Abstract
We report a novel, modular approach to immuno-detection based on antibody recognition and PCR read-out that employs antibody-conjugated bacteriophage, easily-manipulated nonpathogenic viruses, as affinity agents. Our platform employs phage genetically tagged for in vivo biotinylation during phage maturation that can easily be linked, through avidin, to any biotinylatable affinity agent, including full-length antibodies, peptides, lectins or aptamers. The presence of analyte is reported with high sensitivity through real-time PCR. This approach avoids the need to clone antibody-encoding DNA fragments, allows the use of full-length, high affinity antibodies and, by having DNA reporters naturally encapsulated inside the bacteriophage, greatly reduces nonspecific binding of DNA. We validate the efficacy of this new approach through the detection of VEGF (Vascular Endothelial Growth Factor), a known angiogenic cancer biomarker protein, at attomolar concentrations in bronchoalveolar lavage (BAL) fluid.
Keywords: Bacteriophage, biomarker, ELISA, immuno-PCR, immuno-phage assay
Introduction
Over the last 30 years, improvements in the ultra-sensitive detection of nucleic acids through DNA amplification (e.g., PCR) have revolutionized diagnosis and research, but there is still no direct equivalent for equally-sensitive protein detection. Immuno-PCR reagents have been constructed from nanoparticles decorated with antibodies and DNA oligonucleotides, resulting in assays that require fewer steps to detection, and show a lower detection limit (Nam et al. 2003; Barletta et al. 2009; Thaxton et al. 2009; Malou et al. 2011).
Recently, a sensitive immunoassay for small molecule detection using phage-displayed peptides, which bind to antibody-analyte complexes in either ELISA-based (Kim et al. 2009; Kim et al. 2010) or real-time PCR-based (Kim et al. 2011) detection has been published. Here we report an improved modular approach to immuno-detection based on PCR that employs intact antibodies, (rather than single-chain variable fragment antibodies (scFvs), which must be cloned and which usually have lower affinity than the parent antibody), avidin-linked to biotinylated bacteriophage as affinity agents. These SAM-AviTag phage are derivatives of the Escherichia coli phage M13 where the N-terminus of the phage tail protein III contains the enzymatically-biotinylatable AviTag peptide (GLNDIFEAQKIEWHE) (Scholle et al. 2006). The lysine residue (K) in the AviTag is a substrate for biotinylation by the E. coli biotin ligase (birA) enzyme. Using streptavidin or NeutrAvidin, any biotinylated affinity agent (including full-length uncloned antibodies, aptamers, lectins, etc.) can then easily be linked to these enzymatically-biotinylated phage particles. We have recently demonstrated the great utility of these antibody-functionalized AviTag phage in a lateral-flow immuno-chromatographic assay (Adhikari et al. 2013) and here demonstrate their use in detecting Vascular Endothelial Growth Factor (VEGF) at atto- to femtomolar concentrations in bronchoalveolar lavage (BAL) fluid in an assay based on PCR amplification of the phage DNA.
Materials and Methods
Materials
AviTag bacteriophage were a gift from Prof. Brian Kay at the University of Illinois at Chicago. De-identified bronchoalveolar lavage (BAL) fluid samples were provided through an Institutional Review Board (IRB) approved protocol at The Houston Methodist Research Institute, Houston, TX.
Preparation of antibody reagents
Biotinylation of the antibody used in this study, Ranibizumab, a recombinant G1κ isotype antibody Fab fragment, MW: 48 kDa, (Chen et al. 1999), was performed using the EZ-Link Sulfo-NHS-SS-Biotin Kit as described in the manufacturer’s protocol. The reaction was allowed to proceed for 1 h before unincorporated biotin was removed using 7 kDa Zeba spin desalting columns. The degree of biotinylation was estimated to be 1.5 biotins per antibody using 4′-hydroxyazobenzene-2-carboxylic acid (HABA).
Capture particle functionalization
Magnetic capture particles (60 μl, ~6×109 Dynabeads® MyOne (1 μm), Tosyl activated, Life Technologies) were antibody-modified according to the manufacturer’s protocol. The particles were washed, resuspended in coating buffer (1×107 particles in 1 ml 0.1 M sodium borate, pH 9.5) and mixed with 240 μl polyclonal anti-human Vascular Endothelial Growth Factor (VEGF) antibody (1 mg/ml, AB-293-NA, R&D Systems) and 240 μl 3 M (NH4)2SO4. After 24 h incubation at 37 °C with slow tilt rotation, the particles were collected and resuspended in 500 μl phosphate-buffered saline (PBS) containing 0.5% (w/v) Bovine Serum Albumin (BSA) and 0.05% (v/v) Tween 20, and incubated for 24 h at 37 °C. The particles were then washed three times with 1 ml PBS containing 0.1% (w/v) BSA and 0.05% (v/v) Tween 20, and finally resuspended in 200 μl PBS containing 0.1% (w/v) BSA.
