PEGylation is the most common and successful surface-chemistry strategy for reducing nonspecific accumulation and prolonging blood circulation of inorganic nanoparticles (NPs), so that the NPs can effectively target tumors through well-known “enhanced permeability and retention (EPR)” effect.[1] These strengths fundamentally arise from the fact that poly(ethylene glycol) (PEG) moiety on the particle surface creates steric hindrance for the serum protein (opsonin) adsorption and slows down the NP uptake by the reticuloendothelial system (RES) organs (liver, spleen etc.).[2] However, the majority of PEGylated NPs still end up in RES organs after the circulation,[3] resulting in low targeting specificity (defined as the amount of NPs in tumor vs that in liver).[4] For instance, even though PEGylated AuNPs with a 2 nm core size can circulate in the body at a high concentration, they were found to severely accumulate in the liver (78 %ID/g) and spleen (15.2 %ID/g) at 24 h post-injection (p.i.).[5] Such long-term severe accumulation in RES potentially induces health hazards, hampering their clinical translation. Therefore, developing PEGylated inorganic NPs that not only can retain strong EPR effect but also can be eliminated from the urinary system like clinically used small molecular contrast agents[6] is highly desired but remains a big challenge.
High RES uptake of PEGylated inorganic NPs essentially results from their large hydrodynamic diameters (HDs) above the kidney filtration threshold (~5.5 nm).[7] For instance, for 2 nm AuNPs, their HDs were increased to 9~10 nm after PEGylation.[5] To develop renal clearable PEGylated quantum dots (QDs), Choi and coworkers investigated the influences of PEG lengths (PEG-n, n= 2, 3, 4, 8, 14, 22) on the renal clearance of QDs and observed efficient renal clearance from the QDs conjugated with PEG-4.[8] PEG ligands with other lengths failed to enhance renal clearance of QDs due to either large HDs or low physiological stability.[8] However, tumor targeting of the renal clearable PEGylated QDs is still not clear. Silica NPs of ~7 nm coated with 0.5 kDa PEG were also renal clearable,[9] but the passive tumor-targeting efficiency was only 0.9 %ID/g at 4 h p.i..[10]
To avoid significant increases in HDs, zwitterionic ligand-based surface chemistry was used in the development of renal clearable NPs.[11] For example, HDs of 3 nm QDs and 2.5 nm luminescent AuNPs were only 4.9 nm and 3.4 nm in the presence of serum protein after being coated with cysteine (CS-QDs)[11a] or glutathione (GS-AuNPs),[11h] respectively; as a result, they were effectively eliminated from the urinary system (CS-QDs: >65 %ID, 4 h p.i.; GS-AuNPs: >60 %ID, 48 h p.i.).[11a,g] However, short retention time and low concentration of these renal clearable NPs in the blood sacrificed their effectiveness in passive tumor targeting through EPR effect. Consequently, the tumor contrast was only enhanced ~80% over normal tissues of the mice after being injected with CS-QDs.[11b] Overall tumor targeting efficiency of GS-AuNPs was only 2.3 %ID/g at 12 h p.i..[11h]
Low tumor-targeting efficiencies of these known renal clearable zwitterionic NPs raise a new challenge in the delivery of inorganic NPs into clinical practices. In addition, fundamental understandings of how PEGylation and zwitterionization based surface chemistries influence renal clearance and tumor targeting of renal clearable NPs are still missing. To address these challenges, we created a renal clearable PEGylated near-IR-emitting AuNP (NIR-emitting PEG-AuNPs) with photophysical properties, core sizes, low affinity to serum protein, and high physiological stability identical to our previously reported zwitterionic NIR-emitting GS-AuNPs (Figure S1).[11h] By conducting head-to-head comparison of these two NPs in renal clearance and tumor targeting, we were able to unravel pro and cons of these two surface chemistries in tumor imaging of luminescent AuNPs. Our results show that NIR-emitting PEG-AuNPs exhibit efficient renal clearance and low RES accumulation comparable to NIR-emitting GS-AuNPs: >50 %ID were excreted in the urine and only <4 %ID/g of NPs were accumulated in the liver 24 h p.i.. However, these two types of renal clearable luminescent AuNPs are significantly different in tumor targeting: (1) PEG-AuNPs targeted the tumor with an efficiency of ~8 %ID/g at both 1 and 12 h p.i., which is ~3 times higher than that of GS-AuNPs and comparable to the high tumor targeting efficiencies of reported non-renal clearable NPs;[1b,12] (2) PEG-AuNPs also exhibited a higher passive targeting specificity (2.4, at 12 h p.i.) than GS-AuNPs (1.6, at 12 h p.i.) and other known renal clearable or non-renal clearable NPs;[1a,12b,13] (3) accumulation of PEG-AuNPs in the tumor and their clearance from normal tissues were 7 and 5 times slowly than those of GS-AuNPs, respectively; (4) contrast index (CI) of PEG-AuNPs reached a threshold for substantial tumor detection (CI = 2.5) much slowly than that of GS-AuNPs (12 h vs 3 h); (5) Similar differences in renal clearance and tumor targeting between PEG-AuNPs and GS-AuNPs were also observed in PC-3 prostate tumors, indicating the observed surface-chemistry effects on tumor targeting of luminescent AuNPs can be generalized to other tumor models. The fundamental understandings of the strengths and limitations of these two surface chemistries in tumor targeting of renal clearable AuNPs provide a foundation for design of new generation of renal clearable AuNPs for future clinical practices.
