Abstract
Background
Dyslipidemia is a risk factor for premature cardiovascular morbidity and mortality in renal transplant recipients (RTR). Pharmacotherapy with mTOR inhibitors aggravates dyslipidemia thus necessitating lipid-lowering therapy with fluvastatin, pravastatin or atorvastatin. These agents may not sufficiently lower lipid levels and therefore a more potent agent like rosuvastatin maybe needed.
Methods
We have aimed to assess the lipid-lowering effect of rosuvastatin as compared to fluvastatin in RTR receiving everolimus. Safety was assessed as the pharmacokinetic (PK) interaction potential of a rosuvastatin/everolimus combination in RTR. A 12-hour everolimus PK-investigation was performed in twelve stable RTR receiving everolimus and fluvastatin (80 mg/day). Patients were then switched to rosuvastatin (20 mg/day) and a follow-up 12/24-hour PK-investigation of everolimus/rosuvastatin was performed after one month. All other drugs were kept unchanged.
Results
In RTR already receiving fluvastatin, switching to rosuvastatin further decreased LDL-cholesterol and total cholesterol by 30.2±12.2% (p<0.01) and 18.2±9.6% (p<0.01), respectively. Everolimus AUC0-12 was not affected by concomitant rosuvastatin treatment, 80.3±21.3 μg*h/mL before and 78.5±21.9 μg*h/mL after, respectively (p=0.61). Mean rosuvastatin AUC0-24 was 157±61.7 ng*h/mL, about 3-fold higher than reported in the literature for non-transplants. There were no adverse events and none of the patients had or developed proteinuria.
Conclusions
Rosuvastatin showed a superior lipid-lowering effect compared to fluvastatin in stable RTR receiving everolimus. The combination of everolimus/rosuvastatin appears to be as safe as the everolimus/fluvastatin combination.
Keywords: Renal transplantation, everolimus, rosuvastatin, lipid lowering, pharmacokinetic, drug-drug interaction
Introduction
Despite a significant improvement in rejection rates and short-term graft survival in renal transplant recipients (RTR), long-term survival of these patients has remained essentially unchanged, and cardiovascular disease continues to be a major cause of death in this patient population (1-4). Hyperlipidemia is one of the major risk factors for developing cardiovascular disease and is a frequent complication post-transplantation, occurring in up to 60% of the patients (5-8). Lipid lowering therapy with the HMG-CoA reductase inhibitors (statins) is generally recommended and may reduce the overall cardiovascular risk (9-11).
The mammalian target of rapamycin (mTOR) inhibitor, everolimus, is a relatively new and increasingly used immunosuppressive drug in RTR. Everolimus provides similar graft survival rates, but has a different cardiovascular risk profile compared to the more commonly used calcineurin inhibitors (CNIs) (12-14). Studies indicate that everolimus may have a favorable effect on renal function and reduce post transplant hypertension (15, 16) but induces a considerable dyslipidemia. Fluvastatin is considered to be a safe statin to use in RTR due to its low interaction potential (9, 17, 18). However, fluvastatin has a modest lipid-lowering effect in patients receiving everolimus, in comparison to those receiving fluvastatin in combination with CNIs (19, 20). Consequently, there might be a need of a more potent lipid-lowering drug in RTR receiving everolimus. In non-transplant patients rosuvastatin has been shown to be a more potent lipid-lowering drug than fluvastatin (21). In addition, rosuvastatin is minimally metabolized and similar to fluvastatin has a low risk for metabolic pharmacokinetic (PK) interactions. However, rosuvastatin has a high affinity for several drug transporters (22-24) and since everolimus has been shown in vitro to inhibit various of these drug transporters (25), the possibility of drug-drug interaction between rosuvastatin and everolimus at transporter level cannot be ruled out.
The aims of the present study were to assess the lipid-lowering effect of rosuvastatin in comparison with fluvastatin and to assess the drug-drug interaction potential of the rosuvastatin and everolimus combination in RTR by performing 12/24-hour PK investigations.
Results
Patients
The twelve patients (5 men and 7 women) had a mean age of 61±10 years and all completed the study. Demographic data at inclusion are summarized in Table 1. The patients were treated with 20 mg rosuvastatin per day for an average of 30±4 days with 100% compliance. The rosuvastatin 23- and 24-hour sample was not obtained in six of the twelve patients, while all other everolimus and rosuvastatin concentrations were obtained successfully.
Table 1. Patients' characteristics at baseline.
| Characteristics | n=12 |
|---|---|
| Age (years, mean ± SD) | 61 ± 10 |
| Gender (male/female, n) | 5/7 |
| Weight (kg, mean ± SD) | 84 ± 28 |
| Height (m, mean ± SD) | 1.72 ± 0.10 |
| BMI (kg/m2 , mean ± SD) | 27.8 ± 6.4 |
| HLA-AB mismatch (mean ± SD) | 2.1 ± 1.4 |
| HLA-DR mismatch (mean ± SD) | 0.9 ± 0.5 |
| Time after transplantation (years, median range) | 3 (1-38) |
| LD/DD (n) | 8/4 |
| Everolimus dose (mg/day, mean ± SD) | 2.5 ± 1.0 |
| Treated with MPA (n) | 11/12 |
| Prednisolone dose (mg/day, mean ± SD) | 4.6 ± 1.0 |
| Fluvastatin dose (mg/day, mean ± SD) | 80 ± 0.0 |
| P-creatinine (μmol/L, mean ± SD) | 104 ± 36 |
BMI, body mass index; LD, living donor; DD, deceased donor; HLA, human leucocyte antigen; MPA, mycophenolate acid.
