Abstract
Background
Adult offspring of Holocaust survivors comprise an informative cohort in which to study intergenerational transmission of the effects of trauma exposure. Lower cortisol and enhanced glucocorticoid sensitivity have been previously demonstrated in Holocaust survivors with PTSD, and in offspring of Holocaust survivors in association with maternal PTSD. In other work, reduction in the activity of the enzyme 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD-2), which inactivates cortisol, was identified in Holocaust survivors in comparison to age-matched, unexposed Jewish controls. Therefore, we investigated glucocorticoid metabolism in offspring of Holocaust survivors to evaluate if similar enzymatic decrements would be observed that might help to explain glucocorticoid alterations previously shown for Holocaust offspring.
Methods
Holocaust offspring (n=85) and comparison subjects (n=27) were evaluated with clinical diagnostic interview and self-rating scales, and asked to collect a 24-hr urine sample from which concentrations of cortisol and glucocorticoid metabolites were assayed by GCMS. 11β-HSD-2 activity was determined as the ratio of urinary cortisone to cortisol.
Results
Significantly reduced cortisol excretion was observed in Holocaust offspring compared to controls (p=.046), as had been shown for Holocaust survivors. However, 11β-HSD-2 activity was elevated for offspring compared to controls (p=.008), particularly among those whose mothers had been children, rather than adolescents or adults, during World War II (p=.032). The effect of paternal Holocaust exposure could not be reliably investigated in the current sample.
Conclusions
The association of offspring 11β-HSD-2 activity with maternal age at Holocaust exposure is consistent with the influence of glucocorticoid programming. Whereas a long standing reduction in 11β-HSD-2 activity among survivors is readily interpreted in the context of Holocaust related deprivation, understanding the directional effect on offspring will require replication and further exploration.
Keywords: 11β-hydroxysteriod dehydrogenase type 2, Cortisol, Glucocorticoid metabolism, Developmental programming, Intergenerational, Holocaust, Offspring, biomarkers, PTSD, HPA axis
1. Introduction
Disruptions in glucocorticoid metabolism and activity represent enduring consequences of extreme trauma that appear to be at least partially related to age of exposure (Seckl and Holmes, 2007; Yehuda et al., 2010; Yehuda and Seckl, 2011). The persistence of these effects was demonstrated in elderly Holocaust survivors who showed relative deficiencies in the activities of 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD-2) and 5α-reductase (Yehuda et al., 2009). In contrast to previously reported endocrine findings in Holocaust survivors, such as reduced basal cortisol levels and increased sensitivity to glucocorticoids (Yehuda et al., 2005b; Yehuda et al., 2002), the enzymatic alterations observed were not related to the presence or absence of PTSD or other psychiatric disorders in the survivor. Rather, the observed changes were associated with Holocaust exposure. Furthermore, they were particularly prominent in survivors who were youngest at age of exposure to the Holocaust, suggesting a critical developmental window during which adversity could permanently down-regulate glucocorticoid metabolism (Yehuda et al., 2009).
The impacts of Holocaust exposure and related psychopathology have also been examined in the adult offspring of Holocaust survivors. Adult offspring of Holocaust survivors with PTSD, born a median of 15 years after the end of WWII, demonstrated similar reductions in glucocorticoid levels and enhanced glucocorticoid receptor sensitivity (Lehrner et al., 2014; Yehuda et al., 2007a) as had been observed in Holocaust and other trauma survivors with PTSD (Yehuda et al., 2000; Yehuda et al., 2002). Among adult offspring of Holocaust survivors, lower ambient cortisol was observed specifically in association with maternal PTSD (Yehuda et al., 2007b), an association that has also been demonstrated in offspring of survivors of other traumatic experiences. For example, infants born to mothers who developed PTSD following exposure to the 9/11 attacks in the second and third trimesters of pregnancy also showed lower cortisol compared to infants of similarly exposed mothers who did not develop PTSD (Yehuda et al., 2005a). The trimester effect may reflect differences in maternal 11β-HSD-2 or other placental hormones, peptides, or enzymes that were altered in association with maternal stress. Interestingly, the effects on infants can also be observed if the maternal trauma exposure occurred early in life. Higher cortisol reactivity has been shown for infants of mothers with PTSD as a consequence of childhood abuse (Brand et al., 2010).
In the adult 11β-HSD-2 is largely confined to the kidney, where it contributes to blood pressure regulation. In pregnant women, however, 11β-HSD-2 is highly expressed in the placenta, where it regulates fetal glucocorticoid levels by catalyzing conversion of cortisol to inert cortisone, thus reducing access of high concentrations of maternal cortisol to the umbilical vein (Bertram et al., 2001; Chapman et al., 2013; Drake et al., 2012; Reynolds, 2013). Placental 11β-HSD-2 thus functions to protect the fetus from potentially deleterious effects of maternal stress during pregnancy (Edwards et al., 1993), although this ‘barrier’ is incomplete since 10–20% of maternal cortisol crosses intact to the fetus (Seckl, 2008). Thus, a relative deficiency in placental 11β-HSD-2, whether as a consequence of exogenous glucocorticoid treatment, maternal stress or nutritional deprivation during pregnancy, or direct inhibition of 11β-HSD-2, results in fetal overexposure to maternal glucocorticoids. Glucocorticoids of fetal and maternal origin play a key role in inducing terminal maturation pathways in fetal organs to prepare for extrauterine life. However, excessive glucocorticoid exposure during gestation may result in the programming of offspring vulnerability to subsequent disease states that become apparent with age. Among these are behavioral disturbances, psychiatric disorders, and conditions related to metabolic syndrome and to cardiometabolic risk, including hypertension (Drake et al., 2007; O'Donnell et al., 2009; Räikkönen et al., 2010; Seckl, 2008; Tang et al., 2011).
The purpose of the current study was to examine 11β-HSD-2 in the adult offspring of Holocaust survivors. Since prior work in Holocaust survivors demonstrated reduced activity of 11β-HSD-2 in association with younger age at exposure, we examined the influence of age of maternal exposure on offspring 11β-HSD-2. We also examined the effect of maternal PTSD on offspring 11β-HSD-2 based on the finding that PTSD severity was a significant correlate of 11β-HSD-2 among Holocaust survivors (Yehuda et al., 2009), and on the significant association of maternal PTSD with glucocorticoid-related alterations in Holocaust offspring (Lehrner et al., 2014; Yehuda et al., 2007a; Yehuda et al., 2007b). We hypothesized that greatest alterations in 11β-HSD-2 would be present in Holocaust offspring with younger mothers and with mothers with PTSD.
