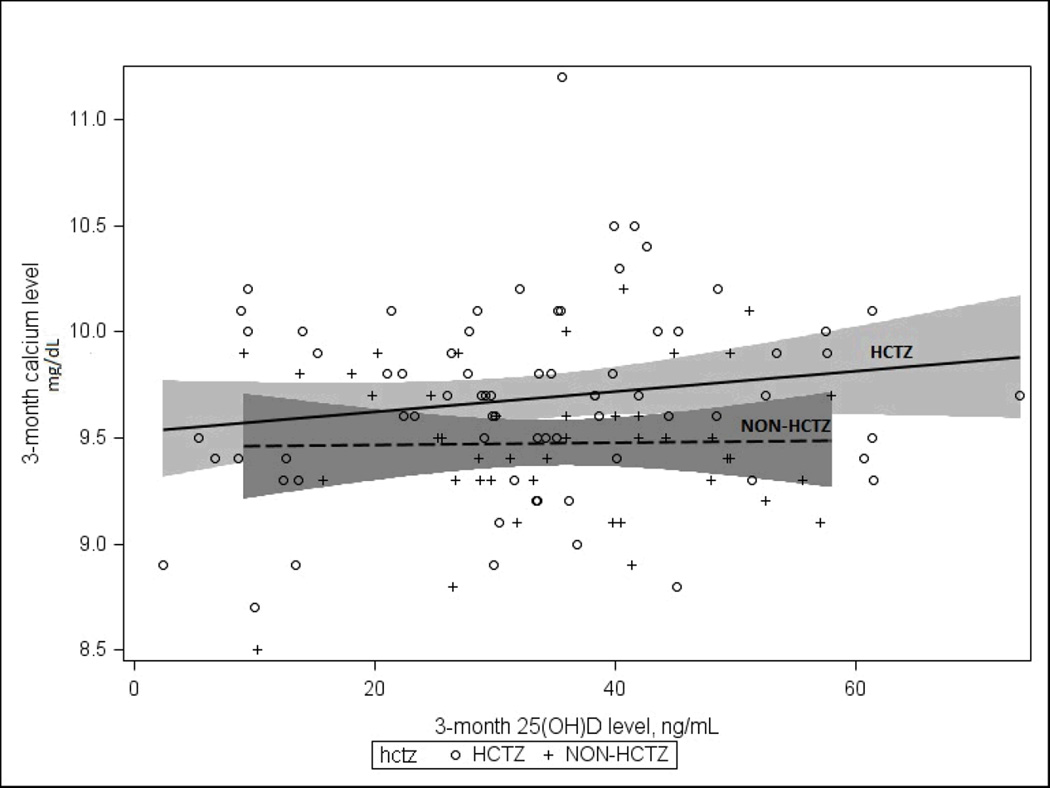
After 3 months of supplementation, the serum calcium levels for the HCTZ [mean(SD):9.7(0.4) mg/dL] and non-HCTZ participants [mean(SD)9.5(0.6) mg/dL] remained below the upper normal calcium limit of 10.5 mg/dL. However, this difference between the serum calcium between the HCTZ and non-HCTZ participants was statistically significant (p=0.01). Furthermore, there is not significant interaction by HCTZ (p for interaction=0.89), on the relationship between plasma 25(OH)D level at 3-months and serum calcium at 3-months (the two axes). This HCTZ non-interaction is indicated by parallel slopes between the two regression lines for HCTZ and non-HCTZ users (Figure 1).
Figure 1.
Serum 25(OH)D at 3-Months versus Serum Calcium at 3-Months with 95% CI for Hydrochlorothiazide (HCTZ) and Non-Hydrochlorothiazide (Non-HCTZ) Groups
Using a stepwise selection regression model with vitamin D dose and baseline 25(OH)D forced into the model, we identified only HCTZ use (p=.009) as a significant independent predictor for serum calcium level among the participants in our study. In a linear regression model to identify significant independent predictors of serum calcium at 3 months, only HCTZ use [Estimate (SE):0.05(0.01) p=0.01] was a significant predictor in a model that included the covariates: age, sex, baseline 25(OH)D and 25(OH)D at 3 months, dietary calcium, dietary vitamin D, and vitamin D dose per 1000 IU/day. HCTZ use was not modified by vitamin D dose or baseline 25(OH)D level, p for interaction (p=0.53 and p=0.87 respectively).
