Abstract
Background/aims
In terms of blind-person years, the worldwide burden of childhood blindness is second only to cataracts. In many developing countries, 30–72% of childhood blindness is avoidable. The authors conducted this study to determine the causes of childhood blindness and visual impairment (VI) in Botswana, a middle-income country with limited access to ophthalmic care.
Methods
This study was conducted over 4 weeks in eight cities and villages in Botswana. Children were recruited through a radio advertisement and local outreach programmes. Those ≤15 years of age with visual acuity <6/18 in either eye were enrolled. The WHO/Prevention of Blindness Eye Examination Record for Children with Blindness and Low Vision was used to record data.
Results
The authors enrolled 241 children, 79 with unilateral and 162 with bilateral VI. Of unilateral cases, 89% were avoidable: 23% preventable (83% trauma-related) and 66% treatable (40% refractive error and 31% amblyopia). Of bilateral cases, 63% were avoidable: 5% preventable and 58% treatable (33% refractive error and 31% congenital cataracts).
Conclusion
Refractive error, which is easily correctable with glasses, is the most common cause of bilateral VI, with cataracts a close second. A nationwide intervention is currently being planned to reduce the burden of avoidable childhood VI in Botswana.
INTRODUCTION
By WHO criteria, there are 1.26 million children worldwide who are blind, 0.42 million of whom live in sub-Saharan Africa.1 While the estimated number of blind children has improved or at least stabilised in other parts of the developing world, in sub-Saharan Africa, this number has increased by 31% over the past 10 years.1 Children who are blind have a lifetime of visual impairment (VI) ahead of them, with all the associated emotional, social and economic costs to the child, the family and the society. The worldwide burden of childhood blindness is second only to cataracts after accounting for duration of disability.2
Thus, childhood blindness is a major public health concern. In order to help nations combat childhood blindness and VI, it is important to determine the specific aetiologies by region. This will enable each nation to better understand its specific needs, and better ensure that appropriate resources are efficiently allocated for prevention and treatment. Botswana is a middle-income country in southern Africa with limited access to ophthalmic care. We conducted this study to determine avoidable causes of childhood blindness and VI in Botswana so that a nationwide intervention can be planned.
METHODS
Population of children in Botswana at risk for blindness or VI
In Botswana, the population aged 0–15 years is approximately 720 000.3 Under 5 mortality rates (U5MR) are used to estimate prevalence of childhood blindness. For Botswana, this is 112/1000 children. However, if we estimate that 60% of deaths are due to HIV, as in neighbouring Zambia, the U5MR due to non-HIV causes is 40% of 112 or 45/1000 children. The estimated prevalence of blindness is, therefore, approximately 0.5/1000 children which translates to 360 blind children in Botswana (C Gilbert, personal communication, 2009).1
Children were recruited for this study from the community, schools and eye clinics. Radio announcements were made on Radio Botswana (national radio, with over 90% national coverage) in Setswana (the language spoken by more than 80% of the population, and the primary language of instruction in primary schools), throughout the day 2 weeks prior to the survey and throughout the survey period. Parents, guardians and other care givers were asked to bring children with ‘difficulty seeing’ to be examined on the dates that the study team would be in their area. In addition, the Ministry of Education, through the Special Education Division, mobilised district education officers, schools and district rehabilitation officers to identify children with ‘difficulty seeing’ for examination during the survey. The schools transported the children to examination sites. The Ministry of Health, through the Prevention of Blindness Programme, requested all ophthalmologists and ophthalmic nurses to keep a record of all the children with VI seen at their clinics so they could be recalled for examination by the research team. Botswana has one primary school for the blind in the north (Francistown) and one primary, three junior and one senior secondary schools in the south (Mochudi), which accept blind and severely visually impaired children. All the children in these schools who met the recruitment criteria were enrolled in the study. Screening was also done at a school in the far north of the country (Maun) which accepts children with multiple disabilities. The plan was for children to be recruited from the whole country.
This study was approved by the Institutional Review Board of the Children’s Hospital of Philadelphia, the Ministry of Health of Botswana and the Institutional Review Board of Princess Marina Hospital in Gaborone, Botswana and conformed to the US Health Insurance Portability and Accountability Act.
