Abstract
Reduced intensity conditioning/non-myeloablative conditioning regimens are increasingly used in allogeneic hematopoietic cell transplantation (HCT). Reports have shown CD34+ dose to be important for transplant-outcome using myeloablative conditioning. The role of CD34+ dose of peripheral blood progenitor cells (PBPC) has not been previously analyzed in a large population undergoing reduced intensity conditioning/non-myeloablative HCT. We studied 1,054 patients aged 45–75 years, with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) transplanted between 2002 and 2011. Results of multivariate analysis showed that PBPC from HLA-matched siblings containing <4 × 106 CD34+/kg were associated with higher non-relapse mortality (HR 2.03, p=0.001), overall mortality (HR 1.48, p=0.008), and lower neutrophil (OR 0.76, p=0.03) and platelet (OR 0.76, p=0.03) recovery. PBPC from unrelated donors with CD34+ dose <6 × 106 CD34+/kg were also associated with higher non-relapse (HR 1.38, p=0.02) and overall mortality (HR 1.20, p=0.05). In contrast to reports after myeloablative HCT, CD34+ dose did not affect relapse or graft-versus-host disease with either donor type. An upper cell dose limit was not associated with adverse outcomes. These data suggest that PBPC CD34+ dose >4 × 106 CD34+/kg and >6 × 106 CD34+/kg are optimal for HLA-matched sibling and unrelated donor HCT, respectively.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) for patients with acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS) can be curative.1 In the setting of myeloablative transplantation, nucleated cell dose is important for several outcome parameters including survival, decreased graft rejection, decreased incidence of infections, relapse, and graft-versus-host disease (GVHD).2–6 However, a high CD34+ dose has been associated with an increased risk of acute GVHD and in some instances, chronic GVHD.7–10 Reduced intensity conditioning and non-myeloablative regimens are increasingly used for older patients and for those with pre-existing co-morbidities.11–14 Reduced intensity/non-myeloablative conditioning regimens rely more on the anti-leukemic effect of the transplanted cells, than on the anti-leukemic effects of the radiation and chemotherapy given during conditioning and peripheral blood progenitor cells (PBPC) the predominant graft used for these transplantations. Although there are several reports detailing the effect of CD34 dose on outcomes after HLA-matched sibling transplants, there are few reports that describe its effect after unrelated donor transplants.15,16 In the largest report to-date with about 932 recipients of unrelated donor PBPC transplantation, mortality risks were lower with CD34+ dose greater than 4.5 × 106/kg but without increasing graft versus host disease risks.16 Although this report included all transplant conditioning regimens, the lesser intense regimens accounted for only a third of transplantations. A separate analysis of the lesser intense conditioning regimens was not performed and the study period was prior to 2003. As lesser intense conditioning regimens now account for about 40% of allogeneic transplants in adults, we sought to determine the optimal CD34+ dose for reduced intensity and non-myeloablative conditioning regimens that may improve survival in 1054 patients aged 45 – 75 years with AML or MDS in a more recent period.
Patients and Methods
Data source
The Center for International Blood and Marrow Transplant Research is a voluntary working group of more than 450 transplant centers that contribute data on consecutive allogeneic and autologous transplants. Participating centers are required to report all transplants consecutively to avoid any selection bias and compliance is monitored. Patients are followed longitudinally until death or lost to follow-up. All patients provided written informed consent for data submission and research participation. The Institutional Review Boards of the Medical College of Wisconsin and the National Marrow Donor Program approved this study.
Eligibility criteria
Patients aged 45 – 75 years with AML or MDS who received their first allogeneic transplant with reduced intensity/non-myeloablative conditioning regimens during the interval between 2002 and 2011 were included in the analysis. The study population was limited to those with AML or MDS, the most common indications for allogeneic transplantation in older patients. All patients received PBPC. CD34+ dose was reported by transplant centers and the dose determined by the Cell Processing laboratories at these centers. Regimens included low dose total body irradiation (TBI; 200 cGy), busulfan (≤ 8 mg/kg oral or ≤6.5 mg/kg intravenous) or melphalan ≤140 mg/m2.17 GVHD prophylaxis included tacrolimus or cyclosporine with mycophenolate or methotrexate. Patients received PBPC from HLA-matched siblings (n=370) or unrelated adult donors, HLA matched at the allele-level at HLA-A, -B, -C and –DRB1 (8/8; n=521) or mismatched at a single HLA-locus (7/8; n=163). Patients aged less than 45 years were excluded as most received myeloablative transplant conditioning regimens.
Outcomes
The primary outcome of interest was non-relapse mortality, defined as death in continuous complete remission. Secondary outcomes examined included hematopoietic recovery (time to neutrophil recovery, ≥500/μl for 3 consecutive days, and platelet recovery, ≥20,000/μL without transfusion for 7 days), acute GVHD18, chronic GVHD19, relapse, and overall mortality. Relapse was defined as morphologic, molecular or cytogenetic evidence of disease recurrence. Death from any cause was considered an event for overall survival and surviving patients were censored at last follow up.
