Abstract
In eosinophilic esophagitis (EoE), remodeling changes are manifest histologically in both the epithelium as well as in the subepithelium where lamina propria (LP) fibrosis, expansion of the muscularis propria and increased vascularity occur. The major clinical symptoms and complications of EoE are largely consequences of esophageal remodeling. Important mediators of the process include IL-5, IL-13, TGFβ1, mast cells, fibroblasts and eosinophils. Methods to detect remodeling effects include upper endoscopy, histopathology, barium esophagram, endoscopic ultrasonography, esophageal manometry, and functional luminal imaging. These modalities provide evidence of organ dysfunction that include focal and diffuse esophageal strictures, expansion of the mucosa and subepithelium, esophageal motor abnormalities and reduced esophageal distensibility. Complications of food impaction and perforations of the esophageal wall have been associated with reduction in esophageal caliber and increased esophageal mural stiffness. The therapeutic benefits of topical corticosteroids and elimination diet therapy in resolving mucosal eosinophilic inflammation of the esophagus are evident. Available therapies, however, have demonstrated variable ability to reverse existing remodeling changes of the esophagus. Systemic therapies that include novel, targeted biologic agents have the potential of addressing subepithelial remodeling. Esophageal dilation remains a useful, adjunctive therapeutic maneuver in symptomatic adults with esophageal stricture. As novel treatments emerge, it is essential that therapeutic endpoints account for the fundamental contributions of esophageal remodeling to overall disease activity.
Keywords: Eosinophilic esophagitis, Remodeling, Fibrosis, Gastroesophageal reflux disease, dysphagia, endoscopy, esophagitis
Introduction
Since the initial case descriptions two decades ago, eosinophilic esophagitis (EoE) has emerged as an important clinical entity with steadily rising prevalence.[1] In children, EoE is an increasingly recognized etiology for feeding disorders and manifests with poor weight gain, anorexia, vomiting, regurgitation, abdominal pain, and dysphagia. In adult patients, EoE is one of the most common causes of dysphagia. An increasing number of studies have shown that the primary symptoms in children and adults as well as clinical complications of EoE are consequences of esophageal remodeling and fibrostenosis. This article focuses on the current understanding of the pathogenesis, clinical detection and therapeutic implications of esophageal remodeling in EoE.
Definition of esophageal remodeling
The concept of eosinophil associated tissue remodeling stems from diseases such as the hypereosinophilic syndrome and asthma. Remodeling can be defined as tissue changes in target organs that result in end organ dysfunction. Remodeling is associated with histologic alterations such as fibrosis and angiogenesis which are caused by changes in cellular function, phenotype, and products. Remodeling itself may not be a pathogenic process as it could be considered to represent a protective mechanism akin to wound healing. However, when remodeling is not controlled, presumably due to unbridled inflammation, there are negative consequences for organ function. Indeed, the natural history of untreated EoE is to progress to stricture formation, at least in adults. [2, 3]
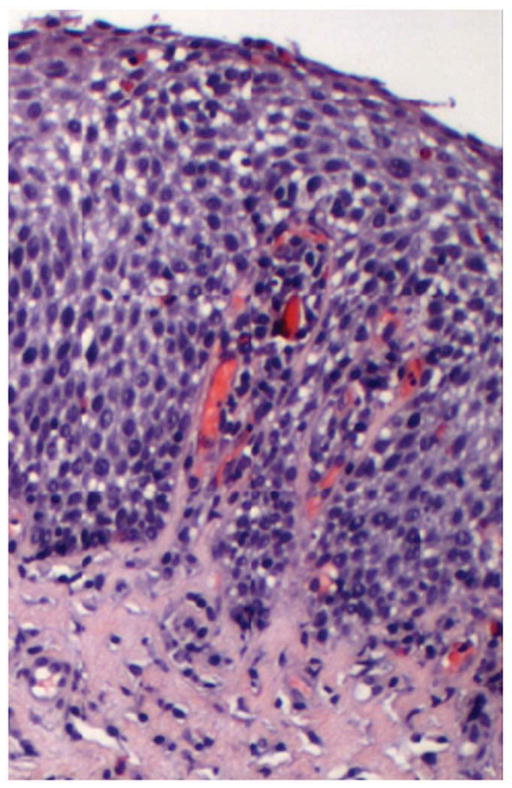
In EoE remodeling changes are seen histologically in both the epithelium and subepithelium (Figure 1). Epithelial changes include basal zone hyperplasia and increased length of the vascular papillae. The papillae are intrusions of the sub-epithelium into the epithelial space and, as such, are likely a further reflection of subepithelial expansion. Subepithelial changes include lamina propria fibrosis with increased collagen deposition and thickness and increased vascularity with vascular activation. Muscularis remodeling changes include smooth muscle hypertrophy and hyperplasia. Together these tissue changes are the likely mechanisms for the esophageal dysfunction that characterizes EoE and underlies the clinical complications of dysphagia, strictures, food impactions, esophageal rigidity and dysmotility. Ultimately it is the potential control of the clinical consequences of remodeling that motivates practitioners to treat EoE. In this vain, the assumption is that control of inflammation is equated to control of remodeling. However, this has yet to be systematically proven.
Figure 1.
Histopathology of remodeling changes in eosinophilic esophagitis. The squamous epithelium shows basal zone hyperplasia and lamina propria shows increased collagen density in EoE.
While it is recommended that there is recurrent tissue procurement for EoE management, this is not the case in other eosinophil associated diseases. This paucity of repeatedly acquired human tissue has limited our understanding of the true clinical implications of tissue remodeling. For this reason, EoE provides a unique opportunity to understand the clinical complications, natural history, and reversibility of eosinophil associated tissue remodeling. This is further underscored by the fact that young children have recurrent tissue assessments, allowing us to investigate the long term effects of tissue architectural changes on esophageal function and EoE progression. If EoE is akin to asthma, a person’s fibrotic phenotype may be defined very early in life.
For the purposes of this review, tissue remodeling as it relates to EoE is considered to be comprised of epithelial changes including basal cell hyperplasia and epithelial mesenchymal transition, subepithelial changes of fibrosis and angiogenesis, and smooth muscle hypertrophy. We provide a summary of the current molecular and clinical data that support the hypothesis that esophageal tissue remodeling 1) is driven by EoE associated esophageal inflammation and 2) is the underlying etiology for major EoE clinical symptoms and complications.
Pathogenesis of esophageal remodeling in EoE
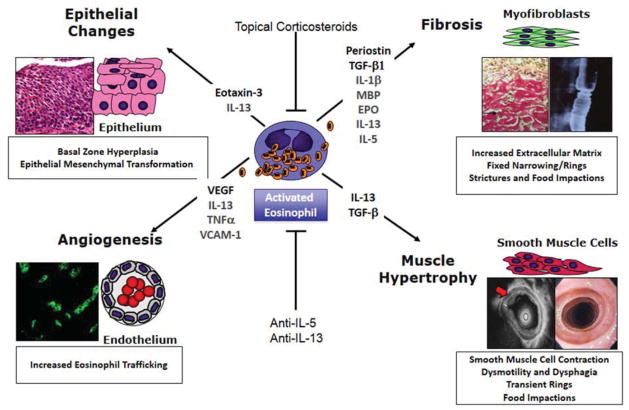
Inflammatory mediators and cells clearly play a role in driving esophageal remodeling (Table 1, Figure 2). Animal models demonstrate that mice lacking eosinophils or the eosinophilopoetic cytokine IL-5, have significantly less collagen deposition and fibronectin expression than their wild type littermates.[4] [5] In addition, mice that have decreased esophageal eosinophils also have decreased basal zone hyperplasia.[5] Importantly, a lack of eosinophils, even in the presence of IL-5 overexpression, leads to decreases in stricture formation. In contrast, there is no effect of eosinophil loss on esophageal dysmotility.[6] Overexpression of IL-13 causes esophageal stricture that is not reversible by the subsequent removal of IL-13.[6] This underscores a number of important concepts. First, there is a dependence on eosinophilic inflammation in order to drive strictures. Second, interleukins in the absence of subsets of cellular inflammation can have distinct effects. Third, various esophageal remodeling features can be uncoupled and can utilize distinct mechanistic pathways (Figure 2).