Study team and eye examinations
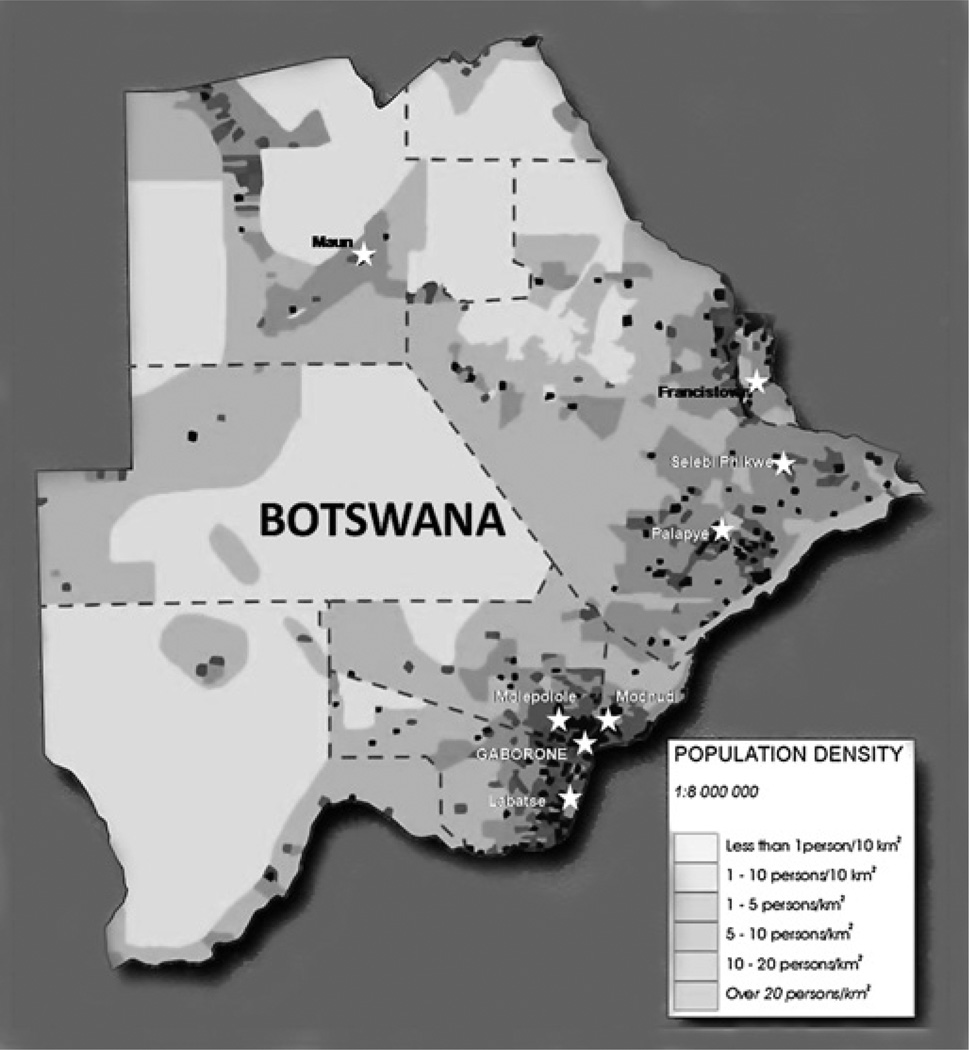
The study was conducted over a 4-week period in February to March 2009 in eight cities and villages in Botswana (figure 1). The core study team consisted of three ophthalmologists (SN, WVA and ON), one refractionist, one ophthalmic nurse and low vision officer from the Ministry of Education. One day was used to train the core team. Additional personnel were recruited and trained locally at each site.
Figure 1.
Population density map of Botswana showing examination sites. Each examination site is indicated with a star.
Children ≤15 years of age with visual acuity <6/18 in one or both eyes were enrolled. Parental or guardian informed consent and child assent was obtained at the time of examination with a translator, as necessary. No payment was made to subjects or their families.
Tumbling ‘E’ method with 6/18 and 6/60 prototypes was used for visual acuity examination at 6 m The criteria for vision at a certain level was four correct consecutive showings, five correct out of six showings, or six correct out of eight showings. If the child failed 6/18 level, the 6/60 was tested. If the child failed the 6/60 level, the child was moved to 3 m and tested with the 6/60 ‘E’. In young children, matching LEA symbols was tested if they did not understand the tumbling ‘E’. In those children in whom visual acuity could not be assessed, fix and follow, fixation preference and clinical history were used to determine whether the child was ‘believed blind’, ‘believed visually impaired’ or ‘believed sighted’. The study ophthalmologists (SN, WVA and ON) came to a consensus prior to categorising these children.
Once a child was enrolled, further eye examination was performed, including: pupils, extraocular motility, intraocular pressure with Goldmann applanation tonometer as necessary, anterior segment examination by handheld light or slit lamp and dilation of eyes for cycloplegic refraction by retinoscopy and funduscopic examination with a direct or indirect ophthalmoscope. For dilation, phenylephrine hydrochloride 2.5%, tropicamide 1% and cyclopentolate hydrochloride 1% were instilled in all patients with the following exceptions: (1) patients with cardiac problems were not given phenylephrine and (2) children <6 months of age or children with a seizure history were not given cyclopentolate. Drops were repeated after 30 min if the patient was not adequately cyclopleged.