Statistical methods
Exploratory analysis of the effect of CD34+ dose on non-relapse mortality was performed separately for HLA-matched sibling and unrelated donor transplantations. The optimal cut-point for CD34+ dose above and below which there were significant differences was determined by maximizing the partial likelihood from the Cox’s proportional hazards model for non-relapse mortality, the primary study endpoint.20 For HLA-matched sibling transplantation, survival rates were lower when the CD34+ dose was fewer than 4 × 106/kg. For unrelated donor transplantation, survival rates were lower when the CD34+ dose was fewer than 6 × 106/kg. Therefore, for all subsequent analyses, the CD34+ dose was considered based on the threshold dose described above and separate analyses undertaken for HLA-matched sibling and unrelated donor transplantations. The characteristics of patients, their disease and transplantation are shown in Tables 1A and 1B. Categorical variables were compared using the X2 test. The probability of overall survival was calculated using the Kaplan-Meier estimator21. The probabilities of neutrophil and platelet recovery, acute and chronic GVHD, non-relapse mortality and relapse were calculated using the cumulative incidence method.22
Table 1.
| Table 1A. Characteristics of patients, their disease and transplantation | |||
|---|---|---|---|
| CD34+ dose | |||
| Variable | < 4 × 106/kg | ≥ 4 × 106/kg | P-value |
| Number | 93 | 277 | |
| Age | 0.86 | ||
| 45 – 60 years | 43 (46%) | 131 (47%) | |
| 61 – 75 years | 50 (54%) | 146 (53%) | |
| Sex, male | 59 (63%) | 168 (61%) | 0.63 |
| Recipient CMV serostatus | 0.98 | ||
| Positive | 63 (68%) | 188 (68%) | |
| Negative | 28 (30%) | 84 (30%) | |
| Not reported | 2 ( 2%) | 5 ( 2%) | |
| Performance score | 0.02 | ||
| 90 – 100 | 52 (56%) | 144 (52%) | |
| ≤ 80 | 32 (34%) | 124 (45%) | |
| Not reported | 9 (10%) | 9 ( 3%) | |
| Body mass index | 0.01 | ||
| Normal | 24 (26%) | 98 (35%) | |
| Overweight | 34 (37%) | 118 (43%) | |
| Obese | 35 (38%) | 61 (22%) | |
| Disease | 0.30 | ||
| Acute myeloid leukemia | 79 (85%) | 222(80%) | |
| Myelodysplastic syndrome | 14 (15%) | 55 (20%) | |
| Disease status | 0.02 | ||
| 1st complete remission/refractory anemia | 61 (66%) | 148 (53%) | |
| 2nd complete remission | 13 (14%) | 30 (11%) | |
| Relapse/refractory anemia with excess blast | 19 (20%) | 99 (36%) | |
| Cytogenetic risk | 0.47 | ||
| Good | 8 ( 9%) | 32 (12%) | |
| Intermediate | 54 (58%) | 158 (57%) | |
| Poor | 29 (31%) | 73 (26%) | |
| Not reported | 2( 2%) | 14 ( 5%) | |
| Interval from diagnosis to transplantation | 0.41 | ||
| 1 – 5 months | 44 (47%) | 136 (49%) | |
| 6 – 11 months | 24 (26%) | 84 (30%) | |
| ≥ 12 months | 25 (27%) | 57 (21%) | |
| Conditioning regimen | 0.78 | ||
| TBI (200 cGy) + fludarabine | 17 (18%) | 63 (23%) | |
| TBI (200 cGy) + cyclophosphamide or busulfan | 4 ( 4%) | 13 ( 5%) | |
| Alkylating agent + fludarabine + ATG | 20 (22%) | 57 (21%) | |
| Alkylating agent + fludarabine without ATG | 52 (56%) | 144 (52%) | |
| Graft versus host disease prophylaxis | 0.04 | ||
| Calcineurin inhibitor + methotrexate | 57 (61%) | 135 (49%) | |
| Calcineurin inhibitor + mycophenolate | 36 (39%) | 142 (51%) | |
| Donor-recipient sex match | 0.03 | ||
| Female donor/male recipient | 35 (38%) | 72 (26%) | |
| Other | 58 (62%) | 205 (74%) | |
| Donor-recipient blood group ABO match | 0.53 | ||
| Matched | 60 (65%) | 166 (60%) | |
| Minor mismatch | 14 (15%) | 39 (14%) | |
| Major mismatch | 18 (19%) | 61 (21%) | |
| Not reported | 1 ( 1%) | 11 ( 4%) | |
| Transplant period | 0.31 | ||
| 2002 – 2005 | 36 (39%) | 124 (45%) | |
| 2006 – 2011 | 57 (61%) | 153 (55%) | |
| Median follow-up (range), months | 50 (12–97) | 60 (4–144) | |
| Table 1B. Characteristics of patients, their disease and transplantation | |||
|---|---|---|---|
| CD34+ dose | |||
| Variable | < 6 × 106/kg | ≥ 6 × 106/kg | P-value |
| Number | 281 | 406 | |
| Age | 0.54 | ||
| 45 – 60 years | 120 (43%) | 183 (45%) | |
| 61 – 75 years | 161 (57%) | 223 (55%) | |
| Sex, male | 185 (66%) | 245 (60%) | 0.14 |
| Recipient CMV serostatus | 0.01 | ||
| Positive | 154 (55%) | 257 (63%) | |
| Negative | 121 (43%) | 148 (36%) | |
| Not reported | 6 ( 2%) | 1 (<1%) | |
| Performance score | 0.87 | ||
| 90 – 100 | 158 (56%) | 227 (56%) | |
| ≤ 80 | 109 (39%) | 155 (38%) | |
| Not reported | 14 ( 5%) | 26 ( 6%) | |
| Body mass index | 0.61 | ||
| Normal | 71 (25%) | 116 (29%) | |
| Overweight | 108 (38%) | 153 (38%) | |
| Obese | 102 (36%) | 137 (34%) | |
| Disease | 0.82 | ||
| Acute myeloid leukemia | 229 (81%) | 328 (81%) | |
| Myelodysplastic syndrome | 52 (19%) | 78 (19%) | |
| Disease status | 0.66 | ||
| 1st complete remission/refractory anemia | 140 (50%) | 203 (50%) | |
| 2nd complete remission | 54 (19%) | 68 (17%) | |
| Relapse/refractory anemia with excess blast | 87 (31%) | 135 (33%) | |
| Cytogenetic risk | 0.42 | ||
| Good | 8 ( 9%) | 32 (12%) | |