Lipid parameters
The absolute lipid levels and percent change from baseline are summarized in Table 2. From baseline, while patients were on steady state fluvastatin therapy, to one-month of rosuvastatin treatment LDL-cholesterol and total cholesterol decreased by 30.2±12.2% (p<0.01) and 18.2±9.6% (p<0.01), respectively. In addition, triglycerides also decreased by 18.2±17.7% (p=0.01) and HDL-cholesterol was increased by 5.4±10.4% (p=0.15).
Table 2.
Mean (SD) lipid levels on steady state fluvastatin and rosuvastatin treatment, and percent change from steady state fluvastatin treatment to one month of treatment with 20 mg rosuvastatin per day in renal transplant recipients.
| Lipid parameter | On fluvastatin treatment | On rosuvastatin treatment | % Change | P-value |
|---|---|---|---|---|
| LDL-cholesterol | ||||
| mmol/L | 3.54 (0.93) | 2.45 (0.72) | -30.2 (12.2) | < 0.01 |
| mg/dL | 137 (36.0) | 94.6 (27.6) | ||
| Total cholesterol | ||||
| mmol/L | 5.98 (1.05) | 4.82 (0.81) | -18.2 (9.59) | < 0.01 |
| mg/dL | 231 (40.7) | 187 (31.3) | ||
| HDL-cholesterol | ||||
| mmol/L | 1.63 (0.42) | 1.71 (0.48) | 5.38 (10.4) | 0.14 |
| mg/dL | 62.7 (16.4) | 65.9 (18.5) | ||
| Triglycerides | ||||
| mmol/L | 2.38 (1.04) | 1.85 (0.71) | -18.2 (17.7) | 0.01 |
| mg/dL | 210 (91.9) | 164 (63.0) |
LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Everolimus pharmacokinetics
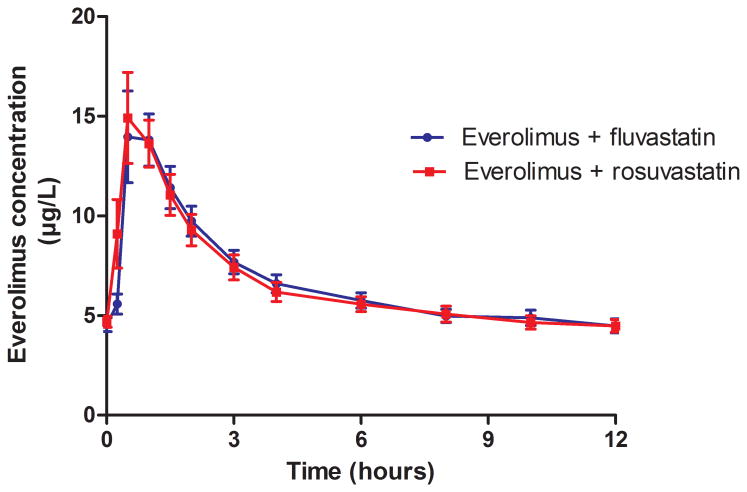
The mean whole blood concentrations versus time curves of everolimus before and after co-administration with rosuvastatin were superimposable as shown in Figure 1. Everolimus pharmacokinetics fulfilled the bioequivalence criteria when co-administered with rosuvastatin (Table 3). The 90% confidence intervals for AUC0-12 and Cmax after:before-ratio were 0.91-1.05 and 0.96-1.15, respectively.
Figure 1. Mean (± SEM) everolimus whole-blood concentration-time profiles before and after concomitant treatment with rosuvastatin.
Table 3.
Everolimus pharmacokinetic variables during concomitant treatment with either 80 mg fluvastatin or 20 mg rosuvastatin daily. All variables except Tmax were ln-transformed before statistical analysis with paired Student's t-test. Tmax was analyzed with Wilcoxon signed rank test. Data are presented as mean (SD).
| Everolimus + fluvastatin | Everolimus + rosuvastatin | Ratio | P-value | |
|---|---|---|---|---|
| AUC0-12 (μg*h/L) | 80.3 (21.3) | 78.5 (21.9) | 0.98 | 0.61 |
| Cmax (μg/L) | 15.6 (7.48) | 16.2 (7.37) | 1.05 | 0.39 |
| CL/F (L/h) | 16.5 (8.52) | 17.4 (7.53) | 1.08 | 0.25 |
| T1/2 (h) | 20.4 (9.63) | 19.9 (7.47) | 1.02 | 0.42 |
| Tmax (h) | 0.79 (0.26) | 0.69 (0.28) | 0.96 | 0.37 |
| C0 (μg/L) | 4.54 (1.19) | 4.69 (0.96) | 1.05 | 0.35 |
AUC0-12, area under the plasma concentration versus time curve from zero to 12 hours; Cmax, maximum plasma concentrations; T1/2, half-life; Tmax, time to Cmax; C0, concentrations before the dose.
Rosuvastatin pharmacokinetics
The mean steady state AUC0-24 of rosuvastatin was 156±61.8 ng*h/mL and the mean Cmax was 17.1±6.49 ng/mL. Individual AUC0-24 values ranged from 75.3-265 ng*h/mL (3.5-fold range) and values for Cmax ranged from 6.91-28.9 ng/mL (4.2-fold range). Mean CL/F was estimated to be 148 L/h. The estimated T1/2 from the six patients with all concentrations available was 9.5±6.7 hr.