Study definitions
Blindness was defined as presenting distance visual acuity <3/60, severe VI as <6/60 to 3/60 and VI as <6/18 to 6/60 with available correction. Both unilateral and bilateral cases were recruited. Infants and toddlers in whom visual acuity could not be assessed were categorised as ‘believed blind’, ‘believed visually impaired’ or ‘believed sighted’. Those children ‘believed blind’ were placed in the ‘blind’ group. Unilateral amblyopia typically causes more severe VI than bilateral amblyopia. Thus, the unilateral ‘believed visually impaired’ eyes were placed in the ‘severe VI group’ and the bilateral ‘believed visually impaired’ eyes were placed in the ‘VI’ group.
Data collection
The WHO/Prevention of Blindness Eye Examination Record for Children with Blindness and Low Vision was used to record data for enrolled children. This included personal/demographic information, medical history, presence of other disabilities, previous eye surgery and eye examination data (detailed above).
Both an anatomic and an aetiologic classification of visual loss were determined for each eye and each child as a whole. The anatomic classification attempts to locate the part of the eye affected and categories included: whole globe, cornea, lens, uvea, retina, optic nerve and normal globe. Children with normal globes were further classified as having refractive error, amblyopia, cortical blindness or normal vision. Whole globe includes microphthalmia, anophthalmia and phthis bulbi. The most treatable or most preventable anatomic cause was chosen as the primary cause. If neither a treatable nor preventable cause existed, the ophthalmologists used clinical judgement to agree upon the primary anatomic cause. The aetiologic classification attempts to determine the stage in the child’s development when the injury leading to visual loss happened. The aetiologic categories included: hereditary disease, intrauterine factors, perinatal/neonatal factors, postnatal/infancy/childhood factors and unclassifiable factors.
There were no follow-up visits for purposes of this study. However, if a treatable condition or previously undiagnosed problem was identified, the names of those children were recorded, and parents or guardians were notified and referred to appropriate treatment facilities. Samples of topical or oral medications were offered when available, medically warranted and agreed to by the child’s parent or guardian. Donated spectacles were distributed if available in an adequate prescription.
The following were categorised as treatable causes of VI and blindness: cataract, glaucoma, lens subluxation, refractive error, amblyopia, uveitis, infection and vernal keratoconjunctivitis. Preventable causes included: corneal scar, vitamin A deficiency, trauma and intrauterine infections. Any cause that was either treatable or preventable was deemed avoidable.
Data were entered into Excel files and causes of blindness and VI quantified as percentages.
RESULTS
A total of 241 children were enrolled and examined, of whom 79 had unilateral and 162 had bilateral VI or blindness. Demographics of the entire group are shown in table 1. Slightly more males than females and older, school-age children were enrolled. Just under half of the children were in schools for the blind or special mixed schools. The mean age was 10.9±3.5 years. The vast majority were Tswana-speaking. For many subjects, the age of onset for VI and blindness was unknown. In cases of severe VI or blindness, the age of onset was most commonly <1 year for both unilaterally and bilaterally affected children. Nineteen of the 241 children (7.9%) had other disabilities, including: developmental delay, learning disability, mental retardation, physical handicaps and epilepsy.
Table 1.
Demographics of entire group
| Number | Percentage of total (n=241) |
|
|---|---|---|
| Sex | ||
| Male | 124 | 51.5% |
| Female | 117 | 48.5% |
| Age (years) | ||
| 0–1 | 5 | 2.1% |
| 1–5 | 13 | 5.4% |
| 6–10 | 70 | 29.0% |
| 11–15 | 153 | 63.5% |
| School attendance | ||
| In school | 19 | 7.9% |
| Not in school | 222 | 92.1% |
| In school for the blind or special mixed schools | 116 | 48.7% |
| Ethnic group | ||
| Tswana-speaking | 167 | 69.3% |
| Kalanga/Ndebele | 28 | 11.6% |
| Basarwa/Bakgalagadi | 14 | 5.8% |
| Other | 21 | 8.7% |
| Unknown | 11 | 4.6% |
Anatomic classification
Unilateral blindness and VI
Using anatomic classification, 39 of 79 children with unilateral blindness and VI had normal globes (49.4%); 30 (38%) had untreated refractive error and 21 (26.6%) had untreated amblyopia. Seventeen children (21.5%) had lens-related blindness or VI. Refractive error was most common in the visually impaired group (74.2%; 23/31), while lens-related issues were more common among blind children (36.8%; 14/38).
Bilateral blindness and VI
Among the 162 children with bilateral blindness and VI, refractive error was most common in the visually impaired group (49.2%; 31/63). However, among blind children, whole globe issues were most common (33.8%; 23/68), and lens-related issues were the second most common (22.1%; 15/68). Predictably, amblyopia was less common among bilaterally affected children (11.1%; 18/162) compared with those who were unilaterally affected (26.6%; 21/79).
