Abstract
Schizophrenia remains a major burden1. The dopamine (DA) and neurodevelopmental hypotheses attempt to explain the pathogenic mechanisms and origins of the disorder respectively2-4. Recently an alternative, the cognitive model, has gained popularity5. However the first two theories have not been satisfactorily integrated, and the most influential iteration of the cognitive model makes no mention of DA, neurodevelopment, or indeed the brain5. Here we show that developmental alterations secondary to variant genes, early hazards to the brain and childhood adversity, sensitise the DA system, and result in excessive presynaptic DA synthesis and DA release. Social adversity biases the cognitive schema that the individual uses to interpret experiences towards paranoid interpretations. Subsequent stress results in dysregulated DA release, causing the misattribution of salience to stimuli, which are then misinterpreted by the biased cognitive processes. The resulting paranoia and hallucinations in turn cause further stress, and eventually repeated DA dysregulation hard-wires the psychotic beliefs. Finally we consider the implications of this model for understanding and treating schizophrenia.
Schizophrenia: no longer a mystery, merely a puzzle
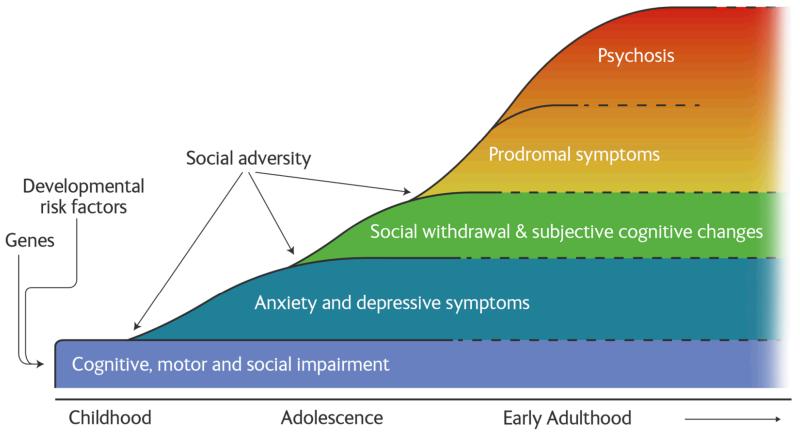
Schizophrenia affects about 1% of the population, and is one of the top ten causes of health burden in the World1. The clinical presentation is characterised by psychotic (positive) and negative symptoms, and cognitive impairments (see box 1 for further details). People who develop schizophrenia tend to show subtle cognitive, social and motor impairments in childhood. This is followed, in adolescence/early adulthood, by anxiety, low mood and social withdrawal, and then the emergence of prodromal symptoms of psychosis leading to the onset of the first psychotic episode (figure 1). Schizophrenia frequently follows a fluctuating course with enduring residual positive and negative symptoms interspersed by acute exacerbations of positive symptoms. Antipsychotics, the main pharmacological treatment, are effective at treating psychosis and reducing the risk of relapse but largely fail to treat other aspects of the disorder and have troubling side-effects6. There has been little progress in developing alternatives, and, unfortunately, all current antipsychotics essentially use the same mechanism as drugs discovered in the 1950s7. This highlights the importance of understanding the pathogenesis of the disorder. Fortunately, understanding of schizophrenia has advanced from earlier data free, stigmatising theories such as the idea that bad parenting caused the disorder (the ‘schizophrenogenic mother’8). It has become clear that dopaminergic and neurodevelopmental alterations, and biases in thinking and in appraising experiences underlie the disorder. Here we review how recent advances help us to at last understand the onset and course of schizophrenia.
Box 1.
| Term | Explanation |
|---|---|
| Delusion | A fixed implausible, preoccupying belief, such as that a microchip has been implanted behind the ear and is controlling their thoughts |
| Hallucination | A voice, vision or other percept in the absence of a stimulus. In schizophrenia these characteristically take the form of voices commenting on the sufferer’s actions |
| Passivity delusions | Delusions that an external agency is controlling thoughts, actions or perceptions |
| Positive symptoms | Psychotic symptoms such as delusions and hallucinations |
| Prediction error | Used in computational models to describe the mismatch between what is expected and what actually happens that drives learning |
| Psychosis | A syndrome characterised by one or more of the following symptoms: delusions, hallucinations, thought disorder, catatonia |
| Psychotic disorders | Schizophrenia is the most common psychotic disorder, but psychosis is also seen in bipolar and unipolar affective disorders |
| Negative symptoms | Symptoms such as apathy, reduced social interactions, poor self-care |
| Schizophrenia | A chronic mental illness characterised by persistent psychotic and negative symptoms and relatively subtle cognitive impairment. |
Figure 1. The trajectory to schizophrenia showing the evolution of symptoms and the main risk factors.
Dopamine dysfunction in schizophrenia (box 2)
Box 2. Summary of evidence for the dopamine hypothesis of schizophrenia.
