Abstract
Objective
Differential effects of maternal and paternal PTSD have been observed in adult offspring of Holocaust survivors in both glucocorticoid receptor sensitivity and vulnerability to psychiatric disorder. The current study examined the relative influences of maternal and paternal PTSD on DNA methylation of the exon 1F promoter of the glucocorticoid receptor gene (NR3C1) in peripheral blood mononuclear cells (PBMCs), and its relationship to glucocorticoid receptor sensitivity, in Holocaust offspring.
Method
Adult offspring with at least one Holocaust survivor parent (n=80), and demographically similar participants without parental Holocaust exposure or PTSD (n=15) completed clinical interviews, self-report measures, and biological procedures. Blood samples were collected for analysis of glucocorticoid receptor gene exon 1F (GR-1F) promoter methylation and cortisol levels in response to low-dose dexamethasone, and two-way analysis of covariance was performed using maternal and paternal PTSD as main effects. Hierarchical-clustering analysis was used to permit visualization of maternal vs. paternal PTSD effects on clinical variables.
Results
A significant interaction demonstrated that in the absence of maternal PTSD, offspring with paternal PTSD showed higher GR-1F promoter methylation, whereas offspring with both maternal and paternal PTSD showed lower methylation. Lower GR-1F promoter methylation was significantly associated with greater post-dexamethasone cortisol suppression. The clustering analysis confirmed that maternal and paternal PTSD effects were differentially associated with clinical indicators.
Conclusions
This is the first study to demonstrate alterations of GR-1F promoter methylation in relation to parental PTSD and neuroendocrine outcomes. The moderation of paternal PTSD effects by maternal PTSD suggests different mechanisms for the intergenerational transmission of trauma-related vulnerabilities.
Keywords: Holocaust, epigenetic, methylation, intergenerational, glucocorticoid receptor, NR3C1 gene, GR-1F promoter, PTSD, HPA axis, cortisol
Introduction
Offspring of trauma survivors are at increased risk for mental and physical illness (1-3). Although studies of the intergenerational transmission of trauma traditionally emphasize the influence of exposure (1, 2), there is evidence for the effects of parental trauma-related psychopathology. Parental PTSD, but not Holocaust exposure, associates with alterations in hypothalamic-pituitary-adrenal (HPA) axis function, including enhanced cortisol suppression following dexamethasone administration (4) and lower baseline cortisol levels (5) in offspring. Cortisol levels in infants of mothers who developed PTSD following exposure to the 9/11 World Trade Center attacks were lower than those of mothers who did not develop PTSD (6). These neuroendocrine findings on offspring of trauma survivors are similar to observations in Holocaust survivors and other trauma exposed persons with PTSD (7).
Transmission of effects from parents to children is thought to be mediated by developmental programming of glucocorticoid signaling (8). Variations in maternal care in the rat predict hippocampal glucocorticoid receptor gene expression and levels of cytosine methylation of the exon 17 promoter of the rat glucocorticoid receptor gene (9). Studies of postmortem human brain tissue demonstrated an association between methylation status of the orthologous, exon 1F promoter (GR-1F) region in the human glucocorticoid receptor gene (NR3C1) in the hippocampus and history of childhood abuse (10). This same association between childhood adversity and GR-1F methylation has been observed in whole blood (leukocytes) (11, 12).
Although the majority of studies on transgenerational transmission of trauma effects highlight maternal influences, few studies have directly compared maternal and paternal effects on the offspring (1, 2). Among Holocaust offspring, maternal PTSD was associated with increased risk for developing PTSD, whereas paternal PTSD was associated with greater risk for Major Depressive Disorder (MDD), an effect that emerged after controlling for the impact of maternal PTSD (13). Maternal PTSD has been more strongly associated with lower cortisol levels in Holocaust offspring than has paternal PTSD (14), and we recently demonstrated that maternal PTSD related to increased glucocorticoid receptor sensitivity (15). A longitudinal study of adopted children reported differential and interacting maternal and paternal effects on children’s cortisol variability (16). Inconsistent, over-reactive parenting by mothers predicted lower cortisol variability, whereas a similar parenting style in fathers generally predicted higher cortisol variability in offspring, but only in the absence of low maternal cortisol variability. Similarly, we recently observed that both 24-hr urinary cortisol excretion and cortisol non-suppression following dexamethasone administration were higher in offspring with paternal PTSD in the absence of maternal PTSD, but lower in those with maternal PTSD (15). The current study examined the distinct influences of maternal and paternal PTSD on DNA methylation of the exon 1F promoter of the glucocorticoid receptor gene in Holocaust survivor offspring. We hypothesized that maternal PTSD would be associated with lower offspring GR-1Fpromoter methylation, and paternal PTSD would be associated with higher GR-1F promoter methylation. We further hypothesized that GR-1F promoter methylation would inversely correlate with glucocorticoid receptor sensitivity.
Methods
Participants
120 participants were recruited over a two-year period (2010-2012) as previously described (15), and 95 completed study procedures. The study was approved by the Institutional Review Board (Icahn School of Medicine at Mount Sinai), and written informed consent was obtained. Parental Holocaust exposure was defined as: 1) being interned in a Nazi concentration camp, 2) having witnessed/experienced torture, or 3) having to flee for one’s life or hide during the Nazi era. Although the main questions concerned the impact of maternal and/or paternal PTSD, a small group of demographically similar Jewish participants without parental PTSD, whose parents were not in Nazi-occupied Europe before and during WWII, were recruited in order to control for potential effects of Holocaust exposure.