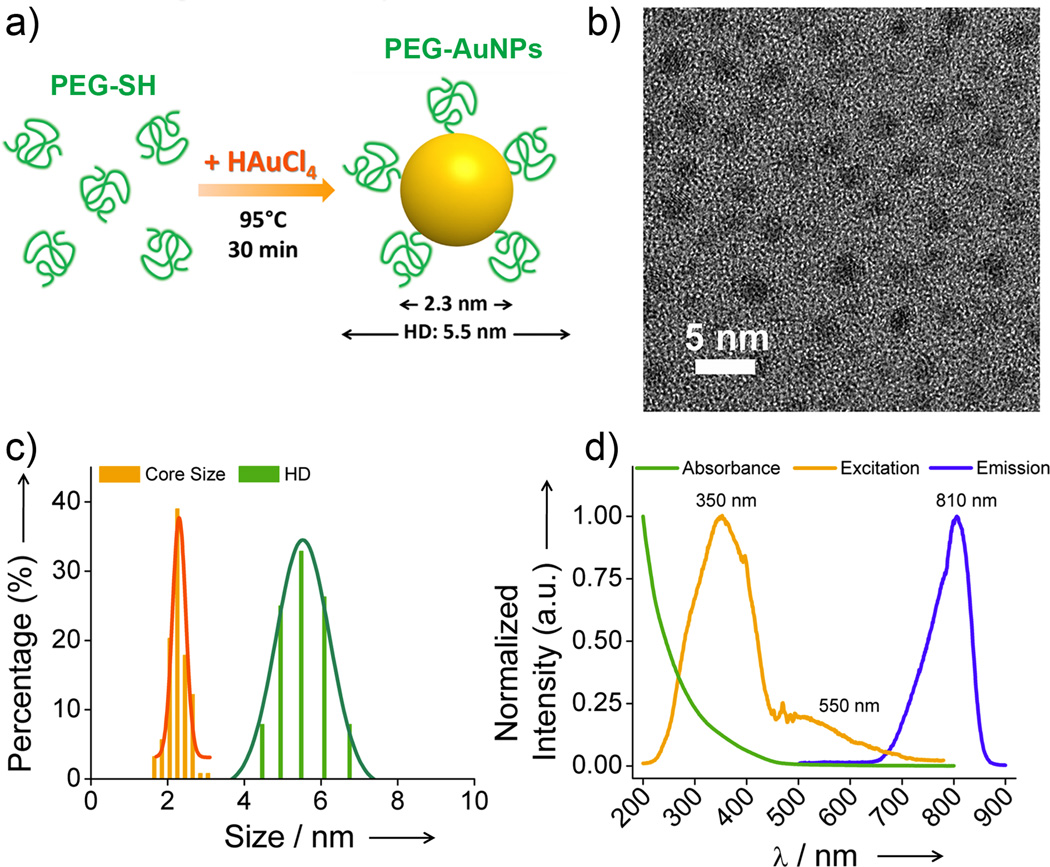
NIR-emitting PEG-AuNPs were created through a facile one-step synthesis by thermally reducing HAuCl4 in the presence of thiolated PEG (PEG-SH) ligands with molecular weight (MW) of 1 kDa (~21 units) in aqueous solution (Figure 1a and Figure S2, and Supporting Information). Neither shorter (0.35 kDa) nor longer (5 kDa) PEG ligands can generate highly luminescent (Figure S3) or stable AuNPs (Figure S4). The core size of PEG-AuNPs was measured to be 2.3 ± 0.3 nm, very similar to that of GS-AuNPs (2.5 nm), but their HD (5.5 ± 0.4 nm) was slightly larger than the HD of GS-AuNPs (3.3 nm) (Figure 1b&c). Such a larger increase in HD after PEGylation in comparison with zwitterionic ligand coating was in agreement with previous findings.[14] However, the HD layer of PEG-AuNPs (1.6 nm) is much thinner than those of PEGylated 2 nm AuNPs (3.5 – 4 nm),[5] and comparable to the calculated Flory radius (F, ~1.9 nm) of PEG (MW, 1 kDa) in “mushroom” conformation on the AuNP surface,[1c] suggesting a relatively low-density structure of PEG on the particle surface rather than a high-density extended structure.[14]
Figure 1.