Table 1.
Mediators of esophageal remodeling in eosinophilic esophagitis
| Mediator | Remodeling Effects | Evidence |
|---|---|---|
| IL-5 | Increased collagen Increased smooth muscle contraction force Increased epithelial TGFβ1 Increased tenascin C |
Animal models Animal models Human anti-IL-5 trials Human anti-IL-5 trials |
| IL-13 | Increased collagen deposition Increased esophageal thickness Stricture formation |
Animal models |
| Periostin | Increased periostin deposition in lamina propria Increased eosinophil trafficking |
Human in vitro studies |
| Siglec-8 | Increased fibronectin Increased angiogenesis |
Animal models |
| Smad3 | Increased fibronectin Increased angiogenesis |
Animal models |
| TGFβ1 | Increased fibrosis Smooth muscle cell contraction |
Human in vitro studies |
| TSLP | Increased food impactions | Animal models |
| Eosinophils | Increased fibrosis Strictures Increased tenascin Increased epithelial TGFβ1 |
Animal models Human anti-IL-5 studies |
| Mast Cells | Smooth muscle hypertrophy and hyperplasia | Animal studies |
| Basophils | Increased food impactions | Animal studies |
Figure 2.
Schematic representation of eosinophil induced esophageal remodeling, key interleukins and cytokines and its clinical consequences. Adapted from Aceves, S.S. and S.J. Ackerman, Relationships between eosinophilic inflammation, tissue remodeling, and fibrosis in eosinophilic esophagitis. Immunol Allergy Clin North Am, 2009. 29(1): p. 197–211, xiii–xiv; with permission.
Esophageal eosinophils in EoE produce the profibrotic factor, TGFβ1 which can increase the production of collagen, fibronectin, and other extracellular matrix proteins [7–9]. TGFβ1 mRNA and protein levels are elevated in the epithelium and subepithelium of pediatric and adult EoE subjects when compared with control subjects. In addition to TGFβ1 other profibrotic molecules including CCL-18 and FGF-9 are increased in EoE subjects, suggesting that there are also TGFβ1independent pathways to fibrosis [10, 11]. Increased numbers of lamina propria (LP) cells that express phosphorylated Smad2/3, part of the canonical TGFβ1 transcription factor complex, are also found in the LP of pediatric EoE subjects. Profibrotic factors such as FGF-9 and TGFβ1 can also have effects on the function of epithelial cells including proliferation and epithelial mesenchymal transformation [11] Kagalwalla, 2012 #585}
Mast cells are important in EoE pathogenesis. Indeed the numbers of mast cells infiltrating the deeper esophageal layers such as the muscularis mucosa can exceed the numbers of eosinophils. [12]. Both eosinophils and mast cells also provide a source of TGFβ1 in EoE and a distinct mast cell transcript signature is present in both pediatric and adult EoE subjects.[12–14] Mast cells are found in couplets with eosinophils in the esophagus of EoE subjects and eosinophils produce the mast cell survival and recruitment factor, IL-9, suggesting that there is an intricate balance between eosinophilia and mastocytosis in EoE.[15] Animal studies have shown that mast cells and eosinophils travel together in EoE models. There is a decrease in smooth muscle hypertrophy and proliferation in mast cell deficient mice. [16] As such, it is likely that mast cells not only promote fibrosis but also alter smooth muscle function during the process of esophageal remodeling.
IL-13 is a master regulator in EoE, functioning to increase both IL-5 and eotaxin-3 in the esophagus [17, 18]. Pulmonary over-expression of IL-13 using a Clara cell promoter demonstrates increased fibrosis and esophageal circumference. [19] The effects of IL-13 can be independent of eosinophils and can promote the formation of irreversible strictures. [6, 19] Both IL-13 and TGFβ1 increase the levels of periostin, an extracellular matrix protein that promotes the migration and adherence of eosinophils, thus propagating inflammation in EoE. [20]
Subepithelial angiogenesis is present in EoE. [7] [5] [21] Consistent with this, there are elevated levels of pro-angiogenic factors including VEGF and angiotensin in the esophagus of pediatric EoE subjects.[21] Increased vascularity provides elevated numbers of conduits for the transport of inflammatory cells into the esophagus. Elevated levels vascular activation factors described in EoE, such as VCAM-1, allows vessels to have increased tethering and transmigration of inflammatory cells. [7, 21] Indeed, mice deficient in eosinophils have diminished levels of angiogenesis. [5]
Human studies using endoscopic ultrasound demonstrates increased esophageal thickness through all the esophageal layers including the concentric and longitudinal muscle layers in pediatric and adult EoE subjects.[9, 22] Subsets of EoE subjects have altered esophageal motility on manometric studies that assess concentric muscle function and studies that analyze both concentric and longitudinal muscle layers demonstrate significant changes in the coordination between these smooth muscle layers.[23–26] Functionally, TGFβ1 can cause direct contraction of primary esophageal smooth muscle cells in culture, suggesting that inflammatory cell derived growth factors can alter esophageal muscle cell function.[12, 27] In addition transgenic mice that over-express IL-5 have increased longitudinal and circular smooth muscle contraction force.[6] Interestingly, these IL-5 transgenic mice that lack eosinophils continue to have increased contraction force demonstrating that although strictures depend on the presence of eosinophils, dysmotility utilizes other inflammatory cells and/or factors.[6] Human data demonstrates that there is transmural inflammation with both eosinophilia and mastocytosis of the muscularis propria.[28, 29] It is likely that the presence of such inflammatory cells and their chemical mediators would have functional consequences in EoE. Consistent with this concept, mice deficient in TSLP or basophils are protected from food impactions in an experimental EoE model, demonstrating that TSLP and basophils play significant roles in esophageal dysfunction. [30]
Relationship of esophageal remodeling with clinical manifestations and complications
The clinical presentations of EoE reflect esophageal dysfunction. These functional changes in the esophagus likely reflect esophageal remodeling. In adults, EoE is dominated by symptoms of dysphagia and food impaction while in children symptoms more commonly mimic GERD with dysphagia and food impactions becoming more prominent in adolescence. In adult subjects, there are two determinants for esophageal food impaction risk: 1) a reduction in luminal diameter and 2) limitation in esophageal mural distensibility. These features can occur concurrently or separately. Esophageal strictures, defined as a reduction of normal caliber, can be identified in 30–80% of adults with EoE while decreased distensibility is reported in over 70 % of EoE adults. It is important to note that the rates of esophageal mural rigidity have not been defined in pediatric EoE and, as such, the disease duration of EoE that causes decreased esophageal compliance in children is not clear. Certainly, esophageal strictures are uncommonly identified in children (<5% of EoE subjects), even though food impactions occur in up to 30% of subjects. In adults, strictures defined as a reduction in luminal diameter to less than 10 mm have been reported in 38% [2]. The strictures can involve any portion of the esophagus, with many patients demonstrating diffusely compromised esophageal diameter, a condition termed “narrow or small caliber” esophagus. [31]. It is possible that lower grade esophageal stenosis is under-reported in the literature due to a lack of sensitivity for such luminal narrowing using the currently available endoscopic and radiographic techniques. Duration of untreated disease has been associated with increased risk of esophageal stricture, supporting the concept of progressive esophageal remodeling in EoE that may explain phenotypic differences between children and adults (Figure 3).[2, 32, 33]
Figure 3.