| Intermediate | 54 (58%) | 158 (57%) | |
| Poor | 29 (31%) | 73 (26%) | |
| Not reported | 2( 2%) | 14 ( 5%) | |
| Interval from diagnosis to transplantation | 0.79 | ||
| 1 – 5 months | 104 (37%) | 158 (39%) | |
| 6 – 11 months | 97 (35%) | 136 (33%) | |
| ≥ 12 months | 78 (28%) | 111 (27%) | |
| Not reported | 2 (<1%) | 1 (<1%) | |
| Conditioning regimen | 0.06 | ||
| TBI (200 cGy) + fludarabine | 40 (14%) | 79 (19%) | |
| TBI (200 cGy) + cyclophosphamide or busulfan | 13 ( 5%) | 28 ( 7%) | |
| Alkylating agent + fludarabine + ATG | 113 (40%) | 170 (42%) | |
| Alkylating agent + fludarabine without ATG | 115 (41%) | 129 (32%) | |
| Graft versus host disease prophylaxis | 0.13 | ||
| Calcineurin inhibitor + methotrexate | 121 (43%) | 198 (49%) | |
| Calcineurin inhibitor + mycophenolate | 160 (57%) | 208 (51%) | |
| Donor-recipient sex match | 0.002 | ||
| Female donor/male recipient | 63 (22%) | 72 (26%) | |
| Other | 214 (76%) | 205 (74%) | |
| Not reported | 4 ( 1%) | 10 ( 2%) | |
| Donor-recipient blood group ABO match | 0.51 | ||
| Matched | 120 (43%) | 154 (38%) | |
| Minor mismatch | 69 (25%) | 118 (29%) | |
| Major mismatch | 91 (33%) | 132 (33%) | |
| Not reported | 1 (<1%) | 2 (<1%) | |
| Donor-recipient HLA-match | 0.60 | ||
| 8/8 HLA-matched | 211 (75%) | 312 (77%) | |
| 7/8 HLA-matched | 70 (25%) | 94 (23%) | |
| Donor age | 0.12 | ||
| 18 – 32 years | 102 (36%) | 178 (44%) | |
| 33 – 50 years | 138 (49%) | 188 (46%) | |
| > 50 years | 22 ( 8%) | 21 ( 5%) | |
| Not reported | 19 ( 7%) | 19 ( 5%) | |
| Transplant period | 0.32 | ||
| 2002 – 2005 | 73 (26%) | 92 (23%) | |
| 2006 – 2011 | 208 (74%) | 314 (77%) | |
| Median follow-up (range), months | 60 (12–101) | 49 (4–97) | |
Abbreviation: TBI = total body irradiation; ATG = anti-thymocyte globulin
To study the association between clinical outcomes and CD34+ dose, Cox regression models20 were built for acute and chronic GVHD, non-relapse mortality, relapse and overall mortality. Results generated are expressed as hazard ratio (HR) together with the 95% confidence interval (CI).
The variable of primary interest, CD34+ dose, was held in all steps of model building. Other variables tested included: age (45 – 60 vs. >60 years), sex, performance score (≤ 80 vs. 90 – 100), recipient CMV serostatus (positive vs. negative), body mass index (normal vs. overweight vs. obese), disease (AML vs. MDS), disease status (1st complete remission/refractory anemia vs. second remission vs. refractory anemia with excess blasts/relapse), cytogenetic risk (good vs. intermediate vs. poor risk), interval from diagnosis to transplantation (<6 vs. 6 – 12 vs. >12 months), conditioning regimen (TBI-containing vs. alkylating agent + fludarabine + in vivo T-cell depletion vs. alkylating agent + fludarabine), GVHD prophylaxis (calcineurin inhibitor + methotrexate vs. calcineurin inhibitor + mycophenolate), donor age (18 – 32 vs. 33 – 50 vs. >50 years), donor-recipient HLA match for unrelated donor transplantation only (8/8 vs. 7/8 HLA-matched), donor-recipient ABO match (matched vs. minor vs. major mismatch) and transplant period (2002 – 2005 vs. 2006 – 2011). The variable of CD34+ dose was retained in all models regardless of level of significance. For other factors, only those associated with the outcome of interest at the 5% level or less were retained in the final model. All p-values were two-sided. Analyses were done using SAS 9.3 (Cary, NC).
Results
Patient, disease and transplant characteristics
Among the 370 recipients of HLA-matched sibling transplantation (Table 1A), 93 (25%) received PBPC grafts that contained CD34+ fewer than 4 × 106/kg (median CD34+ dose 5 × 106/kg). The characteristics of patients who received PBPC with CD34+ dose fewer than 4 × 106/kg and those who received a higher CD34+ dose were similar except that those who received a CD34+ dose fewer than 4 × 106/kg were more likely to report performance scores of 90 or 100, to be obese as determined by body mass index, and receive calcineurin inhibitor with methotrexate for GVHD prophylaxis. Additionally, male recipients were more likely to receive PBPC from female donors that contained fewer than 4 × 106/kg CD34+ cells. The median follow up of patients who received grafts that contained fewer than 4 × 106/kg CD34 cells was 50 months and for those who received higher CD34, 60 months.
Among the 687 recipients of unrelated donor transplantation, 281 (41%) received PBPC that contained CD34+ fewer than 6 × 106/kg (median CD34+ dose 7 × 106/kg). The characteristics of the two treatment groups were similar except that patients who received CD34 cells fewer than 6 × 106/kg were more likely to be CMV seronegative. Male recipients were more likely to receive PBPC from female donors that contained fewer than 6 × 106/kg CD34+ cells. The median follow up of patients who received grafts with CD34+ dose fewer than 6 × 106/kg CD34 cells was 60 months and for those who received higher CD34+ dose, 49 months.