Offspring of Holocaust survivors had to have been born after WWII or after their parents had escaped to safety, and have at least one Holocaust survivor parent. Exclusion criteria included any history of psychotic disorder or bipolar illness, significant current alcohol or drug use, and the presence of current PTSD (to distinguish effects of parental PTSD from those associated with expressed PTSD). Participants were also excluded if they had a major medical condition or were taking systemic steroids.
Clinical Evaluation
Axis I diagnoses were determined by clinical psychologists using the Structured Clinical Interview for the DSM-IV (SCID; (17)). Parental PTSD was determined by consensus of at least three clinicians based on the Parental PTSD Questionnaire , completed by the offspring, and a semi-structured interview. The Parental PTSD Questionnaire was previously validated against direct clinician assessment of the parent (18), and includes offspring perceptions of the impact of the Holocaust on the offspring. Participants also completed measures to assess relevant psychiatric symptoms and early life experiences, including the Beck Depression Inventory (BDI) (19), the Spielberger State Trait Anxiety Inventory (STAI) (20), the Dissociative Experiences Scale (21), the Relationship Scales Questionnaire (22), the Childhood Trauma Questionnaire (23), and perceived emotional health (24).
Cytosine Methylation Assessment
Basal morning blood samples were collected for assessment of GR-1F promoter methylation and GR-1F expression, and plasma cortisol levels. Peripheral blood mononuclear cells (PBMCs) were purified from EDTA-pretreated blood, and DNA was extracted as previously described (25). Cytosine methylation was estimated across the 39 C—phosphate—G (CpG) sites in the GR-1F promoter, using 30 clones per sample, in four batches (10, 25). Variability in the DNA bisulfate treatment between batches did not exceed 2%. The number of methylated clones at each of the 39 CpG sites was converted to a percentage and summed across the GR-1F promoter sequence to create a total methylation percentage. As expected, when methylation in CpG islands is low, the distribution of this variable is positively skewed, and it was transformed (natural logarithm) for analytic purposes. The number of CpG sites (out of a possible 39) showing methylation in any clone was also determined for each subject. The percent methylation and number of methylated sites were highly correlated (r=.820, p<.0005, n=95).
Leukocyte type determination
Since PBMC-type composition may affect estimates of DNA methylation (26), the ratio of lymphocytes to monocytes was calculated (PBMC-ratio) as a proxy of PBMC-type and used as a covariate in methylation analyses.
GR-1F expression
GR-1F transcript expression was run as a validation of methylation estimates of the corresponding promoter. RNA, from Trizol-dissolved PBMCs, was extracted and used for determination of the GR-1F expression by quantitative polymerase chain reaction as previously described (25) .The primers and probes used are included in Suppl.Table 1. Data analysis was performed using qBase v2.5 (Biogazelle NV, Belgium).
Hormone determination
Day 1 cortisol levels and Day 2 cortisol and dexamethasone levels obtained in association with the low dose (0.5 mg) dexamethasone suppression test were determined by radioimmunoassay as previously described (25).
Statistical Analyses
A two-way analysis of covariance (ANCOVA) was used to examine the effects of the presence or absence of maternal and paternal PTSD on offspring GR-1F promoter methylation. Because a significant percentage of Holocaust offspring indicated no lifetime parental PTSD, non-Holocaust offspring were compared with the Holocaust offspring with no parental PTSD on key demographic and clinical variables (see Suppl.Table 2). As no relevant differences were observed, participants from both groups were coded as having no maternal or paternal PTSD. To identify effects of parental PTSD, rather than Holocaust exposure, the presence or absence of maternal and paternal Holocaust exposure were included as covariates. Other covariates were age (27), and lifetime smoking (pack-years; associated with percent methylation: r= −.315, n=95, p=.002, and number of methylated sites: r=−.301, n=95, p=.003), and the PBMC-type. There were no associations of GR-1F promoter methylation with gender, and including gender as a covariate did not affect any of the analyses. Bonferroni post-hoc tests were conducted to investigate significant effects. Pearson’s correlational analysis investigated the association of GR-1F promoter methylation with gene expression. A correlation between GR-1F promoter methylation and cortisol decline following dexamethasone administration was performed, partialling out the effects of dexamethasone levels, body mass index, age, smoking history and PBMC-type.
Phenotypic Clustering
Psychological/clinical data were subject to an unsupervised hierarchical clustering analysis, which allowed phenotypic and group clustering in relation to maternal and paternal PTSD. Data derived from the clinical rating scales, described in Table 2, were log2 transformed, and z-scores were calculated. Contrast was enhanced by ensuring that high numbers reflected more deleterious outcomes. An initial clustering analysis was performed using an average linkage-clustering algorithm with the output expressed using Euclidean distance (CIMminer). This was followed by a clustering analysis adding GR-1F promoter percent methylation to determine whether this measure would cluster with any of the identified phenotype-by-group-clusters. The data are presented in a heatmap that includes dendrograms of the phenotype and group clusters. Two-way ANOVAs were conducted to test the relationships of the phenotype measures included in the clustering analysis with maternal and paternal PTSD and are presented in Table 2.
