Abstract
Background
Modular lower extremity (LE) robotics may offer a valuable avenue for restoring neuromotor control after hemiparetic stroke. Prior studies show that visually-guided and visually-evoked practice with an ankle robot (anklebot) improves paretic ankle motor control that translates into improved overground walking.
Objective
Assess the feasibility and efficacy of daily anklebot training during early sub-acute hospitalization post-stroke.
Methods
Thirty-four inpatients from a stroke unit were randomly assigned to anklebot (N=18) or passive manual stretching (N=16) treatments. All suffered a first stroke with residual hemiparesis (ankle manual muscle test grade 1/5 to 4/5), and at least trace muscle activation in plantar- or dorsiflexion. Anklebot training employed an “assist-as-needed” approach during > 200 volitional targeted paretic ankle movements, with difficulty adjusted to active range of motion and success rate. Stretching included >200 daily mobilizations in these same ranges. All sessions lasted 1 hour and assessments were not blinded.
Results
Both groups walked faster at discharge, however the robot group improved more in percent change of temporal symmetry (p=0.032) and also of step length symmetry (p=0.038), with longer nonparetic step lengths in the robot (133%) vs. stretching (31%) groups. Paretic ankle control improved in the robot group, with increased peak (p≤ 0.001) and mean (p≤ 0.01) angular speeds, and increased movement smoothness (p≤ 0.01). There were no adverse events.
Conclusion
Though limited by small sample size and restricted entry criteria, our findings suggest that modular lower extremity robotics during early sub-acute hospitalization is well tolerated and improves ankle motor control and gait patterning.
INTRODUCTION
There is now convergent evidence that central nervous system (CNS) injuries can be at least partially offset through experience dependent plasticity in the neural networks that control movements.1–3 However, motor practice with the affected limb(s) is needed to shape the emergent networks. This notion of time on task, along with goal setting and performance feedback, is a fundamental principle of motor learning.4 The goal of central neural reorganization sub-serving functional movement has been associated with repetitive, goal-oriented practice using the impaired limb.5–9 Thus high-volume task-oriented training has been a major research focus for restoring motor function in individuals with hemiparetic stroke.
The use of robotic devices to augment usual therapy has gained considerable attention because it could offer a platform to achieve many of the objectives inherent to motor learning.10,11 Along with capabilities for individualized programming and precise measurement, robots can enable thousands of goal-oriented movements with performance feedback. In the area of robotic gait therapy post-stroke, most studies have targeted whole body locomotor training to promote task specificity.12–18 In contrast, modular robotics directed at single joints19–24 or limbs invites a focus on addressing impairments in underlying motor control and learning, while affording opportunities to explore effects on gait. Potential for transfer to locomotor performance is reported in recent studies of robotic ankle training in chronic stroke.25,26 Given these positive results in chronic hemiparesis, one might hypothesize that an ideal time to push the CNS toward more effective reorganization is during the earlier periods of sub-acute stroke recovery when the biological milieu may be poised for remodeling. While there are concerns based on rodent models and with human participants that imposing too much activity too early after stroke may be detrimental, 27–30 recent evidence suggests that initiating robotic therapy 48-hours after stroke, including with lower extremity (LE) robotics, is not harmful and may accelerate functional gains.31–34 Thus, our objective is to investigate if there are measurable benefits from providing modular LE robotics early in the recovery period.
Here we report initial results on the feasibility and efficacy of a modular, impedance-controlled ankle robot 24 (“anklebot”) in the early sub-acute phase of stroke. The first goal was to determine how well patients in the rehabilitation hospital setting would tolerate daily use of anklebot and how well this protocol would mesh with usual inpatient care. We also determined if daily anklebot training improves paretic ankle motor control and spatio-temporal gait parameters in patients that initially have at least a trace of ankle muscle activation, compared to controls receiving dose-matched amounts of manual stretching of the paretic ankle.
METHODS
Subjects
A convenience sample of consecutive patients with hemiparetic stroke and at least trace activation of paretic plantar- or dorsi-flexors (PF-DF), and who were available to participate five days weekly, was recruited from the inpatient Stroke Unit at the University of Maryland Rehabilitation & Orthopedics Institute (UMROI). Recruitment and informed consent procedures were approved by University of Maryland, Baltimore Institutional Review Board, Baltimore Veterans Affairs Research and Development Committee, Massachusetts Institute of Technology Committee on the Use of Humans as Experimental Subjects, and the Medical Executive Committee for UMROI.
Inclusion criteria included (1) first ischemic or hemorrhagic stroke; (2) residual LE hemiparesis involving the ankle, defined by manual muscle testing (strength range grade 1/5 to 4/5, capable of generating at least trace muscle activation in PF-DF; (3) adequate language and neurocognitive function (e.g., follow two-step commands) to participate in training, testing, and to give informed consent; and (4) clinical, neurological, and hemodynamic stability to sit in the chair for 30–60 minutes per session of ankle training.
Exclusion criteria included (1) total plegia (0/5) at paretic ankle; (2) fixed or painful contractures or other LE pain syndrome that could impede participation; (3) dementia, based on clinical diagnosis; (4) orthopedic, arthritic, or inflammatory condition limiting ankle movement; (5) known or suspected current or recent (< 3 months) signs or symptoms of deep venous thrombosis or pulmonary thrombo-embolism; (6) vision impairment to preclude visual tracking needed for training; and (7) severe receptive or global aphasia confounding testing and training.
Experimental design
After obtaining informed consent and completion of baseline testing, subjects were block randomized to either robotics or the stretching group. Because participants were made aware of potential assignment to either group, blinding to treatment was not possible. All subjects received usual physical therapy in accordance with inpatient standards of care.