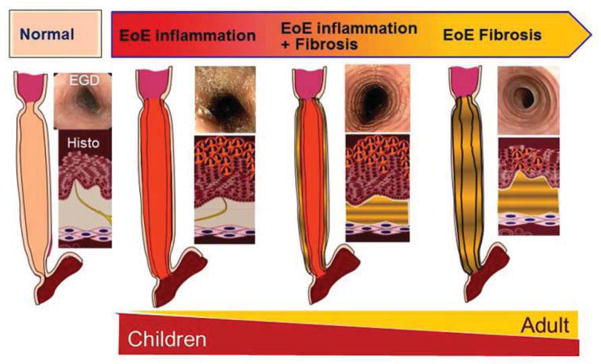
Conceptual model of the consequences of esophageal remodeling. Esophageal remodeling over time leads to increasing subepithelial fibrosis that is associated with progressive esophageal structuring and narrow caliber esophagus. This model may explain phenotypic differences between pediatric and adult presentations of eosinophilic esophagitis.
Both food impactions and strictures have significant complications. For example, food impactions that require emergency room visits and urgent endoscopic extraction is reported in 30–55% of adult cohorts with complications that include chest pain, as well as the risks of aspiration, esophageal tears and esophageal perforation.[1] Esophageal perforation is also a recognized complication of endoscopic extraction of food impactions, particularly when food extraction is performed using rigid endoscopy. Furthermore, several reports of esophageal perforation related to esophageal dilation of strictures have led to reluctance in performing this therapy in EoE.[34]
Additional potential complications of EoE as they relate to esophageal remodeling include impaired quality of life and risk of nutritional deficiency due to dietary restriction. The majority of aspects of diminished quality of life in EoE adults are related to the need for dietary modification and social embarrassment as well as anxiety created by choking episodes.[35] Nutritional concerns in EoE can be related to food aversion that may be secondary to the inflammatory response to specific food antigens. In adults, decreased esophageal mural compliance and distensibility may limit the tolerability of specific foods due to texture, most commonly meat. [25, 36]
Clinical methods to assess remodeling in EoE
Barium radiography
A variety of methods have been used in clinical practice and investigative studies to demonstrate the remodeling consequences of EoE (Table 2). One of the oldest methods to evaluate the structure of the gastrointestinal tract is barium radiography. Early case series demonstrated the association of marked restriction of the esophageal luminal caliber with EoE, characterized as a narrow caliber or small caliber esophagus [31]. Figure 4 illustrates the diffuse nature of this finding. The multiple, ring-like stenoses spanning lengths of the esophagus were initially confused with congenital esophageal stenosis but were subsequently recognized to be a characteristic feature of EoE [37]. Most recently, Alexander characterized restriction of the esophageal diameter in a cohort of adults with EoE, demonstrating a reduction in both the maximum and minimum diameters compared with controls.[38] Radiologic assessment of esophageal remodeling is clinically feasible but does not assess for variations in diameter as a function of intraluminal distension forces. A small volume of barium with low intrabolus distension pressure will have a tendency to provide falsely low estimates of the diameter of an esophageal stricture since the stiffness of the esophageal wall limits the ability of the wall to expand. Limited studies have used cross sectional imaging modalities such as computed tomography (CT) or magnetic resonance imaging (MRI) to characterize the intramural effects of EoE [39]( Figure 5).
Table 2.
Clinical methods to detect esophageal remodeling in eosinophilic esophagitis
| Method | Findings | Advantages | Disadvantages |
|---|---|---|---|
| Barium esophagram | Esophageal rings, strictures, narrow caliber esophagus | Availability, ease of testing, cost | Radiation exposure, two dimensional imaging, measures diameter but not distensibility or compliance |
| Upper endoscopy | Esophageal rings, strictures, narrow caliber esophagus | Availability, routine use in management of EoE, capability of therapeutic dilation, validated classification, biopsy evaluation of histologic remodeling | Cost, limited accuracy in determination of stricture diameter |
| Esophageal manometry | Esophageal motor dysfunction including peristaltic integrity, esophageal pressurization and esophagogastric junction function | Availability, cost, potential physiologic biomarker of esophageal remodeling | Patient tolerance, limited sensitivity and specificity of identified abnormalities, limited to measurement of circular and not longitudinal smooth muscle function |
| Esophageal biopsy | Lamina propria fibrosis, basal zone hyperplasia, rete peg elongation | Availability, routine use in management of EoE | Limited sensitivity for evaluable lamina propria, validation of methodology used for procurement and analysis needed, potential for sampling variability |
| Endoscopic ultrasonography | Increased thickness of mucosa, submucosa, muscularis propria | Availability, assessment of esophageal intramural remodeling | Cost, endoscope diameter may exceed esophageal diameter, validation of methodology needed, non-functional/non-quantifiable output |
| Functional luminal imaging | Reduction in esophageal distensibility and compliance | Objective quantification of organ-level esophageal remodeling consequences with demonstrated correlation with clinical outcomes | Limited availability, utility in pediatric patients unknown |
| Tissue biomarkers | TGFβ1, periostin, tenacin C, fibronectin | Potential increased sensitivity for remodeling, potential predictor of targeted therapies, may correlate with TGFβ1 genotypes/pharmacogenomics responses | Validation of methodology needed |
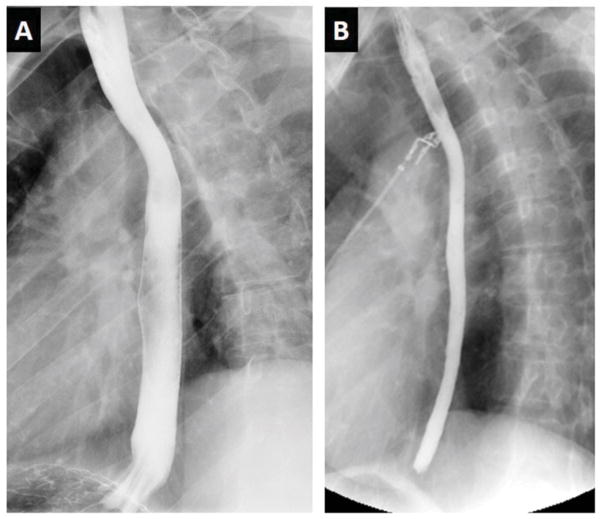
Figure 4.
Radiologic imaging in eosinophilic esophagitis. Barium esophagram in eosinophilic esophagitis. Panel A depicts a normal caliber esophagus in a patient with gastroesophageal reflux disease. Panel B shows an over 50% reduction in luminal diameter of the entire esophagus in an adult with eosinophilic esophagitis, a manifestation referred to as a narrow or small caliber esophagus.
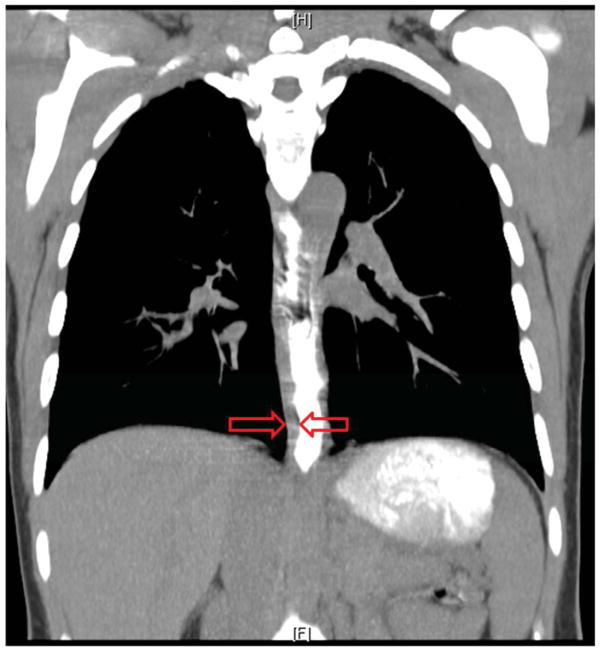
Figure 5.