All participants that experienced hypercalcemia after taking vitamin D3 were female (Table 3). Three did not experience any symptoms of elevated calcium, although their safety tests at 1-month of supplementation revealed modestly elevated blood calcium levels. For the participant that experienced symptoms, her symptoms resolved within 1 week of discontinuation of the study medication (Vitamin D 1000IU/day) (Table 3) and repeat serum calcium was normal.
DISCUSSION
Both HCTZ(23, 24) and vitamin D(25–27) have the potential to increase serum calcium levels. In this post-hoc analysis, we evaluated the hypothesis that hypercalcemia may occur with concurrent high dose vitamin D supplementation in hypertensive individuals using HCTZ. In our review of the literature, we found no studies examining the risk of hypercalcemia associated with concurrent HCTZ and vitamin D (2000 IU to 4000 IU) treatment in blacks. Although we do not know what the optimal 25(OH)D level is for blacks, this study is critical because lower doses of vitamin D may be insufficient to correct vitamin D deficiency that is common in blacks.(28–31) Additionally, vitamin D supplementation may lower blood pressure in blacks.(10)
In the setting of osteoporosis, hypercalcemia with vitamin D and thiazide use has been reported(32). Prior case reports suggest that the combination of thiazide and vitamin D supplementation may cause hypercalcemia in at-risk individuals such as the elderly(32), those using high dose calcium supplements (33, 34), or those with compromised renal function or uncontrolled entry of calcium into the extracellular fluid (e.g. hyperparathyroidism or sarcoidosis).(35–41) Hypercalcemia resolved after hydration and withdrawal of thiazide, vitamin D and calcium supplements.(41)
This is the first analysis to directly analyze the effect of concurrent use of vitamin D and HCTZ in hypertensive but otherwise healthy individuals. Although HCTZ can decrease calcium excretion by the kidney(42, 43) and vitamin D can increase absorption of calcium in the intestines(44–47), both resulting in higher blood levels of calcium, we report that only 5.9% of HCTZ users experienced hypercalcemia with concurrent use of HCTZ and vitamin D supplements. For the 4 HCTZ users with hypercalcemia at 1-month, more subjects had hypercalcemia in the 1000 IU group than in the higher dose groups (2000 IU or 4000 IU) at 1-month. At 3-months, only one participant in the HCTZ group had hypercalcemia. The lack of a dose response effect of hypercalcemia with vitamin D supplementation suggests that participants that experienced in hypercalcemia in month 1 of vitamin D supplementation had hypercalcemia prior to initiation of vitamin D supplementation or that the appearance of early hypercalcemia is transient. The late occurrence of one case of hypercaclemia at month 3 suggests hypercalcemia may occur later during the course of therapy or it may have been a random event given our small sample sizes. Previous studies have already established that serum calcium levels are elevated in persons taking HCTZ. The role of dietary calcium and supplemental calcium, age, and gender in risk for hypercalcemia and higher serum calcium levels should be investigated in larger randomized control trials. Yet, these findings suggest that high dose vitamin D is not toxic over long periods of time.
Our study of a convenience sample of blacks taking HCTZ within a randomized clinical trial of vitamin D supplementation was limited by sample size. Moreover, our trial did not collect HCTZ dose, baseline calcium, or parathyroid hormone (PTH) levels on any of the participants, and serum calcium levels were only assess in a subset of non-HCTZ users. The available data does not permit estimation of within-subject change in serum calcium concentration with vitamin D supplementation. Thus, in all participants, symptoms relating to hypercalcemia were assessed every 2 weeks, and reports of symptoms possibly related to hypercalcemia mandated serum calcium measurement. The strength of this study is that it characterizes the time of onset of hypercalcemia in patients that do manifest hypercalcemia during vitamin D and HCTZ treatment. Another important result obtained from this study is that most cases of hypercalcemia were asymptomatic. The patient that developed symptomatic hypercalcemia at one month was not continued in the trial with vitamin D supplementation and her hypercalcemia spontaneously resolved. It is not known whether the cases of hypercalcemia documented at 1 month were already present at baseline.