Characterization of NIR-emitting PEGylated AuNPs (PEG-AuNPs). a) Scheme of the particle synthesis. b) Typical TEM image of the synthesized PEG-AuNPs. c) The core size measured by TEM, and hydrodynamic diameter (HD) of PEG-AuNPs in PBS measured by dynamic light scattering (DLS). d) Absorption and luminescent spectra of PEG-AuNPs in PBS at pH 7.4.
The PEG-AuNPs exhibited strong emission with a maximum at 810 nm and broad excitation with two peaks at 350 and 550 nm (Figure 1d), exactly identical to those of GS-AuNPs,[11h] suggesting that surface chemistries have little influence on luminescence from AuNPs. In addition, like GS-AuNPs,[11h] NIR-emitting PEGylated AuNPs also exhibit high physiological stability (Figure S5) and high resistance to serum protein adsorption after being incubated in mouse serum protein at 37 °C for 48 h (Figure S6). These similarities in photophysical properties between PEG-AuNPs and GS-AuNPs make it feasible to conduct head-to-head comparison on their renal clearance and tumor targeting under the same imaging conditions.
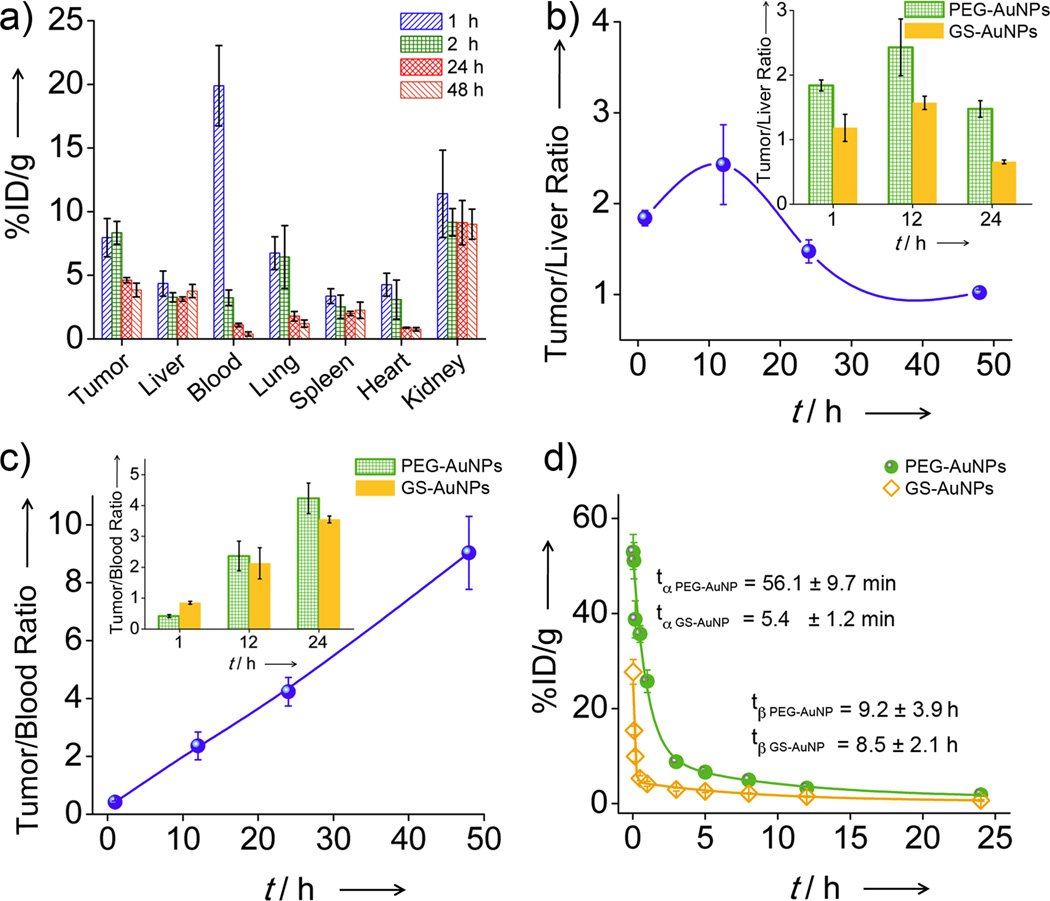
In order to quantify the tumor-targeting efficiency and specificity, MCF-7 tumor-bearing mice were used as a model system and biodistribution of PEG-AuNPs in the mice at the selected p.i. time points (1, 12, 24, and 48 h) was measured with ICP-mass spectrometry (ICP-MS, Figure 2a). The tumor-targeting efficiencies of PEG-AuNPs were determined to be 8.0 ± 1.5 and 8.3 ± 0.9 %ID/g at 1 and 12 h p.i., respectively, which were ~3 and 10 times higher than those of GS-AuNPs (2.3 %ID/g at 12 h p.i.)[11h] and renal clearable silica NPs (0.9 %ID/g),[10] respectively. The targeting efficiency of ~8 %ID/g was also comparable to those of reported non-renal clearable NPs with strong EPR effect, such as PEGylated 20 nm AuNPs (6.63 %ID/g),[12b] and six-armed PEGylated Ag2S QDs (10 %ID/g).[12c] In terms of liver uptake, the maximum accumulation was 4.35 %ID/g at 1 h p.i. and the amount remained roughly constant (3.27~3.76 %ID/g) during subsequent 48 h, which is comparable to the liver accumulation of GS-AuNPs.[11g,h] Since the clearance of PEG-AuNPs in tumor and liver was different, the targeting specificity was time-dependent (Figure 2b) and reached its maximum of 2.4 ± 0.4 at 12 p.i., higher than that of GS-AuNPs (1.6 ± 0.1)[11h] and nearly two orders better than any known non-renal clearable AuNPs.[1a,12b,13] The difference in tumor and liver uptake behaviors between PEG-AuNPs and GS-AuNPs, suggested that PEGylation of renal clearable AuNPs could further enhance tumor- targeting specificity without sacrificing low RES uptake.