Hematopoietic recovery
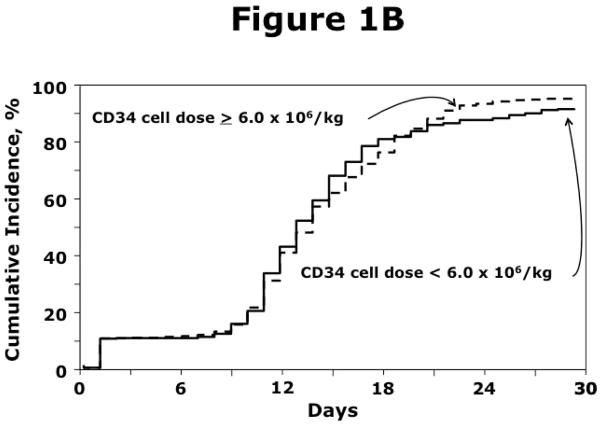
The effects of CD34+ dose on hematopoietic recovery are shown in Table 2A, 2B (Figure 1A, 1B). For HLA-matched sibling donor transplantation, PBPC containing fewer than 4 × 106/kg CD34 cells was associated with lower likelihoods of neutrophil (OR 0.76, 95% CI 0.60 – 0.98, p=0.03) and platelet (OR 0.76, 95% CI 0.58 – 0.98, p=0.03) recovery (Table 2A). For unrelated donor transplantations, hematopoietic recovery was not associated with CD34+ dose (Table 2B).
Table 2A.
Results of multivariate analysis: HLA-matched sibling transplantation
| Hazard Ratio (95% confidence interval) | P-value | |
|---|---|---|
| Neutrophil recovery* | ||
| CD34 dose ≥ 4 × 106/kg | 1.00 | |
| CD34 dose < 4 × 106/kg | 0.76 (0.60 – 0.98) | 0.03 |
| Platelet recovery** | ||
| CD34 dose ≥ 4 × 106/kg | 1.00 | |
| CD34 dose < 4 × 106/kg | 0.76 (0.58 – 0.98) | 0.03 |
| Grade 2 – 4 acute GVHD*** | ||
| CD34 dose ≥ 4 × 106/kg | 1.00 | |
| CD34 dose < 4 × 106/kg | 0.98 (0.64 – 1.49) | 0.91 |
| Chronic GVHD**** | ||
| CD34 dose ≥ 4 × 106/kg | 1.00 | |
| CD34 dose < 4 × 106/kg | 1.09 (0.75 – 1.58) | 0.65 |
| Non-relapse mortality# | ||
| CD34 dose ≥ 4 × 106/kg | 1.00 | |
| CD34 dose < 4 × 106/kg | 2.03 (1.26 – 3.27) | 0.004 |
| Relapse## | ||
| CD34 dose ≥ 4 × 106/kg | 1.00 | |
| CD34 dose < 4 × 106/kg | 1.06 (0.73 – 1.52) | 0.77 |
| Overall mortality### | ||
| CD34 dose ≥ 4 × 106/kg | 1.00 | |
| CD34 dose < 4 × 106/kg | 1.48 (1.11 – 1.99) | 0.008 |
Adjusted for conditioning regimen- recovery was less likely with low dose irradiation regimens (HR 0.61, p=0.0002).
Adjusted for performance score, disease and transplant period – recovery was less likely with performance scores 80 or lower (HR 0.68, p=0.0009), diagnosis of MDS (HR 0.64, p=0.002) and transplantations between 2002 and 2005 (HR 0.68, p=0.0007).
Adjusted for conditioning regimen and GVHD prophylaxis - risks were lower with irradiation-containing (HR 0.50p=0.006), non-irradiation regimens with in vivo T-cell depletion (HR 0.54, p=0.02) and with GVHD prophylaxis that included calcineurin inhibitor with methotrexate (HR 0.53, p=0.003).
Adjusted for conditioning regimen - lower risks after non-irradiation regimens with in vivo T-cell depletion (HR 0.42, p=0.0005) compared to non-irradiation regimens without in vivo T-cell depletion.
Adjusted for disease status at transplantation - transplantation in relapse was associated with higher non-relapse mortality (HR 1.94, p=0.006).
Adjusted for performance score, disease, disease status at transplantation, cytogenetic risk, conditioning regimen, GVHD prophylaxis and transplant period - risks were higher for those with AML (HR 1.74, p=0.03), transplantation in relapse (HR 1.82, p=0.001), poor risk cytogenetics (HR 2.03, p=0.03), performance scores of 80 or lower (HR 1.56, p=0.006), non-irradiation regimens with in vivo T-cell depletion (HR 1.93, p=0.0004), calcineurin inhibitor with methotrexate GVHD prophylaxis (HR 1.83, p=0.003) and transplants performed prior to 2006 (HR 1.54, p=0.01).
Adjusted for performance score, disease status at transplantation, cytogenetic risk and transplant period - higher overall mortality for performance scores of 80 or lower (HR 1.72, p<0.0001), poor risk cytogenetic abnormalities (HR 2.13, p=0.002), transplantations in relapse (HR 1.34, p=0.04) and those performed prior to 2006 (HR 1.44, p=0.008).
Table 2B.
