Abstract
We examined the ability to discriminate facial expressions among 8-year-old children who had been abandoned and placed in institutions in infancy and children with no institutional rearing (Never Institutionalized Group; NIG). Following a baseline assessment (average age=22 months), half the institutionalized children were randomly assigned to a foster care intervention (foster care group; FCG) and half to remain in the institution (care as usual group; CAUG). All three groups had a more difficult time recognizing fearful as compared to neutral expressions. However, the NIG and FCG were both better at inhibiting responses to neutral and fearful faces than the CAUG. Regarding ERPs, the P1 was biggest to angry faces for the NIG, smallest among the CAUG and intermediate for the FCG. The N170 and the P300 were biggest to fear in all groups. Although the children in foster care showed improvements in their ability to recognize fear and neutral faces, and their P1 to angry was midway between the NIG and CAUG, we observed no timing of placement effects. These findings support the view that institutional rearing leads to deficits in the ability to process facial emotion, and placement in foster care partially, although incompletely, ameliorates these deficits.
An important function of the brain is to scan incoming sensory information for the presence of relevant signals and act on this information. For humans, the most salient signals are often social in nature, such as the identity and the emotional expression of the faces we encounter in our everyday lives. Indeed, it can be argued that our survival as a species depends in large measure on these skills.
Although there is now considerable research that describes the development and neural bases of facial emotion processing, much less work has been done to examine children whose emotion recognition skills have been altered or compromised by exposure to species-atypical early experiences. As has been the case with the literature on experience-dependent changes in brain function in general (see Fox, Levitt, & Nelson, 2010), the role of experience in the development of face processing has historically been examined by studying the effects of deprivation and/or abnormal or atypical early experience. For example, Mondloch, Maurer and colleagues studied the face processing abilities of children with congenital cataracts who were deprived of patterned visual input for the first months of life, and then had their vision restored. These children showed normal processing of facial features (e.g., subtle differences in the shape of the eyes and mouth), but impairments in processing facial configuration (i.e., the spacing of features within the face; Le Grand et al., 2001; Mondloch et al., 2002). These and related studies suggest that visual input during early infancy is necessary for the normal development of at least some aspects of face processing.
In a similar vein, maltreated children generally perform more poorly on emotion recognition tasks than do non-maltreated children (Camras, Grow, & Ribordy, 1983; Camras, Ribordy, & Hill, 1988). For example, Pollak and colleagues reported that perception of the facial expression of anger, but not other expressions, was altered in children who had experienced physical abuse. Compared to children with no history of abuse, Pollak and colleagues report that abused children showed a response bias for anger (Pollak, Cicchetti, Hornung, & Reed, 2000), identified anger based on less perceptual input (Pollak & Sinha, 2002), and showed altered category boundaries for anger (Pollak & Kistler, 2002). These results suggest that exposure to a limited range of facial emotion and/or altered emotional interactions with caregivers results in a change in the basic perception of emotional expressions in abused children.
A dramatic example of adverse early experience for young children is institutional rearing. Over the past 10 years we have been conducting a randomized controlled trial of foster care for early institutionalization (see Zeanah et al., 2003; Nelson et al., 2007 for study details). In the Bucharest Early Intervention Project (BEIP), three groups of children have been studied: those abandoned, placed and then raised in one of six institutions in Bucharest, Romania; those abandoned and placed in one of these institutions but then placed in high-quality foster care created, maintained and monitored by the study team; and never institutionalized children who live with their parents in Bucharest. Over time, we consistently reported that children with a history of institutionalization show impairments and delays in a variety of areas, including diminished intellectual function (Nelson et al., 2007; Fox et al., 2011), language (Windsor et al., 2007, 2011), and growth (Johnson et al., 2010); reductions in brain activity (Marshall et al.2004, 2008; Vanderwert et al., 2010); deficits in executive functions (Bos et al., 2009); a high prevalence of stereotypies (Bos et al., 2010) and of psychopathology (Zeanah et al., 2009); and disturbances of attachment (Smyke et al., 2010; Gleason et al., in press). In some of these domains we also observed considerable recovery if children are placed in high quality foster care before the age of approximately 2 years. Not surprisingly, the precise age cutoff varies by domains, with, for example, the sensitive period being earlier for language than for IQ (see Nelson, Bos, Gunnar, & Sonuga-Barke, in press). In other domains, however, we have seen little effect of foster care regardless of age at entry – for example, the prevalence of externalizing problems such as conduct disorder or disruptive behavior disorder is as high among children placed in foster care as among children living in institutions (see Zeanah et al., 2009), as is the prevalence of executive function problems (Bos et al., 2009).
There has been one domain, however, that appears to show relative sparing, and that pertains to the discrimination of facial identity and facial emotion. Across a series of studies, beginning in infancy and culminating at 3.5 years, using both behavioral and electrophysiological assays, we reported relatively few differences in how institutionalized children vs. never institutionalized children discriminate facial identity and facial emotion. For example, at age of entry into the study (average age 22 months; range = 5–31 months), using a preferential looking paradigm, we (Nelson et al., 2006) reported that institutionalized children performed just as well as never institutionalized children on a looking time task that tapped the ability to discriminate happy, sad, neutral and fearful faces; moreover, both groups showed the typical profile of looking longer at fearful faces vs. other faces (see Leppanen & Nelson, 2009 for discussion). We then followed up this sample after randomization to foster care, testing them again at 42 months, using the same paradigm and stimuli. Once again, we observed no group differences (see Jeon et al., 2010).