Table 2. Clinical Characteristics of Offspring with Maternal versus Paternal PTSD.
| No Parental PTSD (n=31) |
Paternal PTSD (n=11) |
Maternal PTSD (n=22) |
Both parents PTSD (n=31) |
Analysis | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal PTSD | Paternal PTSD | Maternal PTSD x Paternal PTSD |
|||||||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | df | p | F | df | p | F | df | p | |
| Depressiona | 7.9 | 7.67 | 10.6 | 7.63 | 12.7 | 7.67 | 13.4 | 7.79 | 5.00 | 91 | .028 | .92 | 91 | ns | .35 | 91 | ns |
| Childhood traumab | 6.9 | 2.19 | 9.1 | 2.32 | 7.6 | 2.40 | 9.3 | 2.23 | .71 | 90 | ns | 13.87 | 90 | .000 | .256 | 90 | ns |
| Dissociationc | |||||||||||||||||
| Absorption | 5.6 | 7.12 | 8.6 | 7.30 | 6.2 | 7.67 | 11.4 | 7.24 | 1.05 | 89 | ns | 2.86 | 89 | .095 | .40 | 89 | ns |
| Amnesia | 1.4 | 3.29 | 1.8 | 3.65 | 1.5 | 3.84 | 3.8 | 3.34 | 1.63 | 89 | ns | 6.10 | 89 | .015 | 1.31 | 89 | ns |
| Derealization/Depersonalization | .9 | 4.93 | 2.3 | 5.31 | 1.7 | 5.28 | 4.0 | 5.01 | 1.18 | 89 | ns | 2.44 | 89 | ns | .15 | 89 | ns |
| Dissociation | 1.6 | 5.48 | 3.6 | 5.31 | 2.5 | 5.75 | 5.2 | 5.57 | 1.07 | 89 | ns | 3.80 | 89 | .055 | .08 | 89 | ns |
| Perceived emotional health | 3.7 | 1.10 | 3.5 | .99 | 3.1 | .96 | 3.1 | 1.11 | 4.71 | 91 | .033 | .19 | 91 | ns | .07 | 91 | ns |
| PPQd | |||||||||||||||||
| Psychological scarse | 2.4 | 1.64 | 3.7 | 1.33 | 3.8 | 1.44 | 4.2 | 1.11 | 7.94 | 75 | 006 | 7.15 | 75 | .009 | 2.0, | 75 | ns |
| Sensitivity to violence/injusticef | 3.4 | 1.64 | 3.7 | .99 | 3.8 | .96 | 4.3 | 1.11 | 3.57 | 75 | .063 | 2.38 | 75 | ns | .19 | 75 | ns |
| Sensitivity to stressg | 2.4 | 2.19 | 3.3 | 1.66 | 2.8 | 1.44 | 3.3 | 1.67 | .39 | 75 | ns | 3.98 | 75 | .050 | .21 | 75 | ns |
| Vicarious traumah | 2.9 | 1.64 | 3.7 | 1.33 | 3.6 | 1.44 | 4.2 | 1.11 | 3.78 | 75 | .056 | 5.01 | 75 | .028 | .27 | 75 | ns |
| RSQi | |||||||||||||||||
| Dismissing attachment | 3.1 | 0.55 | 3.6 | .66 | 3.1 | .48 | 3.7 | .56 | .068 | 89 | ns | 11.27 | 89 | .001 | .08 | 89 | ns |
| Fearful attachment | 2.6 | 1.10 | 3.5 | .99 | 2.9 | .96 | 3.5 | 1.11 | .347 | 89 | ns | 10.29 | 89 | .002 | .62 | 89 | ns |
| Secure attachment | 3.4 | 0.55 | 2.8 | .66 | 3.1 | .96 | 2.8 | .56 | .80 | 89 | ns | 8.17 | 89 | .005 | .19 | 89 | ns |
| Preoccupied attachment | 2.8 | 0.55 | 2.7 | .66 | 2.9 | .96 | 3.0 | .56 | 1.56 | 89 | ns | .10 | 89 | ns | .66 | 89 | ns |
| Anxietyj | |||||||||||||||||
| State anxiety | 14.0 | 11.50 | 21.3 | 1.13 | 21.2 | 11.99 | 18.4 | 11.14 | .73 | 89 | ns | .74 | 89 | ns | 3.88 | 89 | .052 |
| Trait anxiety | 17.0 | 11.50 | 21.7 | 1.16 | 23.6 | 11.99 | 26.9 | 11.69 | 5.13 | 89 | .026 | 2.38 | 89 | ns | .07 | 89 | ns |
Analyses represent 2×2 ANOVA of maternal and paternal PTSD on clinical outcomes, with statistics for main effects and the interaction term reported.
Beck Depression Inventory
Childhood Trauma Questionnaire total score
Dissociative Experiences Scale (DES)
Parental PTSD Questionnaire (PPQ);
PPQ item: “I believe that I have psychological scars as a result of the fact that I was raised by parent(s) that survived the Holocaust”
PPQ item: “I believe that I am more sensitive to violence and injustice because of my parents’ experiences in the Holocaust”
PPQ item: “I believe that I am more likely to be affected by stress than other individuals my age (who were not raised by Holocaust survivors)”
PPQ item: “Although I did not directly undergo the Holocaust, I have vicariously experienced and have been deeply troubled by the trauma of the Holocaust”
Relationship Scales Questionnaire (RSQ)
Spielberger State Trait Anxiety Inventory (STAI)
Results
Demographic and Clinical Characteristics
Table 1 presents demographic and clinical data based on presence or absence of maternal and paternal PTSD. 75% of the Holocaust offspring had two Holocaust exposed parents. PTSD was reported for 55.8% (n=53) of mothers and 44.2% (n=42) of fathers; only 15 Holocaust offspring reported no parental PTSD. Participants with maternal PTSD were slightly older than those with paternal PTSD, and those with paternal PTSD only were the youngest. There were no differences in gender, body mass index, education, or current Axis I psychopathology. There were differences in lifetime rates of depression and anxiety disorders, such that those without parental PTSD had lower rates of these disorders than those with maternal or paternal PTSD.