| Dopaminergic index | Link to schizophrenia | Strength of evidence | |
|---|---|---|---|
| Drug studies | Effect of DA agonist drugs (eg amphetamine) |
Induces/ worsens psychotic symptoms | + |
| Effect of DA receptor antagonists (eg antipsychotics) |
Reduces symptoms | ++ | |
| Effect of DA depleting drugs (eg reserpine) |
Reduces symptoms | + | |
| Peripheral markers | DA metabolites in CSF and plasma | Increased in schizophrenia | ~ may depend on phase of illness and influenced by peripheral catecholamine metabolism |
| Ex vivo studies | Dopamine and DA metabolite levels in brain | Increased in striatum in schizophrenia | + potentially confounded by prior antipsychotic treatment |
| Brain imaging studies | DA synthesis and DA release capacity, and baseline DA levels | Increased in striatum in schizophrenia | ++ |
++=found in meta-analysis; += found in well-controlled studies; ~inconsistent findings
The DA hypothesis was built on the findings that antipsychotics work by blocking DA D2/3 receptors, and that drugs that activate the DA system such as amphetamine can induce psychotic symptoms 7, 9-11. However, meta-analysis of over 50 molecular imaging studies of the DA system in schizophrenia has now shown that the alterations in D2/3 receptor availability are inconsistent and small at most 12, whilst there is no difference in transporter availability 12, 13. In contrast, meta-analysis has found robust evidence for elevated DA synthesis capacity, increased DA release and indeed increased baseline synaptic DA levels in schizophrenia, all with large effect sizes (cohen’s d>0.8) 12. In short, molecular imaging pin-points presynaptic dysregulation as the major locus of DA dysfunction in the disorder7, 12, 14.
The specificity of dopaminergic dysfunction to schizophrenia and its link to psychosis
The presynaptic DA abnormality is not simply a non-specific mark of psychiatric illness - DA synthesis capacity and DA release are not elevated in people with other common psychiatric disorders15- and even has potential as a diagnostic test for schizophrenia16. However, elevated DA synthesis capacity has been reported in people with psychosis linked to temporal lobe epilepsy17; furthermore, individuals with schizotypal personality disorder, who have psychotic-like symptoms (and an increased risk of developing schizophrenia18), show both increased amphetamine-induced DA release relative to controls19, and increased DA synthesis capacity20.
The onset of schizophrenia is frequently preceded by a prodromal phase of sub-clinical psychotic symptoms. People who present with these “at risk” features have, on average, increased DA synthesis capacity21, 22 but, of course, not all “at risk” individuals are truly prodromal. Elevated dopamine synthesis capacity is specific to those who go on to develop frank psychosis20. Furthermore, greater DA synthesis capacity is associated with greater severity of sub-clinical symptoms but again only in those who go on to develop clinical psychosis - there is no relationship between DA synthesis capacity and symptoms in those who do not develop a psychotic disorder20. Many of the latter group continue to experience sub-clinical psychotic symptoms. In this respect, they are similar to people in the general population who experience sub-clinical psychotic symptoms for many years without either developing a psychotic disorder or showing DA elevation23, 24. Another group in whom sub-clinical psychotic symptoms are seen is relatives of people with schizophrenia, but here the findings are contradictory25, 26.
Studies using radiotracers selective for DA D2/3 receptors to index DA release following amphetamine in patients with schizophrenia indicate that greater release is associated with greater induction of psychotic symptoms27. The opposite also holds: greater depletion of DA levels following inhibition of DA synthesis is associated with greater reduction in psychotic symptoms28. DA release is greater in patients who are acutely psychotic than in stable, remitted patients27. Furthermore, a longitudinal study where patients were scanned in the prodrome and then again after they developed acute psychosis, found an increase in DA synthesis capacity during the progression from the prodrome to the first psychotic episode29.
These findings indicate a link between greater DA dysfunction and the development of more severe psychotic symptoms, and suggest that the DA dysfunction is dynamic, increasing with the worsening of the disorder. However, whilst DA dysfunction appears most marked in acute psychosis, it is not confined to schizophrenia per se, but is also seen in people with other psychotic disorders (box 2) and people with sub-clinical psychotic symptoms.
The neurodevelopmental hypothesis (box 3)
Box 3. Summary of evidence for the neurodevelopmental hypothesis of schizophrenia.
| Indicator | Link to schizophrenia | Strength of evidence | |
|---|---|---|---|
| Pre or perinatal risk factors | Obstetric complications |  |
++ |
| Low birth weight |  |
++ | |
| In utero infection |  |
++ | |
| Developmental trajectory | Motor delay |  |
++ |
| Social alterations |  |
++ | |
| Cognitive impairments |  |
++ | |
| Brain structural alterations | Ventricular enlargement |  |
++ |
| Grey matter reductions |  |
++ | |
| White matter disruption |  |
+ |
++=found in meta-analysis; += found in well-controlled studies, ~inconsistent findings
When the neurodevelopmental hypothesis was first articulated, it was based on three main lines of evidence3, 4. First there were the associations between pre- and peri-natal hazards on the one hand, and later schizophrenia on the other. Second, there was an excess of neuromotor, minor physical, and other markers of developmental deviance in children destined to develop schizophrenia. Third, imaging studies showed that structural brain defects were present at onset of schizophrenia, whilst post-mortem studies showed no evidence of neurodegeneration.
Since then much more evidence has accrued supporting these associations. Thus obstetric complications, such as low birth weight, Caesarean-section, hypoxia and other perinatal hazards, are linked to increased risk of schizophrenia, as is prenatal exposure to infection30-32. Those children who develop schizophrenia not only show an excess of markers of disordered neurodevelopment33, but also of neurological, cognitive and social problems, all with at least moderate effect sizes (odds ratios of 2 or more34, 35). The evidence for structural brain alterations at onset of psychosis has continued to amass36, 37, and there is now also evidence that it is present prior to the onset of schizophrenia 38, 39.
Weinberger originally highlighted the role of the dorsolateral prefrontal cortex4. Although this cortical lesion was postulated to be present from early neurodevelopment, its effects were thought to only become clinically apparent as a result of normal adolescent maturational changes combining to result in subcortical disinhibition3, 4. The cognitive impairments and negative symptoms seen in schizophrenia were accounted for by the cortical deficits, whilst the subcortical disinhibition, beginning in adolescence, caused the emergence of positive symptoms. Dopamine dysfunction was regarded as a manifestation of the subcortical hyperfunction and was considered as secondary to the interaction between the primary cortical lesion and normal maturational processes3, 4. However, several new lines of evidence, discussed below, have emerged that redefine the nature of the link between the neurodevelopmental damage and dopaminergic dysfunction.