Radiologic imaging in eosinophilic esophagitis. Coronal section of computed tomographic imaging illustrating the marked expansion of the esophageal wall in an adult with eosinophilic esophagitis. The imaging was obtained during ingestion of radiopaque contrast that clearly demarcates the inner lumen of the esophagus. The red arrows demarcate the mural thickness.
Endoscopy
Endoscopically detected esophageal features of EoE include longitudinal furrows, white exudates (plaques), edema (loss of vascular markings), rings (trachealization), and strictures. Prospective studies in EoE have identified endoscopic abnormalities in 93% of patients with EoE.[40] Endoscopic findings in patients with EoE have been shown to vary by age. Younger patients are more likely to have findings of exudates, furrows, edema, or a normal appearing esophagus whereas adult patients are more likely to have strictures, rings, narrow caliber esophagus, and crepe-paper mucosa.[32, 41] Fibrostenotic features including strictures and lumen compromising rings are commonly identified in adults with EoE but only among a minority of pediatric EoE patients (Figure 6). The extensive lacerations of the esophageal wall following esophageal dilation provide evidence of the longitudinal extent of the reduced esophageal elasticity in adults with EoE (Figure 6).[42] Pediatric studies demonstrate that features of exudates, furrows and loss of vascular markings correlate with histologic epithelial features that include eosinophilic inflammation while only loss of vascular markings and furrows correlate with lamina propria (LP) features of fibrosis [43]. In alignment with the concept that pediatric disease is more inflammatory and potentially less fibrotic in nature, endoscopy in pediatric EoE is commonly characterized by inflammatory features with severe mucosal exudates. The presence of furrows and edema has been shown to be similar between age groups. The endoscopic findings correlate with typical clinical presentations that are characterized by anorexia/early satiety, GERD-like symptoms and dysphagia in children and dysphagia with food impaction in adults [44, 45]. These observations support an important distinction in the prevalence of fibrostenotic consequences of esophageal eosinophilia in different age groups and the concept of progressive remodeling with duration of disease (Figure 3).
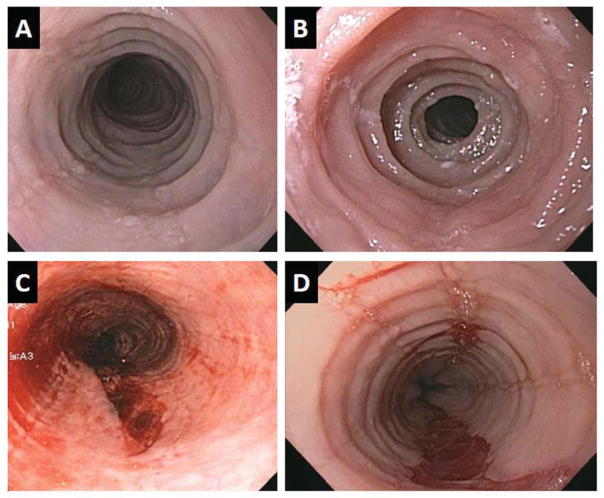
Figure 6.
Endoscopic imaging of 4 adult patients with eosinophilic esophagitis. Panels A and B illustrate remodeling changes of esophageal rings and stricture. Panels C and D illustrate esophageal mural tears that occurred following esophageal dilation likely indicative of diffuse loss of esophageal elasticity.
A classification and grading system to assess the endoscopic findings in EoE has been proposed.[46] The acronym for the Endoscopic REFerence Scoring system, EREFS, designates the five major features of EoE (Edema, Rings, Exudates, Furrows, and Stricture). This instrument was created to standardize and grade the endoscopic assessment performed by gastroenterologists. While most features have good inter-observer agreement amongst gastroenterologists, some of the endoscopic features that reflect esophageal remodeling were eliminated due to lack of sensitivity or problems with definition of terminology. Specifically EREFS does not include either narrow caliber esophagus or crepe paper esophagus, which may be more specific signs of EoE remodeling likely related to the loss of tissue elasticity.[42] EREFS is an important tool that accounts for aspects of esophageal remodeling that are not currently captured in routine pathology reports. To emphasize the significance of endoscopically detected remodeling, the occurrence of food impaction, a clinically relevant symptom outcome of EoE, has recently been shown to be associated with the assessment of ring severity using the EREFS system [45].
Endoscopic and endoluminal ultrasonography
Endoscopic ultrasonography depiction of expansion of the muscularis propria was described in an early case report of an elderly patient with eosinophilic esophagitis. [39] A small pediatric case series demonstrated significant increases in thickness of the combined mucosal-submucosa as well as muscularis propria in children with EoE [47]. Most recently, Straumann utilized endoscopic ultrasonography in a controlled trial of topical budesonide and demonstrated significant remodeling effects in adults with EoE [48]. Doubling of the thickness of the mucosa and a 50% increase in the thickness of the muscularis propria were found with the most marked difference being a 3-fold increase in submucosal thickness. Consistent with the natural history of EoE being a chronic, progressive disease associated with submucosal remodeling, the magnitude of the relative increases in mural thickness demonstrated in adults is greater than found in children with EoE (Figure 3). This observation supports the concept that remodeling in EoE is an ongoing process with progression based on duration of disease.
Esophageal manometry
The expansion of the muscularis propria on imaging as well as reports of dysphagia in EoE in the absence of identified esophageal stricture have led to the concept that esophageal motor function may be affected in EoE. Of note, the first two cases of “eosinophilic esophagitis” were reported in adults with major esophageal motility disorders; one having achalasia and the second having esophageal spasm.[49, 50] While these patients would be excluded from the current definition of EoE due to the presence of a major esophageal motility disorder and concomitant eosinophilic gastroenteritis [1], the concept was introduced regarding potential for esophageal eosinophilia to result in esophageal motor dysfunction. A recent case report described an adult with more characteristic features of EoE with manometric findings consistent with achalasia [51]. The patient’s dysphagia and manometry improved, but did not normalize, following treatment of systemic corticosteroids. Subsequent esophageal motility studies in adults with EoE have demonstrated hypertensive or weak peristaltic function in a subset of EoE patients. [24, 52] Nurko and colleagues utilized prolonged ambulatory esophageal manometry to demonstrate a higher frequency of high amplitude contractions and ineffective peristalsis in children with EoE compared with GERD or controls.[53] Peristaltic dysfunction was observed during episodes of dysphagia, although cause and effect could not be differentiated since the presence of a food bolus could itself secondarily alter esophageal motor function.
An investigation of adults utilizing high resolution esophageal manometry and Chicago classification systematically compared a cohort of 50 patients with EoE, 50 patients with GERD and 50 healthy controls and demonstrated normal peristalsis in 64%, with 36 % demonstrating nonspecific esophageal motor patterns dominated by weak and failed peristalsis[23][23][23]. While such abnormalities could contribute to dysphagia, they are not accepted as major motility disorders due to limited direct correlation with symptoms. Furthermore, the frequency of these abnormal patterns was not significantly different from the motility abnormalities in the cohort of patients with GERD. A novel finding in this study was abnormal esophageal pressurization, characterized by pan esophageal pressurization in 16% and distal esophageal pressurization in 18%. Demonstration of this finding was accentuated by utilizing higher volume of swallowed boluses. Another study from Spain substantiated this observation through the demonstration of pan esophageal pressurization in 48% of EoE patients and none of a control group.[54] The esophageal pressurization events in EoE may reflect reduced esophageal mural compliance secondary to the trans mural remodeling demonstrated on EUS imaging or alterations in motility that may occur secondary to EoE associated inflammation and remodeling.