Table 1. Demographic and Clinical Characteristics of Offspring with Maternal versus Paternal PTSD.
| Characteristic | No Parental PTSD |
Paternal PTSD |
Maternal PTSD |
Both parents PTSD |
Analysis | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n=31) | (n=11) | (n=22) | (n=31) | Maternal PTSD | Paternal PTSD | Maternal PTSD X Paternal PTSD |
||||||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | --- | F | df | p | F | df | p | F | df | p | |||
| Age | 57.13 | 7.78 | 47.64 | 7.93 | 57.36 | 8.10 | 58.68 | 7.91 | --- | 10.10 | 1,95 | .002 | 5.31 | 1,95 | .023 | 9.28 | 1,95 | .003 | ||
| Body Mass Index | 27.32 | 4.87 | 24.37 | 4.97 | 26.84 | 5.04 | 25.87 | 4.96 | --- | .21 | 1,95 | ns | .3.15 | 1,95 | ns | .80 | 1,95 | ns | ||
| Education (years) | 18.00 | 2.46 | 17.73 | 2.49 | 17.18 | 2.54 | 17.45 | 2.51 | --- | .97 | 1,95 | ns | .00 | 1,95 | ns | .24 | 1,95 | ns | ||
| N | % | N | % | N | % | N | % | χ 2 | df | p | --- | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | 16 | 51.6 | 7 | 63.6 | 18 | 81.8 | 25 | 80.6 | 6.52 | 3 | ns | --- | ||||||||
|
Major Depressive
Disorder currenta |
1 | 3.2 | 1 | 9.1 | 3 | 13.6 | 0 | 0.0 | 5.06 | 3 | ns | --- | ||||||||
|
Major Depressive
Disorder lifetime |
11 | 35.5 | 8 | 72.7 | 16 | 72.7 | 26 | 83.9 | 15.75 | 3 | .001 | --- | ||||||||
|
Anxiety Disorde
currentb |
9 | 29.0 | 6 | 54.5 | 13 | 59.1 | 15 | 48.4 | 4.50 | 3 | ns | --- | ||||||||
|
Anxiety Disorder
lifetime |
9 | 29.0 | 7 | 63.6 | 16 | 72.7 | 18 | 58.1 | 9.71 | 3 | .021 | --- | ||||||||
current SCID diagnosis of Major Depressive Disorder
current SCID diagnosis of Axis I Anxiety Disorder
Maternal PTSD, Paternal PTSD and GR-1F promoter methylation
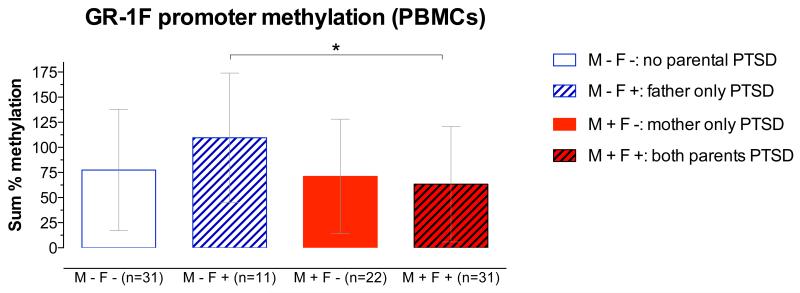
ANCOVA revealed a significant interaction of maternal and paternal PTSD on GR-1F promoter methylation (F1,86=5.97, p=.017, Figure 1). Bonferroni post-hoc tests revealed that the effects of paternal PTSD were moderated by the presence or absence of maternal PTSD. Thus, in the absence of maternal PTSD, offspring with paternal PTSD only showed higher GR-1F promoter methylation, whereas offspring with both maternal and paternal PTSD showed lower GR-1F promoter methylation (t=3.49, df= 86, p<.05). For number of methylated sites, ANCOVA also revealed a significant interaction of maternal and paternal PTSD using the same covariates (F1,86 =4.2, p=.045). When the ANCOVAs were repeated without controlling for maternal and paternal Holocaust exposure the interaction effect of maternal and paternal PTSD was unchanged for percent GR-1F promoter methylation, controlling for age, smoking, and PBMC-type (F1,88=4.60, p=.035). For number of methylated sites, there was a main effect of maternal PTSD (F1,88=5.51, p=.021), but the interaction effect was reduced (F1,88=3.54, p=.063), with the same covariates. Given that severe trauma exposure itself may result in epigenetic modifications, a 2-way ANCOVA was conducted to investigate the influence of maternal vs. paternal Holocaust exposure status alone on offspring methylation. Results of this ANCOVA, using the same covariates as above, did not reveal any significant main effects of exposure or a significant interaction between maternal and paternal exposure.
Figure 1. Percent methylation in the exon 1F promoter of the glucocorticoid receptor gene (GR-1F promoter) in Peripheral Blood Mononuclear Cells (PBMCs).
based on the presence or absence of maternal and paternal PTSD, controlling for parental Holocaust exposure, age, smoking history and PBMC-type. The represented data (mean ± SD) are based on an analysis of co-variance using raw data. The post-hoc statistic was from the analysis using transformed (natural logarithm) data.
Correlations between GR-1F promoter methylation and GR-1F expression and functional outcomes
GR-1F promoter methylation was negatively correlated with GR-1F expression (for percent methylation: r=−.346, n=73, p=.003; for number of methylated sites: r=−.361, n=73, p=.002), indicating the validity of the GR-1F promoter methylation measures.