The effect of developmental insults on the dopaminergic system
Early developmental insults to rodents have effects that mirror the changes seen in schizophrenia. Thus animals exposed to inflammatory challenges in utero show increased striatal levels of DA and its metabolites, and increased levels of DA synthetic enzymes in adulthood40. They also demonstrate increased behavioural responses to amphetamine41, another indicator of greater DA release. Perinatal hypoxia models increase brain DA synthesis capacity, and DA levels42, 43, while Caesarean-section is also associated with increased DA levels44, enhanced DA release45 and increased DA synthesis capacity in response to stress42. Other studies have targeted the ventral hippocampus46 as neonatal hypoxia has been frequently associated with damage to the hippocampus in both healthy humans and in schizophrenia47. These studies also show increased striatal DA levels and DA release46, increased behavioural responses to amphetamine48, and a greater DA response to stress49.
The effects of developmental insults are evident despite cross-fostering, indicating they are not due to the mother maltreating offspring as a result of the in utero exposure or post-natal influences42, 44. A key element is that these dopaminergic changes persist into adulthood40; indeed, in some models the altered dopaminergic function only becomes evident later in development46.
Social risk factors
In recent years, evidence for the effects of social factors on schizophrenia has become well-established. Thus, being an immigrant is associated with a relative risk of 2.9 and a risk of over 4 if the migrant lives in an area where he/she is readily identifiable as being in the minority50. Similarly having grown up in a city, is associated with increased risk (pooled odds ratio of 1.9)51. Childhood adversity -such as loss of a parent, or abuse- is also associated with an increased risk of schizophrenia with an odds ratio of 2.852. Whilst it is easy to see that childhood adversity, or being part of a minority group exposed to discrimination could have long-term effects on an individual’s stress response, the effect of urbanicity is less obvious. However, a recent study in healthy volunteers found that city-living was associated with greater brain responses to a stress task, suggesting (although not proving) that city-living could alter the brain response to stress53. These findings have led to the original neurodevelopmental hypothesis being extended to include social stressors54-56. Furthermore, new real-time sampling techniques have demonstrated that patients with schizophrenia show greater sensitivity to everyday life hassles than controls and have linked even mild stress to increases in psychotic symptoms57. Similarly, higher cortisol levels have been linked to a greater likelihood of going on to develop psychosis in people at risk of schizophrenia58, although this should be considered preliminary given the relatively modest sample sizes in studies to date.
The effects of social stressors on striatal dopamine
Social isolation is well-established as a chronic stressor in social animals. Isolation rearing leads to increased striatal synaptic DA levels in adult animals, and increased striatal DA release to subsequent environmental and drug challenges, including cocaine and amphetamine, as adults59. Interestingly, position in the social hierarchy influences the recovery of the DA system after isolated animals are returned to the social group: dominant, but not sub-ordinant, monkeys show reversal of the striatal DA changes60. Acute stressors have also been found to activate dopaminergic transmission in the striatum in rodent models resulting in DA release and increased DA synthesis61, 62. Whilst many of these studies have used physical stressors such as tail-pinches or electric shocks, which have less obvious parallels with what patients experience, increased DA release is also seen to social stressors63, 64. For example, social instability, produced by repeatedly switching cage partners, and social defeat, where an animal loses an interaction with an aggressive animal, are associated with elevated sensitivity to amphetamine, increased striatal DA release and increased DA neuron firing65.
Stress also increases striatal DA release in humans66, 67, although not in all studies68. This inconsistency may reflect the severity of the stressor - animal studies indicate that mild stressors do not always increase striatal DA levels. Furthermore, greater DA release is associated with greater cortisol response to a challenge66. For obvious reasons, it is not possible to isolate children to determine if this has lasting effects on the DA system, but healthy adults who report low maternal care as children show increased DA release to a social stressor69. This same psychosocial stress test has been used in patients with schizophrenia and individuals at ultra-high risk of psychosis70. Both groups showed greater DA release to social stress than matched controls, providing evidence that people with schizophrenia, and those at risk of it, show an enhanced dopaminergic response to psychosocial stress.
The sensitivity, and sensitisation, of the dopamine system
The effects of neurodevelopmental insults on different neurotransmitter systems have been compared. Caesarean-section and mild hypoxia both affect the dopaminergic but not the serotonergic system42 ; indeed, whilst caesarean-section increases DA levels, it decreases norepinephrine levels, at least in male rats44. Furthermore, although dexamethasone exposure in utero, a prenatal stress model, increases both brain serotonin and DA levels, the effect is more marked for the DA system71. Similarly isolation rearing is associated with increased DA release but reduced serotonin release to subsequent challenges72.
An additional factor is sensitisation - the marked amplification in a response after repeated stimulation that persists over time. The DA system shows sensitisation to a number of drugs and stressors73, 74. Furthermore prior exposure to one challenge leads to an elevated subsequent dopaminergic response to a different challenge - there is cross-sensitization75-77. Thus animals exposed to an inflammatory challenge in utero also show a greater sensitization to repeated amphetamine administration than control animals41. Similarly, adult rats who had been subject to transient perinatal anoxia show greater sensitisation to the effects of subsequent stress on dopamine release in the striatum, and greater amphetamine-induced locomotor activity45. Cross-sensitisation has also been seen with adult rats who had been previously subject to social isolation78, and has also been found in humans79.