Figure 2.
Biodistribution analysis of the passive tumor targeting in MCF-7 tumor-bearing mice. a) The biodistributions of PEG-AuNPs at 1, 12, 24, and 48 h p.i.. b) The time-dependent ratios of PEG-AuNPs concentration in tumor to that in liver within 48 h p.i. (Inset: a comparison of PEG-AuNPs and GS-AuNPs in tumor/liver ratios at 1, 12, and 24 h p.i.). c) The time-dependent tumor/blood ratios of PEG-AuNPs within 48 h p.i. (Inset: a comparison of the two probes in tumor/blood ratios at 1, 12, and 24 h p.i.). d) Pharmacokinetics of these two probes after intravenous (IV) injection (Data presented as mean ± SD, n = 3).
The origin of high tumor-targeting efficiency of these NIR-emitting PEG-AuNPs is attributed to their strong EPR effect enhanced by PEGylation. To determine how well EPR effect functions, the tumor/blood ratio of probes is a typical parameter to be measured.[1e] Shown in Figure 2c, the tumor/blood ratio of PEG-AuNPs monotonously increased with time and reached 9.0 ± 1.3 at 48 h p.i., comparable to many known non-renal clearable NPs with strong EPR effect. For example, the tumor/blood ratio (4.2 ± 0.5) of the PEG-AuNPs at 24 h p.i. was higher than those of PEG coated 33 nm gold nanocages (3.81 ± 1.08)[12a] and GS-AuNPs at the same time point (Figure 2c), suggesting that PEGylation can enhance EPR effect than zwitterionization. While EPR effect is often considered as a unique strength of non-renal clearable NPs because they can escape the rapid kidney filtration and remain in the blood at a high concentration,[1f,15] the observed high tumor/blood ratio of PEG-AuNPs clearly indicated that strong EPR effect can also be achieved by tuning the surface chemistry of renal clearable NPs.
The enhanced EPR effect of PEG-AuNPs is fundamentally due to prolonged retention time and high concentration of the AuNPs in the blood. A classical two-compartment pharmacokinetics was observed for PEG-AuNPs, which were 56.1 ± 9.7 min and 9.2 ± 3.9 h for distribution half-life (t1/2α) and elimination half-life (t1/2β), respectively (Figure 2d). The t1/2α of PEG-AuNPs was one order longer than that of GS-AuNPs (5.4 ± 1.2 min) even though the t1/2β of PEG-AuNPs and GS-AuNPs (8.5 ± 2.1 h) were comparable. In addition, area under the cure (AUC) of PEG-AuNPs (142.8 %ID·h/g) was 3 times larger than that of GS-AuNPs (47.2 %ID·h/g) 24 h p.i.. Such large AUC of PEG-AuNPs in the blood is fundamentally responsible for their high tumor-targeting efficiency.