Preparation of immuno-phage reagents
Growth and titer estimation of M13 phage were performed as previously described (Lee et al. 2007). Phage were found to be roughly 50% biotinylated during growth in E. coli BL21. This could be raised to nearly 100% by additional in vitro biotinylation using recombinant biotin ligase. The enzyme was expressed in inclusion bodies from cells (4 l) grown in the Overnight Express Autoinduction System 1 (Novagen). The cells were harvested by centrifugation (30 min, 3,000 x g, 4 °C), washed in 50 ml TBS (20 mM Tris-HCl, pH 8, 150 mM NaCl), and resuspended in 150 ml resuspension buffer (20 mM Tris-HCl, pH 8, 10 mM β-mercaptoethanol). After a 1 h incubation at 4 °C with 0.1 mg/ml lysozyme and 1.25% (v/v) Serratia nuclease solution (Miller et al. 1991) the cells were sonicated for 5 min, and 2 ml 70% NP-40 solution, and 40 ml B-PER were added. After 1 h at 4 °C, the cell lysate was centrifuged (30 min, 16,000 x g, 4 °C), and the pellet containing the inclusion bodies was denatured using 7.5 M urea, 0.4 M L-arginine, 10 mM DTT, 50 mM Tris-HCl, pH 8, 500 mM NaCl. The inclusion bodies were refolded in 50 mM Tris-HCl, pH 8, 500 mM NaCl, 5 % (v/v) glycerol, 5% (v/v) sucrose, and applied to a chelating Sepharose column charged with 0.1 M NiSO4. The hexahistidine-tagged biotin ligase was eluted using an imidazole gradient (0.02-0.25 M). Peak fractions were checked for homogeneity using SDS-PAGE.
For in vitro biotinylation, 100 μl of 1×1011 phage/ml were mixed with 14.3 μl Bicine (0.5 M, pH 8.3), 14.3 μl of a solution containing 100 mM ATP, 100 mM MgO(Ac)2 and 500 μM Biotin, 10 μl D-biotin (500 μM), and 10 μl biotin ligase (2 mg/ml) in Tris-HCl, pH 8 and incubated for 1 h at 25°C. Phage were precipitated from the reaction by adding 250 μl of 20% PEG in 2.5 M NaCl to 1 ml of biotinylated phage, followed by 1 h incubation on ice and centrifugation at 11,000 x g for 20 min. The phage pellet was resuspended in 1 ml PBS.
To prepare the NeutrAvidin/biotinylated phage construct, NeutrAvidin (A-2666, Life Technologies) and biotinylated phage were incubated at a molar ratio of 100:1 in 500 μl PBS, pH 7.7 on a rotary mixer for 40 min at 25 °C. Unincorporated NeutrAvidin was removed using an Amicon 100 kDa centrifugal filter according to the manufacturer’s instructions. Phage were collected in a final volume of 20 μl, and were stored at 4 °C.
To prepare the antibody/NeutrAvidin/biotinylated phage construct, the biotinylated Ranibizumab and NeutrAvidin/biotinylated phage were mixed at a molar ratio of 10:1 in 500 μl of PBS, pH 7.7, and incubated for 24 h at 25°C with continuous rotation. Free antibodies were removed using an Amicon 100 kDa filter as described above. The concentrated antibody/NeutrAvidin/biotinylated phage construct (1×1011 phage constructs/ml) was stored at 4°C, and was diluted just prior to use.
Immuno-phage assay format
Antibody-functionalized magnetic capture particles are added to the target solution, and, after a single wash step, the integrated immuno-phage reagent is added for detection (Fig. 1). 100 μl VEGF at concentrations from 26 aM to 2.6 pM in 50% BAL fluid (in PBS) were mixed with magnetic particles (0.5 μg, ~5×106) functionalized with the polyclonal anti-VEGF antibody, incubated on a shaker for 2 h at 25 °C and then washed three times with PBS. The reaction was blocked with 100 μl 3% BSA in PBS. The antibody/NeutrAvidin/biotinylated phage construct was added to the reaction at 60 pM, and the reaction was allowed to incubate for 2 h at 25 °C on a shaker. After two washes with 0.3% Tween 20 in PBS to remove unbound phage and two washes with PBS, the particles were analyzed by PCR.