Aetiologic classification
Unilateral blindness and VI
The majority of children with unilateral blindness and VI (64.6%; 51/79) had an unclassifiable aetiology, with refractive error (26.6%; 21/79) and amblyopia (20.2%; 16/79) being most common. The next most frequent cause was postnatal/infancy/childhood factor (26.6%; 21/79), caused mainly by trauma (19.0%; 15/79). Although refractive error predominated in the unilaterally visually impaired group, trauma predominated among the unilaterally blind group.
Bilateral blindness and VI
The aetiology could not be classified in 65.4% (106/162) of children with bilateral blindness and VI with refractive error (19.1%; 31/162) and cataract (12.3%; 20/162) contributing most of the cases. The next most frequent cause was hereditary (24.7%; 40/162) and the mode of inheritance was unknown in half of the children and autosomal dominant in 38% (15/40). Hereditary causes were more frequent in the severely visually impaired and blind groups.
Avoidable blindness and VI
Among the 79 unilaterally affected children, 88.6% of the causes of blindness and VI were avoidable (table 2), of which 22.8% were preventable and 65.8% treatable. Trauma was the commonest preventable cause (83%; 15/18), whereas the commonest treatable causes included refractive error (40%; 21/52) and amblyopia (31%; 16/52). Of 162 bilaterally affected children, 62.9% were avoidable (table 3), of which 4.9% were preventable and 58.0% treatable. The most common treatable causes were refractive error (33%; 31/94) and cataract (31%; 29/94).
Table 2.
Causes of unilateral blindness and visual impairment
| <6/18–6/60 (VI) | <6/60–3/60 (SVI) | <3/60 (Blind) | All subjects | |||||
|---|---|---|---|---|---|---|---|---|
| Avoidable | 27 | 87.1% | 8 | 80.0% | 35 | 92.1% | 70 | 88.6% |
| Preventable | 2 | 6.5% | 1 | 10.0% | 15 | 39.5% | 18 | 22.8% |
| Trauma | 1 | 3.2% | 0 | 0.0% | 14 | 36.8% | 15 | 19.0% |
| Intrauterine infection | 1 | 3.2% | 1 | 10.0% | 0 | 0.0% | 2 | 2.5% |
| Corneal scar | 0 | 0.0% | 0 | 0.0% | 1 | 2.6% | 1 | 1.3% |
| Treatable | 25 | 80.6% | 7 | 70.0% | 20 | 52.6% | 52 | 65.8% |
| Refractive error | 18 | 58.1% | 1 | 10.0% | 2 | 5.3% | 21 | 26.6% |
| Amblyopia—refractive | 4 | 12.9% | 0 | 0.0% | 4 | 10.5% | 8 | 10.1% |
| Amblyopia—strabismic | 2 | 6.5% | 4 | 40.0% | 2 | 5.3% | 8 | 10.1% |
| Cataract-related | 0 | 0.0% | 2 | 20.0% | 6 | 15.8% | 8 | 10.1% |
| Corneal infection | 0 | 0.0% | 0 | 0.0% | 4 | 10.5% | 4 | 5.1% |
| Vernal keratoconjunctivitis | 1 | 3.2% | 0 | 0.0% | 1 | 2.6% | 2 | 2.5% |
| Subluxed lens | 0 | 0.0% | 0 | 0.0% | 1 | 2.6% | 1 | 1.3% |
| Unavoidable | 4 | 12.9% | 2 | 20.0% | 3 | 7.9% | 9 | 11.4% |
| Optic nerve atrophy | 3 | 9.7% | 1 | 10.0% | 2 | 5.3% | 6 | 7.6% |
| Albinism | 0 | 0.0% | 1 | 10.0% | 0 | 0.0% | 1 | 1.3% |
| Anterior segment dysgenesis | 0 | 0.0% | 0 | 0.0% | 1 | 2.6% | 1 | 1.3% |
| Unknown | 1 | 3.2% | 0 | 0.0% | 0 | 0.0% | 1 | 1.3% |
| Total | 31 | 100.0% | 10 | 100.0% | 38 | 100.0% | 79 | 100.0% |
VI, visual impairment; SVI, severe visual impairment.
Table 3.