We have also examined the electrophysiological correlates of emotion processing in BEIP children. For example, we (Parker et al., 2005) presented infants at the initial assessment point (baseline) with alternating images of happy, fear, anger and sad faces while recording event-related potentials (ERPs). Although the institutionalized infants showed reduced amplitude of all ERP components, for the most part, there were only minor differences in emotion processing. Specifically, we found differences primarily in a number of early (sensory) components (N170, P250), but no differences in later, more perceptual, cognitive components (NC, PSW). In a follow up to this study, when the children were 42 months of age, the same paradigm was used, with essentially the same result -- no group differences in discriminating happy, sad, fearful or angry faces (Moulson et al., 2009a). Moreover, as in the behavioral study at 42 months (Jeon et al., 2010), and as Nelson & de Haan (1996) have observed in typically developing 6 month olds, all three groups of children showed a larger NC component (indicative of attention allocation) to the fearful face, a typical developmental pattern. Of particular interest in this study are two ERP components linked to early visual processing – P1 and N170, and two components linked to attention and inhibitory control – N2 and P300. Both the P1 and N170 have been shown to possess both sensitivity and specificity to face processing (with the N170 possessing great specificity than the P1). The N2 has been shown to be manipulated by attentional demands, whereas the P300 has been shown to be manipulated by both attentional and processing demands.
We originally hypothesized that institutional rearing would lead to deficits in facial emotion processing due to the effects such experiences have on the amygdala and orbitofrontal cortex, key structures involved in processing facial emotion (particularly fear; see Leppanen & Nelson, 2009; Leppanen & Nelson, in press). In fact, recent evidence indicates both structural (Mehta et al., 2009) and functional (Tottenham et al, 2011) consequences of such experience on the amygdala. However, because the cortical specialization that underpins facial emotion processing depends so heavily on experience, if children in institutions have adequate exposure to faces and facial emotion, a competing hypothesis is that discriminating facial expressions might be intact. Indeed, across our 4 studies to date examining this ability (reviewed above), we have found this to be the case. Nevertheless, discriminating one expression from another to some degree only requires relatively low-level perceptual abilities, which may be partially spared by institutional rearing. In the current study we were more interested in the recognition of facial emotion, not simple discrimination. Based on the work of Pollak and others, we anticipated that recognizing basic facial emotion (e.g. neutral) would be impaired in children with histories of institutionalization, however highly salient affective cues, such as those denoting anger or fear, might be spared as minimal exposure to these cues can induce adequate processing. However, whether there is sufficient plasticity in this system such that deficits in processing emotion can be compensated for by placement in foster care is unknown. Finally, although behavioral deficits were anticipated in processing facial emotion among our institutionalized children, we were less certain what to expect when it came to our ERP data. On the one hand we expected all three groups to be comparable in detecting the task relevant stimulus – an angry face – and thus show a normal P300. On the other hand, we did expect to observe a reduction in amplitude and prolonged latency of the P1 and N170 to the different emotions among the care as usual children compared to the never institutionalized children, although we were uncertain what to expect among the FCG. A similar pattern of findings was anticipated for the P1 and N2 components (i.e., reduced amplitude, longer latency). Regarding brain and behavior associations, we had several predictions. First, we predicted that there would be a positive correlation between the amplitude of the P300 (invoked by the task relevant stimulus, angry) and response accuracy; and second, that these correlations would be highest for the NIG, second highest for the FCG and worst for the CAUG.
To test these hypotheses, in the current study we report on the BEIP sample at age 8 years, using a somewhat more demanding task of emotion recognition. Specifically, we asked children to keep track of one particular emotion – anger – and to ignore two others – neutral and fear. On the assumption that a history of institutional rearing perturbs the development of the amygdala and its many projections, we hypothesized that children with a history of institutionalization would show impaired recognition of facial emotion both behaviorally and electrophysiologically.
Methods
The BEIP has been extensively described in a series of papers, and thus, we refer the reader to two papers in particular that describe in great detail our experimental design (Zeanah et al., 2003; Nelson et al., 2007) and the ethical issues that confronted the investigative team (Zeanah et al, 2006). Here, we offer only a brief description of the overall project and then discuss the design of the current study.
Participants
From a sample of 187 children abandoned at or near the time of birth and placed in one of six institutions throughout Bucharest, 136 were selected for participation in this study. These 136 children were deemed to be free of major genetic or neurological disease/disorders and not to have shown signs of fetal alcohol syndrome. Following an extensive baseline assessment when they were 6–30 months of age, half of these children were randomly assigned to remain in institutional care – what we refer to as the Care as Usual Group (CAUG) – and the other half were randomly assigned to a high-quality foster care program we created, maintained, and monitored – the Foster Care Group (FCG) (see Smyke et al., 2010 for details of the foster care program). We also enrolled an additional group of never institutionalized children (NIG) who had never spent time in an institution and who were recruited from pediatric clinics in Bucharest.