Results of multivariate analysis: Unrelated donor transplantation
| Hazard Ratio (95% confidence interval) | P-value | |
|---|---|---|
| Neutrophil recovery* | ||
| CD34 dose ≥ 6 × 106/kg | 1.00 | |
| CD34 dose < 6 × 106/kg | 1.02 (0.87 – 1.19) | 0.81 |
| Platelet recovery** | ||
| CD34 dose ≥ 6 × 106/kg | 1.00 | |
| CD34 dose < 6 × 106/kg | 0.87 (0.74 – 1.02) | 0.09 |
| Grade 2 – 4 acute GVHD*** | ||
| CD34 dose ≥ 6 × 106/kg | 1.00 | |
| CD34 dose < 6 × 106/kg | 1.11 (0.88 – 1.41) | 0.39 |
| Chronic GVHD**** | ||
| CD34 dose ≥ 6 × 106/kg | 1.00 | |
| CD34 dose < 6 × 106/kg | 0.87 (0.70 – 1.09) | 0.22 |
| Non-relapse mortality# | ||
| CD34 dose ≥ 6 × 106/kg | 1.00 | |
| CD34 dose < 6 × 106/kg | 1.38 (1.06 – 1.79) | 0.02 |
| Relapse## | ||
| CD34 dose ≥ 6 × 106/kg | 1.00 | |
| CD34 dose < 6 × 106/kg | 0.96 (0.76 – 1.22) | 0.74 |
| Overall mortality### | ||
| CD34 dose ≥ 6 × 106/kg | 1.00 | |
| CD34 dose < 6 × 106/kg | 1.20 (1.00 – 1.43) | 0.05 |
Adjusted for age, donor-recipient HLA-match and conditioning regimen - recovery was less likely in patients aged less than 60 years (HR 0.84, p=0.03), low dose irradiation regimens (HR 0.61, p<0.0001), in vivo T-cell depletion with alkylating agents and fludarabine (HR 0.82, p=0.03), and 7/8 HLA-matched transplants (HR 0.78, p=0.007).
Adjusted for performance score, disease status, time from diagnosis to transplant, conditioning regimen and transplant period – recovery was less likely with performance score 80 or lower (HR 0.76, p=0.002), interval from diagnosis to transplantation is 6 – 12 months (HR 0.78, p=0.01) or longer than 12 months (HR 0.72, p=0.006) compared to less than 6 months, and transplantation in relapse compared to complete remission (HR 0.80, p=0.02). Platelet recovery was more likely with TBI-containing regimens (HR 1.43, p=0.001) and for transplants performed after 2005 (HR 1.28, p=0.01).
Adjusted for body mass index, disease status and GVHD prophylaxis - Grade 2 – 4 acute GVHD risks after unrelated donor transplantation were lower in obese (HR 0.72, 95% CI 0.64 – 0.96, p=0.03) and overweight (HR 0.72, 95% CI 0.54 – 0.96, p=0.02) patients compared to those with normal body mass index, transplantations in second compared to first complete remission (HR 0.63, 95% CI 0.43 – 0.90, p=0.01) and calcineurin inhibitor with methotrexate compared to mycophenolate GVHD prophylaxis (HR 0.58, 95% CI 0.45 – 0.74, p<0.0001). Acute GVHD risks were higher for transplantations in relapse or refractory anemia with excess blasts (HR 1.35, 95% CI 1.05 – 1.74, p=0.02).
Adjusted for conditioning regimen and GVHD prophylaxis - risks were lower after non-irradiation regimens with in vivo T-cell depletion (HR 0.54, p<0.0001) and with calcineurin inhibitor and methotrexate GVHD prophylaxis (HR 0.64, p=0.0007).
Adjusted for disease status at transplantation, time from diagnosis to transplant, HLA-match - higher non-relapse mortality risks for transplantation of grafts from donors who were HLA-mismatched to the patient (HR 1.47, p=0.009) and when the interval from diagnosis to transplantation was longer than 12 months (HR 1.76, p=0.004). Risks were lower for transplantations in second complete remission compared to first complete remission (HR 0.43, p=0.0004).
Adjusted for performance score, body mass index, disease, disease status at transplantation, cytogenetic risk - risks were higher for those with AML (HR 1.61, p=0.01), transplantation in relapse (HR 1.77, p=0.001) and performance scores of 80 or lower (HR 1.56, p=0.0004).
Adjusted for age and performance score – higher mortality with performance scores of 80 or lower (HR 1.38, p=0.003) and patients older than 60 years (HR 1.27, p=0.01)
Figure 1.
Figure 1A. The incidence of neutrophil recovery after transplantation of PBPC from HLA-matched siblings
Figure 1B. The incidence of neutrophil recovery after transplantation of PBPC from unrelated donors
Graft-versus-host disease
CD34+ dose was not associated with grade 2 – 4 acute GVHD after HLA-matched sibling or unrelated donor transplantation (Table 2A, 2B). CD34+ dose was also not associated with chronic GVHD after HLA-matched sibling transplants (Table 2A) except when the dose was greater than 10 × 106/kg (n=38). Transplantation of grafts that contained CD34+ dose greater than 10 × 106/kg was associated with lower risks of chronic GVHD compared to grafts that contained <4 × 106/kg (HR 0.46, 95% CI 0.23 – 0.89, p=0.02) or 4 – 10 × 106/kg (HR 0.45, 95% CI 0.24 – 0.83, p=0.01). CD34+ dose was not associated with chronic GVHD after unrelated donor transplantation (Table 2B). We tested for an effect of higher CD34+ dose (>10 × 106/kg) and found none (data not shown).
Relapse and Non-relapse mortality
CD34+ dose was not associated with relapse after HLA-matched sibling or unrelated donor transplantation (Table 2A, 2B). However, CD34+ dose was associated with non-relapse mortality risks (Table 2A, 2B; Figures 2A, 2B). Transplantation of PBPC from HLA-matched siblings containing CD34+ fewer than 4 × 106/kg was associated with higher non-relapse mortality. Similarly, transplantation of PBPC from unrelated donors containing CD34+ fewer than 6 × 106/kg was associated with higher non-relapse mortality.
Figure 2.