2. Methods
2.1. Subjects
112 Holocaust survivor offspring and comparison subjects were recruited through advertisements requesting Jewish volunteers to participate in a study examining the effects of the Holocaust. Holocaust survivors who had previously participated in related research were informed of the study in order to recruit their adult offspring. All procedures were approved by the Institutional Review Boards of the Mount Sinai School of Medicine and the James J. Peters Veterans Affairs Medical Center. Written informed consent was obtained from all participants. Data pertaining to the health complaints and medication usage from 66 subjects included in the current study (52 offspring and 14 controls) were reported previously (Flory et al., 2011).
Holocaust survivor parents were defined as having been interned in a Nazi concentration camp during World War II or as having faced comparably severe threats in hiding or during escape to a safe country prior to liberation. Holocaust offspring were defined as being born after World War II and being raised through adolescence by their biological parents. Comparison subjects were Jewish adults of a similar age whose parents were not exposed to the Holocaust, with the majority born in Canada, the US, or Middle East. Holocaust survivor offspring were further subdivided based on maternal age of Holocaust exposure and the presence or absence of maternal and paternal PTSD. In this sample, all Holocaust offspring had fathers who met the definition of Holocaust survivor; 69 were the offspring of two Holocaust survivors (i.e., 16 had mothers born in Canada, the US, Middle East or other non-occupied countries).
Participants received a comprehensive psychiatric evaluation to determine the presence of any current or lifetime psychiatric disorder, and completed several psychological and symptom severity measures. PTSD was assessed using the Clinician-Administered PTSD Scale (CAPS; (Blake et al., 1995)), other diagnoses were made using the Structured Clinical Interview for DSM-IV (SCID; (Spitzer et al., 1995)). Self-rated measures included the Civilian Mississippi Scale for PTSD (Keane et al., 1990) for the assessment of trauma related symptoms, the Beck Depression Inventory (BDI; (Beck et al., 1961)), the Spielberger State Trait Anxiety Inventory (STAI; (Spielberger, 1968)) the Symptom Checklist 90 (SCL-90; (Derogatis, 1975)) which details 9 symptom dimensions and three global symptom indices, and the Childhood Trauma Questionnaire (CTQ; (Bernstein et al., 1994)) for the assessment of the childhood care environment.
Exclusion criteria were: history or evidence of psychotic illness or bipolar disorder; current alcohol or substance abuse or dependence; major medical, endocrinologic, or neurologic illness likely to interfere with HPA axis function or assessment. Presence of current or lifetime mood or anxiety disorders were not exclusionary. Parental PTSD was established using the Parental PTSD Questionnaire (PPQ), a self-report measure that asks offspring to rate the presence and severity of each of the 17 items that comprise the DSM-IV PTSD diagnostic criteria for each parent, and contains additional items pertaining to perceived effects of being raised by a Holocaust survivor (Yehuda et al., 2006). The scale has been previously validated in direct interviews of survivors using the CAPS and was shown to have good convergent validity for PTSD diagnosis and subscale ratings (Yehuda et al., 2006). A diagnosis of parental PTSD was assigned on the basis of a positive endorsement of symptoms distributed appropriately across the three symptom domains as required by DSM-IV criteria (APA, 2000).
2.2 Procedures
Following the psychological evaluation, participants were given sterile containers with instructions to collect a 24-hr urine sample at home (beginning after the first voided urine following awakening, and continuing through the first voided urine on the following day), on a day that was expected to be relatively quiet and free from stress or strenuous exercise. Urine was stored frozen during the collection period to prevent degradation of cortisol and its metabolites. Following the collection, a research coordinator inquired about adherence to instructions and completeness of the collection procedure. Frozen samples were thawed, urine volume recorded, and aliquots were refrozen until assay. Glucocorticoids were measured by electron impact GCMS providing direct assessment of free cortisol, and its metabolites, from which estimates of glucocorticoid metabolic enzyme activities were derived. Samples were batched for assay using methods described elsewhere (Best and Walker, 1997).
2.3. Dependent variables and statistical analysis
Glucocorticoid enzyme determinations
The following measures were obtained: urinary free cortisol (F) and free cortisone (E), and their metabolites, 5α- and 5β-tetrahydrocortisol (5α-THF, 5β-THF) and tetrahydrocortisone (THE). Total urinary glucocorticoids were calculated as the sum of these five measures. Enzyme activity was estimated based on the ratios of glucocorticoid metabolites to precursors. 11β-HSD-2 activity was inferred from the urinary E/F ratio (Best and Walker, 1997) which primarily reflects activity of the renal enzyme in adults. Exploratory analyses were performed for other enzyme determinations. 11β-HSD total activity, i.e., the sum of 11β-HSD-1 and 11β-HSD-2 activities, was calculated from the ratio of cortisol reduction products ((α-THF + β-THF)/ THE). 11β-HSD-1 functions predominantly as an 11β-reductase, responsible for regenerating cortisol from its inert 11-keto metabolites, notably in liver. 5α-reduction was inferred from the ratio of 5α-THF/F, and 5β-reductase activity from the ratio of 5β-THF/F. The A-ring reductase ratio of 5α-reductase to 5β-reductase activities was calculated as 5α-THF/ 5β-THF.
Statistical transformation and covariate determinations
All of the metabolites and enzyme determinations were substantially kurtotic (2.4 to 15.4) and skewed (1.3 to 7.9), with the exception of cortisone. Therefore, all metabolites, total glucocorticoids, and enzyme estimates were log-transformed for analyses, which uniformly normalized their distributions. Analyses were conducted on log-transformed variables, but raw data is reported and represented in the figures for ease of interpretation. Prior to conducting the analyses, potential covariates were examined (e.g., age, gender, BMI, cigarette smoking, and medication usage), and used as appropriate in analyses of covariance (ANCOVA).
Analytic methods
Comparisons of Holocaust offspring and comparison subjects were performed using chi-square analyses for categorical variables and analyses of variance (ANOVA) and covariance for dimensional data. Based on prior studies demonstrating specific contributions of both maternal trauma exposure and age of Holocaust exposure to glucocorticoid metabolism, the sample was divided into four groups based on whether mothers were Holocaust exposed, and if so, whether they were children (11 years or younger), adolescents (12 to 18 years of age) or adults (18 years or greater) at the time of exposure. ANCOVA was used to examine the contribution of maternal age of exposure to offspring 11β-HSD-2 activity. We also examined the effect of maternal PTSD on offspring 11β-HSD-2 activity. Holocaust offspring were subdivided into three groups: 1) maternal Holocaust exposure without maternal PTSD, 2) maternal Holocaust exposure with maternal PTSD, and 3) comparison subjects (i.e., no parental exposure or parental PTSD), excluding offspring without maternal exposure (n=16). One-way ANCOVA was used to examine the influence of maternal exposure versus maternal PTSD on offspring 11β-HSD-2. Finally, linear regression was used to test the relative importance of maternal exposure versus maternal PTSD in explaining the variance in offspring 11β-HSD-2 activity. Covariates were entered in the first step, maternal age at Holocaust exposure in the second step, and maternal PTSD in the final step. Then the regression was repeated with covariates in the first step, maternal PTSD in the second step, and maternal age at Holocaust exposure in the final step.