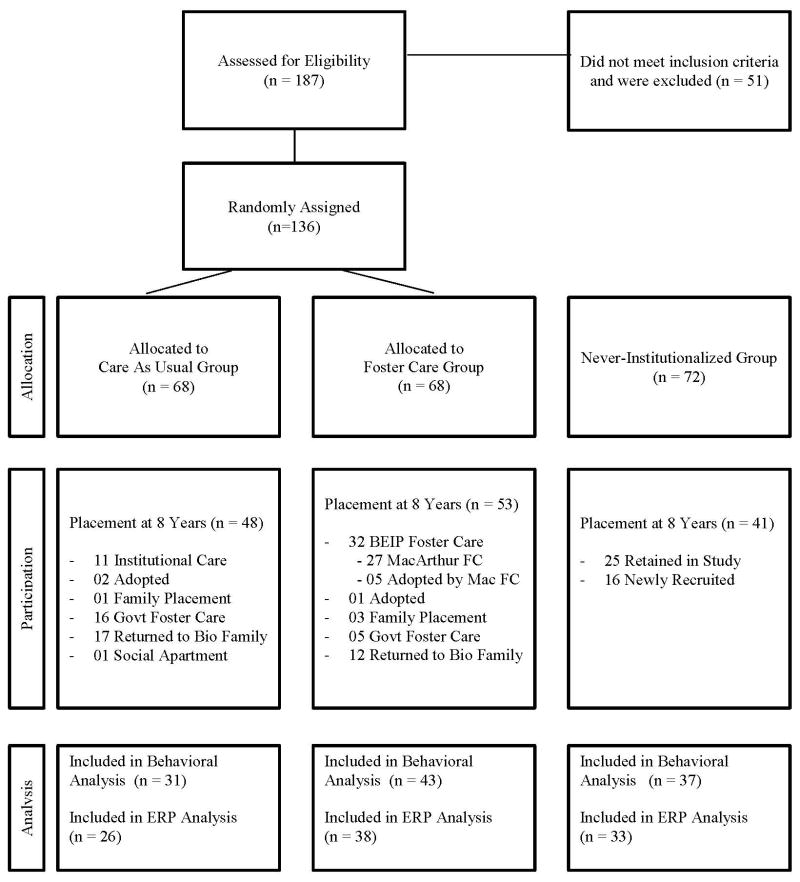
Between study entry and age 8 years, there were many changes in children’s group assignment. Thus, many children initially assigned to either the CAUG or FCG had changed living arrangements. Figure 1 illustrates the initial status of the children, as well as their status at age 8 years. At 8 years, 53 FCG children and 48 CAUG children agreed to participate in the testing session. In addition, 41 children from the NIG were included as a comparison group; of these, 16 were not part of the original longitudinal sample. Children were excluded from further analysis if their full-scale IQ, as measured on the Wechsler Test of Intelligence was less than 70 (14 CAUG, 10 FCG, 1 NIG). An additional 5 children were excluded due to equipment failure (3 CAUG and 2 NIG), and 1 child (NIG) was excluded for task non-compliance. The final sample for analysis of behavioral data included 31 (12 female) CAUG children, 43 (21 female) FCG children and 37 (16 female) NIG children. Children were excluded from electrophysiological analysis if they had fewer than 10 artifact-free ERP trials per condition or excessive eye or body movement artifact. The final sample for analysis of ERP data included 26 (11 female) CAUG, 38 (18 female) FCG, and 33 (14 female) NIG children.
Figure 1.
Group assignment over time: current status at 8 years of age
As in previous reports, all data were analyzed by adopting an intent-to-treat design; that is, we maintained the original group assignment of children regardless of where such children were residing at the time of the study. In so doing, the advantages of randomization were preserved, and thus estimates of the effects of the intervention were conservative. In addition, this approach permits the strongest test of our hypothesis that it is early experience that most powerfully contributes to subsequent development.
Informed consent was signed by the local Commission on Child Protection for each child participant living in his sector of Bucharest, as dictated by Romanian law. Further assent for each procedure was obtained from each caregiver who accompanied the child to the visit. The Institutional Review Boards of all three US Universities (representing Fox, Nelson & Zeanah) also approved the protocol.
Stimuli and Procedure
Drawing from the NimStim set of facial emotions (Tottenham et al., 2009), children viewed color photographs of Caucasian females portraying angry, fearful, and neutral expressions. Among this stimulus set, the Caucasian faces were most representative of the types of faces that Romanian children frequently encounter. We focused our attention on these three emotions for several reasons. First, it has been our intuition that children in institutions (at least in our institutions) are exposed to considerably less positive affect than negative affect, and thus, we sought to juxtapose two negative emotions to determine whether there would be a differential response. Second, a great deal is known about the neural substrate for fearful faces, although less is known about anger and neutral faces. Thus, we wanted to be certain to include fear as one of our stimuli (given its links to the amygdala, for example). Third, we used a neutral expression as a template against which the other expressions could be compared. Finally, although it would seem desirable to have included additional emotions (e.g., sad, happy), we were well aware of the performance limitations of many of our children and thus felt compelled to limit the task to just three emotions. Each emotion category was presented 48 times, randomly and equally distributed across 144 total trials. Each trial consisted of a 100-ms baseline, a 500-ms stimulus presentation, and a 700-ms post-stimulus recording, resulting in a total trial length of 1300-ms. The intertrial interval randomly varied between 500 and 1000 ms. Children were instructed to press a button whenever they saw an angry face, and not to press the button when they saw either a neutral or fearful face. The faces were presented on a gray background centered on a 15” computer screen and subtended a visual angle of approximately 5.3º x 6.5º. Every child completed the entire experiment.