Figure 2A. The incidence of non-relapse mortality after transplantation of PBPC from HLA-matched siblings
Figure 2B. The incidence of non-relapse mortality after transplantation of PBPC from unrelated donors
Overall survival
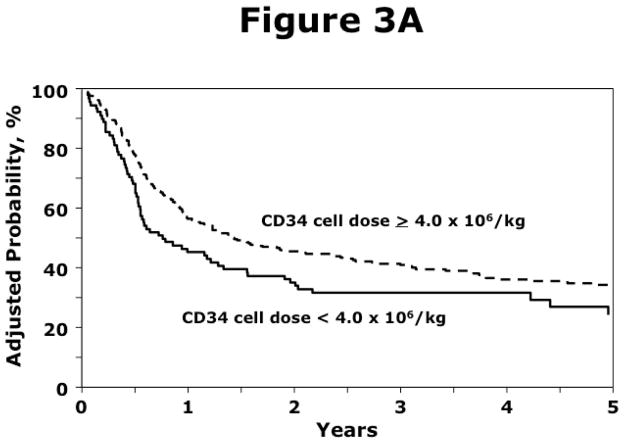
Transplantation of PBPC from HLA-matched siblings with CD34+ dose fewer than 4 × 106/kg was associated with higher overall mortality (Table 2A; Figure 3A). CD34+ dose was only marginally associated with overall mortality after unrelated donor transplantation (Table 2B; Figure 3B). There were no differences in causes of death between the two CD34+ dose groups for either donor source. For recipients of HLA-matched sibling transplants, approximately 50% of deaths were attributed to recurrent disease and the remaining attributed to transplant-related causes. Similarly, for recipients of unrelated donor transplants, approximately 40% of deaths were attributed to recurrent disease and the remaining attributed to transplant-related causes.
Figure 3.
Figure 3A. The probability of overall survival after transplantation of PBPC from HLA-matched siblings
Figure 3B. The probability of overall survival after transplantation of PBPC from unrelated donors
Subset Analysis
The Sorror co-morbidity score23 was ascertained for patients transplanted between 2008 and 2011. This score is not available for patients transplanted prior to 2008. One hundred and forty-six recipients of HLA-matched sibling transplantation were evaluable and there were no differences in co-morbidity score between the two CD34+ dose groups (p=0.70). Two hundred and eighty-two recipients of unrelated donor transplantation were evaluable and there were no differences in co-morbidity score between the two CD34+ dose groups (p=0.43). Among recipients of HLA-matched sibling transplants, co-morbidity score of 2 or 3 was not associated with higher overall mortality (data not shown). However, in recipients of unrelated donor transplants, co-morbidity score of 2 and 3 or higher was associated with higher overall mortality (HR 1.63, 95% CI 1.02–2.61, p=0.04 and HR 1.75, 95% CI 1.13–2.70, p=0.01, respectively), independent of CD34+ dose.
Discussion
Published literature support CD34+ dose is associated with transplantation outcomes after bone marrow or mobilized peripheral blood HLA-matched sibling and unrelated donor transplantation.2–10,15,16 However, most of these reports included primarily recipients of myeloablative transplant conditioning regimens and bone marrow graft. Increasing number of reduced intensity/non-myeloablative conditioning regimens for allogeneic transplantation are being performed as this treatment is now offered to older individuals and those who are unable tolerate myeloablative regimens. It is in this group of transplant recipients the potential effect of CD34+ dose on transplant outcomes is not well described. Therefore, in the current analysis, we assessed the effect of PBPC CD34+ dose on transplantation outcomes in over 1000 patients with AML and MDS, the two most common indications for allogeneic transplantation in patients over the age of 45 years. An important finding of the current analyses is the independent predictive value of higher CD34+ dose for improvements in survival after HLA-matched and unrelated donor transplantation. Notably, the threshold of CD34+ dose associated with better survival was lower, at 4 × 106/kg for HLA-matched sibling transplants than for unrelated transplant recipients where the threshold, was 6 × 106/kg. We did not detect additional lower dose thresholds that were associated with worse survival. Although our findings to some extent confirm those reported by others, there are also notable differences such as the lack of association between higher cell dose and disease recurrence.5,15,24 Our inability to observe differences in relapse risks may be explained by differences in regimen intensity between the current analysis and earlier reports.5,15,24 The current analysis is limited to reduced intensity/non-myeloablative regimens with greater reliance on immune modulation and graft-versus-leukemia effects and it may not be possible to identify a CD34+ dose threshold for disease recurrence in this setting. On the other hand, a large series from the National Marrow Donor Program, limited to PBPC transplantations for hematologic malignancy that included transplant conditioning regimens of all intensities also failed to see an effect of CD34+ on disease recurrence, similar to our findings.
Further, we were unable to identify an adverse effect of any upper-limit of CD34+ dose on outcomes after both HLA-matched and unrelated donor transplantations.3,9,10 It is noteworthy that the CD34+ dose threshold above which we observed better survival varied by donor source with higher CD34+ requirement for PBPC from unrelated donors. The observed difference in dose threshold between matched sibling and unrelated donor transplantations may be attributed to differences in HLA-matching between donors and recipients. Although approximately three-quarters of unrelated donor-recipient pairs were HLA-matched at the allele-level at HLA-A, -B, -C and –DRB1 we did not consider HLA-match at the DP locus or HLA-match at the low expression alleles.25,26 It is plausible the cell dose threshold needed to improve survival could be higher with HLA disparity. A single HLA-locus mismatch at one of the high expression alleles (A, B, C or DRB1) was associated with higher mortality but this effect was independent of CD34+ dose. The CD34+ threshold dose of 6 × 106/kg for unrelated donor transplants we observed is higher than that reported in the earlier report from the National Marrow Donor Program.16 In that report, with 600 recipients of myeloablative conditioning and 320 recipients of reduced intensity/non-myeloablative conditioning, survival was better with PBPC grafts that contained CD34+ dose greater than 4.5 × 106/kg. The observed difference in CD34+ dose threshold between that report and the current analysis may be explained by differences in the characteristics of the study population. Ours is a relatively homogenous population with AML and MDS, all patients were older than 45 years, received reduced intensity/non-myeloablative conditioning regimens, and almost all were transplanted after 2003 and received better HLA-matched grafts.