Genotyping
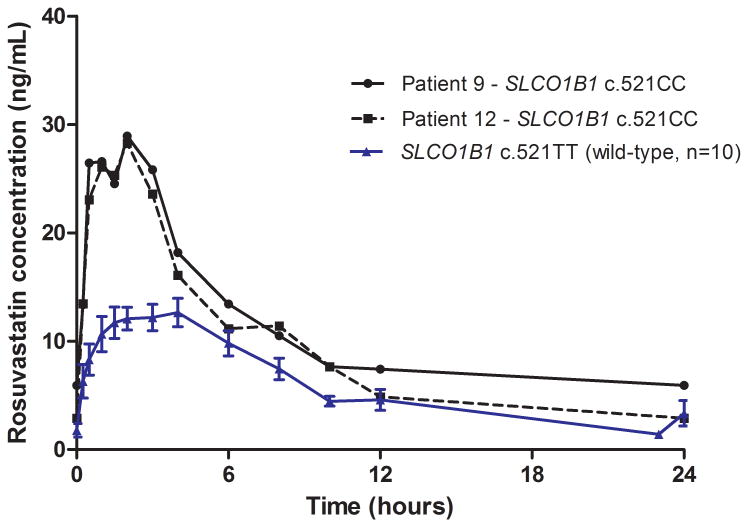
Two of the patients' expressed functional CYP3A5 enzymes (*1/*3) while the other ten patients were not expressing functional CYP3A5 (*3/*3). The two patients with functional CYP3A5 did not show altered pharmacokinetics of everolimus (p=0.67) or rosuvastatin (p=0.52). Only one patient was heterozygote for the variant allele CYP3A5*2, the rest of the patients expressed CYP3A5 (*3/*3). There were no heterozygotes (c.521CT), but two homozygote patients for the SLCO1B1 c.521CC genotype. Rosuvastatin AUC0-24 and Cmax was 74% (p=0.09) and 94% (p=0.03) higher, respectively, in the patients with the c.521CC genotype compared with those with the wild-type genotype (c.521TT) (n=10) (Figure 2). For sequence variant PPARA (rs4253728) G>A three patients were GG, eight were GA and one AA and for the sequence variant PPARA (rs4823616) A>G seven patients were identified as AA, four were AG and one GG. In one patient, homozygote for both PPARA (rs4253728 and rs4823616) variant alleles, everolimus AUC0-12 and Cmax was 36 % and 35 % higher, respectively, compared to the average of homozygote and/or heterozygote wild-type patients. There were no heterozygotes, but three homozygote patients for the POR*28 allele and no significant association were found between this genotype and everolimus/rosuvastatin pharmacokinetics. Polymorphisms of ABCB1 did not appear to influence rosuvastatin or everolimus pharmacokinetics. Allele frequencies for all sequence variant investigated, expect SLC01B1, did not deviate significantly from Hardy-Weinberg distribution.
Figure 2.
Mean (± SEM) rosuvastatin plasma concentration-time profiles in patients with the SLCO1B1 c.521TT variant (wild-type) (n=10) and individual rosuvastatin plasma concentration-time profiles in two patients with the SLCO1B1 c.521CC genotype.
Safety
Rosuvastatin was well tolerated, none of the patients experienced any adverse events and laboratory parameters associated with hepatotoxicity or myelotoxicity (CK, ALAT, ASAT, LD and GT) did not change during the study period (p>0.10). None of the patients had proteinuria, neither on fluvastatin or after 4 weeks of rosuvastatin treatment. The eGFR was 61±20 mL/min at baseline and did not show any significant change (-2.7±8.6 %, p=0.12) during rosuvastatin treatment and no patients experienced any acute rejection episodes during the four-week treatment period.
Discussion
In RTR receiving everolimus based immunosuppression and treated with full dose fluvastatin (80 mg/day), a switch to rosuvastatin (20 mg/day) induced a significant additional lipid-lowering effect. Total cholesterol, LDL-cholesterol and triglycerides were significantly reduced from the fluvastatin treatment values by another 20 to 30% after switching the patients to rosuvastatin. This is the first investigation of this combination in RTR and it appears safe as everolimus pharmacokinetics was unaffected following switch to rosuvastatin, the systemic exposure of rosuvastatin was less than 3-fold higher compared with what is presented for non-transplants in the literature, comparable to what is seen for fluvastatin, and no adverse events were observed (17, 26).
Our results are in agreement with previous findings where rosuvastatin has been consistently found to be the most potent statin in a non-transplant population (27-29). The patients in the current study were already treated with the highest available dose of fluvastatin, and had probably already a LDL-cholesterol reduction of about 38.6 mg/dL (1 mmol/L) from the early post-transplant phase before entering the study (9, 30). Treatment with rosuvastatin reduced LDL-cholesterol further by a mean of 42±21 mg/dL (1.1±0.5 mmol/L). If this effect is due to a more potent drug effect or the increased systemic exposure of rosuvastatin during everolimus treatment remains unanswered. In the Assessment of LEscol in Renal Transplantation (ALERT) study, it was shown that lowering LDL-cholesterol by 38.6 mg/dL (1 mmol/L) reduced cardiac death or myocardial infarction by approximately 30% (30). Implicit this suggests that RTR at high risk for cardiovascular events might benefit from more intensive lipid-lowering therapy. Safely achieving a larger LDL-cholesterol reduction could be of great importance in reducing the cardiovascular risk in these patients. Hence, the additional lipid-lowering effect of rosuvastatin observed in the present study may have a potential to improve long-term outcomes in this population (9, 30).