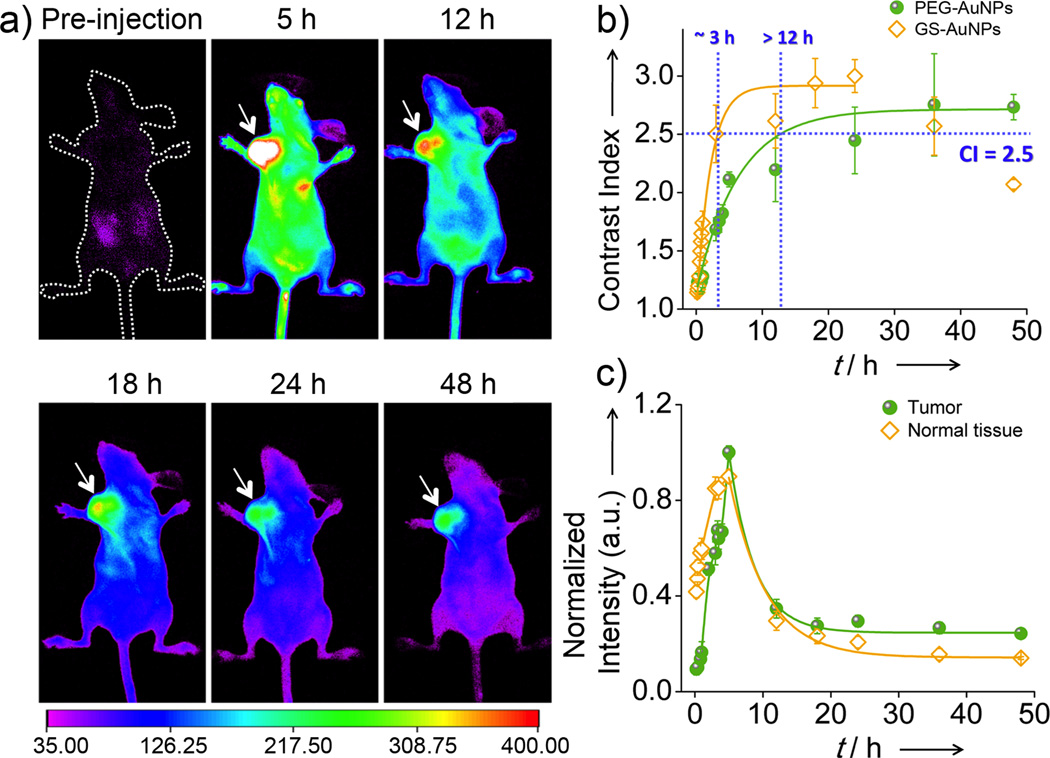
Subsequently, passive tumor-targeting kinetics of NIR-emitting PEG-AuNPs was measured through real-time imaging of the particle accumulation in the MCF-7 tumor. While the tumor area was hardly distinguished right after IV injection of the particles, it became visible with time increasing (Figure 3a). As shown in Figure 3b, after IV injection of PEG-AuNPs, it took > 12 h for the tumor area to reach the contrast index (CI) threshold (CI = 2.5),[16] a general parameter for evaluation of imaging quality,[17] much longer than the time needed for GS-AuNPs (~3 h).[11h] To gain more quantitative understanding of tumor targeting of PEG-AuNPs, we quantified and compared accumulation and clearance kinetics of PEG-AuNPs with those of GS-AuNPs. As shown in Figure 3c, fluorescence intensities of normal tissues reached maximum at 5 h p.i., while it only took < 10 min for GS-AuNPs to reach their maximum.[11h] The clearance kinetics of PEG-AuNPs in the normal tissues exhibited a monoexponential decay with a half-life of 4.1 ± 0.2 h, more than 5 times longer than that of GS-AuNPs (43.4 ± 6.6 min)[11h] (Figure 3c). Subsequent analysis of tumor retention kinetics showed that PEG-AuNPs reached their maximum accumulation at the tumor site at 5 h p.i., more than 7 times slowly than that of GS-AuNPs (~40 min)[11h] and consistent with their slow diffusion in the normal tissues. Once the emission intensity of the tumor reached its maximum, PEG-AuNPs, like many other NPs[13a] or nanosized proteins,[1e] slowly released back to the blood stream. Tumor clearance kinetics of PEG-AuNPs can be fitted with monoexponential decay with half-life of 3.7 ± 0.8 h. Interestingly, unlike GS-AuNPs, of which more than 76% remain in the tumor at 24 h p.i., only ~30% of the PEG-AuNPs were retained in the tumor.
Figure 3.