The limitations of this task must be acknowledged. Although we could readily compare children’s overt responses to angry faces, because children were not asked to push a button to fear or neutral, we would have to infer accuracy information about these emotions from the lack of a button press. This in and of itself presented children with essentially two tasks: push to angry and do not push to fear or neutral. If we had been testing adults or a sample of typically developing children, we may well have elected to do a task that required three button presses (i.e., push one button to angry, another to fear, yet another to neutral). However, it is important to note that many of the children in this study are cognitively challenged (e.g., the Mean IQ of the CAUG is in the mid to upper 70s), and we were not confident that these children could perform such a complicated task. Thus, our compromise was to require children to push only one button. Second, by electing to make angry the target emotion we were unable to compare one target emotion to another (e.g., anger vs. fear). Again, it would have placed undue demands on our children to extend the task to all three emotions and if we had counterbalanced the task (for one group angry is the target, for another fear and for yet another, neutral), we would have lacked statistical power. These limitations notwithstanding, we felt that the current task would provide invaluable information about emotion processing, and would shed light on the impact of early psychosocial deprivation on this ability.
Procedure and Experimental Design
Children were seated in front of a computer screen and an electrode cap (Electro-Cap International, Inc., Eaton, OH) was fitted to their head (for details, see Moulson et al., 2009a,b; Vanderwert, 2010). Based on the International 10/20 system (Jasper, 1958), electrode location corresponded to 13 scalp locations (Fz, F3, F4, Cz, C3, C4, Pz, P3, P4, T7, T8, O1, O2) and left and right mastoids. The electro-oculogram (EOG) was recorded from electrodes placed directly above and below the left eye to record blinks and other eye movements. Cz was the reference electrode during acquisition. Following electrode placement, a mildly abrasive gel was inserted into each of the electrode sites, after which the scalp under each electrode site was gently abraded. A small amount of electrolytic gel was then inserted into each electrode. Impedances were at or below 10 kω for each electrode. EEG and EOG signals were amplified by factors of 5,000 and 2,500 respectively, with a 0.1 to 100 Hz band-pass filter, using custom bioelectric amplifiers from SA Instrumentation Company (San Diego, CA). All channels were digitized at 512 Hz onto the hard drive of a PC using a 12-bit analog-to-digital converter (+/− 2.5 V input range) and Snap-Master acquisition software (HEM Data Corporation, Southfield, MI).
A 30-Hz digital lowpass filter was applied using the ERP Analysis Systems software from James Long Company (Caroga Lake, NY). Subsequent data processing was carried out using the ERP32 data analysis software package (Version 3.82; New Boundary Technologies, Minneapolis, MN). Channels that exceeded +/− 100 microvolts were marked as bad in a particular trial. After the data were re-referenced to an average mastoids configuration, individual averages were created for each of the three stimulus conditions (anger, fear, neutral) using 100 ms prior to stimulus onset for baseline correction. During the averaging process, a trial was rejected if there were more than two channels marked bad due to artifact. Additionally, a blink correction algorithm was applied based on methods described in the literature (Gratton, Coles, & Donchin, 1983). Participants with fewer than 10 good trials per condition were excluded from further analysis. Only trials where a correct behavioral response was made within 1200-ms following stimulus onset were included for analyses. On average, participants contributed 32 trials per emotion condition (SD = 7.7). Grand means were created by averaging the individual averages together.
Grand means were inspected to identify components of interest (these components were targeted based on past face processing research). Two occipital components (P1, N170), one frontocentral component (N2), and one midline parietal component (P300) were analyzed. Peak amplitude (μV) and latency to peak amplitude (ms) were automatically extracted for the P1 (80–160 ms) and N170 (150–300 ms) at the right and left occipital electrodes (O1, O2), and for the N2 (300–500 ms) at central electrodes (C3, Cz, C4). Mean amplitude was extracted for the P300 (500–800 ms) at the midline parietal electrode (Pz).
Results
All statistical analyses were conducted using IBM SPSS Statistics 18.0. Because children were pressing a button to angry faces and inhibiting a button press to fearful and neutral faces, we considered this analogous to two tasks, and as a result, conducted separate analyses by trial type (i.e., one analysis on the “push-to-angry” trials and another to “do not push-to-fear/neutral” trials). Group differences in behavioral accuracy and reaction times for angry faces were examined using univariate analysis of variance (ANOVA). Behavioral accuracy (as inferred by the correct inhibition of a button press) for neutral and fearful faces were analyzed using a 2 emotion x 3 group (CAUG, FCG, NIG) repeated-measures (RM) ANOVA.
Electrophysiological measures (amplitudes and latencies) were also analyzed using RM ANOVA. Greenhouse-Geisser corrected degrees of freedom were used when the assumption of sphericity was violated. As in the behavioral data, ERP responses to angry faces were analyzed separately from neutral and fearful faces. Except for the P300, all analyses included group (CAUG, FCG, NIG) as a between-subjects factor and electrode as a within-subjects factor. Specifically, two levels of electrode (O1, O2) were included for the P1 and N170 analyses, and 3 levels of electrode (C3, Cz, C4) were included for the N2 analyses. The P300 is maximal at electrode Pz, therefore group comparisons were conducted for the mean amplitude at this electrode only. When the omnibus ANOVA revealed significant main or interaction effects (p ≤ .05), post hoc comparisons were carried out and bonferroni corrections for multiple comparisons were applied.
There were no associations between gender and any of the behavioral or electrophysiological dependent measures, and thus, gender was not included as a covariate in any of the analyses.