Pharmacokinetic interactions make immunosuppressive therapy in RTR a challenge and it is important to control potential interactions. Everolimus is extensively metabolized via CYP3A and is a substrate for P-glycoprotein (P-gp) (31, 32). Rosuvastatin is subjected to a minimal degree of metabolism and has not been reported to be a P-gp substrate (22, 33, 34) and based on this does not seem to be a potential pharmacokinetic risk together with everolimus. However, organic anion-transporting polypeptide 1B1 (OATP1B1) has been shown to be an important transporter when it comes to interactions between other immunosuppressive drugs and statins (17, 35). Previous single dose studies in healthy volunteers investigating the interaction between everolimus and simvastatin, atorvastatin or pravastatin have not shown any evidence of clinically relevant interactions (36, 37). Our results support the previous findings, indicating that rosuvastatin does not influence everolimus pharmacokinetics to any relevant degree in RTR.
Elevated plasma concentrations of statins have been associated with an increased risk of adverse events like myopathies (38). Cyclosporine significantly increases the plasma concentrations of all statins and thus increase the risk of adverse events (17). Recently, Simonsen et al. reported a 7-fold increase in the steady state AUC and an 11-fold increase in Cmax of rosuvastatin in heart transplant recipients on cyclosporine based immunosuppression (39). The marginally higher systemic exposure of fluvastatin (about 2-fold) in combination with cyclosporine is considered safe in RTR and a main reason for it being the most commonly used combination in these patients (17). In the present study, mean rosuvastatin steady state AUC0-24 and Cmax values were 2.8-fold and 2.5-fold higher, respectively, compared to literature data in non-transplant patients, showing AUC0-t and Cmax of 56.8 ng*h/mL and 6.79 ng/mL, respectively (26). Even though a slight increase in risk of statin induced side effects cannot be ruled out, these data indicate that rosuvastatin treatment should be safe in combination with everolimus in RTR. Treatment with high doses (e.g. 80 mg/day) of rosuvastatin has been associated with new onset proteinuria (40). This was not confirmed in the present study and substantiate studies where the frequency of dipstick-positive proteinuria at rosuvastatin doses ≤ 20 mg was comparable to that seen with other statins or with placebo (41). Although none of the patients in the present study developed proteinuria, interpretation of this should be made with caution due to the low number of patients included and the short duration of treatment with rosuvastatin.
Consistent with previous findings, no effect of the presence of functional CYP3A5 enzymes or polymorphisms in ABCB1 on everolimus disposition was observed (42, 43). The large interindividual pharmacokinetic variability observed with statin therapy has at least in part been associated with altered expression and/or function of OATP1B1 (SLCO1B1) (44). The SLCO1B1 c.521T>C variant is associated with reduced activity of OATP1B1 (45) and the two patients in the present study with c.521CC genotype, had a substantial higher AUC0-24 of rosuvastatin compared to the patients expressing the wild-type genotype. These results mirror previous studies and suggest that patients carrying the c.521CC variant could be more susceptible to adverse events of rosuvastatin. Recent clinical data has identified polymorphisms in PPARA (rs4253728 and rs4823613) as potential sources of variability in CYP3A4 activity (46). Interestingly, one patient was homozygote carrier for both PPARA variant alleles (rs4253728 and rs4823613) and showed higher systemic exposure of everolimus compared to heterozygote and/or homozygote wild type genotypes. As expected, polymorphisms in CYP3A5, ABCB1, POR*28, PPARA (rs4253728 and rs4823613) had no influence on rosuvastatin pharmacokinetics.
Even though the sample size is adequate for the investigation of the drug-drug interaction, it is a limitation to only investigate twelve patients for one month when it comes to assessing side effects. Another potential weakness is the lack of washout period between the discontinuation of fluvastatin and the introduction of rosuvastatin. It was however considered unethical to take these patients off statin treatment and due to the short half-life of fluvastatin (2.3 h) no residual lipid-lowering effect of fluvastatin is anticipated after 4 weeks on rosuvastatin. In addition, the use of literature data for comparison of systemic exposure of rosuvastatin is obviously not an optimal study design. We believe however that it is an informative comparison considering the ethical and practical difficulties to obtain data from transplanted patients with and without their main immunosuppressive drugs.
In conclusions, rosuvastatin showed a superior lipid-lowering effect to fluvastatin in everolimus treated RTR. The combination of everolimus and rosuvastatin seems to be safe, but a slightly increased risk of statin-induced side effects cannot be ruled out.
Patients and Methods
Patients and study design
Twelve RTR receiving everolimus, mycophenolate acid and steroid-based immunosuppression were included in this open label, single-center prospective study. Inclusion criteria included >18 years of age, stable renal function (plasma creatinine <200 μmol/L and <20 % change in the last two weeks) and having received both everolimus and fluvastatin (80 mg/day) for a minimum of three months. Everolimus was subjected to therapeutic drug monitoring (TDM), aiming for trough concentrations in the range 4-8 μg/L. Doses of all concomitantly used drugs, including everolimus, were to be kept unchanged from at least two weeks prior to the first 12-hour PK investigation and throughout the study. Patients with a known hypersensitivity to rosuvastatin were excluded.
At the first PK investigation day, baseline measurement of fasting plasma lipid levels and a 12-hr pharmacokinetic investigation of everolimus were performed. The following day the patients were switched from fluvastatin therapy (80 mg/day) to 20 mg rosuvastatin daily. After one month of concomitant everolimus and rosuvastatin treatment, measurement of plasma lipid levels was repeated and the second 12-hr pharmacokinetic investigation of everolimus was performed. In addition, a 24-hr pharmacokinetic investigation of rosuvastatin was performed. An EDTA whole blood sample was also drawn during the study, for determination of the recipients' genotypes (CYP3A5, ABCB1, POR*28, PPARA and SLCO1B1). Proteinuria was examined by urine dipstick before and after treatment with rosuvastatin. Tablet count was performed after four weeks of rosuvastatin treatment.