Hematopoietic recovery was better after transplantation of PBPC grafts with CD34+ dose greater than 4 × 106/kg from HLA-matched siblings. This is not surprising as better graft composition is associated with better recovery rates and fewer graft failures.27
In the setting of unrelated donor transplants we did not observe an association between CD34+ dose and hematopoietic recovery. The lack of association is surprising but is consistent with the other large report on CD34+ dose and outcomes after unrelated donor transplantation.16
The association between CD34+ dose and acute and chronic GVHD are mixed with some studies reporting higher GVHD rates, others lower rates, and several reports that have failed to show an association.3,5,6,9,15,16,27,28 We did not observe differences in acute GVHD rates by CD34+ dose with either donor source. However, we observed lower rates of chronic GVHD with PBPC that contained CD34+ dose greater than 10 × 106/kg after HLA-matched sibling transplantation only. This was observed in 38 patients (10% of recipients of HLA-matched sibling transplant), a relatively small cohort warranting further confirmation in larger study population. It is plausible that grafts containing low CD34+ dose may trigger infections, which in turn, may trigger acute GVHD. Reduced intensity/non-myeloablative conditioning regimens are generally associated with higher acute and chronic GVHD regardless of donor source. In the absence of an adverse effect on survival together with the relatively small numbers of patients who received very high CD34+ dose, we conclude there are no specific advantages to receiving PBPC that contain a substantially higher dose of CD34+ than the threshold dose associated with higher survival. Further, Weisdorf and colleagues examined the effect of acute and chronic GVHD in patients with AML and MDS who had survived disease-free for at least 1-year after reduced intensity/non-myeloablative transplantations.29 Only patients with history of both acute and chronic GVHD experienced lower recurrence but higher non-relapse mortality negated any potential beneficial effects from GVHD. We also tested for an effect of CD34+ dose and GVHD in the setting of in vivo T-cell depleted transplants and found none.
The current analyses used data collected by a transplant registry. Consequently CD34+ dose was not measured in a central laboratory but at individual transplant centers and varying laboratory standards may have influenced, at least in part some of our observations. Another limitation is that we were unable to test for an effect of the Sorror comorbidity index in all patients. These data were available for only about half the patients reported in the current analyses, as this comorbidity index was not routinely assessed prior to 2008. However, in subset analyses, higher comorbidity index was associated with higher mortality, independent of CD34+ dose for recipients of unrelated donor transplantation. Our inability to detect a significant effect for recipients of HLA-matched sibling transplantation may be attributed to a relatively small sample size of 146. Importantly, recipients of HLA-matched sibling transplantation were equally likely to report worse co-morbidity score (2 or more) in both CD34+ dose groups. Although we controlled for known risk factors associated with transplant outcomes and ensured the effect of these risk factors were independent of the effect of CD34+ dose, there may be several unknown or unmeasured factors that influenced outcomes. Nevertheless, we were able to show the independent effect of higher CD34+ dose on non-relapse and overall mortality after reduced intensity/non-myeloablative conditioning HLA-matched sibling and unrelated donor transplantation in a relatively homogenous population. The data offer the opportunity to establish standards for PBPC collection from related and unrelated donors when contemplating reduced intensity conditioning transplantation for older patients with AML or MDS such that some of the excess mortality associated with allogeneic transplantation may be alleviated.
Acknowledgments
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-12-1-0142 and N00014-13-1-0039 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics, Inc.; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;*Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children’s Foundation; Kiadis Pharma; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick’s Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *TerumoBCT; *Teva Neuroscience, Inc.; *THERAKOS, Inc.; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government. *Corporate Members
Footnotes
Conflict-of-Interest: The authors have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thomas ED, Buckner CD, Banaji M, et al. One hundred patients with acute leukemia treated by chemotherapy, total body irradiation and allogeneic marrow transplantation. Blood. 1977;49:511–533. [PubMed] [Google Scholar]
- 2.Sierra J, Storer B, Hansen JA, et al. Transplantation of marrow cells from unrelated donors for treatment of high-risk leukemia: the effect of leukemia burden, donor HLA-matching and marrow cell dose. Blood. 1997;89:4226–4235. [PubMed] [Google Scholar]
- 3.Przepiorka D, Smith TL, Folloder J, et al. Risk factors for acute graft-versus-host disease after allogeneic blood stem cell transplantation. Blood. 1999;94:1465–1470. [PubMed] [Google Scholar]
- 4.Rocha V, Labopin M, Gluckman E, et al. Relevance of bone marrow cell dose on allogeneic transplantation for patients with acute myeloid leukemia in first complete remission: results of a European survey. J Clin Oncol. 2002;20:4324–4330. doi: 10.1200/JCO.2002.11.058. [DOI] [PubMed] [Google Scholar]