Behavioral Data
Univariate analyses revealed no differences between the groups for accuracy in identifying angry faces, F(2,110) = .777, p = .462., suggesting that children in all three groups were able to stay on task. Analyses of group and emotion effects for fearful and neutral face trials revealed a main effect of group, F(2,109) = 6.836, p = .002. Follow-up tests indicated that while the NIG (M = 84.2%, SD = 8.3) and FCG (M = 80.0%, SD = 14.3) showed equivalent behavioral performance on trials involving the inhibition of a button press (p = .638), both groups showed significantly better inhibition than children in the CAUG (M = 72.3%, SD = 14.6), p italic> .05 (see Figure 2). Analyses also revealed a main effect of emotion, F (1,109) = 270.184, p bold> .001, whereby all children showed better inhibition performance for neutral faces (M = 90.5%, SD = 9.9) compared to fearful faces (M = 68.1%, SD = 19.3). This last finding permits the inference that children had a harder time recognizing fear than they did neutral.
Figure 2.
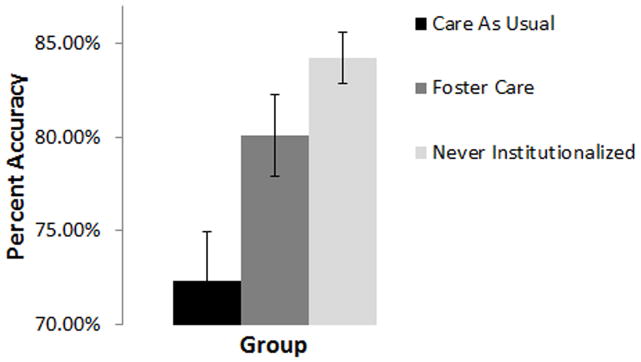
Main effect of group for behavioral accuracy (collapsed across fearful and neutral faces). Error bars represent +/− 1 standard error of the mean.
Univariate analyses of mean reaction times for correctly identified angry face trials revealed no differences between the groups (CAUG M = 634 ms, SD = 82; FCG M = 613 ms, SD = 89; NIG M = 633 ms, SD = 87), p = .507.
ERP Data
P1
Analysis of peak amplitude for angry faces revealed a marginal group effect, F(2,90) = 2.864, p = .06) such that angry faces elicited larger P1 amplitudes from children in the NIG (M = 16.19 μV, SD = 4.7) than for those in the CAUG (M = 12.70 μV, SD = 6.2); children in the FCG (M = 14.28 μV, SD = 5.6) showed responses that were not significantly different from the NIG or CAUG. Analyses of neutral and fearful face trials revealed a main effect of electrode, F(1,90) = 4.323, p = .04, which was qualified by an electrode x group interaction, F(2,90) = 4.269, p = .017. Post hoc comparisons revealed that only children in the CAUG showed larger P1 amplitudes at electrode O2 (M = 14.52 μV, SD = 6.6) compared to O1 (M = 12.38 μV, SD = 6.4), whereas children in the FCG (O1 M = 15.0 μV, SD = 5.6; O2 M = 14.64 μV, SD = 5.3) and NIG (O1 M = 15.63 μV, SD = 5.4; O2 M = 16.02 μV, SD = 5.9) showed similar amplitudes across both occipital electrodes.
Analysis of latency to the P1 component for angry faces revealed no main or interaction effects of group or electrode. There were also no main or interaction effects of group, electrode or emotion type for P1 latency on neutral and fearful face trials.
N170
Analysis of N170 peak amplitude for angry faces showed no significant main or interaction effects of group or electrode. Analyses of neutral and fearful face trials revealed a main effect of electrode, F (1,90) = 4.728, p = .032. A comparison of the electrode means indicated that data from O1 (M = −1.18 μV, SD = 4.1) showed larger peak amplitudes compared to O2 (M = −.60 μV, SD = 4.1). There was also a main effect of emotion, F (1,90) = 4.156, p = .044, in that fearful faces (M = −1.19 μV, SD = 4.2) elicited significantly larger N170 amplitudes compared to neutral faces (M = −.59 μV, SD = 4.1).
Latency analyses for angry faces revealed a marginally significant group effect, F (2, 90) = 2.810, p = .066, although follow-up analyses were not significant after correcting for multiple comparisons. Analyses of neutral and fearful face trials revealed a main effect of group, F (2,90) = 4.108, p = .02, which was qualified by an emotion x group interaction, F(2,90) = 4.158, p = .019. Post hoc comparisons revealed that only children in the NIG showed faster N170 latency to fearful faces (M = 227 ms, SD = 32) compared to neutral faces (M = 238 ms, SD = 33), whereas children in the CAUG (fearful face M = 235 ms, SD = 36, neutral face M = 229 ms, SD = 33) and FCG (fearful face M = 218 ms, SD = 27, neutral face M = 212 ms, SD = 26) showed similar N170 latencies for both face types.
N2
Analysis of N2 peak amplitude for angry faces revealed a main effect of electrode, F (2,188) = 4.088, p = .02, whereby the N2 amplitude was largest at electrode Cz (M = −10.63 μV, SD = 4.7) compared to C4 (M = −9.80 μV, SD = 4.0); data recorded at electrode C3 (M = −10.28 μV, SD = 4.1) was not significantly different from Cz or C4. Analyses of fearful and neutral face trials also revealed a main effect of electrode, F (2,188) = 19.488, p < .001. A comparison of the electrode means indicated that the largest amplitudes were recorded at electrode Cz (M = −10.16 μV, SD = 3.9) compared to C3 (M = −9.24 μV, SD = 3.5) and C4 (M = −8.82 μV, SD = 3.0).
Analysis of latency to the N2 component for angry faces, as well as for neutral and fearful faces, revealed no main or interaction effects.