Passive tumor-targeting kinetics of PEG-AuNPs in MCF-7 tumor bearing nude mice. a) In vivo NIR fluorescence images of the mouse IV injected with PEG-AuNPs at 5, 12, 18, 24, and 48 h p.i. (arrow: tumor location). b) Time-dependent contrast index (CI) of tumor area after IV injection of PEG-AuNPs and GS-AuNPs, respectively. c) Accumulation and retention kinetics of PEG-AuNPs in tumor and normal tissues.
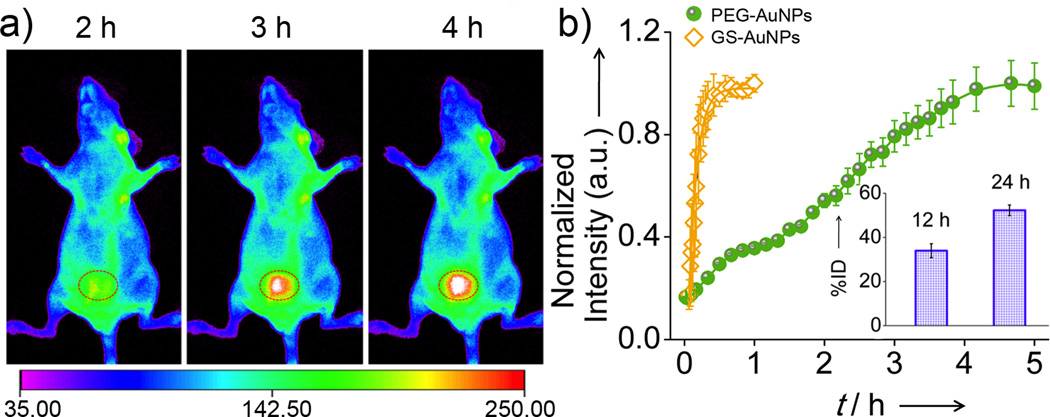
The slow clearance of PEG-AuNPs from the normal tissues was also consistent with the observation in their renal clearance kinetics. While GS-AuNPs were rapidly excreted into the bladder and bladder intensity reached the maximum within 1 h p.i., very little accumulation of PEG-AuNPs in the bladder was observed in the same time window. The bladder area started to become visible at 3 h p.i., and reached the maximum intensity at 5 h p.i. (Figure 4). The urine collected from the mouse IV injected with PEG-AuNPs within the first 5 h p.i. showed a strong NIR emission identical to the pre-injected one, further confirming that the particles were renal clearable and fairly stable during the circulation in the body (Figure S7&S8). Although the PEG-AuNPs showed a slow renal clearance in the initial stage, a comparable amount of PEG-AuNPs and GS-AuNPs[11g] were found in the urine 24 h p.i., suggesting that the slow clearance of PEG-AuNPs from the normal tissues results from their slow diffusion in the body rather than delayed RES uptake.
Figure 4.
Renal clearance kinetics of PEG-AuNPs in nude mice. a) In vivo NIR fluorescence images of the nude mouse IV injected with PEG-AuNPs collected at 2, 3, and 4 h p.i. (circle: bladder area). b) Renal clearance kinetics of PEG-AuNPs and GS-AuNPs (Inset: PEG-AuNPs found in the urine measured by ICP-MS 12 and 24 h p.i.).
In summary, we synthesized the first renal clearable NIR-emitting PEGylated AuNPs through a facile one-step method, which exhibit photophysical properties and core size identical to zwitterionic GS-AuNPs. Systematic studies on renal clearance, pharmacokinetics and passive tumor targeting of PEG-AuNPs and GS-AuNPs in MCF-7 tumor-bearing mice show that PEG-AuNPs can effectively target tumor with an efficiency 3 times higher than that of GS-AuNPs while both of them exhibited comparable low RES uptake. These results were further confirmed in prostate PC-3 tumor xenografts (Figure S9), suggesting that the observed differences in tumor targeting between PEGylation and zwitterionization are a general phenomenon in renal clearable luminescent AuNPs and can be generalized to other tumor models. High tumor-targeting efficiency of PEG-AuNPs is fundamentally because PEGylation can enhance EPR effect of renal clearable luminescent AuNPs than zwitterionization by increasing their retention time and concentration in the blood (large AUC). However, the limitation of PEGylation in tumor imaging is that it took much longer time for PEG-AuNPs to reach desired CI threshold than zwitterionic GS-AuNPs because of their slow tumor accumulation and normal-tissue clearance. These differences in tumor targeting of luminescent AuNPs imply that PEGylated AuNPs are potentially more suitable for cancer therapy due to their high targeting efficiency and long tumor retention while zwitterionic GS-AuNPs have potential in cancer diagnose due to their short detection time and rapid clearance from normal tissues.