Our cohort consists entirely of individuals who were self-identified as black or African-American; as such, these findings may not be generalizable to other populations such as cohorts with higher calcium intake. However, this study is strengthened by the fact that this question is evaluated in an all black cohort, which allows for greater understanding of the potential risk of hypercalcemia with concurrent HCTZ use and vitamin D supplementation in this population – a group that is more likely to be both vitamin D deficient and hypertensive(48).
While further research may be required, this study suggests that, for the majority of HCTZ users, combined HCTZ use with higher doses of vitamin D (2000 IU to 4000 IU) appears safe with a low rate of clinically significant hypercalcemia. Furthermore, vitamin D dose and plasma 25(OH)D level have no significant effect on calcium level in HCTZ users. Identification of optimal levels of plasma 25(OH)D remain to be established (49).
Acknowledgements
We would like to thank Cara Marcus, MSLIS, AHIP, Director of Library Services, Brigham and Women’s Faulkner Hospital for facilitating access to reference articles and YiQing Song, MD, ScD and Meryl S. LeBoff, MD for reviewing manuscript. The authors’ responsibilities were as follows—CSF, ELG, KME, BFD, GGB: conceived and designed the study; PDC JBS CSF ELG: analyzed the study; and all authors contributed to the manuscript.
Sources of Funding
This trial was funded by the National Cancer Institute (P50CA127003; K07CA148894 [Dr Ng]; K22CA126992; 5K05CA124415 [Dr Emmons]; U01CA138962 [Dr. Chandler]), the Department of Defense Prostate Cancer Research Program (PC081669 [Dr Drake]), the National Heart, Lung, and Blood Institute (5R01HL105440 [Dr Forman]), the American Society of Clinical Oncology Career Development Award (Dr Ng), and Pharmavite LLC (Mission Hill, CA). These funding sources had no role in the conception or conduct of the study, took no part in the data collection or analysis, and had no role in the drafting, review, or approval of the article.
Footnotes
No authors declare a conflict of interest.
REFERENCES
- 1.Fiscella K, Holt K. Racial disparity in hypertension control: tallying the death toll. Ann Fam Med. 2008 Nov-Dec;6(6):497–502. doi: 10.1370/afm.873. Epub 2008/11/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper R, Rotimi C. Hypertension in blacks. American journal of hypertension. 1997 Jul;10(7 Pt 1):804–812. doi: 10.1016/s0895-7061(97)00211-2. Epub 1997/07/01. eng. [DOI] [PubMed] [Google Scholar]
- 3.Artaza JN, Contreras S, Garcia LA, Mehrotra R, Gibbons G, Shohet R, et al. Vitamin D and cardiovascular disease: potential role in health disparities. J Health Care Poor Underserved. 2011;22(4 Suppl):23–38. doi: 10.1353/hpu.2011.0161. Epub 2011/12/07. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman AB, Schwartz GL, Boerwinkle E, Turner ST. Predictors of antihypertensive response to a standard dose of hydrochlorothiazide for essential hypertension. Kidney international. 2002 Mar;61(3):1047–1055. doi: 10.1046/j.1523-1755.2002.00200.x. [DOI] [PubMed] [Google Scholar]
- 5.Messerli FH, Makani H, Benjo A, Romero J, Alviar C, Bangalore S. Antihypertensive efficacy of hydrochlorothiazide as evaluated by ambulatory blood pressure monitoring: a meta-analysis of randomized trials. Journal of the American College of Cardiology. Feb 1;57(5):590–600. doi: 10.1016/j.jacc.2010.07.053. [DOI] [PubMed] [Google Scholar]