Robotic Training
Anklebot setup
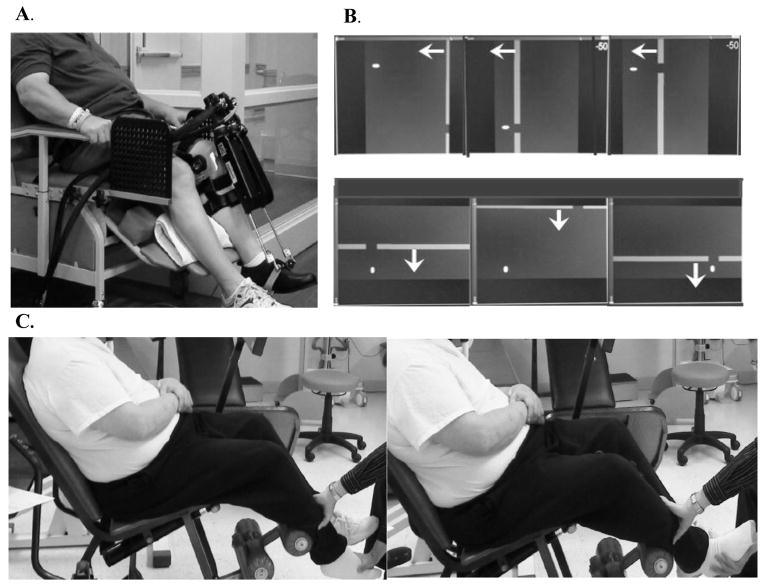
The anklebot’s proximal attachment was mounted anterior to an orthopedic knee brace lined with foam pads and cushioned straps to maximize comfort and provide protection from skin irritations (Fig. 1A). Distal attachments were secured to a modified orthopedic shoe. Additional protection was provided by pads where subjects indicated sensitivity to pressure. Shoe fit was aided with foam insoles and socks as required. Subjects sat in a modified Geri chair with adjustable back-leg rest mechanisms affording optimal postural alignment during training, with a knee brace secured to a mounting plate for support and knee immobilization. A seatbelt was secured around the pelvis to limit proximal hip and thigh motion. The paretic lower leg was positioned at ~45° on a cushioned support, isolating the foot to move freely about the ankle. Subjects were then introduced to the video “racer” game that was subsequently used to assess paretic ankle motor control.
Figure 1.
Training protocol: A. Subject in a seated position for anklebot assessments and training in robotics suite; B. Visual display for dorsi-plantarflexion (top panel) and inversion-eversion (lower panel) formats of the “racer game” used for anklebot training. Arrows are added to denote movement direction of the approaching gates; C. Subject in a seated position for manual passive stretching in a separate clinic.
Performance-based Training
Seated anklebot training, provided for ~ 60 minutes as a supplement to usual therapy, was conducted with volitional ankle movements visually guided by moving targets (“gates”) (Fig. 1A, B). Instructions were to dorsi- or plantar-flex the ankle to move a cursor “up” or “down”, or to invert or evert (INV-EV) to move the cursor “right” or “left” to pass through the gates delivered in blocks of 25 targets. Blocks alternated between DF-PF and INV-EV. Gates appeared every 4 seconds, allowing time to anticipate arrival and attempt to move the cursor for clear passage. Gates were located at different vertical and horizontal levels to span the DF-PF and INV-EV workspaces, with locations scaled to each subject’s paretic ankle active range of motion (AROM) measured prior to each session. Gate locations were scaled within 80% AROM in PF-DF and INV-EV ranges with two intermediate gates at 40% AROM. If subjects’ could not generate an AROM in a particular direction, targets were scaled to 80% passive ROM (PROM). Additional feedback on performance consisted of a cumulative score indicating the number of successful gate passages.
Training sessions began (and ended) with an abbreviated “record only” trial without robotic assistance to assess volitional ankle motor control. Following this, a series of longer “assist as-needed” trials were conducted. Assist-as-needed means that the impedance controller creates a “slot” between the initial position and target. The user can move freely within the slot. If movement is not initiated within 2 seconds, the robot provides graded assistance by closing the backwall of the slot to move the ankle. The amount of assistance depends on user’s ability to move ahead of the collapsing backwall of the slot; however, the robot will not fully complete the task without some volitional movement. Sessions totaled 8–10 blocks as tolerated with adequate rest periods to achieve ≥200 daily repetitions. Task difficulty including gate locations and speed of appearance were adjusted on a session-by-session basis based on AROM and success.
Manual stretching protocol
The stretching group received stretching sessions in a clinic that was physically separate from the robotics training center (Fig. 1C). Subjects remained as relaxed as possible while the paretic ankle was manually moved in DF-PF or INV-EV. This was repeated every 4 seconds with 25 movements per block, approximating the movement rate of the robot group, with the entire session consisting of 8–10 blocks totaling ≥200 movements to approximate the number of daily repetitions received by the robot group.
Assessments
Subjects were assessed at the beginning and completion of training with clinical and robot-based measures to evaluate ankle impairment and function. Assessments included timed floor walks (8 m), the Berg balance test, the motor (ambulatory) component of the Functional Independence Measure (FIM), and indices of volitional paretic ankle control derived using robot-measured positional data. AROMs were measured using the robot in the DF-PF and INV-EV ranges, and muscle strength was evaluated by manual muscle test (MMT). Clinical measures were performed by the same physical therapist; testers were not blinded to treatment owing to limited personnel in this pilot study.
Self-selected walking was performed on an 8-meter instrumented gait mat (CIR Systems, Havertown, PA). Spatio-temporal measures included mean velocity, cadence, stride length, step lengths, step times, and relative times in paretic single limb support and double limb support. A derived measure, interlimb symmetry, was calculated as the ratios of paretic-to-nonparetic (NP) step times and step lengths. All tests were repeated 2–3 times, with steady-state gait observed by starting data collection after two strides. Subjects were asked to first walk at a comfortable self-selected speed and then, if possible, at a fast safe walking speed. During gait assessments, subjects used the least restrictive assistive device with therapist assistance as needed, and seated rests were given between trials.