GR-1F promoter methylation was also associated with the cortisol response to the low dose DST. Partial correlation demonstrated a negative association between GR-1F promoter percent methylation and cortisol decline following dexamethasone administration, such that greater cortisol suppression was associated with lower methylation (r=−.249, df=82, p=.029).
Phenotypic clustering
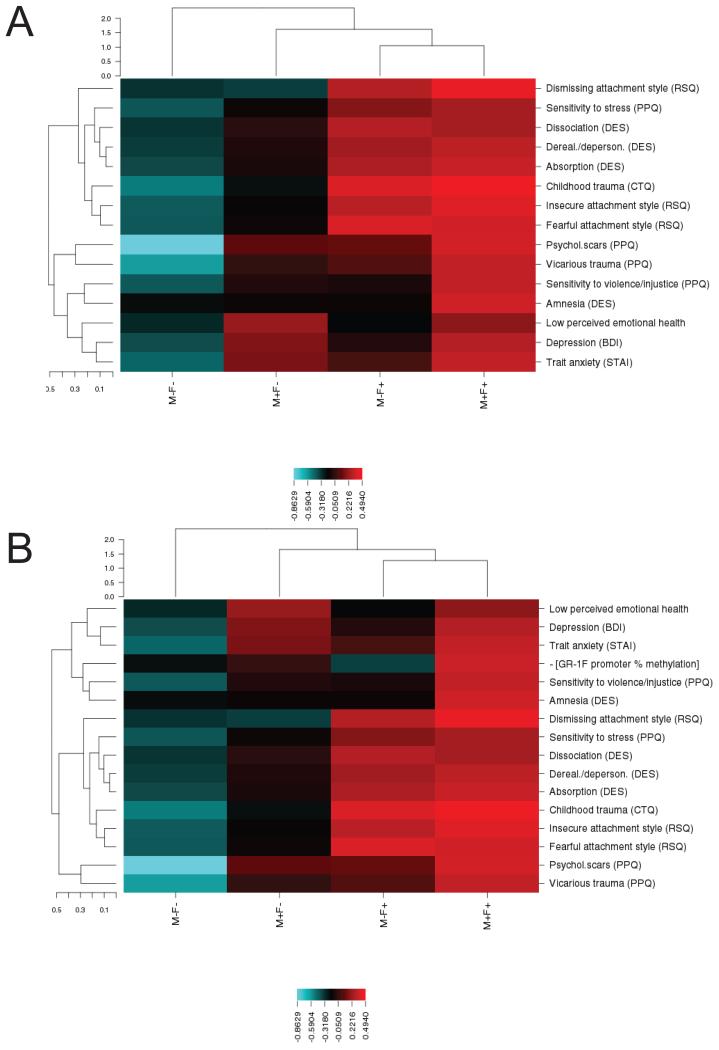
Figure 2A portrays the results of phenotypic clustering associated with presence or absence of maternal PTSD and/or paternal PTSD. Offspring with maternal PTSD demonstrated elevations on poor perceived emotional health, and depression symptoms and trait anxiety. Offspring with paternal PTSD only tended to endorse a dismissing, fearful or insecure attachment style, greater childhood trauma exposure, greater dissociative experiences, and greater sensitivity to violence. Offspring with both maternal and paternal PTSD were more likely to report a subjective feeling of having psychological scars, being affected by vicarious (Holocaust-related) trauma, and having greater sensitivity to violence/injustice as well as more dissociative amnesia. Adding GR-1F promoter methylation to the cluster analysis demonstrated that this variable integrated with maternal PTSD (Figure 2B).
Figure 2. Phenotypic clustering based on the presence or absence of maternal and paternal PTSD.
The unsupervised hierarchical clustering is presented in dendrograms with vertical dendrograms representing phenotypic clustering, horizontal dendrograms representing group clustering and the scales reprsent Euclidean distance. A heatmap depicts phenotype-by-group-clusters with the color scale based on a z-score, where blue represents low score and red represents high. Rows represent different phenotypes that are also presented in Table 2. Columns represent experimental groups. Panel A and panel B contain the same phenotypic variables with the exception of percent methylation in the exon 1F promoter of the glucocorticoid receptor gene (GR-1F promoter), which is presented only in panel B.
To provide further interpretability, a series of separate two-way ANOVAs were conducted to determine the relative influence of maternal and paternal PTSD on the clinical indicators included in the clustering analysis (Table 2). Maternal and paternal PTSD were associated with different clinical and perceived childhood characteristics. Maternal PTSD was associated with higher self-reported depressive symptoms and trait anxiety and lower perceived emotional health. Paternal PTSD was associated with higher reports of childhood trauma and less adaptive attachment styles. There were no significant interactions between maternal and paternal PTSD on any of the clinical measures.
Discussion
This is the first study to demonstrate alterations of GR-1F promoter methylation in relation to maternal and paternal PTSD. Maternal PTSD moderated the effect of paternal PTSD on GR-1F promoter methylation. Paternal PTSD, but only in the absence of maternal PTSD, was associated with higher levels of GR-1F promoter methylation, while offspring with both maternal and paternal PTSD displayed the lowest level of methylation. Although the sample size was limited, findings are consistent with previous reports on the effects of parental PTSD on offspring phenotype. In a different cohort of Holocaust offspring, we observed that maternal PTSD significantly enhanced the risk for PTSD, while paternal PTSD significantly elevated risk for depression when controlling for maternal PTSD (13). The hierarchical-clustering analysis similarly revealed that maternal and paternal PTSD associated with different offspring psychological characteristics, and that lower GR-1F promoter methylation was specifically associated with maternal PTSD.