Conventional esophageal motility evaluates esophageal circular muscle function but does not assess longitudinal muscle contractions that are responsible for axial shortening of the esophagus. Using high frequency ultrasonographic imaging, Korsapati and Mittal assessed longitudinal muscle function in patients with EoE.[26] Compared with healthy controls, patients with EoE showed significantly reduced longitudinal muscle peak thickness as well as duration of contraction. These results are consistent with selective longitudinal but not circular muscle dysfunction in EoE. However, an alternate explanation of the defect identified is that the longitudinal muscle function is intact but that transmural remodeling alterations in EoE mechanically restrict the ability of the esophagus to shorten. Regardless of the underlying cause, impaired esophageal shortening can be a mechanism that limits effective esophageal bolus transport and thereby contributes to dysphagia and food impactions.
In summary, the available studies evaluating esophageal motor function in EoE have demonstrated both hypercontractile and hypocontractile esophageal body functional abnormalities in subsets of children and adults that could impair esophageal bolus transport, especially when combined with structural defects. It should be acknowledged, however, that the manometric patterns identified are non-specific and do not meet criteria for accepted, major esophageal motility disorders. Differences between motor patterns in children and adults are currently unclear but could potentially explain phenotypic distinctions in their clinical presentations. In the majority of adults with EoE with normal peristalsis and lower esophageal sphincter relaxation, dysphagia is likely the result of reduced luminal caliber that is the consequence of decreased esophageal compliance and increased esophageal fibrosis/stiffness due to tissue remodeling. Increased esophageal pressurization events in adults with EoE may reflect the reduced ability of the esophagus to (1) adequately distend in response to an ingested bolus or (2) effectively clear the bolus due to defects in longitudinal muscle function or (3) a combination thereof. In children, esophageal motor defects may have a more substantial role in impairing bolus transit, a concept that needs further investigation. In addition to their possible clinical implications, functional deficits in EoE provide important insights regarding the pathophysiologic effects of esophageal eosinophilic inflammation.
Functional luminal imaging probe
Fibrostenotic consequences of EoE can be visually estimated by endoscopy. However, the severity of esophageal rigidity cannot be quantified using standard endoscopy. As such the novel and quantitative assessment of esophageal mural compliance at the whole organ level utilizing a functional luminal imaging probe (FLIP) is a better approach for assessing not only esophageal thickness but also the functional consequences of remodeling. The FLIP technology incorporates a multichannel electrical impedance catheter and manometric sensor surrounded by an infinitely compliant bag that is filled with an electrode conducting solution. As the bag is filled with the solution, the probe simultaneously ascertains the esophageal luminal diameter and pressure at multiple points along the catheter assembly. The resulting pressure-volume curves provide a detailed interrogation of the distensibility of the esophageal wall. An initial study of FLIP in patients with EoE demonstrated a significant reduction in distensibility in EoE compared with control subjects (Figure 7).[36] A parameter called the distension plateau characterized the maximum ability of the esophagus to expand in spite of increasing intraluminal pressure at the point of minimal luminal diameter of the esophageal body. The distension plateau was reduced by 50% in EoE compared with controls.
Figure 7.
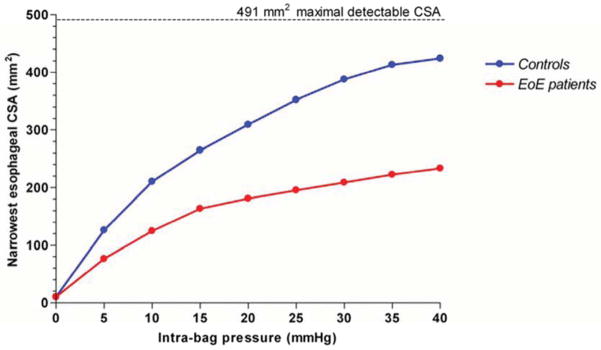
Functional luminal imaging in eosinophilic esophagitis quantified remodeling effects of the esophagus. Esophageal distensibility plots in control subjects (blue) and eosinophilic esophagitis (red) demonstrating diminished distensibility for distension pressures above 5 mm Hg. The calculated value for constant cross sectional area in spite of increasing distension pressure is used to generate the distensibility plateau (DP). Data from Kwiatek, M.A., et al., Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology, 2011. 140(1): p. 82–90.
Nicodème and colleagues recently reported on the assessment of 70 patients with EoE who underwent endoscopy with esophageal biopsy and high-resolution impedance planimetry using a functional lumen-imaging probe.[45] These patients were followed prospectively and rates of food impaction assessed. The study found that patients with a history of food impactions exhibited significantly lower esophageal distensibility, as measured by distensibility plateau values, than those with dysphagia alone. Decreased esophageal distensibility was found to be associated with an increased risk of food impaction and need for dilation during a 4 to 12 month follow-up period. The distensibility plateau was shown to be a more reliable predictor of food impaction risk than findings on endoscopy, although endoscopic estimations of strictures were not included in this comparison. Importantly, no correlation was found between epithelial eosinophil density and food impaction risk, need for dilation, or distensibility. The lack of correlation between esophageal distensibility and mucosal eosinophil density provides a number of potential insights into EoE disease mechanisms. Although epithelial eosinophilic inflammation is used to define one parameter of disease activity, it may not reflect the degree of submucosal disease activity. This is of particular relevance since the pathogenesis of remodeling lies largely below the mucosal surface. Mucosal eosinophilia is likely the harbinger of deeper tissue eosinophilia, as is seen in the LP and muscular layers in EoE. In turn, eosinophilia travels in conjunction with other inflammatory cells and mediators that drive fibrosis, angiogenesis, stenosis, and smooth muscle changes. As such, it appears that while fibrostenosis is an important determinant of clinically relevant symptoms and complications, the histologic finding of eosinophilic inflammation is likely to be the most relevant determinant for the future risk of fibrostenosis.
Effectiveness of available therapies for remodeling in EoE
EoE therapies are rapidly evolving as the mechanisms underlying the disease become better understood. Most drugs are in the early stages of development and none are currently approved by regulatory authorities for use in patients outside of clinical trials. Elimination diet therapy has demonstrated effectiveness similar to medical therapies in terms of resolution of mucosal eosinophilic inflammation. The primary endpoints used to judge the efficacy of therapies are symptoms and esophageal eosinophilic infiltration and to date have not been able to account for effects on esophageal remodeling.[33]
At this time, the most significant therapeutic efficacy on remodeling has been demonstrated with the use of topical, swallowed corticosteroids. The benefits of these agents have been convincing in terms of resolving tissue inflammation [9, 55–57]. Symptom benefits have been more difficult to demonstrate, likely due to limitations in available patient reported outcome instruments, behavior modifications to compensate for symptoms, and the symptom-mucosal eosinophilia dissociation related to esophageal remodeling [33, 58]. Available studies demonstrate heterogeneity regarding the ability to topical steroids to improve esophageal subepithelial fibrosis in EoE. It is likely that the ability to improve fibrotic changes depends on the degree of fibrosis, the duration of the disease, and the age of the EoE subject. Aceves first described significant reduction in severity of fibrosis utilizing topical budesonide in children with EoE.[59] This observation was confirmed by two subsequent pediatric series that utilized diet, topical fluticasone or both as well as in one adult study using topical budesonide.[9, 60, 61] It is important to note that in all of these studies, fibrosis improvement paralleled epithelial inflammatory improvement. In those patients who either did not improve with therapy or who were receiving placebo, both inflammation and fibrosis persisted. [9, 59] Improvement of fibrostenosis with steroids has been less consistent in adult studies of EoE. A significant improvement in esophageal fibrosis using a histopathologic fibrosis score was demonstrated in a randomized controlled trial of adults following 15 days of topical budesonide by Straumann.[9] In contrast, fibrosis score was reduced but did not reach statistical significance in an uncontrolled, prospective study following a year of topical fluticasone utilized in a non-conventional formulation.[9, 10] In a follow up, long-term maintenance, randomized controlled trial, Straumann demonstrated that neither epithelial eosinophilia nor histologic fibrosis control was well retained on low dose budesonide (one fourth of the initial 15 day treatment dose). In addition, although low dose budesonide caused significant improvement in the mucosal thickness following 12 months of therapy, the submucosal and muscularis propria expansion on endoscopic ultrasonography were not significantly improved.[48] While both Aceves (pediatric) and Straumann (adult) have shown reduction in TGFβ1 expression following topical steroid therapy, a study of fluticasone demonstrated decreases only in CCL18.[10] These differences in the results suggest a number of possibilities such as an inability of topical corticosteroids to penetrate the deeper esophageal layers or phenotypic distinctions among subjects who have concordance versus discordance between epithelial inflammation and sub-epithelial fibrosis.