The study was performed in accordance with the Declaration of Helsinki, local laws and other relevant regulation and written informed consent was obtained from all patients prior to inclusion. The study was approved by the Regional Committee for Medical and Health Research Ethics and by the Norwegian Medicines Agency (EudraCT nr: 2011-005212-29). The study is registered on ClinicalTrials.gov (NCT01524601).
Pharmacokinetic investigations
Patients fasted overnight and a standard hospital breakfast was served 2 hr after drug intake. Samples for the PK profiles were collected before administration of everolimus/fluvastatin or rosuvastatin (C0) and at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10 and 12-hr following drug intake. Two additional samples were collected 23-hr and 24-hr after administration of the rosuvastatin dose on the follow-up PK-investigation, after four weeks of rosuvastatin treatment.
Bioanalytical methods
Whole blood concentrations of everolimus
Concentrations of everolimus were determined in EDTA blood with a validated liquid chromatography tandem mass spectrometry (LC-MS/MS) assay as previously described (47). Briefly, the analyte was extracted by protein precipitation with zinc sulfate and acetonitrile. Everolimus was separated chromatographically on a C18 column before MS/MS detection of ammoniated ions using electrospray ionization interface in a positive mode. Stable isotope-labeled (13C2D4) everolimus was used as internal standard. The validated analytical range was between 2.8 and 35 μg/L with coefficients of variation (CV; precision) less or equal to 10% during analyzes of the study samples. Assay accuracy was in agreement with external quality controls from the Analytical Services International proficiency testing program.
Plasma concentrations of rosuvastatin
Rosuvastatin was analyzed with a validated LC-MS/MS method as previously described ((48); see Supplemental Digital Content, Patient and methods).
Genotyping
A 6 mL EDTA blood sample was obtained for each patient and stored at -20°C prior to DNA extraction. Genomic DNA was extracted from the EDTA whole blood using the QIAmp® DNA blood Minikit (Qiagen®, Hilden, Germany).
Genotyping of SLCO1B1 and CYP3A5 was carried out using validated and certified TaqMan®-based real-time PCR methods at Center for Psychopharmacology, Diakonhjemmet Hospital, Oslo, Norway. Designed primers and probes for the detection of SLCO1B1*5 (rs4149056; 521T>C), CYP3A5*2 (rs28365083; 27289C>A), and CYP3A5*3 (rs776746; 6986G>A), were purchased from Applied Biosystems, Foster City, CA. Absence of variant alleles was interpreted as presence of the wild-type allele (*1).
Genotyping of ABCB1 (1199G>A, 1236C>T, 2677G>T, 2677G>A and 3435C>T), POR*28 (rs1057868; C>T), PPARA (rs4253728; G>A) and PPARA (rs4823613; A>G) were performed by polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP) methods, using DNA Engine Dyad® and Tetrad® 2 Thermal Cycler (Bio-Rad Laboratories, Inc.). Specific primers and enzymes were used for the different sequence variants. The different PCR products were digested with 1 Unit of the associated restriction enzyme, and digested products were separated by electrophoresis on a 3% agarose gel, visualized under ultraviolet light after staining with GelRed™.
Calculations and data analyses
Peak concentration (Cmax) and time to Cmax (Tmax) are the actual observed values. The area under the whole blood or plasma concentration versus time curve during a dose interval at steady state, from time zero to 12/24 hr post dose, was calculated in accordance with the log-trapezoidal rule. The terminal half-life (T1/2) was calculated from the slope (kel) of the semi-logarithmic plot of the linear phase (including at least the last three time points) according to the formula T1/2 = ln(2)/kel. Missing drug concentrations were generally not substituted with regards to AUC calculations, but in the case that the last concentration in a dose interval was unavailable it was substituted with the C0 value of that individual.
The lipid lowering effect of rosuvastatin as well as the effect of rosuvastatin on everolimus PK was assessed using each patient as its own control (paired data analysis), comparing the levels at baseline (on steady state fluvastatin treatment) to after one month on rosuvastatin treatment. The safety of rosuvastatin in RTR was assessed by tabulating any adverse events and by comparing the steady state systemic exposure of rosuvastatin (AUC0-24) with levels reported for non-transplant patients in the literature (26). In addition, the individual change in estimated glomerular filtration rate (eGFR) from before to after one month of rosuvastatin treatment was assessed using the Modification of Diet in Renal Disease (MDRD) formula (49, 50).
The influence of different genotypes on everolimus and rosuvastatin PK was tabulated for a descriptive analysis.
Statistics
A sample size of at least twelve patients was calculated to provide 80% power of detecting a 25% difference in AUC0-24 of everolimus.
Statistical analysis was performed using IBM SPSS Statistics version 20.0 (IBM Corp., Armonk, NY). Normality of data was assessed using the Shapiro-Wilk tests. Paired sample t-test and Wilcoxon signed rank test, for parametric and nonparametric data analyses, respectively, were used to compare means of variables before and after treatment with rosuvastatin. A p-value <0.05 was considered statistically significant. All individual AUC and Cmax values were log-transformed and the European Medicines Agency guidelines for bioequivalence studies were used to assess the possible pharmacokinetic interaction (51).
Supplementary Material
Acknowledgments
The authors thank Kirsten Lund, May-Ellen Lauritzen and Hilde Hestvåg in the Department of Transplant Medicine, Oslo University Hospital as well as Siri Johannesen, Beata Mohebi and and Hege Christensen at the School of Pharmacy for their professional assistance during collection and preparation of samples. Partial support of grant # R15GM101599 from National Institutes of Health is gratefully acknowledged.