- 6.Vardan S, Rapacke J, Mookherjee S. Clinical efficacy and cost comparison of an amiloride-hydrochlorothiazide combination versus hydrochlorothiazide and wax-matrix potassium supplement in the treatment of essential hypertension. Clinical therapeutics. 1986;8(4):420–426. [PubMed] [Google Scholar]
- 7.Wright JT, Jr, Dunn JK, Cutler JA, Davis BR, Cushman WC, Ford CE, et al. Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA : the journal of the American Medical Association. 2005 Apr 6;293(13):1595–1608. doi: 10.1001/jama.293.13.1595. Epub 2005/04/07. eng. [DOI] [PubMed] [Google Scholar]
- 8.Cushman WC, Ford CE, Einhorn PT, Wright JT, Jr, Preston RA, Davis BR, et al. Blood pressure control by drug group in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) J Clin Hypertens (Greenwich) 2008 Oct;10(10):751–760. doi: 10.1111/j.1751-7176.2008.00015.x. Epub 2008/12/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen JM, Heran BS, Perez MI, Wright JM. Blood pressure lowering efficacy of beta-blockers as second-line therapy for primary hypertension. Cochrane database of systematic reviews (Online) 2010;(1):CD007185. doi: 10.1002/14651858.CD007185.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Forman JP, Scott JB, Ng K, Drake BF, Suarez EG, Hayden DL, et al. Effect of vitamin D supplementation on blood pressure in blacks. Hypertension. 2013 Apr;61(4):779–785. doi: 10.1161/HYPERTENSIONAHA.111.00659. Epub 2013/03/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal R, Sinha AD. Thiazide diuretics in advanced chronic kidney disease. J Am Soc Hypertens. 2012 Sep-Oct;6(5):299–308. doi: 10.1016/j.jash.2012.07.004. 2012. [DOI] [PubMed] [Google Scholar]
- 12.Aldinger KA, Samaan NA. Hypokalemia with hypercalcemia. Prevalence and significance in treatment. Ann Intern Med. 1977 Nov;87(5):571–573. doi: 10.7326/0003-4819-87-5-571. [DOI] [PubMed] [Google Scholar]
- 13.Aroldi A, Graziani G, Mioni G, Cecchettin M, Brancaccio D, Galmozzi C, et al. Thiazide diuretics in renal hypercalciuria. Proc Eur Dial Transplant Assoc. 1979;16:571–576. [PubMed] [Google Scholar]
- 14.Alon U, Costanzo LS, Chan JC. Additive hypocalciuric effects of amiloride and hydrochlorothiazide in patients treated with calcitriol. Miner Electrolyte Metab. 1984;10(6):379–386. [PubMed] [Google Scholar]
- 15.Desai HV, Gandhi K, Sharma M, Jennine M, Singh P, Brogan M. Thiazide-induced severe hypercalcemia: a case report and review of literature. American journal of therapeutics. Nov-Dec;17(6):e234–e236. doi: 10.1097/MJT.0b013e3181c6c21b. [DOI] [PubMed] [Google Scholar]
- 16.Skendzel LP. How physicians use laboratory tests. JAMA : the journal of the American Medical Association. 1978 Mar 13;239(11):1077–1080. Epub 1978/03/13. eng. [PubMed] [Google Scholar]
- 17.Brickman AS, Massry SG, Coburn JW. changes in serum and urinary calcium during treatment with hydrochlorothiazide: studies on mechanisms. J Clin Invest. 1972 Apr;51(4):945–954. doi: 10.1172/JCI106889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterlik M, Cross HS. Vitamin D and calcium insufficiency-related chronic diseases: molecular and cellular pathophysiology. European journal of clinical nutrition. 2009;63(12):1377–1386. doi: 10.1038/ejcn.2009.105. [DOI] [PubMed] [Google Scholar]