P300
For angry faces, there were no group differences in the mean amplitude of the P300. Analyses of the fearful and neutral faces revealed a main effect of emotion, F (1,94) = 24.327, p < .001. As illustrated in Figure 3, follow-up tests showed that all children showed the largest P300 amplitude to fearful faces (M = 1.47 μV, SD = 2.4) compared to neutral faces (M = 0.24 μV, SD = 2.8).
Figure 3.
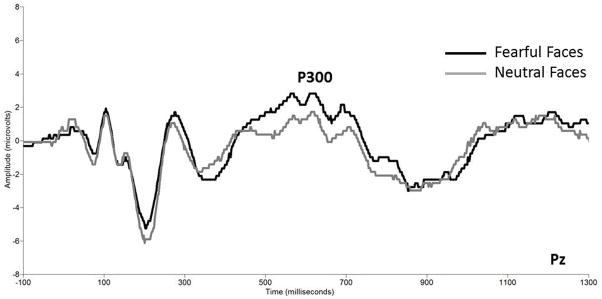
Grand averaged event-related potential waveforms showing the P300 amplitude to neutral and fearful faces at electrode Pz (collapsed across group). The x axis represents latency in milliseconds and the y axis represents amplitude in microvolts.
Timing Effects
In order to explore timing effects in the present study, we examined possible associations between age at placement in foster care and behavioral and electrophysiological data. There were no significant correlations between age at placement and behavioral accuracy in identifying angry, fearful or neutral faces, or with RT to identify angry faces. There were no associations between timing of placement and amplitude or latency for either the P1 or N170 components; there were also no associations between timing and mean amplitude of the P300. There were significant correlations, however, between age at placement and N2 amplitude for angry faces, r(38) = .374, p = .021 and for neutral faces, r(38) = .376, p = .02; the correlation was marginally significant for fearful faces, r(38) = .315, p = .054. Thus, the earlier the child was placed in foster care, the larger the N2 component.
Brain-Behavioral Correlations
We explored possible associations between behavioral task performance (accuracy, RT) and electrophysiological data (amplitude, latency). Correlations were run separately for each group. Contrary to our predictions, there were no significant brain-behavioral correlations revealed in this set of analyses.
Discussion
Let us begin by briefly summarizing the main findings to have emerged from this study, beginning with behavior and then moving to ERPs.
First, all three groups were equally accurate at recognizing anger, which suggests there were no group differences in being able to follow task instructions. Second, all three groups had a more difficult time recognizing fearful faces, which is consistent with the observation that fear is the last expression to be recognized at adult levels (generally in adolescence; see Thomas et al., 2007). Third, the NIG and FCG were both better at inhibiting a button press to neutral and fearful faces than the CAUG, suggesting that the CAUG was generally less accurate at recognizing these two emotions or that they had more difficulty with inhibiting a response generally (which we did not find to be the case in a traditional Go/No Go task we have also used with this sample; see McDermott et al., in press). This finding also suggests that the foster care intervention was effective in improving previously institutionalized children’s ability to recognize the emotions neutral and fear. Finally, there were no group differences in reaction time.
Regarding the ERP data, several intriguing findings emerged. First, the P1, a component that possesses some face sensitivity, was biggest to angry faces for the NIG, smallest among the CAUG and intermediate for the FCG. Contrary to our hypothesis, then, the CAUG showed the smallest response to anger (we anticipated that the CAUG might show the largest responses to anger and fear compared to the other two groups). In addition, as observed more generally with ERP amplitudes at 42 months (Moulson et al., 2009a), this finding also supports the notion that foster care improved the ability to process facial emotion.
In contrast, for the analysis of reactivity to fearful and neutral faces, the magnitude of the P1 did not differ between groups. However, the N170 and the P300 were larger to fearful as compared to neutral faces for all children, regardless of group. So, similar to the pattern of strong neural reactivity to angry faces, the task relevant stimulus, all groups exhibited equivalent responsivity to fearful faces. This pattern supports the assertion put forth by Leppanen and Nelson (2009; in press) that this emotion is of high signal value (even as early as 7 months of life; see Nelson & de Haan, 1996), and certain aspects of neural responses to angry or fearful faces are not strongly impacted by institutional rearing.
Although the children in foster care showed improvements in their ability to recognize fear and neutral faces (to a comparable level of performance as never institutionalized children), and their P1 to angry was midway between the NIG and CAUG, we observed no timing effects for this component, although the amplitude of the N2 was larger the earlier the child was placed in foster care. Our P1 findings are essentially identical to what we observed at 42 months, both for face processing generally (Moulson et al., 2009b) and for processing facial emotion (Moulson et al., 2009a), whereas our N2 finding is novel, and may serve as a marker for timing effects Finally, we observed no relations between our ERP and behavioral data.
These findings stand in stark contrast to our perceptual discrimination observed at earlier ages (Parker et al., 2005a,b; Moulson et al. 2009a,b). Whereas at earlier ages we observed relative sparing in the ability to discriminate facial expressions (as well as differentiating familiar from unfamiliar faces), here we did observe deficits among CAU children in processing facial emotion, both behaviorally and electrophysiologically. Moreover, we observed a number of subtle improvements in processing facial emotion among the foster care group, although we did not elevate their performance to be on par with the never institutionalized group. The fact that virtually no timing effects were observed (with the exception of the N2) suggests that, as with some of our mental health findings (Zeanah et al., 2009), children placed in foster care improve although they do so regardless of the age at which they were placed in foster care for this domain. Of course, the fact that the average age of placement was 22 months urges caution in this interpretation, for it may have been the case that earlier placement might have revealed timing effects.