Fig. 1. Analyte detection using immuno-phage particles.
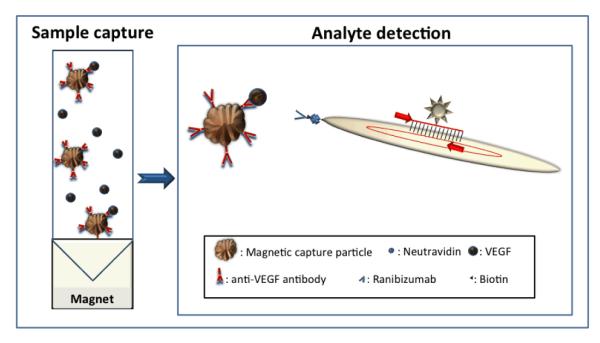
Left panel: Paramagnetic capture particles functionalized with analyte-specific antibodies used to concentrate analyte from solution. Right panel: Pre-made antibody/NeutrAvidin/biotinylated phage affinity agents are detected by real-time PCR of phage DNA; not to scale.
After the final wash of the captured immuno-phage constructs, the magnetic particles were resuspended in 20 μl PBS. 15 μl PCR master mix (0.1 μl of 10 μM forward primer, 0.1 μl of 10 μM reverse primer, 10 μl of 2xPCR mix (Brilliant III Ultra-Fast SYBR mix, Agilent) and 4.8 μl of RNase- and DNase-free DI water) were added to 5 μl of each sample to achieve 20 μl total PCR volume.
The number of retained phage particles was determined by PCR against a standard curve derived from a dilution series (1011 to 105 phage/mL). The AviTag-targeted PCR primers were as following: Forward: 5′-GTTGTTTCTTTCTATTCTCACT-3′, and Reverse: 5′-CAGACGTTAGTAAATGAATTTT-3′. The PCR conditions were: 10 min at 95 °C, 40 cycles of 30 sec at 62 °C and 30 sec at 72 °C, followed by a dissociation step (1 min at 95 °C, 30 sec at 55 °C, and 30 sec at 95 °C).
The presence of the magnetic particles did not affect the PCR (at the levels used here); results were indistinguishable if analyte- and phage-loaded particles were added to the PCR reaction, or if the phage DNA was liberated from the particles by boiling and then added to the PCR.
Results and Discussion
Here we present a novel approach to ultrasensitive immuno-detection using non-pathogenic bacteriophage, easily linkable to common affinity agents such as antibodies, and even to non-proteins such as aptamers or DNA probes. The principle of the method is shown in Fig. 1. Initially, antibody-functionalized magnetic capture particles are used to collect and concentrate the analyte from solution. M13 phage engineered to express the AviTag peptide as a gene III fusion are enzymatically biotinylated, thus allowing the attachment of chemically biotinylated antibodies using NeutrAvidin as a linker. This approach yields affinity agents where the binding of an antibody to its target can be ultra-sensitively reported through real-time amplification of the phage genome, with very low non-specific binding. We have demonstrated the use of these phage immuno-detection reagents using Vascular Endothelial Growth Factor (VEGF) as a model analyte in bronchoalveolar lavage (BAL) fluid.