- 19.Slomski A. IOM endorses vitamin D, calcium only for bone health, dispels deficiency claims. JAMA : the journal of the American Medical Association. 2011;305(5):453–454. 6. doi: 10.1001/jama.2011.50. [DOI] [PubMed] [Google Scholar]
- 20.Avioli LV. The therapeutic approach to hypoparathyroidism. Am J Med. 1974 Jul;57(1):34–42. doi: 10.1016/0002-9343(74)90765-7. [DOI] [PubMed] [Google Scholar]
- 21.McNeill LH, Coeling M, Puleo E, Suarez EG, Bennett GG, Emmons KM. Colorectal cancer prevention for low-income, sociodemographically-diverse adults in public housing: baseline findings of a randomized controlled trial. BMC Public Health. 2009;9:353. doi: 10.1186/1471-2458-9-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollis BW. Quantitation of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D by radioimmunoassay using radioiodinated tracers. Methods Enzymol. 1997;282:174–186. doi: 10.1016/s0076-6879(97)82106-4. [DOI] [PubMed] [Google Scholar]
- 23.More on thiazide-induced hypercalcemia. Am J Psychiatry. 1981 Apr;138(4):534–536. doi: 10.1176/ajp.138.4.aj1384534. [DOI] [PubMed] [Google Scholar]
- 24.Aadland E, Jørgensen H. Hypercalcemia and other metabolic disorders during long-term treatment with thiazides. Tidsskr Nor Laegeforen. 1978 Feb;98(5):256–259. [PubMed] [Google Scholar]
- 25.Assadi F. Hypercalcemia: an evidence-based approach to clinical cases. Iran J Kidney Dis. 2009 Apr;3(2):71–79. [PubMed] [Google Scholar]
- 26.Ackermann D. Hypercalcemia in sarcoidosis--case report, prevalence, pathophysiology and therapeutic options. Ther Umsch. 2007 May;64(5):281–286. doi: 10.1024/0040-5930.64.5.281. [DOI] [PubMed] [Google Scholar]
- 27.Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician. 2003 May;67(9):1959–1966. [PubMed] [Google Scholar]
- 28.Gallagher JC, Peacock M, Yalamanchili V, Smith LM. Effects of vitamin d supplementation in older african american women. J Clin Endocrinol Metab. 2013 Mar;98(3):1137–1146. doi: 10.1210/jc.2012-3106. Epub 2013/02/07. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallagher JC, Sai A, Templin T, 2nd, Smith L. Ann Intern Med. Vol. 156. United States: 2012. Dose response to vitamin D supplementation in postmenopausal women: a randomized trial; pp. 425–437. [DOI] [PubMed] [Google Scholar]
- 30.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab. 2012 Apr;97(4):1153–1158. doi: 10.1210/jc.2011-2601. Epub 2012/03/24. eng. [DOI] [PubMed] [Google Scholar]
- 31.Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey 1988–1994. Am J Clin Nutr. 2002 Jul;76(1):187–192. doi: 10.1093/ajcn/76.1.187. Epub 2002/06/26. eng. [DOI] [PubMed] [Google Scholar]
- 32.Schwartzman MS, Franck WA. Vitamin D toxicity complicating the treatment of senile, postmenopausal, and glucocorticoid-induced osteoporosis. Four case reports and a critical commentary on the use of vitamin D in these disorders. The American journal of medicine. 1987 Feb;82(2):224–230. doi: 10.1016/0002-9343(87)90060-x. Epub 1987/02/01. eng. [DOI] [PubMed] [Google Scholar]
- 33.Drinka PJ, Nolten WE. Hazards of treating osteoporosis and hypertension concurrently with calcium, vitamin D, distal diuretics. J Am Geriatr Soc. 1984 May;32(5):405–407. doi: 10.1111/j.1532-5415.1984.tb02050.x. [DOI] [PubMed] [Google Scholar]