Causes of bilateral blindness and visual impairment
| <6/18–6/60 (VI) | <6/60–3/60 (SVI) | <3/60 (Blind) | All subjects | |||||
|---|---|---|---|---|---|---|---|---|
| Avoidable | 43 | 68.3% | 19 | 61.3% | 40 | 58.8% | 102 | 63.0% |
| Preventable | 0 | 0.0% | 3 | 9.7% | 5 | 7.4% | 8 | 4.9% |
| Optic nerve injury (brain abscess, surgery) | 0 | 0.0% | 1 | 3.2% | 2 | 2.9% | 3 | 1.9% |
| Congenital rubella | 0 | 0.0% | 1 | 3.2% | 1 | 1.5% | 2 | 1.2% |
| Corneal scar | 0 | 0.0% | 1 | 3.2% | 1 | 1.5% | 2 | 1.2% |
| Vitamin A deficiency | 0 | 0.0% | 0 | 0.0% | 1 | 1.5% | 1 | 0.6% |
| Treatable | 43 | 68.3% | 16 | 51.6% | 35 | 51.5% | 94 | 58.0% |
| Refractive error | 26 | 41.3% | 2 | 6.5% | 3 | 4.4% | 31 | 19.1% |
| Cataract | 7 | 11.1% | 8 | 25.8% | 14 | 20.6% | 29 | 17.9% |
| Glaucoma | 0 | 0.0% | 0 | 0.0% | 12 | 17.6% | 12 | 7.4% |
| Amblyopia–refractive | 4 | 6.3% | 3 | 9.7% | 3 | 4.4% | 10 | 6.2% |
| Vernal keratoconjunctivitis | 3 | 4.8% | 2 | 6.5% | 2 | 2.9% | 7 | 4.3% |
| Subluxed lens from Marfan syndrome | 3 | 4.8% | 1 | 3.2% | 0 | 0.0% | 4 | 2.5% |
| Unknown (bilateral enucleation) | 0 | 0.0% | 0 | 0.0% | 1 | 1.5% | 1 | 0.6% |
| Unavoidable | 20 | 31.7% | 12 | 38.7% | 28 | 41.2% | 60 | 37.0% |
| Optic nerve atrophy | 2 | 3.2% | 2 | 6.5% | 8 | 11.8% | 12 | 7.4% |
| Albinism | 6 | 9.5% | 3 | 9.7% | 1 | 1.5% | 10 | 6.2% |
| Retinal dystrophy | 4 | 6.3% | 1 | 3.2% | 2 | 2.9% | 7 | 4.3% |
| Disorganised | 1 | 1.6% | 2 | 6.5% | 4 | 5.9% | 7 | 4.3% |
| Unknown—normal examination | 5 | 7.9% | 1 | 3.2% | 1 | 1.5% | 7 | 4.3% |
| Microphthalmia | 0 | 0.0% | 0 | 0.0% | 5 | 7.4% | 5 | 3.1% |
| Aniridia | 1 | 1.6% | 1 | 3.2% | 2 | 2.9% | 4 | 2.5% |
| Cerebral hypoxia/injury | 0 | 0.0% | 1 | 3.2% | 2 | 2.9% | 3 | 1.9% |
| Optic nerve hypoplasia | 0 | 0.0% | 1 | 3.2% | 1 | 1.5% | 2 | 1.2% |
| Anophthalmos | 0 | 0.0% | 0 | 0.0% | 1 | 1.5% | 1 | 0.6% |
| Cornea plana/sclerocornea | 1 | 1.6% | 0 | 0.0% | 0 | 0.0% | 1 | 0.6% |
| Goldenhar syndrome | 0 | 0.0% | 0 | 0.0% | 1 | 1.5% | 1 | 0.6% |
| Total | 63 | 100.0% | 31 | 100.0% | 68 | 100.0% | 162 | 100.0% |
VI, visual impairment; SVI, severe visual impairment.