3. Results
3.1. Demographic and clinical characteristics of offspring and comparison subjects
Demographic and clinical characteristics of offspring and comparison groups are reported in Table 1. There were no significant differences between the Holocaust offspring and comparison groups in gender, BMI, years of education, age of first and last exposure to traumatic events, or the impact of trauma-related symptoms as assessed by the Mississippi PTSD scale score. Holocaust survivor offspring were an average of 4.3 years older than comparison subjects, and were significantly more likely than comparison subjects to have current and lifetime anxiety disorders as well as higher self-ratings on anxiety scales, as previously observed in an independent offspring sample (Yehuda et al., 2008).
Table 1.
Comparison of demographic and clinical characteristics of Holocaust survivor offspring and comparison subjects
| Comparison Subjects (n=27) |
Holocaust Offspring (n=85) |
F(df)p or χ2 (df) p | |
|---|---|---|---|
| Age | 42.6 ± 10.5 | 46.9 ± 7.6 | F(1,110) = 5.53, p= .021 |
| Gender (Male/Female) | M (48.1%) F (51.9%) |
M (40.0%) F (60.0%) |
χ2 (1) = 0.56, ns |
| BMI | 24.1 ± 4.0 | 24.9 ± 4.4 | F(1,110) = 0.66, ns |
| Years of education | 17.0 ± 2.1 | 17.1 ± 3.2 | F(1,103) = 0.12, ns |
| Maternal age at offspring birth | 27.2 ± 8.5 | 29.7 ± 5.8 | F(1, 89) = 2.13, ns |
| Paternal age at offspring birth | 32.7 ± 6.6 | 35.7 ± 4.8 | F (1,70) = 2.65,ns |
| Age at first trauma a | 16.1 ± 11.4 | 14.0 ± 8.1 | F(1,98) = 0.847, ns |
| Age at last trauma a | 41.0 ± 12.2 | 41.9 ± 9.7 | F(1,98) = 0.11, ns |
| Lifetime anxiety disorder b | 6/21 (22.2%) | 37/48 (44.6%) | χ2 (1) = 4.21, p= .040 |
| Lifetime mood disorder b | 9/18 (33.3%) | 38/47 (45.8%) | χ2 (1) = 1.09, ns |
| Mississippi PTSD Scale c | 66.2 ± 15.2 | 70.0 ± 29.6 | F(1,108) = 0.38, ns |
| Beck Depression Inventory | 4.7 ± 5.6 | 7.3 ± 6.6 | F(1,76) = 2.26, ns |
| Spielberger – Trait anxiety d | 15.7 ± 11.6 | 19.8 ± 10.5 | F(1,72) = 2.06, ns |
| Spielberger – State anxiety d | 9.9 ± 8.8 | 15.9 ± 12.2 | F(1,75) = 3.96, (p=.050) |
| CTQ – Total score e | 36.6 ± 12.3 | 41.1 ± 13.3 | F(1,102) = 2.34, ns |
Assessed using the Clinician-Administered PTSD Scale
DSM-IV based on clinical interview
Civilian Mississippi Scale for PTSD
Spielberger State-Trait Anxiety Inventory (STAI)
Childhood Trauma Questionnaire (CTQ)
Maternal and paternal years of birth were highly correlated in the entire cohort (r=.78, p<.0005) and for Holocaust offspring (r=.64, p<.0005). Offspring fathers tended to be approximately 6 years older than offspring mothers: offspring fathers’ year of birth was 1918.5±8.6; offspring mothers’ year of birth was 1924.3 ± 7.7 years. Likewise, maternal and paternal age at offspring birth were highly correlated (r=.64, p<.0005). The absence in this sample of offspring with maternal but no paternal exposure compromised our ability to identify distinct contributions of parental exposure by parental gender. Further, given the degree of correlation between maternal and paternal age of exposure, apparent effects of paternal age could not be distinguished from artifacts of maternal age. As a consequence of the uneven distributions of maternal and paternal exposures, therefore, apparent findings related to paternal age of exposure are not reported.
3.2. Comparison of glucocorticoid metabolite and enzyme activities between offspring and comparison subjects
Table 2 demonstrates that Holocaust offspring showed significantly lower levels of F and lower 5α-THF, the major metabolite of F, at a trend level of significance, but did not show differences for E, β-THF, or THE. Total glucocorticoids, principally composed of 5α-THF, is also lower among the offspring at a trend level of significance. Holocaust offspring had higher estimated activity of 11β-HSD-2 than controls, but showed no significant differences from controls in any other estimated enzyme activity level.
Table 2.