Abbreviations
- ALERT
Assessment of LEscol in Renal Transplantation
- AUC
Area under the curve
- BCRP
Breast cancer resistance protein
- Cmax
Peak concentration
- CNI
Calcineurin inhibitor
- MDRD
Modification of Diet in Renal Disease
- mTOR
The mammalian target of rapamycin
- LC-MS/MS
Liquid chromatography tandem mass spectrometry
- PCR-RFLP
polymerase chain reaction restriction fragment length polymorphism
- P-gp
P-glycoprotein
- PK
Pharmacokinetic
- OATP1B1
Organic anion transporting polypeptide 1B1
- RTR
Renal transplant recipients
- T1/2
Terminal half-life
- Tmax
Time to Cmax
- TDM
Therapeutic Drug Monitoring
Footnotes
Authors' Contributions: IR, AÅ, HH and KM participated in research design. IR, AÅ and KM participated in performance of the research. KM, HH, TG and MRN recruited patients. IR, AÅ, NTV, FA, MG and EM participated in data analysis. IR, AÅ and KM wrote the paper, whereas all authors have been involved in discussion of results and have contributed to, read and approved the final manuscript.
Disclosure: We received financial support (10 000 euro) from AstraZeneca to perform the rosuvastatin analysis. The authors have no conflict of interest.
References
- 1.Ojo AO. Cardiovascular complications after renal transplantation and their prevention. Transplantation. 2006;82:603. doi: 10.1097/01.tp.0000235527.81917.fe. [DOI] [PubMed] [Google Scholar]
- 2.Ojo AO, Hanson JA, Wolfe RA, Leichtman AB, Agodoa LY, Port FK. Long-term survival in renal transplant recipients with graft function. Kidney Int. 2000;57:307. doi: 10.1046/j.1523-1755.2000.00816.x. [DOI] [PubMed] [Google Scholar]
- 3.Lindholm A, Albrechtsen D, Frodin L, Tufveson G, Persson NH, Lundgren G. Ischemic heart disease - Major cause of death and graft loss after renal transplantation in Scandinavia. Transplantation. 1995;60:451. doi: 10.1097/00007890-199509000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Arend SM, Mallat MJK, Westendorp RJW, vanderwoude FJ, vanEs LA. Patient survival after renal transplantation; more than 25 years follow-up. Nephrol Dial Transplant. 1997;12:1672. doi: 10.1093/ndt/12.8.1672. [DOI] [PubMed] [Google Scholar]
- 5.Hjelmesaeth J, Hartmann A, Midtvedt K, Aakhus S, Stenstrom J, Morkrid L, et al. Metabolic cardiovascular syndrome after renal transplantation. Nephrol Dial Transplant. 2001;16:1047. doi: 10.1093/ndt/16.5.1047. [DOI] [PubMed] [Google Scholar]
- 6.Andany MA, Kasiske BL. Dyslipidemia and its management after renal transplantation. J Nephrol. 2001;14:S81. [PubMed] [Google Scholar]
- 7.Pannu HS, Singh D, Sandhu JS. Lipid profile before and after renal transplantation - A longitudinal study. Ren Fail. 2003;25:411. doi: 10.1081/jdi-120021153. [DOI] [PubMed] [Google Scholar]
- 8.Riella LV, Gabardi S, Chandraker A. Dyslipidemia and Its Therapeutic Challenges in Renal Transplantation. Am J Transplant. 2012;12:1975. doi: 10.1111/j.1600-6143.2012.04084.x. [DOI] [PubMed] [Google Scholar]
- 9.Holdaas H, Fellstrom B, Jardine AG, Holme I, Nyberg G, Fauchald P, et al. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo-controlled trial. Lancet. 2003;361:2024. doi: 10.1016/S0140-6736(03)13638-0. [DOI] [PubMed] [Google Scholar]
- 10.Jardine AG, Gaston RS, Fellstrom BC, Holdaas H. Prevention of cardiovascular disease in adult recipients of kidney transplants. Lancet. 2011;378:1419. doi: 10.1016/S0140-6736(11)61334-2. [DOI] [PubMed] [Google Scholar]
- 11.Stevens PE, Levin A. Evaluation and Management of Chronic Kidney Disease: Synopsis of the Kidney Disease: Improving Global Outcomes 2012 Clinical Practice Guideline. Ann Intern Med. 2013;158:825. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 12.Nashan B, Curtis J, Ponticelli C, Mourad G, Jaffe J, Haas T, et al. Everolimus and reduced-exposure cyclosporine in de novo renal-transplant recipients: A three-year phase II, randomized, multicenter, open-label study. Transplantation. 2004;78:1332. doi: 10.1097/01.tp.0000140486.97461.49. [DOI] [PubMed] [Google Scholar]
- 13.Vitko S, Tedesco H, Eris J, Pascual J, Whelchel J, Magee JC, et al. Everolimus with optimized cyclosporine dosing in renal transplant recipients: 6-month safety and efficacy results of two randomized studies. Am J Transplant. 2004;4:626. doi: 10.1111/j.1600-6143.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 14.Carmellini M, Collini A, Ruggieri G, Garosi G, Bernini M. Excellent Long-Term Results in De Novo Renal Transplant Recipients Treated With Proliferation Signal Inhibitors and Reduced Calcineurin Inhibitors Exposure. Transplant Proc. 2008;40:1858. doi: 10.1016/j.transproceed.2008.05.047. [DOI] [PubMed] [Google Scholar]