While we successfully unraveled different effects of zwitterionization and PEGylation surface chemistries on tumor targeting and renal clearance of NIR-emitting AuNPs, the fundamental understandings of the origin of these differences still demand more efforts. In addition, a recent interesting observation of renal clearance of silica NPs with size > 50 nm,[18] much larger than kidney filtration threshold (~ 5.5 nm), encourages more thorough understandings of renal clearance of NPs. Nevertheless, the observed differences of these two distinct surface chemistries in the tumor targeting and renal clearance are expected to help further develop a new generation of renal clearable inorganic NPs that can be eventually translated into the clinical practices with minimized nanotoxicity.
Supplementary Material
Acknowledgments
This work was supported in part by the NIH (R21EB009853 to J.Z.), CPRIT (RP120588) and the start-up fund from the University of Texas at Dallas (J.Z.). N.X. acknowledged Office of Sponsored Research for undergraduate research award.
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.a) Wang Y, Liu Y, Luehmann H, Xia X, Brown P, Jarreau C, Welch M, Xia Y. Acs Nano. 2012;6:5880–5888. doi: 10.1021/nn300464r. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Chen JY, Glaus C, Laforest R, Zhang Q, Yang MX, Gidding M, Welch MJ, Xia YN. Small. 2010;6:811–817. doi: 10.1002/smll.200902216. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanomedicine-Uk. 2011;6:715–728. doi: 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Della Rocca J, Huxford RC, Comstock-Duggan E, Lin WB. Angew. Chem. Int. Edit. 2011;50:10330–10334. doi: 10.1002/anie.201104510. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Matsumura Y, Maeda H. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]; f) Iyer AK, Khaled G, Fang J, Maeda H. Drug Discov. Today. 2006;11:812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]; g) Hong H, Zhang Y, Sun J, Cai W. Nano Today. 2009;4:399–413. doi: 10.1016/j.nantod.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a) Kommareddy S, Amiji M. J. Pharm. Sci. 2007;96:397–407. doi: 10.1002/jps.20813. [DOI] [PubMed] [Google Scholar]; b) Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Mol. Pharmaceut. 2008;5:505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schipper ML, Iyer G, Koh AL, Cheng Z, Ebenstein Y, Aharoni A, Keren S, Bentolila LA, Li JQ, Rao JH, Chen XY, Banin U, Wu AM, Sinclair R, Weiss S, Gambhir SS. Small. 2009;5:126–134. doi: 10.1002/smll.200800003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a) Aillon KL, Xie Y, El-Gendy N, Berkland CJ, Forrest ML. Adv. Drug Deliv. Rev. 2009;61:457–466. doi: 10.1016/j.addr.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Dobrovolskaia MA, McNeil SE. Nat. Nanotechnol. 2007;2:469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]; c) Kareem H, Sandstrom K, Elia R, Gedda L, Anniko M, Lundqvist H, Nestor M. Tumour Biol. 2010;31:79–87. doi: 10.1007/s13277-009-0011-2. [DOI] [PubMed] [Google Scholar]
- 5.Arvizo RR, Miranda OR, Moyano DF, Walden CA, Giri K, Bhattacharya R, Robertson JD, Rotello VM, Reid JM, Mukherjee P. PLoS One. 2011;6:e24374. doi: 10.1371/journal.pone.0024374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Kelloff GJ, Hoffman JM, Johnson B, Scher HI, Siegel BA, Cheng EY, Cheson BD, O'Shaughnessy J, Guyton KZ, Mankoff DA, Shankar L, Larson SM, Sigman CC, Schilsky RL, Sullivan DC. Clin. Cancer Res. 2005;11:2785–2808. doi: 10.1158/1078-0432.CCR-04-2626. [DOI] [PubMed] [Google Scholar]; b) Colosimo C, Demaerel P, Tortori-Donati P, Christophe C, Van Buchem M, Hogstrom B, Pirovano G, Shen N, Kirchin MA, Spinazzi A. Pediatr. Radiol. 2005;35:501–510. doi: 10.1007/s00247-004-1392-4. [DOI] [PubMed] [Google Scholar]