Phenotypic clustering analysis demonstrated an association of paternal, but not maternal, PTSD with childhood trauma and abuse, consistent with findings in offspring of male war veterans with PTSD (28). Note that the Childhood Trauma Questionnaire assesses a range of potentially traumatic experiences, but does not identify the perpetrator. Thus it cannot be concluded that fathers with PTSD are more likely than mothers to abuse their children. The finding of relatively higher GR-1F promoter methylation with paternal PTSD (in the absence of maternal PTSD) is consistent with a previous finding in post-mortem, human hippocampus of an association between higher GR-1F promoter methylation and childhood abuse (10) and with more recent demonstrations of an association of higher methylation of specific CpG sites within the GR-1F promoter in PBMCs with lower parental care, higher maltreatment, and parental loss in healthy adults (11) and with childhood sexual abuse and extent of childhood maltreatment in borderline personality disorder (12). The type, severity, chronicity and developmental stage of adversity, as well as sex of the parent and offspring, might be critical for these associations. Unfortunately, our sample was too small to investigate the influence of these factors.
The hypothesis that GR-1F methylation would be associated with early adversity was based on an interpretation of differences in offspring methylation status on the orthologous promoter region in the rat based on naturally occurring variations of maternal care (9). The implication of rodent studies of the impact of maternal care on offspring is that early life challenges, including variations in parental care, are capable of producing enduring epigenetic changes resulting in sustained phenotypic outcomes in the offspring, including altered stress reactivity. The impact of these effects will depend on context and the degree to which the resulting phenotypic variation meets the particular demands of the prevailing environment. Chronic stress produces variations in the maternal care of the rat (i.e., reduced pup licking/grooming) that associate with methylation-mediated decreased hippocampal glucocorticoid receptor expression and increased HPA responses to stress (29, 30). This increased stress reactivity may be considered adaptive in highly adverse conditions (31). In Holocaust offspring, a parent may have similarly primed his or her offspring for a highly threatening environment that in a post-Holocaust context results in an overgeneralized and exacerbated fear response.
Although offspring with paternal PTSD reported more childhood trauma, the effect of paternal PTSD differed depending on the presence of maternal PTSD. This raises the possibility of indirect effects, such that PTSD in one parent may affect the parenting of the other, or create a qualitative shift in the family environment. For example, paternal PTSD has been associated with higher levels of family conflict in this sample (15). It seems that paternal PTSD alone produces effects such as higher cortisol excretion, reduced glucocorticoid receptor sensitivity and higher GR-1F promoter methylation, similar to those seen in other samples with MDD and childhood abuse (10, 32, 33). When both parents have PTSD, offspring phenotype including lower GR-1F promoter methylation, appears instead to resemble the biology of PTSD risk or expression (7, 34). It is possible that that when both parents are traumatized or express symptoms there is no buffer for the child, resulting in an unmediated experience of constant or repeated threat imposed by the unpredictability of parental behavior, and resulting in the expression of hypervigilance in offspring. Because most offspring were raised in the social context of the late 1940’s-1960’s, with family structured around traditional gender roles, mothers were more likely to be the primary caregiver, whereas many described fathers who worked long hours and were somewhat distant from the family unit. Thus, social context may moderate the processes underlying the intergenerational transmission of trauma (35). While we were able to show different offspring phenotypes based on maternal versus paternal PTSD, delineations of early parent-child and interparental interactions that may mediate these relationships require further empirical investigation.
The functional relevance of the differences in methylation is apparent in the negative correlation between GR-1F promoter methylation and expression of the respective transcript (i.e., GR-1F transcript). Furthermore, lower levels of GR-1F promoter methylation associated with enhanced glucocorticoid negative feedback inhibition assessed by the dexamethasone suppression test, suggesting that the epigenetic status in blood of offspring resulting from parental PTSD reflects neuroendocrine function. We previously suggested that glucocorticoid receptor sensitivity as assessed by the cortisol response to dexamethasone may be a relatively stable marker of risk in Holocaust offspring (4, 15). Furthermore, it has previously been suggested that glucocorticoid receptor binding in peripheral tissue (PBMCs) is sufficiently stable to associate with PTSD risk as a trait (36). There has only been one other study demonstrating an association between GR-1F promoter methylation and glucocorticoid receptor sensitivity (25), however, associations with reduced negative feedback inhibition were observed in association with higher methylation of specific CpG sites on the GR-1F promoter (11). A similar moderating effect of maternal PTSD on the effects of paternal PTSD was observed in these same participants with respect to both the cortisol response to dexamethasone and 24-hr urinary cortisol excretion. A maternal PTSD effect associated with increased glucocorticoid receptor sensitivity in peripheral tissue was also observed (15). Since DNA methylation is a chemically stable epigenetic mark, these findings suggest that the differences in GR-1F promoter methylation might mediate the variation in glucocorticoid receptor function and HPA-axis feedback sensitivity that associate with the transgenerationally-transmitted effects of Holocaust-related psychopathology trauma on the risk for PTSD in the offspring.
The exact mechanisms underlying this transgenerational transmission are unknown. The offspring in this sample were conceived after, and in some cases, decades after, parental Holocaust exposure. However, studies with rodents demonstrate that pre-conception paternal stress can influence behavior and biology in progeny through epigenetic changes in sperm (37) such that germ-line transmission of epigenetic marks associated with PTSD risk is possible. Additionally, variations in the methylation status of the GR-1F promoter can occur in association with pre-as well as post-natal exposures (10, 11, 38, 39). Moreover, stress in one parent may also exert indirect effects on offspring through its influence on the other parent (e.g., paternally driven maternal effects) (40). Finally, there may be direct effects on parent-offspring interactions that are ultimately reflected in the epigenome.