- 15.Budde K, Becker T, Arns W, Sommerer C, Reinke P, Eisenberger U, et al. Everolimus-based, calcineurin-inhibitor-free regimen in recipients of de-novo kidney transplants: an open-label, randomised, controlled trial. Lancet. 377:837. doi: 10.1016/S0140-6736(10)62318-5. [DOI] [PubMed] [Google Scholar]
- 16.Zeier M, Van Der Giet M. Calcineurin inhibitor sparing regimens using m-target of rapamycin inhibitors: an opportunity to improve cardiovascular risk following kidney transplantation? Transpl Int. 2011;24:30. doi: 10.1111/j.1432-2277.2010.01140.x. [DOI] [PubMed] [Google Scholar]
- 17.Asberg A. Interactions between cyclosporin and lipid-lowering drugs - Implications for organ transplant recipients. Drugs. 2003;63:367. doi: 10.2165/00003495-200363040-00003. [DOI] [PubMed] [Google Scholar]
- 18.Ballantyne CM, Corsini A, Davidson MH, et al. Risk for myopathy with statin therapy in high-risk patients. Arch Intern Med. 2003;163:553. doi: 10.1001/archinte.163.5.553. [DOI] [PubMed] [Google Scholar]
- 19.Kasiske BL, de Mattos A, Flechner SM, Gallon L, Meier-Kriesche HU, Weir MR, et al. Mammalian Target of Rapamycin Inhibitor Dyslipidemia in Kidney Transplant Recipients. Am J Transplant. 2008;8:1384. doi: 10.1111/j.1600-6143.2008.02272.x. [DOI] [PubMed] [Google Scholar]
- 20.Vítko Š, Margreiter R, Weimar W, Dantal J, Kuypers D, Winkler M, et al. Three-Year Efficacy and Safety Results from a Study of Everolimus Versus Mycophenolate Mofetil in de novo Renal Transplant Patients. Am J Transplant. 2005;5:2521. doi: 10.1111/j.1600-6143.2005.01063.x. [DOI] [PubMed] [Google Scholar]
- 21.McKenney JM. Efficacy and safety of rosuvastatin in treatment of dyslipidemia. Am J of Health Syst Pharm. 2005;62:1033. doi: 10.1093/ajhp/62.10.1033. [DOI] [PubMed] [Google Scholar]
- 22.Martin PD, Warwick MJ, Dane AL, Hill SJ, Giles PB, Phillips PJ, et al. Metabolism, excretion, and pharmacokinetics of rosuvastatin in healthy adult male volunteers. Clin Ther. 2003;25:2822. doi: 10.1016/s0149-2918(03)80336-3. [DOI] [PubMed] [Google Scholar]
- 23.Kitamura S, Maeda K, Wang Y, Sugiyama Y. Involvement of multiple transporters in the hepatobiliary transport of rosuvastatin. Drug Metab Dispos. 2008;36:2014. doi: 10.1124/dmd.108.021410. [DOI] [PubMed] [Google Scholar]
- 24.Huang LY, Wang Y, Grimm S. ATP-dependent transport of rosuvastatin in membrane vesicles expressing breast cancer resistance protein. Drug Metab Dispos. 2006;34:738. doi: 10.1124/dmd.105.007534. [DOI] [PubMed] [Google Scholar]
- 25.Picard N, Levoir L, Lamoureux F, Yee SW, Giacomini KM, Marquet P. Interaction of everolimus and sirolimus with the hepatic and intestinal Organic Anion Transporting Polypeptides (OATPs) Fundam Clin Pharmacol. 2010;24:94. [Google Scholar]
- 26.Martin PD, Warwick MJ, Dane AL, Cantarini MV. A double-blind, randomized, incomplete crossover trial to assess the dose proportionality of rosuvastatin in healthy volunteers. Clin Ther. 2003;25:2215. doi: 10.1016/s0149-2918(03)80214-x. [DOI] [PubMed] [Google Scholar]
- 27.Carswell CI, Plosker GL, Jarvis B. Rosuvastatin. Drugs. 2002;62:2075. doi: 10.2165/00003495-200262140-00008. [DOI] [PubMed] [Google Scholar]
- 28.Jones PH, Davidson MH, Stein EA, Bays HE, McKenney JM, Miller E, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* trial) Am J Cardiol. 2003;92:152. doi: 10.1016/s0002-9149(03)00530-7. [DOI] [PubMed] [Google Scholar]
- 29.Cheng JWM. Rosuvastatin in the management of hyperlipidemia. Clin Ther. 2004;26:1368. doi: 10.1016/j.clinthera.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Jardine AG, Holdaas H, Fellstrom B, Cole E, Nyberg G, Gronhagen-Riska C, et al. Fluvastatin prevents cardiac death and myocardial infarction in renal transplant recipients: Post-hoc subgroup analyses of the ALERT study. Am J Transplant. 2004;4:988. doi: 10.1111/j.1600-6143.2004.00445.x. [DOI] [PubMed] [Google Scholar]
- 31.Jacobsen W, Serkova N, Hausen B, Morris RE, Benet LZ, Christians U. Comparison of the in vitro metabolism of the macrolide immunosuppressants sirolimus and RAD. Transplant Proc. 2001;33:514. doi: 10.1016/s0041-1345(00)02116-3. [DOI] [PubMed] [Google Scholar]
- 32.Crowe A, Lemaire M. In vitro and in situ absorption of SDZ-RAD using a human intestinal cell line (Caco-2) and a single pass perfusion model in rats: Comparison with rapamycin. Pharm Res. 1998;15:1666. doi: 10.1023/a:1011940108365. [DOI] [PubMed] [Google Scholar]