Holocaust survivor offspring and control differences in metabolic and 30 enzyme outcome variables
| Comparison Subjects (n=27) |
Holocaust Offspring (n=85) |
F(df) p a | |
|---|---|---|---|
| Metabolites: | |||
| F (cortisol) | 73.68± 6.06 | 57.18 ± 3.37 b | F(1,107) = 4.07, p=.046 c |
| E (cortisone) | 115.37 ± 11.78 | 121.34 ± 6.55 | F(1,108) = 0.11, ns |
| α-THF | 2555.5 ± 407.2 | 1208.2 ± 223.8 | F(1,105) = 3.04, (p=.084) c |
| β-THF | 2896.0 ± 364.6 | 2206.1 ± 202.6 | F(1,107) = 1.43, ns c |
| THE | 3528.7 ± 412.1 | 3082.2 ± 228.9 | F(1,107) = 1.77, ns c |
| Total Glucocorticoids | 9307.5 ± 867.8 | 6706.8 ± 476.9 | F(1,105) = 3.71, (p=.057) c |
| Enzymes: | |||
| 11β-HSD2 | 1.74 ± 0.28 | 2.47 ± 0.15 | F(1,108) = 7.36, p=.008 |
| Total 11β-HSD | 1.47 ± 0.67 | 1.87 ± 0.37 | F(1,106) = 0.19, ns |
| 5α-reductase (5a-THF/F) |
46.82 ± 12.02 | 24.80 ± 6.61 | F(1,105) = 0.24, ns c |
| 5β-reductase (5β-THF/F) |
44.72 ± 3.61 | 43.91 ± 6.49 | F(1,108) .308, ns |
| 5α-THF /5β-THF ratio | 0.91 ± 0.19 | 0.64 ± 0.10 | F(1,105) = 0.76, ns c |
ANCOVA, controlling for age, BMI on log-transformed data
Estimated marginal means (Mean ± S.E.) for unlogged data values
Gender added as covariate
3.3. Relation of 11β-HSD-2 activity to maternal age at Holocaust exposure
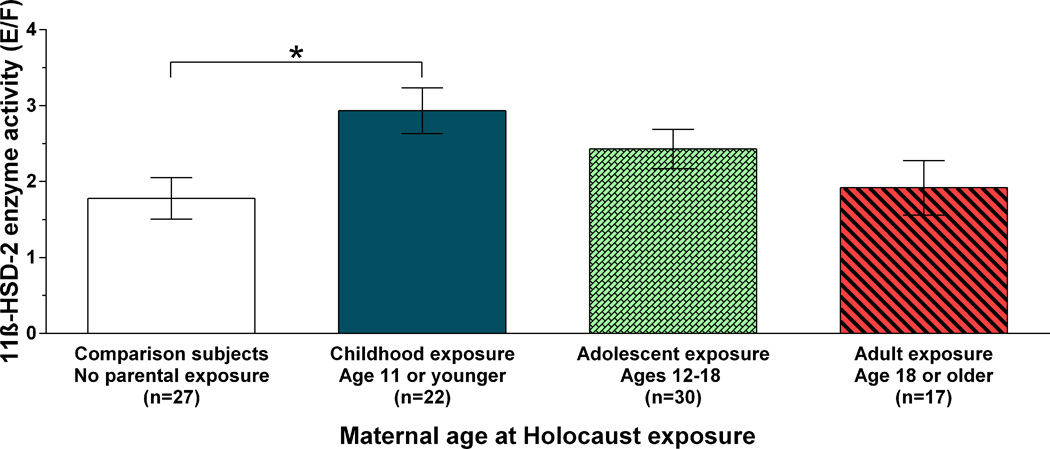
One-way ANCOVA including the three groups of Holocaust offspring, subdivided by maternal age at exposure, and comparison subjects revealed a significant main effect of group (F(3,90)= 3.07; p=.032, controlling for age and BMI). As illustrated in Figure 1, the highest 11β-HSD-2 activity was present in offspring whose mothers were children at the time of the Holocaust, with progressively decreased activity in offspring whose mothers were adolescents and adults. The lowest activity was observed in comparison subjects, i.e., those without any Holocaust exposed parents. Bonferroni post-hoc analysis showed a significant difference in 11β-HSD-2 activity between offspring with maternal exposure in childhood and comparison subjects (p=.029). There were no significant distributions of other metabolites or enzyme indices according to age at maternal exposure, or age of conception.
Figure 1. Association of meternal age at Holocaust exposure with offspring 11β-HSD-2 activity.
Mean 11β-HSD-2 activity for comparison subjects and Holocaust survivor offspring according to age of maternal Holocaust exposure (F(3,90)= 3.07; p=.032, controlling for age and BMI). Bars represent estimated marginal means ± SE of raw data. * (p=.029)
3.4. Relation of 11β-HSD-2 activity to maternal exposure and PTSD
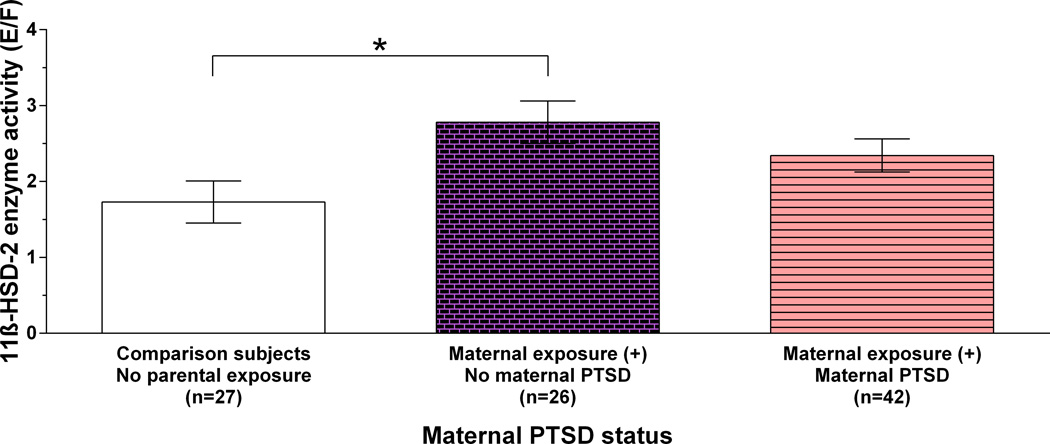
We examined the possibility that maternal PTSD might account for the association of increased 11β-HSD-2 activity with maternal exposure. Offspring were subdivided based on maternal exposure with and without PTSD, and compared to comparison subjects, omitting data for offspring with paternal but without maternal exposure. One-way ANCOVA, controlling for age and BMI, identified significant group differences on offspring 11β-HSD-2 activity (F(2,90)=4.65, p=.012; Figure 2). Bonferroni post-hoc tests showed that 11β-HSD-2 activity differed only between offspring of Holocaust exposed mothers without PTSD and comparison subjects (p=.009). This analysis was also significant when including paternal PTSD as a covariate (F(2,89)=5.03, p=.008), indicating that paternal PTSD did not influence the (negative) relationship of maternal PTSD to offspring 11β-HSD-2 activity.
Figure 2. Relation of 11β-HSD-2 activity to maternal exposure and PTSD status.
Mean 11β-HSD-2 activity for comparison subjects and Holocaust survivor offspring according to maternal PTSD status (F(2,90)=4.65, p=.012; controlling for age and BMI). Bars represent estimated marginal means ± SE of raw data. * (p=.009).