Overall, whilst environmental insults can affect a number of neurotransmitter systems, the DA system seems to be particularly sensitive to them, and furthermore its capacity for cross-sensitization means that insults may have additive, or even multiplicative, effects. Strikingly, DA sensitization in healthy controls reproduces the altered striatal and cortical responses during a cognitive task that are seen in schizophrenia80.
Genes, neurodevelopment and the dopamine system
We have barely mentioned genes until now, a surprising omission for a disorder in which the majority of the variance is considered genetic. Dopamine related genes have also been much studied in schizophrenia, particularly the genes for dopamine receptors and for Catechol-O-Methyl -Transferase (COMT), a key enzyme that degrades DA, but effects have not been large and there are many inconsistencies81. This is not surprising given the imaging studies we reviewed earlier, which showed little or no abnormality in DA receptor or transporter availability, and instead located the major abnormality in presynaptic DA synthesis and release capacity. Unfortunately, there has been less research into genes involved in the synthesis and regulation of presynaptic DA (see82 and http://www.szgene.org/). However, we should probably not expect big main effects even here given that presynaptic DA function shows relatively low heritability and a high contribution from unique environmental factors83. It is important to note that the high heritability of schizophrenia includes gene-environment interactions; failure to account for this may have contributed to some of the inconsistencies in the genetic studies of schizophrenia.
From the beginning, neurodevelopmental impairment in schizophrenia was considered to reflect not only environmental but also genetic risk. Indeed, an early paper was entitled “The genetics of schizophrenia is the genetics of neurodevelopment”84. Subsequently, it has become clear that this is true, though only in part. A number of the susceptibility genes for the disorder for which the best evidence exists are involved in neurodevelopmental processes (e.g. neuregulin1, DISC1, TCF4, mir137, neurogranin, neurexin1)85-90. Most convincingly, an excess of copy number variants (CNVs) has been repeatedly demonstrated in schizophrenia and some of the same copy number variants have been implicated in other neurodevelopmental conditions such as autism, epilepsy and learning disability88, 91-94. Owen et al (2011) point out that all these disorders are also subject to early environmental hazards and suggest that together they constitute a continuum of neurodevelopmental causality95.
Preclinical evidence indicates that altered function in a number of these genes perturbs the DA system. For example, DISC1 knock-down mice showed an increased behavioural response, and increased striatal DA release, to methamphetamine96. Alterations in neuregulin1 and dysbindin have also been found to impact on the DA system. For example, neonatal administration of neuregulin1 resulted in increased striatal tyrosine hydroxylase levels and activity and increased DA levels97, while dysbindin mutant mice showed hyperactivity to DA agonists98. TCF4, a transcription factor, also impacts on the DA system by activating tyrosine hydroxylase transcription99. Finally, further support for the idea that disrupted neurodevelopment and dopaminergic dysfunction combine to underlie psychosis is provided by the 22q11.2 deletion syndrome, a large CNV that includes COMT and developmental genes and is associated with neurodevelopmental abnormalities and a ~25 fold increased risk of schizophrenia91.
Post-synaptic dopamine signalling
We have focused on presynaptic DA but we cannot exclude a role for post-synaptic DA signalling. Indeed a recent finding in patients with both schizophrenia and substance dependence highlights the potential role of post-synaptic DA signal transduction100. In contrast with previous findings in schizophrenia, this study found reduced DA release to amphetamine; but, nevertheless, DA release was still positively associated with the induction of psychotic symptoms101. This suggests that post-synaptic hypersensitivity to DA may contribute to psychosis. In support of a role for post-synaptic factors, schizophrenia is associated with gene variants and altered expression of proteins involved in post-synaptic DA signal transduction, such as AKT1, GSK-3β and DARPP-32102-104. Preclinical studies indicate that alterations in these pathways can dramatically alter DA-related function. For example, an increased behavioural response to amphetamine is seen with genetic or pharmacological manipulations that increase GSK-3 β function or those that reduce AKT1 function105, 106. Furthermore DARPP-32 knockout mice show altered behavioural responses to amphetamine and increased sensitisation to cocaine107, 108. It is important to note that DA sensitisation involves post-synaptic as well as presynaptic changes109.
Thus alterations in these post-synaptic factors could result in a pathologically increased post-synaptic response to DA which may underlie psychosis in dual diagnosis patients, but could also contribute to psychosis in others by amplifying the effects of presynaptic DA dysfunction due to interactions with environmental risk factors. There is already some evidence of such gene-environment interactions; for example SNPs in AKT1 have been found to show an interaction with two of the environmental risk factors that alter presynaptic DA function, obstetric complications and cannabis, to increase the risk of psychosis110-112.
Cognitive theories and their link to dopaminergic dysfunction
Together the DA and developmental hypotheses explain much of what we know concerning the biology of psychosis. However, they do little to help us understand the symptoms that patients suffer. The last decade has seen the rise of cognitive models which attempt to do this5, 113. These suggest that exposure to social adversities (e.g. child abuse, intrusive life events) bias an individual towards developing cognitive schemas that see the world as threatening, and to attributing negative events and experiences to external factors (such as other people)113. In such models, stress is seen as resulting in anomalies of conscious experience which trigger a search for an explanation. Then biased cognitive schema and appraisal processes result in the erroneous judgement that these puzzling experiences are externally driven and uncontrollable - in this way, paranoid delusions are postulated to develop.