We emphasize all data were analyzed using an intent-to-treat design. Because of the ethical requirement of non-interference in placement, by age 8 years only 14 children originally assigned to the CAUG were still actually living in an institution; the rest had been adopted, reunited with their biological families or placed in government foster care. But, because such change in placement occurred, on average, after the age of 2–3 years, the current findings speak to the power of early experiences.
The lack of correspondence between ERP and behavioral data, although not surprising, reinforces the notion that these two measures tap different levels of cognitive function. On the other hand, the ERP data were only examined for correct trials while the behavioral data obviously reflect both correct and incorrect trials. Moreover, the findings of impaired fear processing in the CAUG are in accordance with imaging work suggesting that environmentally driven differences in brain structure and function contribute to alterations in face processing (Mehta et al., 2009; Tottenham et al., 2011).
Overall, the current findings support the work of others (e.g., Wismer Fries & Pollak, 2004) suggesting that institutional care impairs the ability to recognize facial emotion. In addition, deficits in face recognition appear to be remediable, given that we found an intervention effect with high quality foster care leading to improvements in this ability. The data reinforce the need for utilizing multiple tasks examining not only discrimination of faces but recognition as well in order to identify possible subtle deficits that are the result of degraded early environmental input. In addition, although the data speak to the role of early adversity in influencing the recognition of facial emotion, much work remains to be done concerning what precisely is it about early institutionalization that influences the course of emotion processing, and for how long the neural systems that underlie this ability remain plastic.
Contributor Information
Charles A. Nelson, III, Boston Children’s Hospital1, Harvard Medical School2, Harvard Center on the Developing Child3.
Alissa Westerlund, Boston Children’s Hospital.
Jennifer Martin McDermott, University of Massachusetts-Amherst.
Charles H. Zeanah, Tulane University
Nathan A. Fox, University of Maryland
References
- Bos K, Fox N, Zeanah CH, Nelson CA. The effects of early psychosocial deprivation on the development of memory and executive function. Frontiers in Behavioral Neuroscience. 2009;3(16):1–7. doi: 10.3389/neuro.08.016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos KJ, Zeanah CH, Smyke AT, Fox NA, Nelson CA. Stereotypies in children with a history of early institutional care. Archives of Pediatrics & Adolescent Medicine. 2010;164(5):406–411. doi: 10.1001/archpediatrics.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camras LA, Ribordy SC, Hill J, Martino S, Spaccarelli S, Stefani R. Emotion recognition and production by abused children and mothers. Developmental Psychology. 1988;24(6):776–781. [Google Scholar]
- Camras L, Grow JG, Ribordy SC. Recognition of emotional expressions by abused children. Journal of Clinical Child Psychology. 1983;12(3):325–328. [Google Scholar]
- Fox NA, Almas AN, Degnan KA, Nelson CA, Zeanah CH. The effects of severe psychosocial deprivation and foster care intervention on cognitive development at 8 years of age: Findings from the Bucharest Early Intervention Project. Journal of Child Psychology and Psychiatry. 2011 doi: 10.1111/j.1469-7610.2010.02355.x. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SE, Levitt P, Nelson CA. How the timing and quality of early experiences influence the development of brain architecture. Child Development. 2010;81(1):28–40. doi: 10.1111/j.1467-8624.2009.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason MM, Fox NA, Drury S, Smyke AT, Egger HL, Nelson CA, Gregas MG, Zeanah CH. The validity of evidence-derived criteria for reactive attachment disorder: Indiscriminately social/disinhibited and emotionally withdrawn/inhibited types. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50(3):216–231. doi: 10.1016/j.jaac.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogry and Clinical Neurophysiology. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Jeon H, Nelson CA, Moulson M. The effects of early experience on emotion recognition: A study of institutionalized children in Romania. Infancy. 2010;15(2):209–221. doi: 10.1111/j.1532-7078.2009.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DE, Guthrie D, Smyke AT, Koga SF, Fox NA, Zeanah CH, Nelson CA. Growth and associations between auxology, caregiving environment, and cognition in socially deprived Romanian children randomized to foster vs ongoing institutional care. Arxchives of Pediatric Adolescent Medicine. 2010;164(6):507–516. doi: 10.1001/archpediatrics.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grand R, Mondloch CJ, Maurer D, Brent HP. Neuroperception: Early visual experience and face processing. Nature. 2001;410(6831):890. doi: 10.1038/35073749. [DOI] [PubMed] [Google Scholar]
- Leppanen JM, Nelson CA. Tuning the developing brain to social signals of emotions. Nature Reviews Neuroscience. 2009;10(1):37–47. doi: 10.1038/nrn2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppanen JM, Nelson CA. Current Directions in Psychological Science. Early development of fear processing. (in press) [Google Scholar]
- Marshall P, Reeb BC, Fox NA, Nelson CA, Zeanah CH. Effects of early intervention on EEG power and coherence in previously institutionalized children in Romania. Development and Psychopathology. 2008;20:861–880. doi: 10.1017/S0954579408000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, Fox NA the BEIP Core Group. A comparison of the electroencephalogram between institutionalized and community children in Romania. Journal of Cognitive Neuroscience. 2004;16:1327–1338. doi: 10.1162/0898929042304723. [DOI] [PubMed] [Google Scholar]