Measures of paretic ankle motor control were derived using robot-measured unassisted kinematics 26 at baseline and post-training before discharge. Outcomes included peak and mean angular speed averaged across DF-PF movements, movement smoothness quantified by normalized jerk (first derivative of acceleration, divided by peak speed), and task accuracy as the number of successful passages.
Statistical Analyses
Statistical treatment of the data was performed using SPSS ver. 20. For parametric analyses a repeated measures ANOVA was used to test hypotheses that changes in group means over the course of the inpatient stay were significantly different, with post-hoc t-tests applied as warranted. Nonparametric analyses for baseline and discharge data used the Wilcoxon signed-rank test and for ordinal data (e.g., MMT ratings), the Wilcoxon rank-sum test. Paired t-tests were used for within group comparisons and to compare the between group means of relative change on gait parameters. The significance level was set at p < 0.05.
RESULTS
Thirty-nine consecutive subjects were randomized. Five were not included in the analysis for the following reasons: study-unrelated medical complications (N=1), unable to complete 5 training sessions prior to discharge (N=1), time post-stroke > 49 days (N=2), and noncompliance (N=1). Of 34 subjects completing the protocol, 18 were in the robot group and 16 in the stretching group. Both groups were similar in terms of baseline clinical and demographic features (Table 1). They averaged the same number of training sessions (Robot: 10.9±1.0; Stretch: 10.3±0.8; p = 0.620).
Table 1.
Baseline characteristics of robot and stretching groups. (Mean±SE).
| Robot Group (18) | Stretching Group (16) | p-value | |
|---|---|---|---|
| Age (yrs) | 63.3±2.3 | 60.0±3.1 | 0.392 |
| Days post-stroke | 11.9±1.5 | 10.8±1.2 | 0.564 |
| Side of lesion | 9L, 9R | 9L, 7R | |
| FIM-walk (0–7) | 1.1 ±0.1 (range:0–2) | 1.0±0.0 (range:1–1) | 0.317* |
| Gait velocity (cm/s) | 19.1±3.0 | 19.3±3.2 | 0.972 |
| BERG Balance (0–56) | 17.9±2.5 | 16.3±2.9 | 0.659 |
| AROM (deg) | |||
| DF | −17.9±3.6 | −16.3±4.1 | 0.771 |
| PF | 38.2±2.9 | 35.7±3.2 | 0.557 |
| INV | 0.1±2.4 | 1.3±2.7 | 0.755 |
| EV | −1.4±1.4 | −2.9±1.6 | 0.485 |
| MMT (0–5) | |||
| DF | 1.1±0.3 (range:0–3.3) | 1.4±0.3 (range:0–3.3) | 0.417* |
| PF | 1.5±0.2 (range:0–2.7) | 1.8±0.3 (range:0–3.7) | 0.248* |
FIM: functional independence measure for ambulation.
Wilcoxon Sign Rank test. AROM: active range of motion. MMT: manual muscle test.
Clinical assessments
Changes in clinical measures did not achieve significant interactions. Both groups made significant gains in ambulatory FIM scores, with the robot group improving from a mean±SE of 1.1±0.1 to 4.1±0.4 and the stretching group from 1.0±0.0 to 3.9±0.5. Floor walking velocity also increased for both groups (F1,30 = 41.82; p < 0.001), from 19.1±3.0 to 37.2±4.9 cm/s in the robot group and 19.3±3.2 to 33.6±4.9 cm/s for the stretching group. Berg Balance Scale increased for both groups (F1,30 = 43.2, p < 0.001), from 17.9±2.5 to 38.9±3.0 in the robot group and from 16.3±2.9 to 31.1±3.5. AROM in DF-PF changed only in the DF range, with a main effect for time (F1,30 = 20.61; p < 0.001); the robot group’s mean increased 19.1±4.4 deg compared to the stretching group’s gain of 9.8±4.5. Changes in MMT scores in the robot group for the dorsiflexors increased 2.0 points compared to 1.0 point in the stretching group (p = 0.090). This amounts to a change from trace DF to antigravity AROM with robotics, versus trace amount to AROM out of the plane of gravity for controls. Plantarflexor MMT gained 1.2 and 0.8 points for the robot and stretching groups respectively, but the difference in gains was not significant (p = 0.300).
Gait temporal-distance parameters
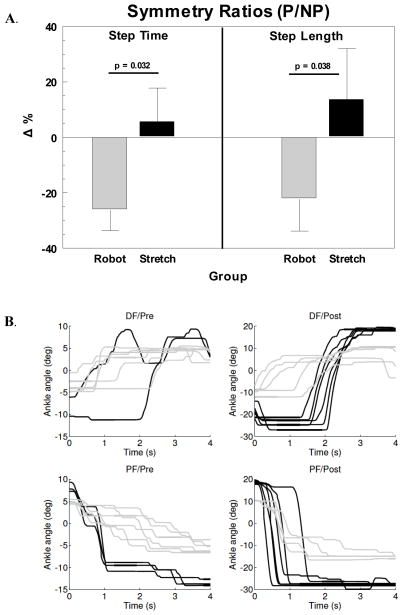
Two subjects in the robot group were not able to complete baseline walks due to severity of gait deficits, and were not included in the analysis. Because nearly all participants were unable to increase walking speed above their self-selected effort, gait analyses were performed on the self-selected speed only. Analysis of interlimb temporal symmetry produced a significant group x time (GxT) interaction favoring the robot group (F1, 30 = 4.50; p = 0.042) along with a main effect for time (F1, 30 = 6.75; p = 0.014). Post-hoc tests indicated a significant decrease toward an ideal 1:1 interlimb temporal symmetry by the robot group (p = 0.005) compared to the stretching group (p = 0.737). Comparisons of the mean percent changes in step time ratios showed a similar effect for the robot group making greater progress toward 1:1 temporal symmetry (p = 0.032; Fig. 2A). The baseline step time symmetry values did not show a difference between groups (p = 0.371).
Figure 2.