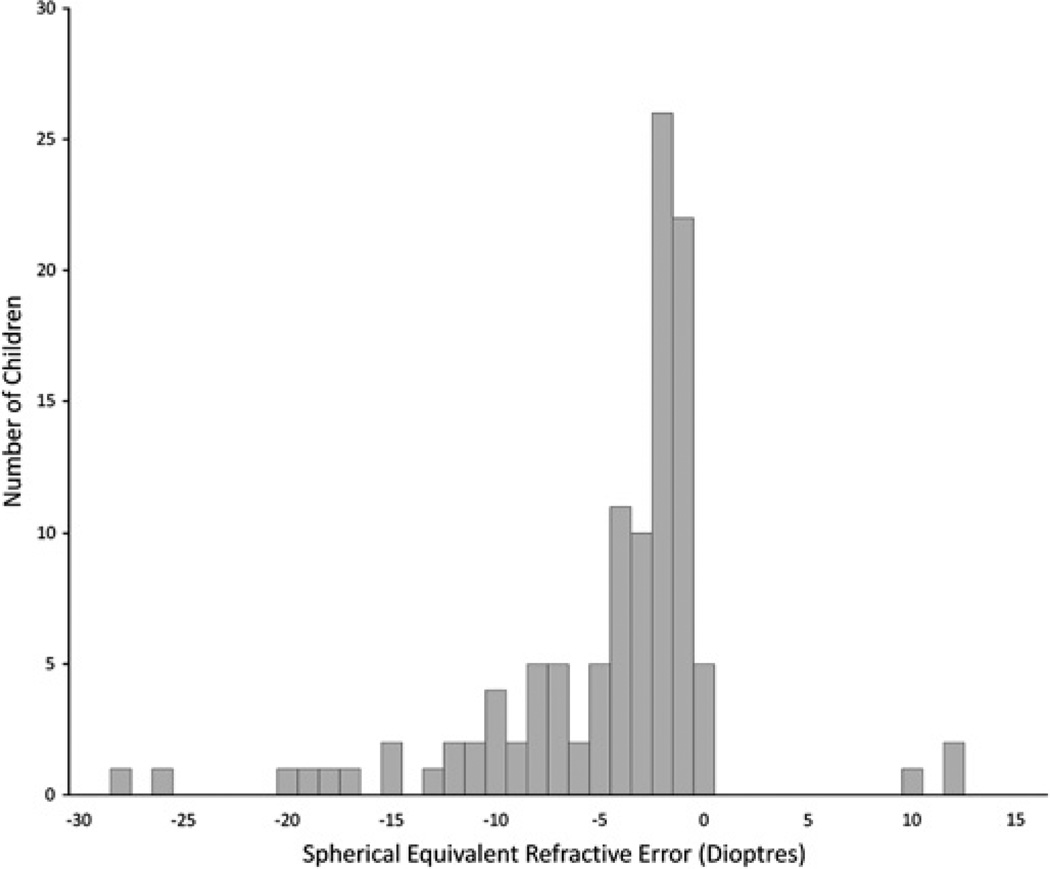
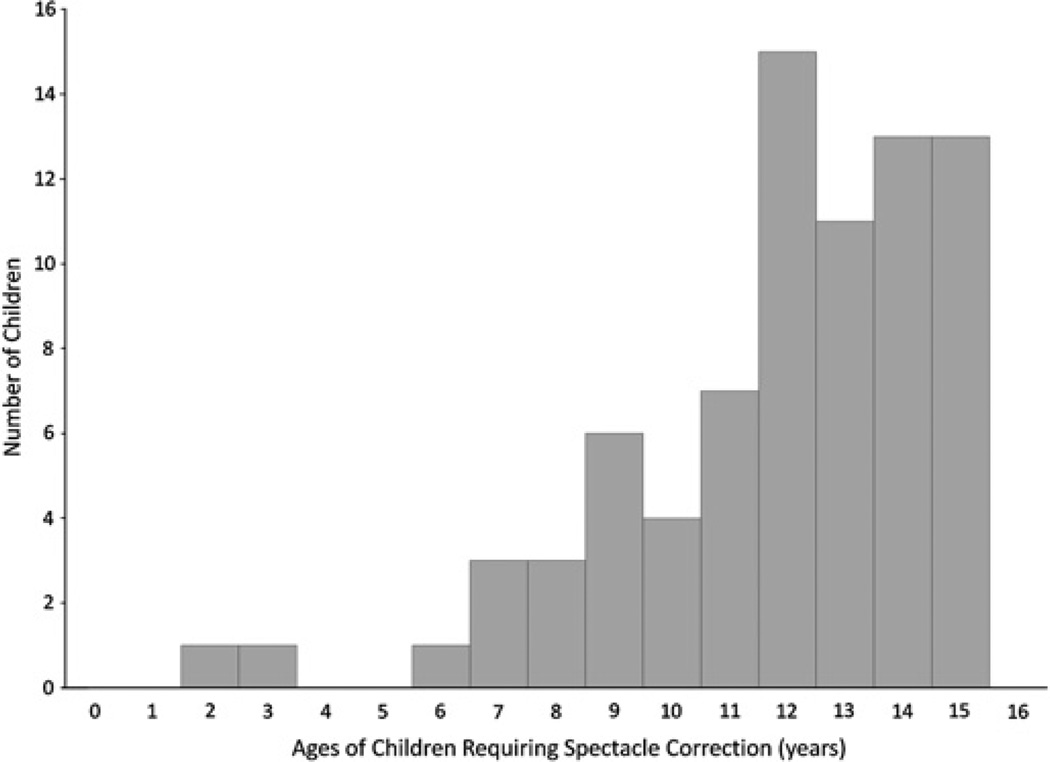
Since refractive error composed such a large burden of disease among visually impaired children, a histogram was created to demonstrate the distribution of spherical equivalent refractive errors requiring correction (excluding aphakic and pseudophakic children) (figure 2). A large amount of uncorrected myopia exists in the study population. The distribution of ages requiring refractive error correction is shown in figure 3. Note that our sample included mostly older children, so this distribution may be skewed.
Figure 2.
Distribution of refractive errors requiring correction.