To further test the relative influences of maternal age at exposure and maternal PTSD on offspring 11β-HSD-2 activity, a linear regression was performed including age, gender, BMI, and two variables reflecting categorical distinctions in age of maternal exposure and the presence or absence of maternal PTSD, respectively. Data for offspring of Holocaust exposed fathers with unexposed mothers were excluded from the analysis. Age of maternal Holocaust exposure was a significant predictor of offspring 11β-HSD-2 when entered, following the covariates, in the second step (β=.254, p=.015), producing a significant adjusted R-squared change (p=.015) that explained 14.6% of the variance, with gender (β =.223, p=.026) also a predictor. When maternal PTSD was entered in a final step, there was no significant change in R-squared, and maternal exposure (β =.342, p=.007) and gender (β =.212, p=.035) remained significant predictors. Thus, after accounting for maternal age of exposure, maternal PTSD did not contribute significantly to the prediction of offspring 11β-HSD-2 activity. An analogous linear regression with maternal PTSD entered in the second step showed no significant R-squared change (β =.033, ns), but the addition of maternal age of exposure in a final step was highly significant (β =.342, p=.007). Taken together these regression analyses squarely identified maternal age of exposure, rather than maternal PTSD, as the significant predictor of offspring 11β-HSD-2 activity.
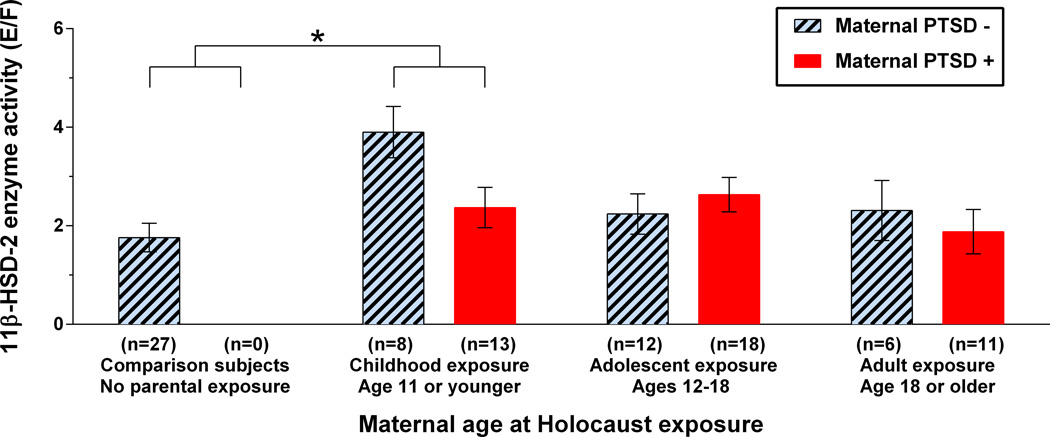
Finally, an ANCOVA for offspring 11β-HSD-2 activity testing both predictors (maternal age of exposure and maternal PTSD), covaried for offspring age, gender, and BMI, was significant for maternal age of exposure (F(3,85)=3.55, p=.018) but not for maternal PTSD (F (1,85)=2.13, ns) or for their interaction (F (2,85)=0.93, ns). The post-hoc comparison between controls and offspring with maternal exposure prior to age 11 was significant (p=.021), but there was no significant post-hoc difference between offspring with and without maternal PTSD (Figure 3).
Figure 3. Association of maternal age at Holocaust exposure and maternal PTSD with offspring 11β-HSD-2 activity.
Mean 11β-HSD-2 activity for comparison subjects and Holocaust survivor offspring according to both maternal age at Holocaust exposure (F(3,85)=3.55, p=.018) and maternal PTSD status (F(1,85)=2.13, ns) with no significant interaction between the two predictors (F(2,85)=0.93, ns), controlling for age, gender, and BMI. Bars represent estimated marginal means ± SE of raw data. * (p=.021).
3.5. Relation of 11β-HSD-2 activity to gender in offspring and comparison subjects
Given the significant association of offspring gender in the above regression models that include maternal age of exposure, gender effects on 11β-HSD-2 activity were compared for offspring and comparison subjects. A significant gender difference in 11β-HSD-2 was apparent for comparison subjects (F(1,23)=4.92, p=.037, controlling for age and BMI; males: 1.37±.21 (M±SE); females: 2.01±.20), whereas no gender difference in 11β-HSD-2 was apparent for offspring (F(1,81)=0.47, ns, controlling for age and BMI; males: 2.46±.27 (M±SE); females: 2.50±.22). The reduction in estimated 11β-HSD-2 activity for males versus females for comparison subjects (32% reduction) is substantially greater than it is for offspring (1.5% reduction).
4. Discussion
In this study we investigated glucocorticoid metabolism among offspring of Holocaust exposed parents and comparison subjects. Holocaust offspring demonstrated significantly lower F, and showed lower levels of αTHF, the major metabolite of F, and of total glucocorticoids, although at trend levels of significance. Most importantly, offspring demonstrated higher 11β-HSD-2 activity relative to comparison subjects. Higher 11β-HSD-2 activity was associated with maternal Holocaust exposure, but not substantially with maternal PTSD. Finally, increased 11β-HSD-2 activity was inversely associated with maternal age at exposure, such that the highest levels of 11β-HSD-2 were associated with maternal exposure to the Holocaust in childhood. Some of these observations mirror findings previously reported for directly exposed Holocaust survivors, whereas others do not.
Like the offspring in this report, Holocaust survivors also showed reductions in F, αTHF and total glucocorticoids – however, with an associated reduction in 5α-reductase activity (Yehuda et al., 2009). Offspring in the current study did not have similarly altered 5α-reductase activity. In the absence of altered 5α-reductase, decrements in F will likely be reflected in relatively lowered levels of αTHF and total glucocorticoids, as suggested for offspring in the current study. However, if cortisol is metabolized more rapidly in the kidney due to elevated 11β-HSD-2, then a compensatory rise in hypothalamic-pituitary-adrenal axis (HPA axis) activity might be expected to maintain F levels. The fact that F levels are lower in the face of unchanged E suggests that the primary cause of lower F is reduced HPA axis drive, possibly as a result of increased glucocorticoid receptor sensitivity or other epigenetically mediated alteration affecting HPA axis regulation.
In striking contrast to the finding in offspring of elevated 11β-HSD-2 activity, Holocaust survivors themselves showed lower 11β-HSD-2 activity. Among the survivors, the greatest decrements in 11β-HSD-2 were evident among those who were youngest at the time of exposure (Yehuda et al., 2009). The specificity of the reduction in 11β-HSD-2 activity for Holocaust ‘child survivors’ was interpreted as an indication of glucocorticoid programming having occurred during a sensitive developmental window, resulting in alterations that remained evident approximately 60 years after exposure. Thus, the effect on 11β-HSD-2 activity was sufficiently enduring to have been present at the time offspring were conceived and carried to term, years after parental Holocaust exposure. In addition to the association with childhood exposure, among Holocaust survivors, the greatest decrements in 11β-HSD-2 activity were associated with the expression of less severe PTSD symptoms (Yehuda et al., 2009). It is indeed consistent with this observation that elevations in offspring 11β-HSD-2 activity in the current report are found for offspring of maternal childhood survivors without PTSD.