Effect of intervention on gait function and ankle targeting: A. Percent change for step time and step length symmetry measured as ratios of paretic-to-nonparetic sides. Decreases in percent change (Δ%) reflect shifts toward greater interlimb symmetry. P = paretic; NP = nonparetic; B. Examples of movement traces from an exemplar patient in the robot (solid black) and the stretching (solid gray) groups without anklebot assistance. Top panels show the changes in dorsiflexion (DF) movement smoothness before (left-Pre) and after (right-Post) the respective interventions. Lower panels show similar changes in plantarflexion (PF). Note that the initial ankle positions (at 0 sec) are influenced by the location of the preceding target. The movement traces also illustrate time-to-target i.e., velocity differences (steeper slope = faster movements) and improved ranges of motion for both groups over the course of hospitalization, although more pronounced for the robot trainee whose data is shown (note scale differences on y-axes Pre-Post in both DF and PF).
The robot group also demonstrated greater improvements in percent change of interlimb spatial symmetry (p = 0.038; Fig. 2A), however the GxT interaction for the absolute step length ratios did not achieve significance (F1,30 = 2.53; p = 0.12). The groups did not differ at baseline (p = 0.173). The main effect for paretic step lengths showed an increase for both groups (F1,30 = 9.01; p = 0.005). The major contributor to the greater relative change in the robot group’s spatial symmetry was a larger increase in the NP step length versus that of the stretching group. A strong trend in the GxT interaction (F1, 30 = 4.10, p = 0.052) and post-hoc t-tests affirmed that both groups increased NP step length, but the robot group’s 133% (p < 0.001) shift toward symmetry was larger than the 31% (p = 0.015) for the stretching group. In absolute terms this amounts to a mean 13.2 cm gain the NP step length with robotics training, compared to only 6.2 cm for controls. Analyses of several other gait parameters (Table 2) reflected substantial improvements in walking over the course of hospitalization. Effect sizes (Cohen’s d) for step time symmetry and nonparetic step length were 0.75 and 0.71 for the robot group respectively, compared to 0.03 and 0.50 for the controls.
Table 2.
Spatio-temporal gait parameters. Means ± SEM.
| Variable | Baseline | Discharge | %Change | Time p-value | G x T p-value | GxT Δ% p-value |
|---|---|---|---|---|---|---|
| P-step Time (s) | 0.000 | 0.172 | 0.212 | |||
| Robot | 2.51±0.35 | 1.34±0.14 | −37 ‡ | |||
| Stretch | 2.41±0.38 | 1.75±0.33 | −25 † | |||
| NP-step Time (s) | 0.008 | 0.365 | 0.174 | |||
| Robot | 1.11±0.11 | 0.91±0.05 | −10 | |||
| Stretch | 1.27±0.20 | 0.87±0.07 | −23 + | |||
| Step-time Symmetry | 0.014 | 0.042 | 0.032 | |||
| Robot | 2.33±0.30 | 1.44±0.14 | −26 † | |||
| Stretch | 1.98±0.23 | 1.89±0.20 | +5 | |||
| P-step Length (cm) | 0.005 | 0.779 | 0.273 | |||
| Robot | 35.5±1.8 | 39.6±2.0 | 13 + | |||
| Stretch | 29.8±2.4 | 34.7±1.9 | 27 | |||
| NP-step Length | 0.000 | 0.052 | 0.065 | |||
| Robot | 18.8±2.6 | 31.6±3.4 | 133 ‡ | |||
| Stretch | 22.1±2.5 | 28.3±3.7 | 31 + | |||
| Step-length Symmetry | 0.125 | 0.122 | 0.038 | |||
| Robot | 3.61±1.25 | 1.63±0.28 | −31 | |||
| Stretch | 1.80±0.36 | 1.81±0.42 | +14 |
G x T: group by time interaction; P: paretic; NP: nonparetic; within group p-values:
p≤0.05,
p≤0.01,
p≤0.001
Paretic ankle motor control measures
Evaluations of paretic ankle motor control were based on participants’ performances on unassisted trials of the robot racer game at baseline and at discharge (Fig. 2B). Because their strokes were relatively recent (on average, 11 days post onset) some subjects were not able to produce sufficient movements to yield adequate baseline measures for these analyses. Results for the angular velocity and success measures used data from 18 subjects in the robot group and 12 subjects in the stretching group. Analysis of the normalized jerk data was further limited to 13 in the robot group and 9 in the stretching group due to the fact that it was impossible to calculate normalized jerk when baseline velocity was zero. Between group t-tests on the baseline variables established that the groups did not differ on peak angular velocity (p = 0.951), mean angular velocity (p = 0.529), or percentage of successful passages (p = 0.447). The groups were different on normalized jerk, showing that the stretching group was significantly smoother in the unassisted baseline attempts (p =0.034).
The peak angular velocity achieved significance in the GxT interaction (F1,28 = 4.42; p = 0.045) and the main effect for time (F1,28 = 18.65; p < 0.001). Post-hoc t-tests showed that the robot group made substantially greater gains (106%; p < 0.001) than the stretching group (33%; p = 0.223). The mean angular velocity values followed a similar trend with the GxT interaction (p = 0.055) and a significant main effect for time (p = 0.003). Paired t-tests showed gains of 141% in the robot group (p = 0.002) vs. 25% in the stretching group (p = 0.312). Movement smoothness (normalized jerk) produced a significant a GxT interaction (F1,20 = 6.45, p = 0.019), and a main effect trend for time (F1,20 = 3.93, p = 0.061). In this measure smoother movements result in lower values. On average the robot group decreased normalized jerk (−24%; p = 0.006), compared to the stretching group who were essentially unchanged (+4%; p = 0.688). Figure 3 depicts examples that compare DF/PF traces of two subjects before and after each intervention to highlight the changes in trajectory smoothness. Effect sizes for peak angular velocity and normalized jerk were 0.77 and 1.12 for the robot group respectively, compared to 0.38 and 0.14 for the controls.