Holocaust offspring who met criteria for PTSD were excluded from the current study in order to better focus on effects associated with transmission of vulnerability and not expressed PTSD. While the presence of psychiatric disorder represents a clear expression of illness vulnerability, it is also useful to identify more subtle or cumulative effects resulting from maternal and paternal PTSD and their interaction. The psychological constructs in the clustering analysis demonstrate aggregate phenotypic differences in both the early environment of the offspring as well as more distal indicators of offspring distress and functioning. In addition to higher reported levels of childhood abuse, paternal PTSD was associated with a dismissing, fearful or insecure attachment style, more dissociative experiences, and greater sensitivity to violence. Maternal PTSD was associated with more self-reported symptoms of depression and higher trait anxiety, consistent with findings that maternal Holocaust exposure associated with higher levels of psychological distress in offspring (24). It is possible that the higher self-reported depression symptoms associated with maternal PTSD are best understood as an index of distress or negative affectivity, particularly in a sample in which PTSD is excluded and with low rates of current Major Depressive Disorder.
Such fine-grained phenotypic distinctions suggest important differences in the expression and impact of maternal and paternal PTSD on offspring phenotype that contribute to psychiatric vulnerability. Research on the intergenerational transmission of trauma requires nuanced assessment of both potential mechanisms of transmission (e.g., parenting and attachment) and of indicators of vulnerability and phenotypic differences that represent cumulative effects of being raised from birth by traumatized, symptomatic parents.
Supplementary Material
Acknowledgements
This work was supported by 1RC1MH088101-01 “Identification of an Epigenetic Risk Marker for PTSD” from the National Institute of Mental Health and in part by a grant (5M01RR00071) for the Icahn School of Medicine at Mount Sinai General Clinical Research Center from the National Institute of Health. The NIMH and NIH had no further role in the study design; in the collection, analysis and interpretation of the data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Financial Disclosures
The authors declare no competing financial interests.
References
- 1.Palosaari E, Punamaki RL, Qouta S, Diab M. Intergenerational effects of war trauma among Palestinian families mediated via psychological maltreatment. Child Abuse Negl. 2013 doi: 10.1016/j.chiabu.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Field NP, Om C, Kim T, Vorn S. Parental styles in second generation effects of genocide stemming from the Khmer Rouge regime in Cambodia. Attach Hum Dev. 2011;13:611–628. doi: 10.1080/14616734.2011.609015. [DOI] [PubMed] [Google Scholar]
- 3.Yehuda R, Schmeidler J, Wainberg M, Binder-Brynes K, Duvdevani T. Vulnerability to posttraumatic stress disorder in adult offspring of Holocaust survivors. Am J Psychiatry. 1998;155:1163–1171. doi: 10.1176/ajp.155.9.1163. [DOI] [PubMed] [Google Scholar]
- 4.Yehuda R, Blair W, Labinsky E, Bierer LM. Effects of parental PTSD on the cortisol response to dexamethasone administration in their adult offspring. Am J Psychiatry. 2007;164:163–166. doi: 10.1176/ajp.2007.164.1.163. [DOI] [PubMed] [Google Scholar]
- 5.Yehuda R, Teicher MH, Seckl JR, Grossman RA, Morris A, Bierer LM. Parental posttraumatic stress disorder as a vulnerability factor for low cortisol trait in offspring of holocaust survivors. Arch Gen Psychiatry. 2007;64:1040–1048. doi: 10.1001/archpsyc.64.9.1040. [DOI] [PubMed] [Google Scholar]
- 6.Yehuda R, Engel SM, Brand SR, Seckl J, Marcus SM, Berkowitz GS. Transgenerational effects of posttraumatic stress disorder in babies of mothers exposed to the World Trade Center attacks during pregnancy. J Clin Endocrinol Metab. 2005;90:4115–4118. doi: 10.1210/jc.2005-0550. [DOI] [PubMed] [Google Scholar]
- 7.Yehuda R. Post-traumatic stress disorder. N Engl J Med. 2002;346:108–114. doi: 10.1056/NEJMra012941. [DOI] [PubMed] [Google Scholar]
- 8.Yehuda R, Seckl J. Minireview: Stress-related psychiatric disorders with low cortisol levels: a metabolic hypothesis. Endocrinology. 2011;152:4496–4503. doi: 10.1210/en.2011-1218. [DOI] [PubMed] [Google Scholar]
- 9.Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 10.McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tyrka AR, Price LH, Marsit C, Walters OC, Carpenter LL. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: preliminary findings in healthy adults. PLoS ONE. 2012;7:e30148. doi: 10.1371/journal.pone.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perroud N, Paoloni-Giacobino A, Prada P, Olie E, Salzmann A, Nicastro R, Guillaume S, Mouthon D, Stouder C, Dieben K, Huguelet P, Courtet P, Malafosse A. Increased methylation of glucocorticoid receptor gene (NR3C1) in adults with a history of childhood maltreatment: a link with the severity and type of trauma. Transl Psychiatry. 2011;1:e59. doi: 10.1038/tp.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yehuda R, Bell A, Bierer LM, Schmeidler J. Maternal, not paternal, PTSD is related to increased risk for PTSD in offspring of Holocaust survivors. J Psychiatr Res. 2008;42:1104–1111. doi: 10.1016/j.jpsychires.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yehuda R, Bierer LM. Transgenerational transmission of cortisol and PTSD risk. Prog Brain Res. 2008;167:121–135. doi: 10.1016/S0079-6123(07)67009-5. [DOI] [PubMed] [Google Scholar]