Figure 3.
Distribution of ages of children requiring refractive error correction.
DISCUSSION
Based on the population of roughly 720 000 children under 15 years in Botswana, we estimated that there would be 360 children with blindness in the country. The main objective of the study was to obtain information relevant to planning vision-related public health initiatives in Botswana. It thus provided data on a convenience sample of 241 children with blindness or VI in one or both eyes. These were children with access to radio and ability to travel to a clinic for an eye examination by the study team or recruited by ophthalmic nurses, teachers and rehabilitation officers.
Based on the results of this survey, there are significant numbers of children with avoidable blindness or VI in Botswana. Refractive error is the most common cause of treatable bilateral VI, with cataracts a close second. The large proportion of children in Botswana with VI due to uncorrected refractive error is likely due to lack of routine vision screening, limited access to refractive services and cost of spectacles. Midwives and nurses at well-baby clinics could be trained to screen for VI and eye diseases in preschool age children. Routine vision screening in schools by trained field workers or teachers would help with early detection of refractive error and other visually impairing eye diseases.
Baltussen et al demonstrated that the incremental cost of screening 5–15-year-old children annually per disability-adjusted life year averted in southern Africa was estimated at 201 International dollars,4 well below Botswana’s per capita GDP (over 13 000 International dollars in 2009) and would be considered very cost-effective for Botswana. As seen in figure 3, this is also the age group most likely to need spectacles.
Cataract-related VI and blindness in children represented a large burden of avoidable diseases in our sample population. This is a more challenging problem to tackle. These children often present late to ophthalmic surgeons, resulting in poor outcomes due to the extended period of visual deprivation. In Tanzania, delay in presentation was related to low socioeducational status of the mother, poor awareness of childhood cataract, difficulty and cost of travelling far distances to specialised institutions, fears about cataract surgery and anaesthesia and lack of knowledge and skills of local health professionals to detect cataracts in children and refer them properly.5 6 It is likely that some of these barriers to care play a role in Botswana. This will need to be studied so that the appropriate interventions can be made. Improving awareness through public education and improving knowledge of primary care staff are essential to improving visual outcomes in these children.
A further issue in paediatric cataract surgery is the need for frequent postoperative follow-up, frequent adjustment of contact lens or glasses prescription as the child grows and the need for amblyopia therapy and intraocular pressure monitoring. In our study, 5 out of 7 unilateral aphakes/pseudophakes and 8 of 18 bilateral aphakes/pseudophakes had amblyopia, demonstrating the need for better follow-up regimens and vigilant amblyopia therapy after cataract surgery in children. In Tanzania, implementation of a counselling service and a tracking system including phoning of a guardian in the event a missed follow-up appointment resulted in significant improvements in 2-week (from 67% to 89%) and 10-week (from 43% to 83%) postoperative follow-up, as well as acquisition of spectacles (94% after intervention).7 The major challenge to direct implementation of such a system in Botswana is the much smaller overall population (the scale of the intervention would have to be smaller to make it cost-effective) and the low population density, necessitating long travel distances to a centralised facility.
Currently in Botswana, all children who present with cataracts have surgery as soon as it is safe to do so. Most children 2 years and older have an intraocular lens (IOL) implanted. If the child is deemed unable to tolerate YAG capsulotomy, surgical posterior capsulotomy is performed. Although contact lens use is generally preferred in infants after cataract surgery, this is often impractical due to the expense, frequent need for replacement and limited availability. Also, environmental challenges may increase risk of corneal infection, especially for those children living in remote areas.
Yorston et al8 and Wilson et al9 advocated for IOL use in children of all ages (including infants as young as 4 weeks of age) to promote the development of useful vision by partial refractive error correction when contact lenses are impractical.9 Wilson et al also recommended anterior vitrectomy in at least all children ≤8 years of age.9 Technical challenges to successful outcome after cataract surgery in Botswana include inadequate biometry and lack of vitrectomy machines. Bilateral paediatric cataract surgery during the same admission, but not same list, may need to be given consideration due to the hardship that may be encountered for families travelling long distances for surgery.
Amblyopia comprised a large burden of disease among children with unilateral blindness and VI in our study. There are very few orthoptists in sub-Saharan Africa, and none in Botswana, which adds to the challenge of amblyopia treatment. Raising awareness among eye care practitioners and making resources available to recognise and treat amblyopia appropriately with refractive correction and patching should be part of a comprehensive strategy to prevent childhood blindness. Parents must be adequately educated on the reason for patching and the necessity of adherence for it to be effective. In addition, improved low vision services are integral to improving the lives of children with visual loss that cannot be corrected by medical, surgical, refractive or amblyopia therapy.