Targeting success produced a strong main effect for time (F1,28 = 49.32; p < 0.001) indicating that both groups improved, but without significant GxT interaction. The robot group increased successful targeting by 168% compared to 117% by the stretching group.
DISCUSSION
This randomized pilot study is the first to investigate the use of impedance-controlled ankle robotics in the early sub-acute phase of stroke, showing that anklebot training produces superior gains in paretic ankle motor control and spatio-temporal measures of interlimb symmetry, compared to reference treatment controls that received matched amounts of ankle stretching therapy. These group differences were within the context of large improvements for both groups in the ambulatory FIM, walking velocity, and Berg Balance measures. Our findings suggest that seated, modular joint specific robotics superimposed on early stroke rehabilitation can be utilized by patients across a wide range of paretic ankle deficits to reduce motor impairments that translate into improved gait patterning.
Though upper extremity impedance-controlled robotics has proven successful in randomized controlled trials for chronic stroke,35 there is controversy regarding the lower extremity, with current recommendations against the routine use of robotic-based therapy because of evidence that such interventions are either “ineffective” or that the “harms outweigh benefits”. 11 While such findings have steered many investigators toward robotics as an adjunct, not replacement, for conventional rehabilitation, prior LE robotics studies are limited from a neuromotor learning perspective. Though many robotics technologies deployed to date provide highly repeatable locomotor patterns, this approach may constrain motion to an idealized pattern, limiting volitional elements and progression which are key components of motor learning. Furthermore most LE robotics approaches have focused on whole body locomotor patterning and not motor learning across specific joints to address stroke impairments.
Our study utilizes an approach that isolates the ankle in a seated position with therapy administered very early post stroke when many patients have extremely limited ambulatory capacity. This impairment-based approach may contribute to the improvements observed in this early stroke recovery period. In individuals with chronic hemiparetic stroke, robotics interfaced with virtual reality training has been shown to translate to improved whole body mobility function.25 These findings corroborate our prior studies showing seated anklebot training improved both ankle motor control and hemiparetic walking velocity in chronic stroke.26, 36 Thus using modular, joint specific robotic therapy to address fundamental impairments and motor control may improve function across the continuum of care.37
The functional implications from our findings of improved paretic ankle motor control and more symmetric walking patterns suggest that modular robotics may generalize to improve other elements of activities of daily living requiring mobility and balance control. The nearly two-fold increase of the mean peak angular velocity to ~ 60°/s moved the robotics group closer to values observed in slow normal walking, where peak ankle angular velocities between heel strike and foot flat events are about 130–140 °/s and about 80–90 °/s just prior to push-off phase.38 Increased speed of ankle control may also enhance responsiveness to balance perturbations in the anterior-posterior 39–41 and medio-lateral directions.42–44 While our results do not yet indicate a normal ability to deliver sufficient ankle contribution to these real world tasks and challenges, they do suggest that modular robotic training can accelerate recovery of ankle motor control toward better outcomes in other basic mobility and balance domains.
The observed shifts toward greater interlimb symmetry of bilateral step times and step lengths suggest positive changes in the ability to coordinate and control the limbs while walking. Though each group made similar gains in gait velocity, cadence, and stride length, there were differential changes bilaterally in the component step times and step lengths that led to the group differences in interlimb symmetry. The robot group’s reduction in step time symmetry was due to the combined effect of a greater decrease in paretic step time (37%) with a smaller decline in the NP step time (10%). The stretching group also decreased step times, but to about the same degree bilaterally (23–25%), and with no temporal symmetry change. Both groups increased paretic step length about 4–5 cm (when forward propulsion is largely generated by the NP side), but the robot group produced a 133% increase in the NP step length (when propulsion comes from the paretic side) compared to 31% for the stretching group. In absolute terms, the 13 cm vs. 6 cm gain in NP step length suggests the robot group acquired greater paretic side stability and push-off, which is linked to reduced severity of hemiparesis.45 These differences in interlimb ratios may be related to improved motor control at the paretic ankle, and may also signify changes toward a shifting balance of inhibition and disinhibition between cortical hemispheres, as modeled for UE in stroke recovery.46–49 Together these findings suggest a potential for decreased reliance on compensatory strategies in lieu of optimizing neuromotor recovery.
Study limitations
This study is limited by small sample size and entry criteria restricted to patients with at least trace DF-PF ankle motor strength. Our intervention was limited to only 10 sessions (average) during the short inpatient rehabilitation stay. We did not evaluate retention to assess whether these gains were preserved or associated with other metrics of gait or balance function. Further limitations stem from our pilot design in which neither subjects nor testers were blinded to treatment assignment. Hence we cannot generalize our findings to the larger stroke population. Also, the robot group had more training experience with the device, which could influence motor control outcomes in unassisted assessments. Regardless, anklebot training during inpatient stroke rehabilitation appears well-tolerated and effective for improving ankle motor control and inter-limb coordination. Anecdotally, increased awareness and enthusiasm among the clinical staff for anklebot therapy suggests that it can be integrated into inpatient rehabilitation care. While this pilot shows feasibility for selected neuromotor outcomes, further studies are needed to optimize motor learning strategies to exploit visually-evoked and visually-guided training paradigms.20,50 Another consideration is to broaden the entry criteria to include patients having flaccid responses at the ankle, as the robot can still engage them in terms of making efforts to move and assisting as-needed to keep them engaged with the effort. The potential for a more systematic approach is suggested by the challenge point theory that highlights setting optimal levels of task difficulty to promote motor learning.51
Future research needs to examine LE robotics training over a longer time course, beyond the inpatient rehabilitation phase and into the outpatient clinic, and also to monitor durability of responses beyond the period of training. One rationale for deploying robotics as early as possible post-stroke is that an impairment based regimen might shape the naturally occurring neural plasticity toward greater functional benefit, e.g., improved interlimb coordination and control. Precedent for detecting training induced neuroplasticity in LE motor control in the chronic phase of stroke suggests that characterizing the neurophysiological mechanisms across the early subacute phase is warranted.52–55 Another future consideration is that the passive mobilization approach for the control group would not be an ideal reference treatment for a rigorous comparative effectiveness trial, which would consist of blinded evaluations and might show active ankle exercises to be as effective as anklebot.