The down-regulation of 5α-reductase (a nutritionally-sensitive hepatic enzyme) and of 11β-HSD-2 in directly exposed survivors was interpreted as an adaptive change to minimize catabolism of cortisol in liver and kidney without elevation of circulating steroid levels, thus maximizing the output of glucose and other metabolic fuels from liver and the renal retention of sodium. These changes are a presumed advantage in a calorie and salt-poor early life environment. That these indicators are either absent (5α-reductase) or in the opposite direction (11β-HSD-2) in the unexposed offspring of Holocaust survivors, despite a similar reduction of cortisol, suggests selection for phenotype and yet separate mechanisms for their generation. Indeed, increased 11β-HSD-2 is unlikely to contribute to lower cortisol since more enzyme activity implies that cortisol is cleared more rapidly in the kidney which would be expected to stimulate HPA axis activity (in order to maintain cortisol levels). The fact that F and its metabolites are lower suggests that the reduced HPA axis drive is the primary cause of lower cortisol, distinct from the elevation in 11β-HSD-2. Similar data have emerged in rodent models where despite phenotypic similarities in directly exposed animals and their second generation offspring, the cellular and molecular mechanisms and the underpinning epigenetic changes are quite distinct in each generation (Drake et al., 2011).
Placental 11β-HSD-2 is principally derived from fetal tissue. Urinary metabolites indicating elevations in 11β-HSD-2 activity in adult offspring principally reflect renal enzyme activity. The extent to which these estimates provide insight into what their placental enzyme activity might have been decades earlier is unknown. However, it is possible that offspring in the present cohort may have been exposed to reduced maternal HPA axis activity and hence lowered cortisol as a consequence of maternal Holocaust exposure, with the effect of programming offspring HPA axis hypofunction. This is plausible since glucocorticoid administration, severe maternal stress, or ingestion of 11β-HSD inhibitors in licorice during gestation associate with offspring HPA axis hyperfunction (Räikkönen et al., 2010). The association of offspring 11β-HSD-2 activity with maternal exposure during childhood provides support for the inference that maternal alterations in 11β-HSD-2 activity may have had intergenerational effects.
Regulation of placental and fetal 11β-HSD-2 activity is the principal mechanism conferring protection of the fetus from exposure to the comparatively elevated levels of maternal glucocorticoids during gestation (Benediktsson et al., 1997; Waddell et al., 1998). There are considerable inter-species differences in the precise timing of feto-placental 11β-HSD-2 expression (Matthews, 2002); in humans, the enzyme increases throughout the second and third trimesters of gestation (Shams et al., 1998; Stewart et al., 1995). Prior to mid-gestation, there is widespread expression of 11β-HSD-2 in fetal tissues, which by parturition, is restricted to specific areas of fetal brain and kidney (Peña et al., 2012). It is possible that prenatal exposure to stress-related elevations in maternal glucocorticoids would be associated with a developmental adaptation that includes alterations in 11β-HSD-2 activity. Indeed, fetal 11β-HSD-2 is itself a target of glucocorticoid programming (Alikhani-Koopaei et al., 2004). Prenatal exposure of rats to elevated levels of glucocorticoids (Tang et al., 2011), or of sheep to maternal undernutrition resulting in glucocorticoid elevations (Whorwood et al., 2001), program reductions in renal 11β-HSD-2 activity and risk for salt-sensitive hypertension (Tang et al., 2011). The HSD11β2 gene promoter and associated regions have several methylation sites that appear to be variably associated with increased and diminished methylation in a tissue specific manner (Peña et al., 2012; Pena et al., 2013). Although the most frequently observed effect of maternal stress is that of increased methylation and decreased expression of11β-HSD-2 in placenta as well as fetal cortex, in rat hypothalamus there is indication of diminished methylation at several sites within the HSD11β2 gene promoter as a consequence of maternal stress (Peña et al., 2012; Pena et al., 2013).
Thus, variations in the nature and timing of maternal stress during pregnancy impact substantially on the direction of effects on fetal 11β-HSD-2. Acute stress, particularly near parturition, is associated with increased placental 11β-HSD-2 activity, which would function to buffer the consequences of more transient maternal exposure-related glucocorticoid elevations, whereas chronic maternal stress generally results in placental 11β-HSD-2 downregulation, resulting in fetal glucocorticoid programming (Stark et al., 2009; Welberg et al., 2005).
Interestingly, the current data suggest a possible gender effect on offspring 11β-HSD-2 activity that will require examination in future studies. The offspring in this report did not show a gender difference in 11β-HSD-2 that was apparent for comparison subjects. Female comparison subjects demonstrated approximately 1.5 × the 11β-HSD-2 activity of the men, whereas for offspring men and women, 11β-HSD-2 activity was essentially equivalent. Thus, it would appear that the programming effect was considerably more pronounced among male than female offspring. It should be noted that a similar gender effect has been observed in animal studies in a parallel direction to the current findings, in which maternal programming has preferential effects on male offspring (Brunton et al., 2013). In another example, sex specific effects, albeit in the reverse direction, were observed in female, but not male, pre-term infants born to women with a history of perinatal stress, who were administered antenatal glucocorticoids (Stark et al., 2009).
In sum, the findings of this study demonstrate a distinct influence of maternal age of exposure in association with adult offspring 11β-HSD-2 activity. These observations support speculations regarding the intergenerational transmission of a set-point for 11β-HSD-2 activity with demonstrated implications for offspring mental and physical illness in adulthood (Cottrell and Seckl, 2009; Drake et al., 2007; Seckl and Holmes, 2007). Whereas effects of in-utero environmental manipulations (e.g., maternal stress, protein deprivation, glucocorticoid administration) have been shown to result in long-lived alterations in 11β-HSD-2 activity in rodent models, this is the first example of such effects in a human sample. The specific observations of elevated 11β-HSD-2 activity among offspring, in concert with prior demonstrations of reduced 11β-HSD-2 activity for Holocaust survivors, both paired with reduced cortisol levels, will require replication in future intergenerational studies of offspring of severely traumatized parents.
Acknowledgements
We would like to thank all study participants for their time and contributions to this research project.
Role of funding source:
This work was supported by 1RC1MH088101-01 “Identification of an Epigenetic Risk Marker for PTSD” and NIMH R01 MH 64675-01 “Biology of Risk and PTSD in Holocaust Survivor Offspring” and in part by a grant (5 M01 RR00071) for the Mount Sinai General Clinical Research Center from the National Institute of Health. The NIMH and NIH had no further role in the study design; in the collection, analysis and interpretation of the data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
None of the authors have any conflicts of interest to report.