Given the conflicting data on improvement in histopathologic and biomarkers for remodeling, the variability of the clinical outcomes of controlled trials of topical steroids is not surprising. Prospective studies in adults with EoE with both topical steroids have demonstrated symptom improvement but persistence of endoscopically detected esophageal features of fibrostenosis including rings and strictures.[9, 33, 62] In some contrast, Alexander and colleagues found improvements in esophageal lumen diameter in the subset of subjects with more restricted pre-treatment esophageal caliber following short term topical budesonide.[38] However, there were no significant improvements in luminal caliber on barium esophagram following 6 weeks of topical steroids among subjects who initially had a normal caliber esophagus. Treatment of adults with elemental formula demonstrated improvements in endoscopic features that reflect inflammation (furrows, plaques) but not in the features of rings or strictures that likely reflect long-term tissue remodeling.[63] Together, the current therapeutic trials suggest that shorter duration EoE and/or less severely remodeled esophagi may be more likely to demonstrate reversal of fibrosis.
While corticosteroids are a frontline therapy for EoE children and adults, there is a subset of EoE patients who are steroid refractory [64–66]. In addition, given its chronic nature, EoE seems to require long-term topical corticosteroids or dietary management. As such corticosteroid or elimination diet sparing agents and/or novel therapeutic strategies are of significant appeal in EoE.[66] To date there have been 3 randomized studies in pediatric and adult EoE using anti-IL-5 and single trial each in adults using anti-IL-13 and a CRTH2 antagonist. As such, these studies are still in their relative infancies. Studies using anti-IL-5 and CRTH2 antagonist have demonstrated statistically significant improvement with incomplete resolution of epithelial eosinophilia.[67] [68, 69]Symptoms were not significantly improved and the impacts on remodeling features were inconsistent with no significant changes in fibrosis in the pediatric anti-IL-5 study (one trial did not report results on fibrosis) but decreases in epithelial TGFβ1 and tenascin C in adult subjects.[68–70]
Animal models have provided preliminary pre-clinical data on potentially novel therapies that could improve EoE.[66] Treatment with anti-Siglec-F, the murine cognate for human Siglec-8[71], causes decreased esophageal eosinophilia and concomitant decreases in remodeling.[5] Both angiogenesis and VEGF producing cells were decreased as were basal zone hyperplasia and fibronectin expression. Smad3 is a member of the transcription factor complex downstream of canonical TGFβ1 signals. Mice deficient in Smad3, and therefore incapable of propagating TGFβ1 signals, have decreased fibrosis and angiogenesis with continued eosinophilia, demonstrating that antigen (ovalbumin) induced esophageal remodeling can be diminished despite persistent eosinophilia in the face of absent TGFβ1signals. As such, both anti-siglec-8 and anti-TGFβ1 therapies could be important for treating esophageal remodeling.[72] Current clinical trials using anti-IL-13 have been completed in adults with EoE but have not yet been published. Given its role in inducing fibrosis, strictures, and increased esophageal thickness in pre-clinical murine models, anti-IL-13 antibodies may represent an important therapeutic avenue in EoE.
The most rapidly effective treatment for symptomatic strictures in adults with EoE is esophageal dilation with either through-the-scope balloon or wire-guided bougie systems.[34] Dilation leads to mechanical disruption of fibrostenotic strictures. Relief of dysphagia is immediate and associated with significant patient reported satisfaction.[73] Dilation, however, does not address the underlying inflammatory process responsible for the development of the stenosis. Although relief of dysphagia can continue for over a year following esophageal dilation even in the absence of anti-inflammatory therapy, EoE is a chronic disease and symptomatic recurrence is expected in most patients [2, 74]. As such, a reduction in esophageal inflammation and/or remodeling, rather than symptom relief may be an appropriate primary endpoint of the therapeutics currently in development. Conceptually, inflammatory control will reduce the risk of ongoing esophageal remodeling and thereby prevent disease progression. While this paradigm is yet unproven, the current emphasis on symptom outcomes only partially recognizes the contribution of remodeling effects and potentially deemphasizes the importance of anti-inflammatory benefits. Utilization of the most accurate therapeutic endpoints that acknowledge the fundamental importance of esophageal remodeling is essential to avoid overlooking valuable treatments for this important and growing disease.
In conclusion, remodeling changes are responsible for the major clinical symptoms and complications of eosinophilic esophagitis. Ongoing studies are investigating the mechanisms behind the chronic inflammation that drives the remodeling process. A variety of existing and novel biomarkers and tests provide important information on remodeling activity in patients with eosinophilic esophagitis. Clinical trials need to account for the presence and reversibility of esophageal remodeling to fully elucidate the potential benefits and limitations of therapeutic interventions.
Table 3.
Eosinophilic esophagitis therapies and effect on esophageal remodeling
| Therapy | Effectiveness | Population |
|---|---|---|
| Topical corticosteroids | Decreased LP fibrosis in subsets of subjects Decreased vascular activation in subset Decreased TGFβ1 in subset Decreased pSmad2/3 in subset |
Adult and Pediatric Pediatric Adult and Pediatric Pediatric |
| Elimination diet | Decreased fibrosis | Pediatric |
| Elemental diet | Decreased thickening, plaques, no change in rings or strictures | Adult |
| Anti-IL-5 | Decreased tenascin, decreased TGFβ1 | Adult |
| Esophageal dilation | No change in inflammation or remodeling; Does not address underlying disease etiology | Adult |
| Systemic steroids Montelukast Anti-IL-13 |
No data No data Results pending publication |
Adult |
Key Points.
Remodeling changes in eosinophilic esophagitis include epithelial basal zone hyperplasia, lamina propria fibrosis, expansion of the muscularis propria and increased vascularity.
Esophageal inflammation in eosinophilic esophagitis drives the remodeling process with mediators that include IL-5, IL-13, TGFβ1, mast cells, fibroblasts and eosinophils.
Recent studies have provided increasing evidence that the primary symptoms of esophageal dysfunction in children and adults as well as clinical complications of eosinophilic esophagitis are consequences of esophageal remodeling and fibrostenosis.
Esophageal remodeling in eosinophilic esophagitis can be demonstrated using widely available tests such as histopathology, barium esophagram, upper endoscopy and endoscopic ultrasonography.
Clinical trials need to account for the presence and reversibility of esophageal remodeling to fully elucidate the potential benefits and limitations of therapeutic interventions.