Conclusions
The present study offers proof of concept that an impairment-based approach for implementing LE robotics in the rehabilitation hospital setting may be a beneficial adjunct to usual therapies. The major benefits include improved ankle motor control and spatio-temporal symmetry of gait. Future studies need to focus on how the intervention can be effectively calibrated to the changing patient deficit levels to enhance motor learning and to determine the intensity, dosage, and temporal profile for LE robotics to improve mobility function after stroke.
Table 3.
Motor control variables measured by the anklebot in the unassisted mode before and after the interventions. Means ± SEM.
| Variable | Baseline | Discharge | %Change | Time p-value | G x T p-value |
|---|---|---|---|---|---|
| Peak Angular Velocity (deg/s) | 0.000 | 0.045 | |||
| Robot | 28.3±5.0 | 58.4±5.6 ‡ | 106% | ||
| Stretch | 31.2±9.1 | 41.6±6.7 | 33% | ||
| Mean Angular Velocity (deg/s) | 0.003 | 0.055 | |||
| Robot | 3.7±0.7 | 8.9±1.6 † | 141% | ||
| Stretch | 4.8±1.4 | 6.0±1.0 | 25% | ||
| Normalized jerk (1/s2) | 0.061 | 0.019 | |||
| Robot | 691.2±53.9 | 528.6±37.6 † | −24% | ||
| Stretch | 495.8±42.2 | 515.9±52.6 | 4% | ||
| Target Success (%) | 0.000 | 0.145 | |||
| Robot | 24.3±4.9 | 65.1±5.9 ‡ | 168% | ||
| Stretch | 22.6±8.5 | 49.0±9.7 † | 117% | ||
Note: lower values for jerk indicate smoother movements. GxT: group by time interaction; P: paretic; NP: nonparetic; within group p-values: + p≤0.05,
p≤0.01,
p≤0.001
Footnotes
Declaration of Conflicting Interests
Dr. H. I. Krebs is a co-inventor in the MIT patents for the robotic devices. He holds equity positions in Interactive Motion Technologies, Inc., the company that manufactures this type of technology under license to MIT.
Funding
Center of Excellence on Task-Oriented Exercise and Robotics in Neurological Diseases, (B3688R); VA RR&D Merit Pilot Award (A7461P); and NIA Claude D. Pepper Older Americans Independence Center (P30-AG028747).
Contributor Information
Larry W. Forrester, Departments of Physical Therapy and Rehabilitation Science and Neurology, University of Maryland School of Medicine, Baltimore, Maryland; VA RR&D Maryland Exercise and Robotics Center of Excellence, Baltimore, Maryland.
Anindo Roy, Department of Neurology, University of Maryland School of Medicine, Baltimore, Maryland; VA RR&D Maryland Exercise and Robotics Center of Excellence, Baltimore, Maryland; Department of Bioengineering, University of Maryland School of Engineering, College Park, Maryland.
Amanda Krywonis, University of Maryland Rehabilitation and Orthopaedics Institute, Baltimore, Maryland.
Glenn Kehs, University of Maryland Rehabilitation and Orthopaedics Institute, Baltimore, Maryland; Department of Neurology, University of Maryland School of Medicine, Baltimore, Maryland.
Hermano Igo Krebs, Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, Massachusetts; Department of Neurology, University of Maryland School of Medicine, Baltimore, Maryland.
Richard F. Macko, Departments of Neurology, Medicine, and Physical Therapy and Rehabilitation Science, University of Maryland School of Medicine, Baltimore, Maryland; Geriatrics Research, Education and Clinical Center, Baltimore Veterans Affairs Medical Center; VA RR&D Maryland Exercise and Robotics Center of Excellence, Baltimore, Maryland.
References
- 1.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10(12):861–72. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 2.Johansson BB. Brain plasticity and stroke rehabilitation. The Willis lecture. Stroke. 2000;31:223–30. doi: 10.1161/01.str.31.1.223. [DOI] [PubMed] [Google Scholar]
- 3.Ziemann U, Muellbacher W, Hallett M, Cohen LG. Modulation of practice-dependent plasticity in human motor cortex. Brain. 2001;124 (6):1171–81. doi: 10.1093/brain/124.6.1171. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt R, Lee TD. Motor control and learning: a behavioral emphasis. Champaign, IL: Human Kinetics; 2005. [Google Scholar]
- 5.Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000 Jun;31(6):1210–6. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- 6.Dobkin BH, Firestine A, West M, Saremi K, Woods R. Ankle dorsiflexion as an fMRI paradigm to assay motor control for walking during rehabilitation. Neuroimage. 2004;23(1):370–381. doi: 10.1016/j.neuroimage.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enzinger C, Dawes H, Johansen-Berg H, Wade D, Bogdanovic M, Collett J, Guy C, et al. Brain activity changes associated with treadmill training after stroke. Stroke. 2009;40(7):2460–2467. doi: 10.1161/STROKEAHA.109.550053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luft AR, Macko RF, Forrester LW, Villagra F, Ivey F, Sorkin JD, Whitall J, McCombe-Waller S, Katzel L, Goldberg AP, Hanley DF. Treadmill exercise activates subcortical neural networks and improves walking after stroke: a randomized controlled trial. Stroke. 2008 Dec;39(12):3341–50. doi: 10.1161/STROKEAHA.108.527531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nudo RJ, Jenkins WM, Merzenich MM, Prejean T, Grenda R. Neurophysiological correlates of hand preference in primary motor cortex of adult squirrel monkeys. J Neurosci. 1992 Aug;12(8):2918–47. doi: 10.1523/JNEUROSCI.12-08-02918.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang VS, Krakauer JW. Robotic neurorehabilitation: a computational motor learning perspective. J NeuroEng Rehabil. 2009;6:5. doi: 10.1186/1743-0003-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller EL, Murray L, Richards L, et al. The comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke patient: a scientific statement from the American Heart Association. Stroke. 2010;41:2402–48. doi: 10.1161/STR.0b013e3181e7512b. [DOI] [PubMed] [Google Scholar]