The assay protocol uses a pre-assembled antibody/NeutrAvidin/biotinylated “antibody-phage” construct. Micrometer-sized magnetic particles, widely used in sample cleanup and concentration (Mani et al. 2011), were used as capture media. The use of magnetic particles and optimized reaction and washing conditions help improve the sensitivity but we believe that the high detection sensitivity is also likely attributable to the low non-specific binding of the immuno-phage reagent; 500-6,000 phage bound non-specifically to the magnetic particles for each million phage offered, with the exact number depending on the matrix and washing conditions used. To validate the analytical sensitivity of the assay in complex samples VEGF was spiked into BAL fluid (50% in PBS), a potential sample source for diagnostic assays, at concentrations ranging from 26 aM to 2.6 pM, Fig. 2. The BAL was not expected to contain any VEGF and, from the linear relationship between Ct values and concentration of spiked VEGF, the level of endogenous VEGF in the BAL was determined to be below the limit of detection (LOD) of this assay. The LOD was calculated to be 200 aM by subtracting three standard deviations from the mean Ct value of the no-VEGF control and calculating the corresponding analyte concentration from the Ct vs. VEGF concentration curve. The r2 value for this curve was 0.97, showing a good representation of the dependence. Operation below the half-saturation concentration of an affinity agent is a general concern for all ultrasensitive measurements, which often employ affinity reagents with lower affinities than those used here. Such operation is feasible because binding is non-zero even at concentrations below the half-saturation concentration. Since the assay can succeed with only a small minority of potential binding sites occupied (due to the great amplification power of PCR), this is sufficient. Moreover, in a recent review of magnetic bead-based assays (Tekin et al. 2013) it is noted that immuno-magnetic particles used in non-competitive sandwich assays similar to ours, enhance immunoassay sensitivity; particles carry an excess of capture antibodies that kinetically drive the reaction towards protein binding (Chang et al. 2012). These numbers are in good accordance with the numbers measured in PBS (LOD 1 fM, data not shown), and also suggest low inherent non-specific binding of the modified phage reagent even in more complex backgrounds; only 0.6 % of the phage offered was found to bind non-specifically in 50 % BAL. This LOD is approximately 100-fold lower than the LOD established for well-optimized commercial ELISAs, (e.g. VEGF ELISA from R&D Systems) emphasizing the improved sensitivity gained through the use of real-time PCR as the read-out. The use of phage greatly reduces the non-specific binding of bare DNA which often troubles immuno-PCR (Malou et al. 2011).
Fig. 2. EGF detection using the immuno-phage assay in 50% BAL fluid.
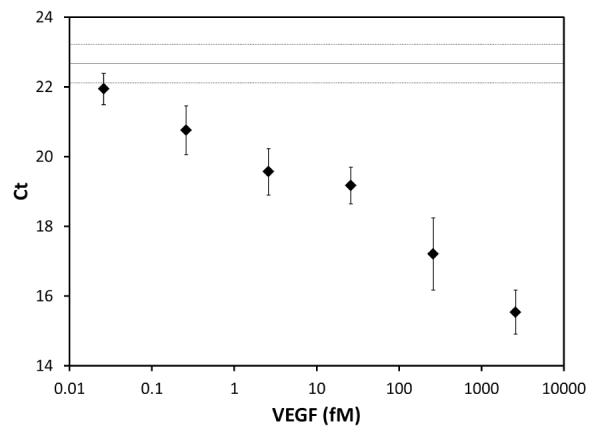
VEGF (26 aM - 2.6 pM) was spiked into 50% BAL fluid (in PBS), and incubated with polyclonal anti-VEGF antibody functionalized magnetic particles. The biotinylated antibody/Neutravidin/phage affinity reagent was added, followed by washing. The sample was then analyzed using real-time PCR with phage-specific primers (n=6, error bars = ±1 SD, Non-template control Ct ≥34). The No-VEGF control (Ct =22.67±0.55) is shown as a solid line with +/− one standard deviation represented by dotted lines.
We believe that the M13 phage used here pose sufficient steric hindrance to give a relatively shallow slope of immuno-phage PCR Ct as a function of analyte. While in its current state of development the assay could not reliably detect small changes in concentration at very low concentrations, it can provide ultrasensitive Yes/No detection of analytes such as toxins, agricultural and industrial chemicals, or the circulating TRPs (Tandem Repeat Protein) of the difficult-to-detect intracellular pathogen Ehrlichia chaffeensis (Luo et al. 2009). The approach might also be useful with rapidly-mutating RNA viruses, in which PCR detection is hindered by the rapid appearance of escape mutants, and the need for RNA reverse transcription before PCR. The versatility of the assay can be extended by using polyclonal antibodies, or non-antibody detection agents, such as lectins and aptamers, avidin-coupled to the biotinylated AviTag phage. Finally, we note that for future applications, our approach lends itself to the detection of multiple analytes in the same sample, using engineered phage populations, each carrying a different antibody, and each containing a unique DNA sequence recognizable by a distinct TaqMan probe.
Acknowledgements
We are grateful to Dr. Brian Kay for providing the phage and plasmid reagents. This research was funded in part by grants from the Welch foundation (Grant E-1264), the Cancer Prevention & Research Institute of Texas (Grant RP110360), the Norman Hackerman Advanced Research Program (Grant 003652-0186-2009) and NIAID/NIH (Grant U54 AI057156), and by the Huffington-Woestemeyer Professorship. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the RCE Programs Office, NIAID, or NIH. A postdoctoral scholarship for AEVH from the Olle Engkvist Byggmästare Foundation is gratefully acknowledged.