Cognitive models were initially almost wholly “brainless” but have now begun to take note of biological theories5, 114. Research highlighting the importance of DA signalling in the salience of stimuli has been crucial to this. DA dysregulation is seen as resulting in aberrant assignment of salience to stimuli, and it is the cognitive interpretation of these excessively salient stimuli that results in psychotic symptoms2, 115. Thus environmental adversity acts both to dysregulate the DA system and to form biased cognitive schema. The biased schema, in turn, result in the excessively salient stimuli being interpreted as threatening. It is easy to see how this can lead to paranoid interpretations. The net result is additional stress, and further DA dysregulation - a vicious cycle that is likely, given the central role of the striatum and dopamine in habit formation, to result in paranoid ideas becoming fixed - effectively hard-wired116.
In earlier versions of the DA hypothesis, it was not clear how DA dysfunction accounted for hallucinations2. However, recent studies in primates indicate that as well as coding the saliency of external stimuli, midbrain DA activity also codes the uncertainty around subjective perceptual decisions about the detection of stimuli117, 118. Importantly this is independent of actual stimulus detection117. Thus DA dysregulation could impair the subjective discrimination of internal from external stimuli, leading to the misattribution of internal stimuli as arising externally. In support of this, patients with schizophrenia show impairments in the ability to detect stimuli and in the normal attenuation of cortical responses to self-made percepts119-122. The failure to attenuate the salience of self-made percepts could also result in the misattribution of their agency, and so account for passivity delusions. The signalling of salience by dopamine plays an important role in reward learning by encoding information about the mismatch between what is expected following a stimulus and what actually happens- the precision of prediction errors in computational models 123. By disrupting reward learning in this way, DA dysregulation could also account for amotivation, apathy and other negative symptoms of schizophrenia. Supporting this, even relatively modest increases in dopaminergic neurotransmission in rodents disrupt reward learning and decrease willingness to work for reward (see review124).
From state to trait: an integrated sociodevelopmental model
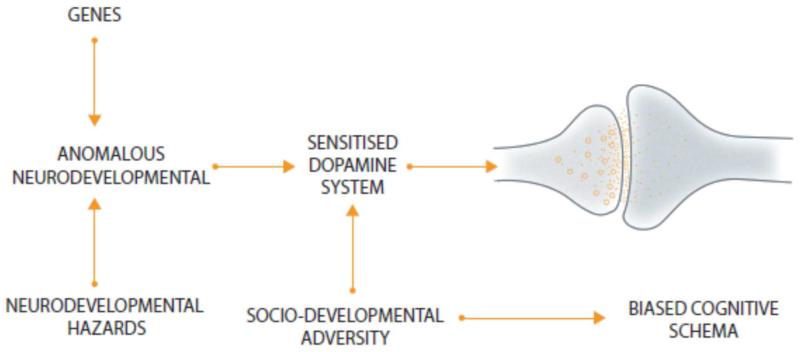
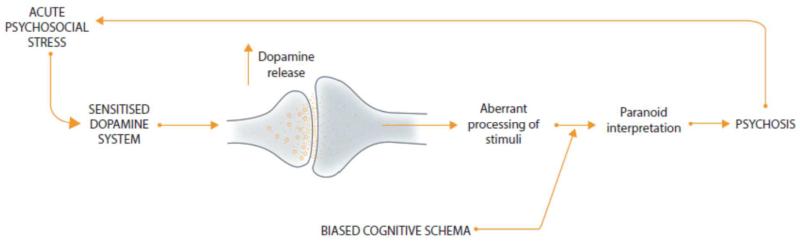
Our model combines aspects of the dopamine, neurodevelopmental and sociodevelopmental hypotheses with cognitive theories. Firstly, developmental deviance secondary to variant genes, hazards to the brain and social adversity in childhood, disrupts the development and sensitises the DA system (figure 2). At the same time social adversity also biases the cognitive schema that the individual uses to interpret experiences, towards psychotic interpretations. Subsequent stress then results in dysregulated DA release, leading to the aberrant assignment of salience, which, when interpreted in the context of biased cognitive schema, contributes to further stress. A vicious cycle is established with stress increasing DA dysregulation, which leads to more stress and so further DA release which eventually hard-wires the psychotic interpretation (figure 3). There is a progressive dysregulation of DA seen from the prodrome to the first and subsequent psychotic episodes.
Figure 2. The effect of neurodevelopmental and sociodevelopmental risk factors for psychosis on the dopamine system and cognitive schema.
Figure 3. Model of the onset of psychosis showing the interaction between acute stress, dopamine dysfunction and biased cognitive schema.
This is a dynamic model in that the degree of dopaminergic dysfunction fluctuates in response to the psychological response to the abnormal DA signalling. This contrasts with previous static versions of the DA hypothesis which could not account for relapses and remissions of the illness. Thus, the DA dysregulation reduces after the acute stressor(s) abate(s), although it does not normalise completely in most patients. This explains a) why some 10% of patients experience no further episodes of psychosis after the first episode125, but also b) why people who have experienced a psychotic episode remain at risk of further episodes even years later and c) the role of social stress in relapse125. Finally, given dopamine’s role in reward learning, the enduring DA dysfunction could account for the negative symptoms that many patients experience between acute episodes.