- 33.Cooper KJ, Martin PD, Dane AL, Warwick MJ, Raza A, Schneck DW. Lack of effect of ketoconazole on the pharmacokinetics of rosuvastatin in healthy subjects. Br J Clin Pharmacol. 2003;55:94. doi: 10.1046/j.1365-2125.2003.01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin PD, Kemp J, Dane AL, Warwick MJ, Schneck DW. No effect of rosuvastatin on the pharmacokinetics of digoxin in healthy volunteers. J Clin Pharmacol. 2002;42:1352. doi: 10.1177/0091270002042012008. [DOI] [PubMed] [Google Scholar]
- 35.Amundsen R, Christensen H, Zabihyan B, Åsberg A. Cyclosporine A, but Not Tacrolimus, Shows Relevant Inhibition of Organic Anion-Transporting Protein 1B1-Mediated Transport of Atorvastatin. Drug Metab Dispos. 2010;38:1499. doi: 10.1124/dmd.110.032268. [DOI] [PubMed] [Google Scholar]
- 36.Kovarik JM, Hartmann S, Hubert M, Berthier S, Schneider W, Rosenkranz B, et al. Pharmacokinetic and pharmacodynamic assessments of HMG-CoA reductase inhibitors when coadministered with everolimus. J Clin Pharmacol. 2002;42:222. doi: 10.1177/00912700222011148. [DOI] [PubMed] [Google Scholar]
- 37.Kovarik JM, Hsu CH, McMahon L, Berthier S, Rordorf C. Population pharmacokinetics of everolimus in de novo renal transplant patients: Impact of ethnicity and comedications. Clin Pharmacol Ther. 2001;70:247. doi: 10.1067/mcp.2001.118022. [DOI] [PubMed] [Google Scholar]
- 38.Bellosta S, Paoletti R, Corsini A. Safety of Statins: Focus on Clinical Pharmacokinetics and Drug Interactions. Circulation. 2004;109:III. doi: 10.1161/01.CIR.0000131519.15067.1f. [DOI] [PubMed] [Google Scholar]
- 39.Simonson SG, Raza A, Martin PD, Mitchell PD, Jarcho JA, Brown CDA, et al. Rosuvastatin pharmacokinetics in heart transplant recipients administered an antirejection regimen including cyclosporine. Clin Pharmacol Ther. 2004;76:167. doi: 10.1016/j.clpt.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 40.Wolfe SM. Dangers of rosuvastatin identified before and after FDA approval. The Lancet. 2004;363:2189. doi: 10.1016/S0140-6736(04)16513-6. [DOI] [PubMed] [Google Scholar]
- 41.Vidt DG, Cressman MD, Harris S, Pears JS, Hutchinson HG. Rosuvastatin-induced arrest in progression of renal disease. Cardiology. 2004;102:52. doi: 10.1159/000077704. [DOI] [PubMed] [Google Scholar]
- 42.Picard N, Rouguieg-Malki K, Kamar N, Rostaing L, Marquet P. CYP3A5 Genotype Does Not Influence Everolimus In Vitro Metabolism and Clinical Pharmacokinetics in Renal Transplant Recipients. Transplantation. 2011;91:652. doi: 10.1097/TP.0b013e31820ae4ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lemaitre F, Bezian E, Goldwirt L, Fernandez C, Farinotti R, Varnous S, et al. Population Pharmacokinetics of Everolimus in Cardiac Recipients: Comedications, ABCB1, and CYP3A5 Polymorphisms. Ther Drug Monit. 2012;34:686. doi: 10.1097/FTD.0b013e318273c899. [DOI] [PubMed] [Google Scholar]
- 44.Niemi M, Pasanen MK, Neuvonen PJ. Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011;63:157. doi: 10.1124/pr.110.002857. [DOI] [PubMed] [Google Scholar]
- 45.Pasanen MK, Fredrikson H, Neuvonen PJ, Niemi M. Different Effects of SLCO1B1 Polymorphism on the Pharmacokinetics of Atorvastatin and Rosuvastatin. Clin Pharmacol Ther. 2007;82:726. doi: 10.1038/sj.clpt.6100220. [DOI] [PubMed] [Google Scholar]
- 46.Klein K, Thomas M, Winter S, Nussler AK, Niemi M, Schwab M, et al. PPARA: A Novel Genetic Determinant of CYP3A4 In Vitro and In Vivo. Clin Pharmacol Ther. 2012;91:1044. doi: 10.1038/clpt.2011.336. [DOI] [PubMed] [Google Scholar]
- 47.Vethe NT, Gjerdalen LC, Bergan S. Determination of cyclosporine, tacrolimus, sirolimus and everolimus by liquid chromatography coupled to electrospray ionization and tandem mass spectrometry: Assessment of matrix effects and assay performance. Scand J Clin Lab Invest. 2010;70:583. doi: 10.3109/00365513.2010.531141. [DOI] [PubMed] [Google Scholar]
- 48.Macwan JS, Ionita IA, Akhlaghi F. A simple assay for the simultaneous determination of rosuvastatin acid, rosuvastatin-5S-lactone, and N-desmethyl rosuvastatin in human plasma using liquid chromatography-tandem mass spectrometry (LC-MS/MS) Anal Bioanal Chem. 2012;402:1217. doi: 10.1007/s00216-011-5548-4. [DOI] [PubMed] [Google Scholar]
- 49.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, et al. A More Accurate Method To Estimate Glomerular Filtration Rate from Serum Creatinine: A New Prediction Equation. Ann Intern Med. 1999;130:461. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 50.Buron F, Hadj-Aissa A, Dubourg L, Morelon E, Steghens JP, Ducher M, et al. Estimating Glomerular Filtration Rate in Kidney Transplant Recipients: Performance Over Time of Four Creatinine-Based Formulas. Transplantation. 2011;92:1005. doi: 10.1097/TP.0b013e3182301602. [DOI] [PubMed] [Google Scholar]
- 51.EMA. Guideline on the investigation of bioequivalence. 2010 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.