- 12.Colombo G. The “Lokomat”-a driven ambulatory orthosis. Med Orth Tech. 2000;6:178–81. [Google Scholar]
- 13.Hesse S, Uhlenbrock D. A mechanized gait trainer for restoration of gait. J Rehabil Res Dev. 2000;37:701–08. [PubMed] [Google Scholar]
- 14.Schmidt H. HapticWalker–A novel haptic device for walking simulation. Proceedings of 2004 EuroHaptics. 2004:60–67. [Google Scholar]
- 15.Hornby TG, Campbell DD, Kahn JH, et al. Enhanced gait-related improvements after therapist- versus robotic-assisted locomotor training in subjects with chronic stroke: a randomized controlled study. Stroke. 2008;39:1786–92. doi: 10.1161/STROKEAHA.107.504779. [DOI] [PubMed] [Google Scholar]
- 16.Hidler J, Nichols D, Pelliccio M, et al. Multicenter randomized clinical trial evaluating the effectiveness of the Lokomat in subacute stroke. Neurorehabil Neural Rep. 2009;23:5–13. doi: 10.1177/1545968308326632. [DOI] [PubMed] [Google Scholar]
- 17.Veneman JF, Kruidhof R, Hekman EE, Ekkelenkamp R, Van Asseldonk EH, van der Kooij H. Design and evaluation of the LOPES exoskeleton robot for interactive gait rehabilitation. IEEE Trans Neural Sys Rehabil Eng. 2007;15(3):379–86. doi: 10.1109/tnsre.2007.903919. [DOI] [PubMed] [Google Scholar]
- 18.Mayr A, Kofler M, Quirbach E, Matzak H, Fröhlich K, Saltuari L. Prospective, Blinded, Randomized Crossover Study of Gait Rehabilitation in Stroke Patients Using the Lokomat Gait Orthosis. Neurorehabil Neural Repair. 2007;21:307–14. doi: 10.1177/1545968307300697. [DOI] [PubMed] [Google Scholar]
- 19.Girone M, Burdea G, Bouzit M, Popsecu V. A Stewart platform-based system for ankle telerehabilitation. Autonomous Robots. 2001;10:203–12. [Google Scholar]
- 20.Zhang LQ, Chung SG, Bai Z, Xu D, Rey EM, Rogers MW, Johnson ME, Roth EJ. Intelligent stretching of ankle joints with contracture/spasticity. IEEE Trans Neural Sys Rehabil Eng. 2002;10(3):149–57. doi: 10.1109/TNSRE.2002.802857. [DOI] [PubMed] [Google Scholar]
- 21.Blaya JA, Herr H. Adaptive control of a variable-impedance ankle-foot orthosis to assist drop-foot gait. IEEE Trans Neural Sys Rehabil Eng. 2004;12(1):24–31. doi: 10.1109/TNSRE.2003.823266. [DOI] [PubMed] [Google Scholar]
- 22.Ferris DP, Czerniecki JM, Hannaford B. An ankle-foot orthosis powered by artificial pneumatic muscles. J App Biomech. 2005;21:189–97. doi: 10.1123/jab.21.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bharadwaj K, Sugar TG, Koeneman JB, Koeneman EJ. Design of a robotic gait trainer using spring over muscle actuators for ankle stroke rehabilitation. Trans Biomech Eng. 2005;127:1009–13. doi: 10.1115/1.2049333. [DOI] [PubMed] [Google Scholar]
- 24.Roy A, Krebs HI, Williams DJ, Bever CT, Forrester LW, Macko RM, Hogan N. Robot-aided neurorehabilitation: A novel robot for ankle rehabilitation. IEEE Trans Robotics. 2009;25:569–582. [Google Scholar]
- 25.Mirelman A, Bonato P, Deutsch JE. Effects of training with a robot-virtual reality system compared with a robot alone on the gait of individuals after stroke. Stroke. 2009;40(1):169–74. doi: 10.1161/STROKEAHA.108.516328. [DOI] [PubMed] [Google Scholar]
- 26.Forrester LW, Roy A, Krebs HI, Macko RF. Ankle training with a robotic device improves hemiparetic gait after a stroke. Neurorehabil Neural Repair. 2011;25(4):369–77. doi: 10.1177/1545968310388291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dromerick AW, Lang CE, Birkenmeier RL, Wagner JM, Miller JP, Videen TO, Powers WJ, Wolf SL, Edwards DF. Very Early Constraint-Induced Movement during Stroke Rehabilitation (VECTORS): A single-center RCT. Neurology. 2009 Jul 21;73(3):195–201. doi: 10.1212/WNL.0b013e3181ab2b27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kozlowski DA, James DC, Schallert T. Use-dependent exaggeration of neuronal injury after unilateral sensorimotor cortex lesions. J Neurosci. 1996;16:4776–86. doi: 10.1523/JNEUROSCI.16-15-04776.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tillerson JL, Cohen AD, Caudle WM, Zigmond MJ, Schallert T, Miller GW. Forced nonuse in unilateral parkinsonian rats exacerbates injury. J Neurosci. 2002;22(15):6790–99. doi: 10.1523/JNEUROSCI.22-15-06790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarkson AN, Huang BS, MacIsaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468(7321):305–9. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ziherl J, Novak D, Olenšek A, Mihelj M, Munih M. Evaluation of upper extremity robot-assistances in subacute and chronic stroke subjects. J NeuroEng Rehabil. 2010;7:52. doi: 10.1186/1743-0003-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergmann J, Krewer C, Muller F, Koenig A, Riener R. Virtual reality to control active participation in a subacute stroke patient during robot-assisted gait training. Proceedings of 2011 IEEE Int Conf on Rehabil Robotics. 2011:1–5. doi: 10.1109/ICORR.2011.5975407. [DOI] [PubMed] [Google Scholar]