A key line of evidence for the original neurodevelopmental hypothesis was that premorbid motor and intellectual abnormalities were evident in pre-schizophrenic children34. At the time it was thought that the dopaminergic dysfunction was mesolimbic rather nigrostriatal. However, subsequent findings indicate that the dopaminergic dysfunction includes the motor and associative parts of the striatum12 , and abnormalities in the latter have been linked to poorer cognitive function in people with prodromal signs of schizophrenia21. Thus motor and cognitive abnormalities could be accounted for by the effect of altered dopaminergic function in the motor and associative striatum respectively. Supporting this, transgenic mouse models show that even a relatively subtle increase in striatal dopaminergic neurotransmission impairs cognitive function126. Of course, our model does not preclude developmental disruption of other systems - this could both contribute to cognitive dysfunction, and underlie the greater sensitivity of the dopaminergic system to subsequent stressors46, 127. Some, albeit tentative, support for this comes from the finding that smaller grey matter volumes are associated with a greater stress-induced increase in a peripheral marker of DA128. Similarly, it is likely that individuals with greater exposure to risk factors, and particularly greater severity of developmental insult, will show more marked dopaminergic dysregulation but also dysfunction of other systems. This explains why patients with more risk factors tend to have a poorer prognosis129, and accounts for heterogeneity in the cognitive impairments seen in patients with schizophrenia130.
The model explains the overlap both in risk factors and brain abnormalities between schizophrenia and neuropsychiatric conditions such as autism and epilepsy as they share neurodevelopmental origins131-133. However, it proposes that it is the impact of these developmental factors and subsequent social stressors on the DA system that determines whether the trajectory is towards progressive dopamine dysregulation, and psychosis, or, where the dopamine system is not progressively dysregulated, another diagnosis or no disorder. Finally it is primarily a theory about psychosis in schizophrenia, and putatively, psychosis in other conditions. Thus, it would account, for example, for the higher rates of psychosis in conditions such as epilepsy, learning disability and autism with similar neurodevelopmental origins.
Strengths and Limitations
The evidence linking neurodevelopmental and sociodevelopmental risk factors to schizophrenia, and for presynaptic DA dysfunction in the disorder, is supported by meta-analyses (see boxes 2 and 3). Similarly the link between developmental risk factors to altered DA function is supported by a large number of preclinical studies. As such it would take a substantial amount of new evidence to refute these aspects of the model. However, the link between the environmental risk factors and DA dysfunction is less well established, particularly in humans, as is the proposal that the DA changes are dynamic - both these components are reliant on a small number of studies and thus warrant further testing. Similarly, whilst the finding that people with schizophrenia show biased cognitive schemas has been replicated in at least two other studies, this is far from established, and evidence to support our proposal that these are biased prior to the onset of psychosis and a consequence of social adversity is needed. The evidence for dopamine’s role in encoding subjective sensory discrimination and that patients show disrupted sensory discrimination is also limited to a handful of studies.
We have not discussed some of the risk factors and neurobiological alterations associated with schizophrenia where more evidence is needed. Some, such as the progressive structural brain loss seen in some patients, may be accounted for within our model by the effects of stress and/or antipsychotic treatment134, 135, or be non-specific correlates of neurodevelopmental disruption. Others may emerge in the fullness of time as key upstream regulators of the dopamine dysfunction. Amongst these, glutamatergic abnormalities, although not always consistent136, have attracted considerable recent interest. Glutamatergic hypofunction could contribute to dopaminergic dysfunction137, although this remains to be determined in patients. The influence of another factor, oestrogens, could explain the later peak age of onset in women, but, whilst estrogens are clearly involved in regulating DA function in preclinical models138, this has yet to be established in humans. Similarly, whilst our model accounts for the link between stimulant use and increased risk of schizophrenia as these drugs are known to induce dopamine sensitisation79, there continues to be some uncertainty over whether abuse of other psychotogenic drugs, such as ketamine and cannabis, operate via dopaminergic pathways139. Finally, whilst the dynamic nature of the proposed DA dysfunction accounts for the fluctuating course of the acute psychotic phases of schizophrenia, the evidence is less clear on how it accounts for the persisting negative symptoms and deficit state that generally persist between acute episodes.
Implications and future directions
This model draws on a number of previous theories4, 55, 101, 140-142, and is likely to be refined with further testing. There are several areas where more evidence may be particularly informative. One is the developmental trajectory of DA function in experimental models of schizophrenia, beginning earlier than previously studied143, and also examining the interactive effects of social risk factors. Another is the interaction between genes impacting on the dopamine system and environmental risk factors. A third is the hypothesised interaction between neurodevelopmental and later social effects on the DA system, and particularly the hypothesised dynamic change and the impact of stressors on this. A fourth is the role of cognitive schema in the transition from experiencing ‘aberrant salience’ to developing psychosis. We have focussed on dopamine in the striatum because this is the region most studied, but this does not preclude effects in other regions- for example, rodent studies show that stress also has effects on DA release in other brain regions61- and this warrants further investigation.
This model highlights that patients are not ‘doomed from the womb’, in contrast to some early interpretations of the neurodevelopmental hypothesis, nor to progressive deterioration. Instead it suggests that life events, and the cognitions associated with them, play a key role, and that by altering cognitive schema, and by reducing stress, psychological therapies and social interventions may interrupt the vicious cycle which is dysregulating DA (figure 4). Evidence from an animal developmental model of schizophrenia also indicates that treatments that reduce stress responsivity prevent the emergence of DA dysregulation144. These interventions are likely to be particularly critical early in the illness, before there has been progressive DA dysregulation, and patterns of interpreting events become hard-wired.
Figure 4. Sites at which psycho-social interventions may act to prevent psychosis.
We have focussed on presynaptic DAergic function but alterations in post-synaptic signal transduction may contribute to further disrupt DA signalling. Whilst further work is needed to determine if post-synaptic signalling is disrupted in schizophrenia, targeting these pathways may nevertheless be therapeutically beneficial to redress presynaptic DA dysfunction. Additionally the model indicates new upstream targets for drugs to reverse the dopamine dysregulation - such as the gamma-aminobutyric acid (GABA)-ergic and glutamatergic regulation of midbrain dopamine neuron firing145-147. Finally, the model indicates that treatment of schizophrenia needs to address psychological, sociodevelopmental and biological factors: it cannot be either wholly brainless or totally mindless.