- McDermott JM, Westerlund A, Zeanah CH, Nelson CA, Fox NA. Early adversity and neural correlates of inhibitory control: Implications for academic adjustment. Developmental Cognitive Neuroscience. doi: 10.1016/j.dcn.2011.09.008. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SCR, et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: The English and Romanian Adoptees Study Pilot. Journal of Child Psychology & Psychiatry. 2009;50:943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- Mondloch CJ, Geldart S, Maurer D, Le Grand R. Developmental changes in face processing skills. Journal of Experimental Child Psychology. 2002;86(1):67–84. doi: 10.1016/s0022-0965(03)00102-4. [DOI] [PubMed] [Google Scholar]
- Moulson MC, Fox NA, Zeanah CH, Nelson CA. Early adverse experiences and the neurobiology of facial emotion processing. Developmental Psychology. 2009a;45(1):17–30. doi: 10.1037/a0014035. [DOI] [PubMed] [Google Scholar]
- Moulson MC, Westerlund A, Fox NA, Zeanah CH, Nelson CA. The effects of early experience on face recognition: An event related potential study of institutionalized children in Romania. Child Development. 2009b;80:1039–1056. doi: 10.1111/j.1467-8624.2009.01315.x. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Bos K, Gunnar MR, Sonuga-Barke E. The neurobiological toll of early human deprivation. In: McCall R, editor. Children without Permanent Parental Care: Research, Practice, and Policy. Monographs of the Society for Research in Child Development. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, Zeanah CH, Fox NA, Marshall PJ, Smyke A, Guthrie D. Cognitive recovery in socially deprived young children: The Bucharest Early Intervention Project. Science. 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Parker SW, Guthrie D the BEIP Core Group. The discrimination of facial expressions by typically developing infants and toddlers and those experiencing early institutional care. Infant Behavior and Development. 2006;29(2):210–219. doi: 10.1016/j.infbeh.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Nelson CA, De Haan M. Neural correlates of infants’ visual responsiveness to facial expressions of emotion. Developmental Psychobiology. 1996;29(7):577–595. doi: 10.1002/(SICI)1098-2302(199611)29:7<577::AID-DEV3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Parker SW, Nelson CA the BEIP Core Group. The impact of deprivation on the ability to discriminate facial expressions of emotion: An event-related potential study. Child Development. 2005;76:54–72. doi: 10.1111/j.1467-8624.2005.00829.x. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Kistler DJ. Early experience is associated with the development of categorical representations for facial expression of emotion. Proceedings of the National Academy of Sciences. 2002;99(13):9072–9076. doi: 10.1073/pnas.142165999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, Sinha P. Effects of early experience on children’s recognition of facial displays of emotion. Developmental Psychology. 2002;38(5):784–791. doi: 10.1037//0012-1649.38.5.784. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Cicchetti D, Hornung K, Reed A. Recognizing emotion in faces: Developmental effects of child abuse and neglect. Developmental Psychology. 2000;36(5):679–688. doi: 10.1037/0012-1649.36.5.679. [DOI] [PubMed] [Google Scholar]
- Smyke AT, Zeanah CH, Fox NA, Nelson CA, Guthrie D. Placement in foster care enhances quality of attachment among young institutionalized children. Child Development. 2010;81(1):212–223. doi: 10.1111/j.1467-8624.2009.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita Y. Face perception in monkeys reared with no experience to faces. Proceedings of the National Academy of Sciences. 2008;105(1):394–398. doi: 10.1073/pnas.0706079105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas LA, De Bellis MD, Graham R, LaBar KS. Development of emotional facial recognition in late childhood and adolescence. Developmental Science. 2007;10:547–558. doi: 10.1111/j.1467-7687.2007.00614.x. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gihooly T, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and differences in emotion recognition. Developmental Science. 2010;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon A, McCarry T, Nurse M, Hare TA, Nelson CA. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwert RE, Marshall PJ, Nelson CA, Zeanah CH, Fox NA. Timing of intervention affects brain electrical activity in children exposed to severe psychosocial neglect. PloS ONE. 2010;5:1–5. doi: 10.1371/journal.pone.0011415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor J, Benigno JP, Wing CA, Carroll PJ, Koga SF, Nelson CA, Fox NA, Zeanah CH. Language recovery in young children. Child Development. doi: 10.1111/j.1467-8624.2011.01604.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor J, Glaze LE, Koga SF the BEIP Core Group. Language acquisition with limited input: Romanian institution and foster care. Journal of Speech, Language, and Hearing Research. 2007;50:1365–1381. doi: 10.1044/1092-4388(2007/095). [DOI] [PubMed] [Google Scholar]
- Wismer Fries AB, Pollak SD. Emotion understanding in postinstitutionalized Eastern European children. Development and Psychopathology. 2004;6:355–369. doi: 10.1017/S0954579404044554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah CH, Egger HL, Smyke AT, Nelson CA, Fox NA, Marshall PJ, Guthrie D. Altering early experiences reduces psychiatric disorders among institutionalized Romanian preschool children. American Journal of Psychiatry. 2009;166:777–785. doi: 10.1176/appi.ajp.2009.08091438. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Koga S, Simion B, Stanescu A, Tabacaru C, Fox N, et al. Ethical considerations in international research collaboration: The Bucharest Early Intervention Project. Infant Mental Health Journal. 2006;27(6):559–576. doi: 10.1002/imhj.20107. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Nelson CA, Fox NA, Smyke AT, Marshall P, Parker SW, Koga S. Designing research to study the effects of institutionalization on brain and behavioral development: The Bucharest Early Intervention Project. Development and Psychopathology. 2003;15:885–907. doi: 10.1017/s0954579403000452. [DOI] [PubMed] [Google Scholar]