- 5.Ringden O, Barrett AJ, Zhang MJ, et al. Decreased treatment failure in recipients of HLA-identical bone marrow or peripheral blood stem cell transplants with high CD34 cell dose. Br Haematol. 2003;121:874–885. doi: 10.1046/j.1365-2141.2003.04364.x. [DOI] [PubMed] [Google Scholar]
- 6.Gorin NC, Labopin M, Boiron JM, et al. Results of genoidentical hematopoietic stem cell transplantation with reduced intensity conditioning for acute myelocytic leukemia: higher doses of stem cells infused benefit patients receiving transplants in second remission or beyond – the Acute Leukemia Working Party of The European Cooperative Group for Blood and Marrow Transplantation. J Clin Oncol. 2006;24:3959–3966. doi: 10.1200/JCO.2006.05.5855. [DOI] [PubMed] [Google Scholar]
- 7.Barrett AJ, Ringden O, Zhang MJ, et al. Effect of nucleated marrow cell dose and survival in identical twin bone marrow transplants for leukemia. Blood. 2000;95:3323–3327. [PubMed] [Google Scholar]
- 8.Remberger M, Mattson J, Hassan Z, et al. Risk factors for acute graft-versus-host disease grades II – IV after reduced intensity conditioning allogeneic stem cell transplantation with unrelated donors: a single center study. Bone Marrow Transplant. 2008;41:399–405. doi: 10.1038/sj.bmt.1705913. [DOI] [PubMed] [Google Scholar]
- 9.Mohty M, Bilger K, Jourdan E, et al. Higher doses of CD34+ peripheral blood stem cells are associated with increased mortality from chronic graft-versus-host disease after allogeneic HLA-identical sibling transplantation. Leukemia. 2003;17:869–875. doi: 10.1038/sj.leu.2402909. [DOI] [PubMed] [Google Scholar]
- 10.Urbano-Ispizua A, Carreras E, Marin P, et al. Allogeneic transplantation of CD34+ selected cells from peripheral blood from human leukocyte antigen-identical siblings: detrimental effect of a higher number of donor CD34+ cells? Blood. 2001;98:2352–2357. doi: 10.1182/blood.v98.8.2352. [DOI] [PubMed] [Google Scholar]
- 11.Slavin S, Nagler A, Naparstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91:756–763. [PubMed] [Google Scholar]
- 12.Giralt S, Estey E, Albitar M, et al. Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft-versus-leukemia without myeloablative therapy. Blood. 1997;89:4531–4560. [PubMed] [Google Scholar]
- 13.Martino R, Caballero MD, Canals C, et al. Allogeneic peripheral blood stem cell transplantation with reduced intensity conditioning: results of a prospective multicenter study. Br J Haematol. 2001;115:653–659. doi: 10.1046/j.1365-2141.2001.03153.x. [DOI] [PubMed] [Google Scholar]
- 14.Luger SM, Ringden O, Zhang MJ, et al. Similar outcomes using myeloablative vs. reduced intensity allogeneic transplant preparative regimens for AML or MDS. Bone Marrow Transplant. 2012;47:203–211. doi: 10.1038/bmt.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura R, Auayporn N, Smith DD, et al. Impact of graft cell dose on transplant outcomes following unrelated donor allogeneic peripheral blood stem cell transplantation: higher CD34 cell doses are associated with decreased relapse rates. Biol Blood Marrow Transplant. 2008;14:449–457. doi: 10.1016/j.bbmt.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pulsipher MA, Chitphakdithai P, Logan BR, et al. Donor, recipient, and transplant characteristics as risk factors after unrelated donor PBSC transplantation: beneficial effects of higher CD34+ cell dose. Blood. 2009;114:2606–2616. doi: 10.1182/blood-2009-03-208355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 19.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 20.Cox DR. Regression model and life tables. J R Stat Soc B. 1972;34:187–200. [Google Scholar]
- 21.Klein JP, Moeschberger ML. Survival Analysis: Statistical Methods for Censored and Truncated Data. 2. New York, NY: Springer-Verlag; 2003. [Google Scholar]
- 22.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.Sorror M, Sandmaier BM, Storer BE, et al. Comorbidity and disease status based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving alloegenic hematopoietic cell transplantation. J Clin Oncol. 2007;25:4246–4254. doi: 10.1200/JCO.2006.09.7865. [DOI] [PubMed] [Google Scholar]
- 24.Perez-Simon JA, Diez-Campelo M, Martino R, et al. Impact of CD34 cell dose on the outcome of patients undergoing reduced intensity conditioning allogeneic peripheral blood stem cell transplantation. Blood. 2003;102:1108–1113. doi: 10.1182/blood-2002-11-3503. [DOI] [PubMed] [Google Scholar]
- 25.Fleischhauer K, Shaw BE, Gooley T, et al. Effect of T-cell-epitope matching at HLA-DPB1 in recipients of unrelated donor haematopoietic cell transplantation: a retrospective study. Lancet Oncol. 2012;13:366–374. doi: 10.1016/S1470-2045(12)70004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernández-Viña MA, Klein JP, Haagenson M, et al. Multiple mismatches at the low expression HLA loci DP, DQ, and DR3/4/5 associate with adverse outcomes in hematopoietic stem cell transplantation. Blood. 2013;121:4603–4610. doi: 10.1182/blood-2013-02-481945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao TM, Shizuru JA, Wong RM, et al. Engraftment and survival following reduced intensity allogeneic peripheral blood hematopoietic cell transplantation is affected by CD8+ T-cell dose. Blood. 2005;105:2300–2306. doi: 10.1182/blood-2004-04-1473. [DOI] [PubMed] [Google Scholar]
- 28.Baron F, Baker JE, Storb B, et al. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2004;104:2254–2262. doi: 10.1182/blood-2004-04-1506. [DOI] [PubMed] [Google Scholar]
- 29.Weisdorf D, Zhang MJ, Arora M, Horowitz MM, Rizzo JD, Eapen M. Graft-versus-host disease induced graft-versus-leukemia effect: greater impact on relapse and disease-free survival after reduced intensity conditioning. Biol Blood Marrow Transplant. 2012;18:1727–1733. doi: 10.1016/j.bbmt.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]