Search strategy and selection criteria.
We searched Pubmed and Embase from 1966 to June 2013 and reviewed article bibliographies using the following search terms: “schizophrenia”, “psychosis”, in combination with “dopamine”, “aetiology”, “risk factors”, “cause”, “theory”, “neurodevelopmental”, and “cognitive”. Where possible we have cited meta-analyses and systematic reviews.
Disclosures and acknowledgments
Oliver Howes and Robin Murray have received speaker bureau honoraria and charitable research funding from pharmaceutical companies, including the following manufacturers of antipsychotic drugs: Astra-Zeneca, BMS, Eli Lilly, Jansenn-Cilag, Roche.
This work was funded by MRC-UK (MC-A656-5QD30) and Wellcome Trust (094849/Z/10/Z) grants to Oliver Howes and the NIHR Biomedical Research Centre, South London & Maudsley NHS Foundation Trust.
Footnotes
The authors report no other potential conflicts of interest.
References
- 1.Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2129–43. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35(3):549–62. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray RM, Lewis SW. Is schizophrenia a neurodevelopmental disorder? Br Med J (Clin Res Ed) 1987;295(6600):681–2. doi: 10.1136/bmj.295.6600.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Archives of general psychiatry. 1987;44(7):660–9. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 5.Garety PA, Kuipers E, Fowler D, Freeman D, Bebbington PE. A cognitive model of the positive symptoms of psychosis. Psychological medicine. 2001;31(2):189–95. doi: 10.1017/s0033291701003312. [DOI] [PubMed] [Google Scholar]
- 6.Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379(9831):2063–71. doi: 10.1016/S0140-6736(12)60239-6. [DOI] [PubMed] [Google Scholar]
- 7.Howes OD, Egerton A, Allan V, McGuire P, Stokes P, Kapur S. Mechanisms underlying psychosis and antipsychotic treatment response in schizophrenia: insights from PET and SPECT imaging. Curr Pharm Des. 2009;15(22):2550–9. doi: 10.2174/138161209788957528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrington A. The fall of the schizophrenogenic mother. Lancet. 2012;379(9823):1292–3. doi: 10.1016/s0140-6736(12)60546-7. [DOI] [PubMed] [Google Scholar]
- 9.Abi-Dargham A. Do we still believe in the dopamine hypothesis? New data bring new evidence. Int J Neuropsychopharmacol. 2004;7(Suppl 1):S1–5. doi: 10.1017/S1461145704004110. [DOI] [PubMed] [Google Scholar]
- 10.Berman SM, Kuczenski R, McCracken JT, London ED. Potential adverse effects of amphetamine treatment on brain and behavior: a review. Molecular psychiatry. 2009;14(2):123–42. doi: 10.1038/mp.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curran C, Byrappa N, McBride A. Stimulant psychosis: systematic review. The British journal of psychiatry. 2004;185:196–204. doi: 10.1192/bjp.185.3.196. [DOI] [PubMed] [Google Scholar]
- 12.Howes OD, Kambeitz J, Kim E, et al. The Nature of Dopamine Dysfunction in Schizophrenia and What This Means for Treatment: Meta-analysis of Imaging Studies. Arch Gen Psychiatry. 2012;69(8):776–86. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen KC, Yang YK, Howes O, et al. Striatal dopamine transporter availability in drug-naive patients with schizophrenia: a case-control SPECT study with [(99m)Tc]-TRODAT-1 and a meta-analysis. Schizophrenia bulletin. 2013;39(2):378–86. doi: 10.1093/schbul/sbr163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyon GJ, Abi-Dargham A, Moore H, Lieberman JA, Javitch JA, Sulzer D. Presynaptic regulation of dopamine transmission in schizophrenia. Schizophrenia bulletin. 2011;37(1):108–17. doi: 10.1093/schbul/sbp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howes OD, Montgomery AJ, Asselin MC, Murray RM, Grasby PM, McGuire PK. Molecular imaging studies of the striatal dopaminergic system in psychosis and predictions for the prodromal phase of psychosis. Br J Psychiatry Suppl. 2007;51:s13–8. doi: 10.1192/bjp.191.51.s13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bose SK, Turkheimer FE, Howes OD, et al. Classification of schizophrenic patients and healthy controls using [18F] fluorodopa PET imaging. Schizophr Res. 2008;106(2-3):148–55. doi: 10.1016/j.schres.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Reith J, Benkelfat C, Sherwin A, et al. Elevated dopa decarboxylase activity in living brain of patients with psychosis. Proc Natl Acad Sci USA. 1994;91(24):11651–4. doi: 10.1073/pnas.91.24.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woods SW, Addington J, Cadenhead KS, et al. Validity of the prodromal risk syndrome for first psychosis: findings from the North American Prodrome Longitudinal Study. Schizophrenia bulletin. 2009;35(5):894–908. doi: 10.1093/schbul/sbp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abi-Dargham A, Kegeles LS, Zea-Ponce Y, et al. Striatal amphetamine-induced dopamine release in patients with schizotypal personality disorder studied with single photon emission computed tomography and [123I]iodobenzamide. Biol Psychiatry. 2004;55(10):1001–6. doi: 10.1016/j.biopsych.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 20.Howes OD, Bose S, Valli I, et al. Dopamine synthesis capacity before onset of psychosis: a prospective [18]-DOPA PET imaging study. Am J Psychiatry. 2011;168(12):1311–7. doi: 10.1176/appi.ajp.2011.11010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howes OD, Montgomery AJ, Asselin MC, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66(1):13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- 22.Egerton A, Chaddock CA, Winton-Brown TT, et al. Presynaptic striatal dopamine dysfunction in people at ultra-high risk for psychosis: findings in a second cohort. Biol Psychiatry. 2013;74(2):106–12. doi: 10.1016/j.biopsych.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Hanssen M, Bak M, Bijl R, Vollebergh W, van Os J. The incidence and outcome of subclinical psychotic experiences in the general population. The British journal of clinical psychology / the British Psychological Society. 2005;44(Pt 2):181–91. doi: 10.1348/014466505X29611. [DOI] [PubMed] [Google Scholar]