Abbreviations
- EoE
Eosinophilic Esophagitis
- eos/hpf
eosinophils/high-power field
- GERD
Gastroesophageal reflux disease
- PPI
Proton pump inhibitor
- LP
lamina propria
- TGFβ1
transforming growth factor-beta 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liacouras CA, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128(1):3–20 e6. doi: 10.1016/j.jaci.2011.02.040. quiz 21–2. [DOI] [PubMed] [Google Scholar]
- 2.Schoepfer AM, et al. Delay in Diagnosis of Eosinophilic Esophagitis Increases Risk for Stricture Formation, in a Time-Dependent Manner. Gastroenterology. 2013 doi: 10.1053/j.gastro.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Dellon ES, et al. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE) Am J Gastroenterol. 2013;108(5):679–92. doi: 10.1038/ajg.2013.71. quiz 693. [DOI] [PubMed] [Google Scholar]
- 4.Mishra A, et al. Esophageal remodeling develops as a consequence of tissue specific IL-5-induced eosinophilia. Gastroenterology. 2008;134(1):204–14. doi: 10.1053/j.gastro.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubinstein E, et al. Siglec-F inhibition reduces esophageal eosinophilia and angiogenesis in a mouse model of eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2011;53 (4):409–16. doi: 10.1097/MPG.0b013e3182182ff8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mavi P, et al. Esophageal functional impairments in experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. 2012;302(11):G1347–55. doi: 10.1152/ajpgi.00013.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aceves SS, et al. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119(1):206–12. doi: 10.1016/j.jaci.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Mishra A. Mechanism of eosinophilic esophagitis. Immunol Allergy Clin North Am. 2009;29(1):29–40. viii. doi: 10.1016/j.iac.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Straumann A, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology. 2010;139(5):1526–37. 1537 e1. doi: 10.1053/j.gastro.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 10.Lucendo AJ, et al. Subepithelial collagen deposition, profibrogenic cytokine gene expression, and changes after prolonged fluticasone propionate treatment in adult eosinophilic esophagitis: a prospective study. J Allergy Clin Immunol. 2011;128(5):1037–46. doi: 10.1016/j.jaci.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Mulder DJ, et al. FGF9-induced proliferative response to eosinophilic inflammation in oesophagitis. Gut. 2009;58(2):166–73. doi: 10.1136/gut.2008.157628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aceves SS, et al. Mast cells infiltrate the esophageal smooth muscle in patients with eosinophilic esophagitis, express TGF-beta1, and increase esophageal smooth muscle contraction. J Allergy Clin Immunol. 2010;126(6):1198–204 e4. doi: 10.1016/j.jaci.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 13.Abonia JP, et al. Involvement of mast cells in eosinophilic esophagitis. J Allergy Clin Immunol. 2010;126(1):140–9. doi: 10.1016/j.jaci.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu Blatman KS, et al. Expression of mast cell-associated genes is upregulated in adult eosinophilic esophagitis and responds to steroid or dietary therapy. J Allergy Clin Immunol. 2011;127(5):1307–8 e3. doi: 10.1016/j.jaci.2010.12.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otani IM, et al. Anti-IL-5 therapy reduces mast cell and IL-9 cell numbers in pediatric patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2013;131(6):1576–82. doi: 10.1016/j.jaci.2013.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niranjan R, et al. Pathogenic role of mast cells in experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. 2013;304(12):G1087–94. doi: 10.1152/ajpgi.00070.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanchard C, et al. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J Allergy Clin Immunol. 2007;120(6):1292–300. doi: 10.1016/j.jaci.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 18.Blanchard C, et al. Coordinate interaction between IL-13 and epithelial differentiation cluster genes in eosinophilic esophagitis. J Immunol. 2010;184(7):4033–41. doi: 10.4049/jimmunol.0903069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuo L, et al. IL-13 induces esophageal remodeling and gene expression by an eosinophil-independent, IL-13R alpha 2-inhibited pathway. J Immunol. 2010;185(1):660–9. doi: 10.4049/jimmunol.1000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanchard C, et al. Periostin facilitates eosinophil tissue infiltration in allergic lung and esophageal responses. Mucosal Immunol. 2008;1(4):289–96. doi: 10.1038/mi.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Persad R, et al. Angiogenic remodeling in pediatric EoE is associated with increased levels of VEGF-A, angiogenin, IL-8, and activation of the TNF-alpha-NFkappaB pathway. J Pediatr Gastroenterol Nutr. 2012;55(3):251–60. doi: 10.1097/MPG.0b013e31824b6391. [DOI] [PubMed] [Google Scholar]
- 22.Fox VL, et al. High-resolution EUS in children with eosinophilic “allergic” esophagitis. Gastrointest Endosc. 2003;57(1):30–6. doi: 10.1067/mge.2003.33. [DOI] [PubMed] [Google Scholar]
- 23.Roman S, et al. Manometric features of eosinophilic esophagitis in esophageal pressure topography. Neurogastroenterol Motil. 2011;23(3):208–14. e111. doi: 10.1111/j.1365-2982.2010.01633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moawad FJ, et al. Esophageal motor disorders in adults with eosinophilic esophagitis. Dig Dis Sci. 2011;56(5):1427–31. doi: 10.1007/s10620-011-1655-5. [DOI] [PubMed] [Google Scholar]
- 25.Read AJ, Pandolfino JE. Biomechanics of esophageal function in eosinophilic esophagitis. J Neurogastroenterol Motil. 2012;18(4):357–64. doi: 10.5056/jnm.2012.18.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korsapati H, et al. Dysfunction of the longitudinal muscles of the oesophagus in eosinophilic oesophagitis. Gut. 2009;58(8):1056–62. doi: 10.1136/gut.2008.168146. [DOI] [PubMed] [Google Scholar]
- 27.Abonia JP, Franciosi JP, Rothenberg ME. TGF-beta1: Mediator of a feedback loop in eosinophilic esophagitis--or should we really say mastocytic esophagitis? J Allergy Clin Immunol. 2010;126(6):1205–7. doi: 10.1016/j.jaci.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saffari H, et al. Patchy eosinophil distributions in an esophagectomy specimen from a patient with eosinophilic esophagitis: Implications for endoscopic biopsy. J Allergy Clin Immunol. 2012;130(3):798–800. doi: 10.1016/j.jaci.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicholson AG, et al. Full thickness eosinophilia in oesophageal leiomyomatosis and idiopathic eosinophilic oesophagitis. A common allergic inflammatory profile? J Pathol. 1997;183(2):233–6. doi: 10.1002/(SICI)1096-9896(199710)183:2<233::AID-PATH936>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 30.Noti M, et al. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat Med. 2013;19(8):1005–13. doi: 10.1038/nm.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasilopoulos S, et al. The small-caliber esophagus: an unappreciated cause of dysphagia for solids in patients with eosinophilic esophagitis. Gastrointest Endosc. 2002;55(1):99–106. doi: 10.1067/mge.2002.118645. [DOI] [PubMed] [Google Scholar]
- 32.Straumann A, Schoepfer AM. Therapeutic concepts in adult and paediatric eosinophilic oesophagitis. Nat Rev Gastroenterol Hepatol. 2012;9(12):697–704. doi: 10.1038/nrgastro.2012.182. [DOI] [PubMed] [Google Scholar]
- 33.Hirano I. Therapeutic end points in eosinophilic esophagitis: is elimination of esophageal eosinophils enough? Clin Gastroenterol Hepatol. 2012;10(7):750–2. doi: 10.1016/j.cgh.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Hirano I. Dilation in eosinophilic esophagitis: to do or not to do? Gastrointest Endosc. 2010;71(4):713–4. doi: 10.1016/j.gie.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 35.Taft TH, et al. Qualitative assessment of patient-reported outcomes in adults with eosinophilic esophagitis. J Clin Gastroenterol. 2011;45(9):769–74. doi: 10.1097/MCG.0b013e3182166a5a. [DOI] [PubMed] [Google Scholar]
- 36.Kwiatek MA, et al. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology. 2011;140(1):82–90. doi: 10.1053/j.gastro.2010.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmerman SL, et al. Idiopathic eosinophilic esophagitis in adults: the ringed esophagus. Radiology. 2005;236(1):159–65. doi: 10.1148/radiol.2361041100. [DOI] [PubMed] [Google Scholar]
- 38.Lee J, et al. Esophageal diameter is decreased in some patients with eosinophilic esophagitis and might increase with topical corticosteroid therapy. Clin Gastroenterol Hepatol. 2012;10(5):481–6. doi: 10.1016/j.cgh.2011.12.042. [DOI] [PubMed] [Google Scholar]
- 39.Stevoff C, et al. EUS and histopathologic correlates in eosinophilic esophagitis. Gastrointest Endosc. 2001;54(3):373–7. doi: 10.1067/mge.2001.116569. [DOI] [PubMed] [Google Scholar]
- 40.Kim HP, et al. The prevalence and diagnostic utility of endoscopic features of eosinophilic esophagitis: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10(9):988–96 e5. doi: 10.1016/j.cgh.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Straumann A, et al. Pediatric and adult eosinophilic esophagitis: similarities and differences. Allergy. 2012;67(4):477–90. doi: 10.1111/j.1398-9995.2012.02787.x. [DOI] [PubMed] [Google Scholar]
- 42.Straumann A, et al. Fragility of the esophageal mucosa: a pathognomonic endoscopic sign of primary eosinophilic esophagitis? Gastrointest Endosc. 2003;57(3):407–12. doi: 10.1067/mge.2003.123. [DOI] [PubMed] [Google Scholar]
- 43.Aceves SS, Ackerman SJ. Relationships between eosinophilic inflammation, tissue remodeling, and fibrosis in eosinophilic esophagitis. Immunol Allergy Clin North Am. 2009;29 (1):197–211. xiii–xiv. doi: 10.1016/j.iac.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aceves SS, et al. A symptom scoring tool for identifying pediatric patients with eosinophilic esophagitis and correlating symptoms with inflammation. Ann Allergy Asthma Immunol. 2009;103(5):401–6. doi: 10.1016/S1081-1206(10)60359-6. [DOI] [PubMed] [Google Scholar]
- 45.Nicodeme F, et al. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2013;11(9):1101–1107 e1. doi: 10.1016/j.cgh.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hirano I, et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2013;62(4):489–95. doi: 10.1136/gutjnl-2011-301817. [DOI] [PubMed] [Google Scholar]
- 47.!!! INVALID CITATION !!!