Author contributions:
RY designed the study. RY and LMB supervised the project and data collection. LMB supervised the clinical assessments, and RY supervised the biological sample collection. IM performed the biological assays. LMB and HNB did primary analyses and drafted the manuscript. NPD and AL assisted with manuscript preparation. JRS edited the manuscript, assisted with data interpretation and was responsible for overseeing analyses of enzyme activities. All the authors discussed the results and commented on the final version of the manuscript.
References
- Alikhani-Koopaei R, Fouladkou F, Frey FJ, Frey BM. Epigenetic regulation of 11 beta-hydroxysteroid dehydrogenase type 2 expression. J. Clin. Invest. 2004;114:1146–1157. doi: 10.1172/JCI21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. American Psychiatric Association. Task Force on DSM-IV. American Psychiatric Association. 2000 [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Benediktsson R, Calder AA, Edwards CRW, Seckl JR. Placental 11β-hydroxysteroid dehydrogenase: a key regulator of fetal glucocorticoid exposure. Clin. Endocrinol. (Oxf.) 1997;46:161–166. doi: 10.1046/j.1365-2265.1997.1230939.x. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am. J. Psychiatry. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Bertram C, Trowern AR, Copin N, Jackson AA, Whorwood CB. The maternal diet during pregnancy programs altered expression of the glucocorticoid receptor and type 2 11betahydroxysteroid dehydrogenase: potential molecular mechanisms underlying the programming of hypertension in utero. Endocrinology. 2001;142:2841–2853. doi: 10.1210/endo.142.7.8238. [DOI] [PubMed] [Google Scholar]
- Best R, Walker BR. Additional value of measurement of urinary cortisone and unconjugated cortisol metabolites in assessing the activity of 11 beta-hydroxysteroid dehydrogenase in vivo. Clin. Endocrinol. (Oxf.) 1997;47:231–236. doi: 10.1046/j.1365-2265.1997.2471061.x. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. J. Trauma. Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Brand SR, Brennan PA, Newport DJ, Smith AK, Weiss T, Stowe ZN. The impact of maternal childhood abuse on maternal and infant HPA axis function in the postpartum period. Psychoneuroendocrinology. 2010;35:686–693. doi: 10.1016/j.psyneuen.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton PJ, Sullivan KM, Kerrigan D, Russell JA, Seckl JR, Drake AJ. Sex-specific effects of prenatal stress on glucose homoeostasis and peripheral metabolism in rats. J. Endocrinol. 2013;217:161–173. doi: 10.1530/JOE-12-0540. [DOI] [PubMed] [Google Scholar]
- Chapman K, Holmes M, Seckl J. 11beta-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol. Rev. 2013;93:1139–1206. doi: 10.1152/physrev.00020.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front. Behav. Neurosci. 2009;3:19. doi: 10.3389/neuro.08.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR. The Symptom Checklist-90-R (SCL-90-R) 1975 [Google Scholar]
- Drake AJ, Liu L, Kerrigan D, Meehan RR, Seckl JR. Multigenerational programming in the glucocorticoid programmed rat is associated with generation-specific and parent of origin effects. Epigenetics. 2011;6 doi: 10.4161/epi.6.11.17942. [DOI] [PubMed] [Google Scholar]
- Drake AJ, McPherson RC, Godfrey KM, Cooper C, Lillycrop KA, Hanson MA, Meehan RR, Seckl JR, Reynolds RM. An unbalanced maternal diet in pregnancy associates with offspring epigenetic changes in genes controlling glucocorticoid action and foetal growth. Clin. Endocrinol. (Oxf.) 2012;77:808–815. doi: 10.1111/j.1365-2265.2012.04453.x. [DOI] [PubMed] [Google Scholar]
- Drake AJ, Tang JI, Nyirenda MJ. Mechanisms underlying the role of glucocorticoids in the early life programming of adult disease. Clin. Sci. 2007;2012;113:219–232. doi: 10.1042/CS20070107. [DOI] [PubMed] [Google Scholar]
- Edwards CR, Benediktsson R, Lindsay RS, Seckl JR. Dysfunction of placental glucocorticoid barrier: link between fetal environment and adult hypertension? Lancet. 1993;341:355–357. doi: 10.1016/0140-6736(93)90148-a. [DOI] [PubMed] [Google Scholar]
- Flory JD, Bierer LM, Yehuda R. Maternal exposure to the holocaust and health complaints in offspring. Dis. Markers. 2011;30:133–139. doi: 10.3233/DMA-2011-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane T, Weathers F, Blake D. Behavioral Science Division. Boston: National Center for Posttraumatic Stress Disorder; 1990. The Civilian Mississippi Scale. [Google Scholar]
- Lehrner A, Bierer LM, Passarelli V, Pratchett LC, Flory JD, Bader HN, Harris IR, Bedi A, Daskalakis NP, Makotkine I, Yehuda R. Maternal PTSD associates with greater glucocorticoid sensitivity in offspring of Holocaust survivors. Psychoneuroendocrinology. 2014;40:213–220. doi: 10.1016/j.psyneuen.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews SG. Early programming of the hypothalamo-pituitary-adrenal axis. Trends Endocrinol. Metab. 2002;13:373–380. doi: 10.1016/s1043-2760(02)00690-2. [DOI] [PubMed] [Google Scholar]
- O'Donnell K, O'Connor TG, Glover V. Prenatal stress and neurodevelopment of the child: focus on the HPA axis and role of the placenta. Dev. Neurosci. 2009;31:285–292. doi: 10.1159/000216539. [DOI] [PubMed] [Google Scholar]
- Peña CJ, Monk C, Champagne FA. Epigenetic effects of prenatal stress on 11β- hydroxysteroid dehydrogenase-2 in the placenta and fetal brain. PLoS ONE. 2012;7:e39791. doi: 10.1371/journal.pone.0039791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena CJ, Neugut YD, Champagne FA. Developmental timing of the effects of maternal care on gene expression and epigenetic regulation of hormone receptor levels in female rats. Endocrinology. 2013;154:4340–4351. doi: 10.1210/en.2013-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Räikkönen K, Seckl JR, Heinonen K, Pyhälä R, Feldt K, Jones A, Pesonen A-K, Phillips DIW, Lahti J, Järvenpää A-L, Eriksson JG, Matthews KA, Strandberg TE, Kajantie E. Maternal prenatal licorice consumption alters hypothalamic–pituitary– adrenocortical axis function in children. Psychoneuroendocrinology. 2010;35:1587–1593. doi: 10.1016/j.psyneuen.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Reynolds RM. Glucocorticoid excess and the developmental origins of disease: two decades of testing the hypothesis--2012 Curt Richter Award Winner. Psychoneuroendocrinology. 2013;38:1–11. doi: 10.1016/j.psyneuen.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Seckl JR. Glucocorticoids, developmental 'programming' and the risk of affective dysfunction. Prog. Brain Res. 2008;167:17–34. doi: 10.1016/S0079-6123(07)67002-2. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Holmes MC. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal 'programming' of adult pathophysiology. Nat. Clin. Pract. Endocrinol. Metab. 2007;3:479–488. doi: 10.1038/ncpendmet0515. [DOI] [PubMed] [Google Scholar]