- 34.Crowe M, Wollner L, Griffiths RA. Hypercalcaemia following vitamin D and thiazide therapy in the elderly. Practitioner. 1984 Mar;228(1389):312–313. [PubMed] [Google Scholar]
- 35.Sharma OP. Vitamin D, calcium, and sarcoidosis. Chest. 1996 Feb;109(2):535–539. doi: 10.1378/chest.109.2.535. Epub 1996/02/01. eng. [DOI] [PubMed] [Google Scholar]
- 36.Heaney RP. Vitamin D depletion and effective calcium absorption. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2003 Jul;18(7):1342. doi: 10.1359/jbmr.2003.18.7.1342. author reply 3. Epub 2003/07/12. eng. [DOI] [PubMed] [Google Scholar]
- 37.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999 May;69(5):842–856. doi: 10.1093/ajcn/69.5.842. Epub 1999/05/08. eng. [DOI] [PubMed] [Google Scholar]
- 38.Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr. 2007 Jan;85(1):6–18. doi: 10.1093/ajcn/85.1.6. Epub 2007/01/09. eng. [DOI] [PubMed] [Google Scholar]
- 39.Heaney RP. Vitamin D: criteria for safety and efficacy. Nutr Rev. 2008 Oct;66(10) Suppl 2:S178–S181. doi: 10.1111/j.1753-4887.2008.00102.x. Epub 2008/12/05. eng. [DOI] [PubMed] [Google Scholar]
- 40.Heaney RP. Vitamin D and calcium interactions: functional outcomes. Am J Clin Nutr. 2008 Aug;88(2):541S–544S. doi: 10.1093/ajcn/88.2.541S. Epub 2008/08/12. eng. [DOI] [PubMed] [Google Scholar]
- 41.Parfitt AM. Thiazide-induced hypercalcemia in vitamin D-treated hypoparathyroidism. Ann Intern Med. 1972 Oct;77(4):557–563. doi: 10.7326/0003-4819-77-4-557. [DOI] [PubMed] [Google Scholar]
- 42.Caló L, Cantaro S, Marchini F, Giannini S, Castrignano R, Gambaro G, et al. Is hydrochlorothiazide-induced hypocalciuria due to inhibition of prostaglandin E2 synthesis? Clin Sci (Lond) 1990 Mar;78(3):321–325. doi: 10.1042/cs0780321. [DOI] [PubMed] [Google Scholar]
- 43.Christensen SE, Nissen PH, Vestergaard P, Mosekilde L. Familial hypocalciuric hypercalcaemia: a review. Curr Opin Endocrinol Diabetes Obes. 2011 Dec;18(6):359–370. doi: 10.1097/MED.0b013e32834c3c7c. [DOI] [PubMed] [Google Scholar]
- 44.Audran M, Legrand E. Hypercalciuria. Joint Bone Spine. 2000;67(6):509–515. doi: 10.1016/s1297-319x(00)00207-4. [DOI] [PubMed] [Google Scholar]
- 45.De Sanctis V, Fiscina B, Ciccone S. Severe hypercalcemia in a patient treated for hypoparathyroidism with calcitriol. Pediatr Endocrinol Rev. 2010 Jun;7(4):363–365. [PubMed] [Google Scholar]
- 46.Kumari M, Khazai NB, Ziegler TR, Nanes MS, Abrams SA, Tangpricha V. Vitamin D-mediated calcium absorption in patients with clinically stable Crohn's disease: a pilot study. Mol Nutr Food Res. 2010 Aug;54(8):1085–1091. doi: 10.1002/mnfr.200900351. Epub 2010/03/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lemann J, Gray RW. Idiopathic hypercalciuria. J Urol. 1989 Mar;141(3 Pt 2):715–718. doi: 10.1016/s0022-5347(17)40993-1. [DOI] [PubMed] [Google Scholar]
- 48.Artaza JN, Mehrotra R, Norris KC. Clin J Am Soc Nephrol. Vol. 4. United States: 2009. Vitamin D and the cardiovascular system; pp. 1515–1522. [DOI] [PubMed] [Google Scholar]
- 49.Bertone-Johnson ER, Chlebowski RT, Manson JE, Wactawski-Wende J, Aragaki AK, Tamimi RM, et al. Dietary vitamin D and calcium intake and mammographic density in postmenopausal women. Menopause. 2010 Nov-Dec;17(6):1152–1160. doi: 10.1097/gme.0b013e3181e102d9. [DOI] [PMC free article] [PubMed] [Google Scholar]