- 15.Lehrner A, Bierer LM, Passarelli V, Pratchett LC, Flory JD, Bader HN, Harris IR, Bedi A, Daskalakis NP, Makotkine I, Yehuda R. Maternal PTSD associates with greater glucocorticoid sensitivity in offspring of Holocaust survivors. Psychoneuroendocrinology. 2014;40:213–220. doi: 10.1016/j.psyneuen.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marceau K, Ram N, Neiderhiser JM, Laurent HK, Shaw DS, Fisher P, Natsuaki MN, Leve LD. Disentangling the effects of genetic, prenatal and parenting influences on children’s cortisol variability. Stress. 2013;16:607–615. doi: 10.3109/10253890.2013.825766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV (SCID) New York State Psychiatric Institute Biometrics Research. 1995 [Google Scholar]
- 18.Yehuda R, Labinsky E, Tischler L, Brand SR, Lavin Y, Blair W, Bierer LM, Goodman RZ, Grossman RA. Are adult offspring reliable informants about parental PTSD? A validation study. Ann N Y Acad Sci. 2006;1071:484–487. doi: 10.1196/annals.1364.047. [DOI] [PubMed] [Google Scholar]
- 19.Beck AT. A systematic investigation of depression. Compr Psychiatry. 1961;2:163–170. doi: 10.1016/s0010-440x(61)80020-5. [DOI] [PubMed] [Google Scholar]
- 20.Spielberger CD. The state-trait anxiety inventory (S T A I) : test manual for Form X. Consulting Psychologists Press; Palo Alto: 1968. [Google Scholar]
- 21.Bernstein EM, Putnam FW. Development, reliability, and validity of a dissociation scale. J Nerv Ment Dis. 1986;174:727–735. doi: 10.1097/00005053-198612000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Griffin DW, Bartholomew K. Models of the self and other: Fundamental dimensions underlying measures of adult attachment. J Pers Soc Psychol. 1994;67:430. [Google Scholar]
- 23.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 24.Flory JD, Bierer LM, Yehuda R. Maternal exposure to the holocaust and health complaints in offspring. Dis Markers. 2011;30:133–139. doi: 10.3233/DMA-2011-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yehuda R, Daskalakis NP, Desarnaud F, Makotkine I, Lehrner AL, Koch E, Flory JD, Buxbaum JD, Meaney MJ, Bierer LM. Epigenetic Biomarkers as Predictors and Correlates of Symptom Improvement Following Psychotherapy in Combat Veterans with PTSD. Front Psychiatry. 2013;4:118. doi: 10.3389/fpsyt.2013.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lam LL, Emberly E, Fraser HB, Neumann SM, Chen E, Miller GE, Kobor MS. Factors underlying variable DNA methylation in a human community cohort. Proc Natl Acad Sci U S A. 2012;109(Suppl 2):17253–17260. doi: 10.1073/pnas.1121249109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, Heine-Suner D, Cigudosa JC, Urioste M, Benitez J, Boix-Chornet M, Sanchez-Aguilera A, Ling C, Carlsson E, Poulsen P, Vaag A, Stephan Z, Spector TD, Wu YZ, Plass C, Esteller M. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A. 2005;102:10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dias A, Sales L, Cardoso R M, R Kleber. Childhood maltreatment in adult offspring of Portuguese war veterans with and without PTSD. European Journal of Psychotraumatology. 2014 doi: 10.3402/ejpt.v5.20198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience. 2008;154:1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Champagne FA, Meaney MJ. Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biol Psychiatry. 2006;59:1227–1235. doi: 10.1016/j.biopsych.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 31.Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, Joels M, Krugers H. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J Neurosci. 2008;28:6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- 33.Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 34.Yehuda R, Flory JD, Bierer LM, Henn-Haase C, Lehrner A, Desarnaud F, Makotkine I, Daskalakis NP, Marmar CR, Meaney MJ. Lower methylation of glucocorticoid receptor gene promoter 1F in peripheral blood of veterans suffering from post-traumatic stress disorder. Biol Psychiatry. doi: 10.1016/j.biopsych.2014.02.006. In Press. [DOI] [PubMed] [Google Scholar]
- 35.Toyokawa S, Uddin M, Koenen KC, Galea S. How does the social environment ‘get into the mind’? Epigenetics at the intersection of social and psychiatric epidemiology. Soc Sci Med. 2012;74:67–74. doi: 10.1016/j.socscimed.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Zuiden M, Geuze E, Willemen HL, Vermetten E, Maas M, Heijnen CJ, Kavelaars A. Pre-existing high glucocorticoid receptor number predicting development of posttraumatic stress symptoms after military deployment. Am J Psychiatry. 2011;168:89–96. doi: 10.1176/appi.ajp.2010.10050706. [DOI] [PubMed] [Google Scholar]
- 37.Franklin TB, Russig H, Weiss IC, Graff J, Linder N, Michalon A, Vizi S, Mansuy IM. Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry. 2010;68:408–415. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 38.Radtke KM, Ruf M, Gunter HM, Dohrmann K, Schauer M, Meyer A, Elbert T. Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Transl Psychiatry. 2011;1:e21. doi: 10.1038/tp.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- 40.Curley JP, Mashoodh R, Champagne FA. Epigenetics and the origins of paternal effects. Horm Behav. 2011;59:306–314. doi: 10.1016/j.yhbeh.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.