- Shams M, Kilby MD, Somerset DA, Howie AJ, Gupta A, Wood PJ, Afnan M, Stewart PM. 11Beta-hydroxysteroid dehydrogenase type 2 in human pregnancy and reduced expression in intrauterine growth restriction. Hum. Reprod. 1998;13:799–804. doi: 10.1093/humrep/13.4.799. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. The state-trait anxiety inventory (S T A I) : test manual for Form X. Palo Alto: Consulting Psychologists Press; 1968. [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV (SCID) New York State Psychiatric Institute. Biometrics Research; 1995. [Google Scholar]
- Stark MJ, Wright IM, Clifton VL. Sex-specific alterations in placental 11betahydroxysteroid dehydrogenase 2 activity and early postnatal clinical course following antenatal betamethasone. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:R510–R514. doi: 10.1152/ajpregu.00175.2009. [DOI] [PubMed] [Google Scholar]
- Stewart PM, Rogerson FM, Mason JI. Type 2 11 beta-hydroxysteroid dehydrogenase messenger ribonucleic acid and activity in human placenta and fetal membranes: its relationship to birth weight and putative role in fetal adrenal steroidogenesis. J. Clin. Endocrinol. Metab. 1995;80:885–890. doi: 10.1210/jcem.80.3.7883847. [DOI] [PubMed] [Google Scholar]
- Tang JI, Kenyon CJ, Seckl JR, Nyirenda MJ. Prenatal overexposure to glucocorticoids programs renal 11β-hydroxysteroid dehydrogenase type 2 expression and saltsensitive hypertension in the rat. J. Hypertens. 2011;29:282–289. doi: 10.1097/HJH.0b013e328340aa18. 210.1097/HJH.1090b1013e328340aa328318. [DOI] [PubMed] [Google Scholar]
- Waddell BJ, Benediktsson R, Brown RW, Seckl JR. Tissue-specific messenger ribonucleic acid expression of 11beta-hydroxysteroid dehydrogenase types 1 and 2 and the glucocorticoid receptor within rat placenta suggests exquisite local control of glucocorticoid action. Endocrinology. 1998;139:1517–1523. doi: 10.1210/endo.139.4.5900. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Thrivikraman KV, Plotsky PM. Chronic maternal stress inhibits the capacity to up-regulate placental 11beta-hydroxysteroid dehydrogenase type 2 activity. J. Endocrinol. 2005;186:R7–R12. doi: 10.1677/joe.1.06374. [DOI] [PubMed] [Google Scholar]
- Whorwood CB, Firth KM, Budge H, Symonds ME. Maternal undernutrition during early to midgestation programs tissue-specific alterations in the expression of the glucocorticoid receptor, 11beta-hydroxysteroid dehydrogenase isoforms, and type 1 angiotensin ii receptor in neonatal sheep. Endocrinology. 2001;142:2854–2864. doi: 10.1210/endo.142.7.8264. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Bell A, Bierer LM, Schmeidler J. Maternal, not paternal, PTSD is related to increased risk for PTSD in offspring of Holocaust survivors. J. Psychiatr. Res. 2008;42:1104–1111. doi: 10.1016/j.jpsychires.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Bierer LM, Andrew R, Schmeidler J, Seckl JR. Enduring effects of severe developmental adversity, including nutritional deprivation, on cortisol metabolism in aging Holocaust survivors. J. Psychiatr. Res. 2009;43:877–883. doi: 10.1016/j.jpsychires.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Bierer LM, Schmeidler J, Aferiat DH, Breslau I, Dolan S. Low cortisol and risk for PTSD in adult offspring of holocaust survivors. Am. J. Psychiatry. 2000;157:1252–1259. doi: 10.1176/appi.ajp.157.8.1252. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Blair W, Labinsky E, Bierer LM. Effects of parental PTSD on the cortisol response to dexamethasone administration in their adult offspring. Am. J. Psychiatry. 2007a;164:163–166. doi: 10.1176/ajp.2007.164.1.163. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Engel SM, Brand SR, Seckl J, Marcus SM, Berkowitz GS. Transgenerational effects of posttraumatic stress disorder in babies of mothers exposed to the World Trade Center attacks during pregnancy. J. Clin. Endocrinol. Metab. 2005a;90:4115–4118. doi: 10.1210/jc.2005-0550. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Flory JD, Pratchett LC, Buxbaum J, Ising M, Holsboer F. Putative biological mechanisms for the association between early life adversity and the subsequent development of PTSD. Psychopharmacology (Berl.) 2010;212:405–417. doi: 10.1007/s00213-010-1969-6. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Golier JA, Kaufman S. Circadian rhythm of salivary cortisol in Holocaust survivors with and without PTSD. Am. J. Psychiatry. 2005b;162:998–1000. doi: 10.1176/appi.ajp.162.5.998. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, Grossman R, Golier JA, Wong C. The cortisol and glucocorticoid receptor response to low dose dexamethasone administration in aging combat veterans and holocaust survivors with and without posttraumatic stress disorder. Biol. Psychiatry. 2002;52:393–403. doi: 10.1016/s0006-3223(02)01357-4. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Labinsky E, Tischler L, Brand SR, Lavin Y, Blair W, Bierer LM, Goodman RZ, Grossman RA. Are adult offspring reliable informants about parental PTSD? A validation study. Ann N. Y. Acad. Sci. 2006;1071:484–487. doi: 10.1196/annals.1364.047. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Seckl J. Minireview: Stress-related psychiatric disorders with low cortisol levels: a metabolic hypothesis. Endocrinology. 2011;152:4496–4503. doi: 10.1210/en.2011-1218. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Teicher MH, Seckl JR, Grossman RA, Morris A, Bierer LM. Parental posttraumatic stress disorder as a vulnerability factor for low cortisol trait in offspring of holocaust survivors. Arch. Gen. Psychiatry. 2007b;64:1040–1048. doi: 10.1001/archpsyc.64.9.1040. [DOI] [PubMed] [Google Scholar]