- 48.Straumann A, et al. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2011;9(5):400–9 e1. doi: 10.1016/j.cgh.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 49.Dobbins JW, Sheahan DG, Behar J. Eosinophilic gastroenteritis with esophageal involvement. Gastroenterology. 1977;72(6):1312–6. [PubMed] [Google Scholar]
- 50.Landres RT, Kuster GG, Strum WB. Eosinophilic esophagitis in a patient with vigorous achalasia. Gastroenterology. 1978;74(6):1298–1301. [PubMed] [Google Scholar]
- 51.Savarino E, et al. Achalasia with dense eosinophilic infiltrate responds to steroid therapy. Clin Gastroenterol Hepatol. 2011;9(12):1104–6. doi: 10.1016/j.cgh.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Lucendo AJ, et al. Manometric findings in adult eosinophilic oesophagitis: a study of 12 cases. Eur J Gastroenterol Hepatol. 2007;19(5):417–24. doi: 10.1097/MEG.0b013e328010bd69. [DOI] [PubMed] [Google Scholar]
- 53.Nurko S, Rosen R, Furuta GT. Esophageal dysmotility in children with eosinophilic esophagitis: a study using prolonged esophageal manometry. Am J Gastroenterol. 2009;104(12):3050–7. doi: 10.1038/ajg.2009.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin Martin L, et al. Esophageal motor abnormalities in eosinophilic esophagitis identified by high-resolution manometry. J Gastroenterol Hepatol. 2011;26(9):1447–50. doi: 10.1111/j.1440-1746.2011.06770.x. [DOI] [PubMed] [Google Scholar]
- 55.Konikoff MR, et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology. 2006;131(5):1381–91. doi: 10.1053/j.gastro.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 56.Dohil R, et al. Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology. 2010;139(2):418–29. doi: 10.1053/j.gastro.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 57.Schroeder S, et al. Successful treatment of eosinophilic esophagitis with ciclesonide. J Allergy Clin Immunol. 2012;129(5):1419–21. doi: 10.1016/j.jaci.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rothenberg ME, et al. Working with the US Food and Drug Administration: progress and timelines in understanding and treating patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2012;130(3):617–9. doi: 10.1016/j.jaci.2012.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aceves SS, et al. Resolution of remodeling in eosinophilic esophagitis correlates with epithelial response to topical corticosteroids. Allergy. 2010;65(1):109–16. doi: 10.1111/j.1398-9995.2009.02142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abu-Sultaneh SM, et al. Fluticasone and food allergen elimination reverse subepithelial fibrosis in children with eosinophilic esophagitis. Dig Dis Sci. 2011;56(1):97–102. doi: 10.1007/s10620-010-1259-5. [DOI] [PubMed] [Google Scholar]
- 61.Chehade M, et al. Esophageal subepithelial fibrosis in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2007;45(3):319–28. doi: 10.1097/MPG.0b013e31806ab384. [DOI] [PubMed] [Google Scholar]
- 62.Alexander JA, et al. Swallowed fluticasone improves histologic but not symptomatic response of adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2012;10(7):742–749 e1. doi: 10.1016/j.cgh.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 63.Peterson KA, et al. Elemental diet induces histologic response in adult eosinophilic esophagitis. Am J Gastroenterol. 2013;108(5):759–66. doi: 10.1038/ajg.2012.468. [DOI] [PubMed] [Google Scholar]
- 64.Caldwell JM, et al. Glucocorticoid-regulated genes in eosinophilic esophagitis: a role for FKBP51. J Allergy Clin Immunol. 2010;125(4):879–888 e8. doi: 10.1016/j.jaci.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wen T, et al. Molecular Diagnosis of Eosinophilic Esophagitis by Gene Expression Profiling. Gastroenterology. 2013 doi: 10.1053/j.gastro.2013.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kern E, Hirano I. Emerging drugs for eosinophilic esophagitis. Expert Opin Emerg Drugs. 2013;18(3):353–64. doi: 10.1517/14728214.2013.829039. [DOI] [PubMed] [Google Scholar]
- 67.Straumann A, et al. Anti-eosinophil activity and clinical efficacy of the CRTH2 antagonist OC000459 in eosinophilic esophagitis. Allergy. 2013;68(3):375–85. doi: 10.1111/all.12096. [DOI] [PubMed] [Google Scholar]
- 68.Assa’ad AH, et al. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology. 2011;141(5):1593–604. doi: 10.1053/j.gastro.2011.07.044. [DOI] [PubMed] [Google Scholar]
- 69.Spergel JM, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2012;129(2):456–63. 463 e1–3. doi: 10.1016/j.jaci.2011.11.044. [DOI] [PubMed] [Google Scholar]
- 70.Straumann A, et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut. 2010;59 (1):21–30. doi: 10.1136/gut.2009.178558. [DOI] [PubMed] [Google Scholar]
- 71.Zimmermann N, et al. Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy. 2008;63(9):1156–63. doi: 10.1111/j.1398-9995.2008.01709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kiwamoto T, et al. Siglec-8 as a drugable target to treat eosinophil and mast cell-associated conditions. Pharmacol Ther. 2012;135(3):327–36. doi: 10.1016/j.pharmthera.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schoepfer AM, et al. Esophageal dilation in eosinophilic esophagitis: effectiveness, safety, and impact on the underlying inflammation. Am J Gastroenterol. 2010;105(5):1062–70. doi: 10.1038/ajg.2009.657. [DOI] [PubMed] [Google Scholar]
- 74.Assa’ad AH, et al. Pediatric patients with eosinophilic esophagitis: an 8-year follow-up. J Allergy Clin Immunol. 2007;119(3):731–8. doi: 10.1016/j.jaci.2006.10.044. [DOI] [PubMed] [Google Scholar]