References
- Adhikari M, Dhamane S, Hagstrom AEV, Garvey G, Chen W-H, Kourentzi K, Strych U, Willson RC. Functionalized viral nanoparticles as ultrasensitive reporters in lateral-flow assays. Analyst. 2013;138:5584–5587. doi: 10.1039/c3an00891f. [DOI] [PubMed] [Google Scholar]
- Barletta J, Bartolome A, Constantine NT. Immunomagnetic quantitative immuno-PCR for detection of less than one HIV-1 virion. J Virol Methods. 2009;157:122–132. doi: 10.1016/j.jviromet.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Chang L, Rissin DM, Fournier DR, Piech T, Patel PP, Wilson DH, Duffy DC. Single molecule enzyme-linked immunosorbent assays: theoretical considerations. J Immunol Methods. 2012;378:102–115. doi: 10.1016/j.jim.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wiesmann C, Fuh G, Li B, Christinger HW, McKay P, de Vos AM, Lowman HB. Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinity-matured Fab in complex with antigen. J Mol Biol. 1999;293:865–881. doi: 10.1006/jmbi.1999.3192. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Ahn KC, Gonzalez-Techera A, Gonzalez-Sapienza GG, Gee SJ, Hammock BD. Magnetic bead-based phage anti-immunocomplex assay (PHAIA) for the detection of the urinary biomarker 3-phenoxybenzoic acid to assess human exposure to pyrethroid insecticides. Anal Biochem. 2009;386:45–52. doi: 10.1016/j.ab.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, McCoy M, Gee SJ, Gonzalez-Sapienza GG, Hammock BD. Noncompetitive phage anti-immunocomplex real-time polymerase chain reaction for sensitive detection of small molecules. Anal Chem. 2011;83:246–253. doi: 10.1021/ac102353z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Rossotti MA, Ahn KC, Gonzalez-Sapienza GG, Gee SJ, Musker R, Hammock BD. Development of a noncompetitive phage anti-immunocomplex assay for brominated diphenyl ether 47. Anal Biochem. 2010;401:38–46. doi: 10.1016/j.ab.2010.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CM, Iorno N, Sierro F, Christ D. Selection of human antibody fragments by phage display. Nat Protoc. 2007;2:3001–3008. doi: 10.1038/nprot.2007.448. [DOI] [PubMed] [Google Scholar]
- Luo T, Zhang X, McBride JW. Major species-specific antibody epitopes of the Ehrlichia chaffeensis p120 and E. canis p140 orthologs in surface-exposed tandem repeat regions. Clin Vaccine Immunol. 2009;16:982–990. doi: 10.1128/CVI.00048-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malou N, Raoult D. Immuno-PCR: a promising ultrasensitive diagnostic method to detect antigens and antibodies. Trends Microbiol. 2011;19:295–302. doi: 10.1016/j.tim.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Mani V, Chikkaveeraiah BV, Rusling JF. Magnetic particles in ultrasensitive biomarker protein measurements for cancer detection and monitoring. Expert Opin Med Diagn. 2011;5:381–391. doi: 10.1517/17530059.2011.607161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MD, Benedik MJ, Sullivan MC, Shipley NS, Krause KL. Crystallization and preliminary crystallographic analysis of a novel nuclease from Serratia marcescens. J Mol Biol. 1991;222:27–30. doi: 10.1016/0022-2836(91)90734-n. [DOI] [PubMed] [Google Scholar]
- Nam JM, Thaxton CS, Mirkin CA. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science. 2003;301:1884–1886. doi: 10.1126/science.1088755. [DOI] [PubMed] [Google Scholar]
- Scholle MD, Kriplani U, Pabon A, Sishtla K, Glucksman MJ, Kay BK. Mapping protease substrates by using a biotinylated phage substrate library. ChemBioChem. 2006;7:834–838. doi: 10.1002/cbic.200500427. [DOI] [PubMed] [Google Scholar]
- Tekin HC, Gijs MA. Ultrasensitive protein detection: a case for microfluidic magnetic bead-based assays. Lab Chip. 2013;13:4711–4739. doi: 10.1039/c3lc50477h. [DOI] [PubMed] [Google Scholar]
- Thaxton CS, Elghanian R, Thomas AD, Stoeva SI, Lee JS, Smith ND, Schaeffer AJ, Klocker H, Horninger W, Bartsch G, Mirkin CA. Nanoparticle-based bio-barcode assay redefines “undetectable” PSA and biochemical recurrence after radical prostatectomy. Proc Natl Acad Sci U S A. 2009;106:18437–18442. doi: 10.1073/pnas.0904719106. [DOI] [PMC free article] [PubMed] [Google Scholar]