Among bilateral cases of blindness and severe VI, a large percentage (26.5% and 32.3%, respectively) was associated with hereditary diseases (most commonly albinism and cataract). It is important that parents of these children are made aware of the possibility of genetic transmission of a disease that may cause blindness or VI in their future children and the need for evaluation soon after birth.
In our study, we only saw one child with a history of vitamin A deficiency and two children with rubella. Although there were few older children with corneal scar of unknown aetiology, there were no acute cases of measles, rubella or vitamin A deficiency.
The use of radio announcements, teachers, rehabilitation officers and eye clinics for recruiting children for the study was an attempt to recruit as nationally representative a sample of blind and visually impaired children as possible and minimise the selection bias associated with blind schools surveys.2 It was hoped that radio announcements and recruitment from eye clinics would increase recruitment of infants and preschool children as those in schools were easily recruited by teachers and in schools for the blind. The study, however, failed to recruit adequate numbers of younger children, who may have different causes of blindness from those in school. In addition, many lower income rural families may not have been reached, which may alter the distribution of causes. Recruitment of children from rural areas by teachers and ophthalmic nurses hopefully minimised this bias. In addition, less mobile children were less likely to be seen, and are likely to have different causes from children with visual loss as their only impairment. Classical population-based surveys are not ideal for the study of childhood blindness because of its relatively low prevalence.2 The newer key informants method10 is more resource intense and not suited for collecting national data over a relatively short period. The strength of this study is that examination was done by a single experienced team of ophthalmologists using study clearly shows that a significant portion of childhood VI in Botswana is preventable and treatable. A number of simple and some more complex interventions may be required to decrease the burden of avoidable childhood blindness in Botswana. Improved eye care systems combined with improved public awareness is integral to the successful implementation of any programme. The major challenge in Botswana is the low population density. Data from this study will assist in the planning and initiation of future programmes to prevent childhood vision loss and assist in the goals of the WHO’s VISION 2020: The Right to Sight initiative.2
Acknowledgments
Funding The study was made possible by the in-kind contributions of the Prevention of Blindness Programme of the Ministry of Health (MoH) and the Special Education Unit of the Ministry of Education and Skills Development (MoESD) in Botswana. The MoESD provided transport and teachers to take children to the examination sites and supported the Low Vision Officer to participate in the field survey for the full four weeks as well as perform post-study follow ups. The MoH supported ophthalmic nurses to conduct pre-study outreach, participate in the study and do post-study follow ups. The ministry also provided transport for the children and the research team as well as subsistence for all their employees who participated in the field work.
Footnotes
Competing interests None.
Ethics approval This study was conducted with the approval of the Children’s Hospital of Philadelphia Institutional Review Board, Princess Marina Hospital Institutional Review Board and Ministry of Health of Botswana.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Chandna A, Gilbert C. When your eye patient is a child. Community Eye Health. 2010;23:1–3. [PMC free article] [PubMed] [Google Scholar]
- 2.Gilbert C, Foster A. Childhood blindness in the context of VISION 2020—the right to sight. Bull World Health Organ. 2001;79:227–232. [PMC free article] [PubMed] [Google Scholar]
- 3. [accessed June 2010];Republic of Botswana Central Statistics Office. http://www.cso.gov.bw/
- 4.Baltussen R, Naus J, Limburg H. Cost-effectiveness of screening and correcting refractive errors in school children in Africa, Asia, America, and Europe. Health Policy. 2009;89:201–215. doi: 10.1016/j.healthpol.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Mwende J, Bronsard A, Mosha M, et al. Delay in presentation to hospital for surgery for congenital and developmental cataract in Tanzania. Br J Ophthalmol. 2005;89:1478–1482. doi: 10.1136/bjo.2005.074146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bronsard A, Geneau R, Shirima S, et al. Why are children brought late for cataract surgery? Qualitative findings from Tanzania. Ophthalmic Epidemiol. 2008;15:383–388. doi: 10.1080/09286580802488624. [DOI] [PubMed] [Google Scholar]
- 7.Kishiki E, Shirma S, Lewallen S, et al. Improving postoperative follow-up of children receiving surgery for congenital or developmental cataracts in Africa. J AAPOS. 2009;13:280–282. doi: 10.1016/j.jaapos.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Yorston D, Wood M, Foster A. Results of cataract surgery in young children in east Africa. Br J Ophthalmol. 2001;85:267–271. doi: 10.1136/bjo.85.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson ME, Pandey SK, Thakur J. Pediatric cataract blindness in the developing world: surgical techniques and intraocular lenses in the new millennium. Br J Ophthalmol. 2003;87:14–19. doi: 10.1136/bjo.87.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalua K, Patel D, Muhit M, et al. Productivity of key informants for identifying blindness in children: evidence from a pilot study in Malawi. Eye. 2009;23:7–9. doi: 10.1038/eye.2008.49. [DOI] [PubMed] [Google Scholar]