- 33.Lum PS, Burgar CG, Van der Loos M, Shor PC, Majmundar M, Yap R. MIME robotic device for upper-limb neurorehabilitation in subacute stroke subjects: A follow-up study. J Rehabil Res Dev. 2006;43(5):631–42. doi: 10.1682/jrrd.2005.02.0044. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz I, Sajin A, Fisher I, Neeb M, Shochina M, Katz-Leurer M, et al. The effectiveness of locomotor therapy using robotic-assisted gait training in subacute stroke patients: a randomized controlled trial. Arch Phys Med Rehabil. 2009;1(6):516–23. doi: 10.1016/j.pmrj.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG, et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. New Eng J Med. 2010;362(19):1772–83. doi: 10.1056/NEJMoa0911341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy A, Forrester LW, Macko RF. Short-term ankle motor performance with ankle robotics training in chronic hemiparetic stroke. J Rehab Res Dev. 2011;8(4):417–29. doi: 10.1682/jrrd.2010.04.0078. [DOI] [PubMed] [Google Scholar]
- 37.Krakauer JW, Carmichael ST, Corbett D, Wittenberg GF. Getting Neurorehabilitation Right What Can Be Learned From Animal Models? Neurorehabil Neural Repair. 2012;26(8):923–31. doi: 10.1177/1545968312440745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palmer ML. Doctoral dissertation. Massachusetts Institute of Technology; 2002. Sagittal plane characterization of normal human ankle function across a range of walking gait speeds. [Google Scholar]
- 39.Kuo AD, Zajac FE. Human standing posture: multi-joint movement strategies based on biomechanical constraints. Prog Brain Res. 1993;97:349–58. doi: 10.1016/s0079-6123(08)62294-3. [DOI] [PubMed] [Google Scholar]
- 40.Kuo AD. An optimal control model for analyzing human postural balance. IEEE Trans Biomed Eng. 1995;42(1):87–101. doi: 10.1109/10.362914. [DOI] [PubMed] [Google Scholar]
- 41.Gatev P, Thomas S, Kepple T, Halett M. Feedforward ankle strategy of balance during quiet stance in adults. J Physiol. 1999;514:915–28. doi: 10.1111/j.1469-7793.1999.915ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mille ML, Rogers MW, Martinez K, Hedman LD, Johnson ME, Lord SR, Fitzpatrick RC. Thresholds for inducing protective stepping responses to external perturbations of human standing. J Neurophysiol. 2003;90(2):666–74. doi: 10.1152/jn.00974.2002. [DOI] [PubMed] [Google Scholar]
- 43.Mille ML, Johnson ME, Martinez KM, Rogers MW. Age-dependent differences in lateral balance recovery through protective stepping. Clin Biomech (Bristol, Avon) 2005;20(6):607–16. doi: 10.1016/j.clinbiomech.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Rogers MW, Mille ML. Lateral stability and falls in older people. Exercise Sport Sc Rev. 2003;31(4):182–87. doi: 10.1097/00003677-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Bowden MG, Balasubramanian CK, Neptune RR, Kautz SA. Anterior-posterior ground reaction forces as a measure of paretic leg contribution in hemiparetic walking. Stroke. 2006;37(3):872–76. doi: 10.1161/01.STR.0000204063.75779.8d. [DOI] [PubMed] [Google Scholar]
- 46.Vines BW, Nair DG, Schlaug G. Contralateral and ipsilateral motor effects after transcranial direct current stimulation. Neuroreport. 2006;17(6):671. doi: 10.1097/00001756-200604240-00023. [DOI] [PubMed] [Google Scholar]
- 47.Vines BW, Cerruti C, Schlaug G. Dual-hemisphere tDCS facilitates greater improvements for healthy subjects’ non-dominant hand compared to uni-hemisphere stimulation. BMC Neurosci. 2008;9(1):103. doi: 10.1186/1471-2202-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, Cohen LG. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128(3):490–99. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- 49.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55(3):400–09. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- 50.Sütbeyaz S, Yavuzer G, Sezer N, Koseoglu BF. Mirror therapy enhances lower-extremity motor recovery and motor functioning after stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2007;88(5):555–59. doi: 10.1016/j.apmr.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 51.Onla-or S, Winstein CJ. Determining the optimal challenge point for motor skill learning in adults with moderately severe Parkinson’s disease. Neurorehabil Neural Repair. 2008;22(4):385–95. doi: 10.1177/1545968307313508. [DOI] [PubMed] [Google Scholar]
- 52.Dobkin BH, Firestine A, West M, Saremi K, Woods R. Ankle dorsiflexion as an fMRI paradigm to assay motor control for walking during rehabilitation. Neuroimage. 2004;23:370–81. doi: 10.1016/j.neuroimage.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luft AR, Macko RF, Forrester LW, Villagra F, Ivey F, Sorkin JD, Whitall J, McCombe-Waller S, Katzell L, Goldberg AP, Hanley DF. Treadmill exercise activates subcortical neural networks and improves walking after stroke: a randomized controlled trial. Stroke. 2008;39 (12):3341–50. doi: 10.1161/STROKEAHA.108.527531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miyai I, Yagura H, Oda I, Konishi I, Eda H, Suzuki T, et al. Premotor cortex is involved in restoration of gait in stroke. Ann Neurol. 2002;52:188–94. doi: 10.1002/ana.10274. [DOI] [PubMed] [Google Scholar]
- 55.Forrester LW, Hanley DF, Macko RF. Effects of treadmill training on TMS-induced excitability to quadriceps after stroke. Arch Phys Med Rehabil. 2006;87(2):229–34. doi: 10.1016/j.apmr.2005.10.016. [DOI] [PubMed] [Google Scholar]