- 7.Longmire M, Choyke PL, Kobayashi H. Nanomedicine-Uk. 2008;3:703–717. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi HS, Ipe BI, Misra P, Lee JH, Bawendi MG, Frangioni JV. Nano Lett. 2009;9:2354–2359. doi: 10.1021/nl900872r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burns AA, Vider J, Ow H, Herz E, Penate-Medina O, Baumgart M, Larson SM, Wiesner U, Bradbury M. Nano Lett. 2009;9:442–448. doi: 10.1021/nl803405h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benezra M, Penate-Medina O, Zanzonico PB, Schaer D, Ow H, Burns A, DeStanchina E, Longo V, Herz E, Iyer S, Wolchok J, Larson SM, Wiesner U, Bradbury MS. J. Clin. Invest. 2011;121:2768–2780. doi: 10.1172/JCI45600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.a) Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI, Bawendi MG, Frangioni JV. Nat. Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Choi HS, Liu WH, Liu FB, Nasr K, Misra P, Bawendi MG, Frangioni JV. Nat. Nanotechnol. 2010;5:42–47. doi: 10.1038/nnano.2009.314. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Zhou C, Sun C, Yu MX, Qin YP, Wang JG, Kim M, Zheng J. J. Phys. Chem. C. 2010;114:7727–7732. doi: 10.1021/jp9122584. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Yu MX, Zhou C, Liu JB, Hankins JD, Zheng J. J. Am. Chem. Soc. 2011;133:11014–11017. doi: 10.1021/ja201930p. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Zheng J, Zhou C, Yu M, Liu J. Nanoscale. 2012;4:4073–4083. doi: 10.1039/c2nr31192e. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Zhou C, Long M, Qin Y, Sun X, Zheng J. Angew. Chem. Int. Edit. 2011;50:3168–3172. doi: 10.1002/anie.201007321. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Zhou C, Hao GY, Thomas P, Liu JB, Yu MX, Sun SS, Oz OK, Sun XK, Zheng J. Angew. Chem. Int. Edit. 2012;51:10118–10122. doi: 10.1002/anie.201203031. [DOI] [PubMed] [Google Scholar]; h) Liu JB, Yu MX, Zhou C, Yang SY, Ning XH, Zheng J. J. Am. Chem. Soc. 2013;135:4978–4981. doi: 10.1021/ja401612x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.a) Wang Y, Liu Y, Luehmann H, Xia X, Wan D, Cutler C, Xia Y. Nano Lett. 2013;13:581–585. doi: 10.1021/nl304111v. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhang G, Yang Z, Lu W, Zhang R, Huang Q, Tian M, Li L, Liang D, Li C. Biomaterials. 2009;30:1928–1936. doi: 10.1016/j.biomaterials.2008.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Hong GS, Robinson JT, Zhang YJ, Diao S, Antaris AL, Wang QB, Dai HJ. Angew. Chem. Int. Edit. 2012;51:9818–9821. doi: 10.1002/anie.201206059. [DOI] [PubMed] [Google Scholar]
- 13.a) Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WCW. Nano Lett. 2009;9:1909–1915. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]; b) Puvanakrishnan P, Park J, Chatterjee D, Krishnan S, Tunnell JW. Int. J. Nanomedicine. 2012;7:1251–1258. doi: 10.2147/IJN.S29147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang M, Thanou M. Pharmacol. Res. 2010;62:90–99. doi: 10.1016/j.phrs.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 15.a) Modi S, Prakash Jain J, Domb AJ, Kumar N. Curr. Pharm. Des. 2006;12:4785–4796. doi: 10.2174/138161206779026272. [DOI] [PubMed] [Google Scholar]; b) Maeda H. Adv. Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 16.a) Andreev OA, Dupuy AD, Segala M, Sandugu S, Serra DA, Chichester CO, Engelman DM, Reshetnyak YK. Proc. Natl. Acad. Sci. USA. 2007;104:7893–7898. doi: 10.1073/pnas.0702439104. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhang EL, Zhang C, Su YP, Cheng TM, Shi CM. Drug Discov. Today. 2011;16:140–146. doi: 10.1016/j.drudis.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Jiang T, Olson ES, Nguyen QT, Roy M, Jennings PA, Tsien RY. Proc. Natl. Acad. Sci. USA. 2004;101:17867–17872. doi: 10.1073/pnas.0408191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.a) Fu C, Liu T, Li L, Liu H, Chen D, Tang F. Biomaterials. 2013;34:2565–2575. doi: 10.1016/j.biomaterials.2012.12.043. [DOI] [PubMed] [Google Scholar]; b) He X, Nie H, Wang K, Tan W, Wu X, Zhang P. Anal. Chem. 2008;80:9597–9603. doi: 10.1021/ac801882g. [DOI] [PubMed] [Google Scholar]; c) Gary-Bobo M, Mir Y, Rouxel C, Brevet D, Basile I, Maynadier M, Vaillant O, Mongin O, Blanchard-Desce M, Morere A, Garcia M, Durand JO, Raehm L. Angew. Chem. Int. Edit. 2011;50:11425–11429. doi: 10.1002/anie.201104765. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.