- 24.Howes OD, Shotbolt P, Bloomfield M, et al. Dopaminergic Function in the Psychosis Spectrum: An [18F]-DOPA Imaging Study in Healthy Individuals With Auditory Hallucinations. Schizophrenia bulletin. 2012 doi: 10.1093/schbul/sbr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huttunen J, Heinimaa M, Svirskis T, et al. Striatal dopamine synthesis in first-degree relatives of patients with schizophrenia. Biol Psychiatry. 2008;63(1):114–7. doi: 10.1016/j.biopsych.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 26.Shotbolt P, Stokes PR, Owens SF, et al. Striatal dopamine synthesis capacity in twins discordant for schizophrenia. Psychol Med. 41(11):2331–8. doi: 10.1017/S0033291711000341. [DOI] [PubMed] [Google Scholar]
- 27.Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry. 1999;46(1):56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- 28.Abi-Dargham A, Rodenhiser J, Printz D, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A. 2000;97(14):8104–9. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howes O, Bose S, Turkheimer F, et al. Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: a PET study. Molecular psychiatry. 2011;16(9):885–6. doi: 10.1038/mp.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. The American journal of psychiatry. 2010;167(3):261–80. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khandaker GM, Zimbron J, Lewis G, Jones PB. Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychological medicine. 2012:1–19. doi: 10.1017/S0033291712000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2002;159(7):1080–92. doi: 10.1176/appi.ajp.159.7.1080. [DOI] [PubMed] [Google Scholar]
- 33.Bramon E, Walshe M, McDonald C, et al. Dermatoglyphics and Schizophrenia: a meta-analysis and investigation of the impact of obstetric complications upon a-b ridge count. Schizophr Res. 2005;75(2-3):399–404. doi: 10.1016/j.schres.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 34.Jones P, Rodgers B, Murray R, Marmot M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344(8934):1398–402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- 35.Reichenberg A, Caspi A, Harrington H, et al. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. The American journal of psychiatry. 2010;167(2):160–9. doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165(8):1015–23. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bose SK, Mackinnon T, Mehta MA, et al. The effect of ageing on grey and white matter reductions in schizophrenia. Schizophr Res. 2009;112(1-3):7–13. doi: 10.1016/j.schres.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 38.Lawrie SM, Whalley HC, Abukmeil SS, et al. Brain structure, genetic liability, and psychotic symptoms in subjects at high risk of developing schizophrenia. Biol Psychiatry. 2001;49(10):811–23. doi: 10.1016/s0006-3223(00)01117-3. [DOI] [PubMed] [Google Scholar]
- 39.Pantelis C, Velakoulis D, McGorry PD, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361(9354):281–8. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 40.Vuillermot S, Weber L, Feldon J, Meyer U. A longitudinal examination of the neurodevelopmental impact of prenatal immune activation in mice reveals primary defects in dopaminergic development relevant to schizophrenia. J Neurosci. 2010;30(4):1270–87. doi: 10.1523/JNEUROSCI.5408-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aguilar-Valles A, Flores C, Luheshi GN. Prenatal inflammation-induced hypoferremia alters dopamine function in the adult offspring in rat: relevance for schizophrenia. PLoS One. 5(6):e10967. doi: 10.1371/journal.pone.0010967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Khodor BF, Boksa P. Effects of birth insult and stress at adulthood on in vivo tyrosine hydroxylase and tryptophan hydroxylase activities in rat brain. Synapse. 2003;47(1):87–9. doi: 10.1002/syn.10147. [DOI] [PubMed] [Google Scholar]
- 43.El-Khodor BF, Boksa P. Long-term reciprocal changes in dopamine levels in prefrontal cortex versus nucleus accumbens in rats born by Caesarean section compared to vaginal birth. Exp Neurol. 1997;145(1):118–29. doi: 10.1006/exnr.1997.6437. [DOI] [PubMed] [Google Scholar]
- 44.El-Khodor BF, Boksa P. Differential vulnerability of male versus female rats to long-term effects of birth insult on brain catecholamine levels. Exp Neurol. 2003;182(1):208–19. doi: 10.1016/s0014-4886(03)00079-7. [DOI] [PubMed] [Google Scholar]
- 45.Brake WG, Noel MB, Boksa P, Gratton A. Influence of perinatal factors on the nucleus accumbens dopamine response to repeated stress during adulthood: an electrochemical study in the rat. Neuroscience. 1997;77(4):1067–76. doi: 10.1016/s0306-4522(96)00543-x. [DOI] [PubMed] [Google Scholar]
- 46.Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27(42):11424–30. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stefanis N, Frangou S, Yakeley J, et al. Hippocampal volume reduction in schizophrenia: effects of genetic risk and pregnancy and birth complications. Biological psychiatry. 1999;46(5):697–702. doi: 10.1016/s0006-3223(99)00089-x. [DOI] [PubMed] [Google Scholar]
- 48.Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry. 2006;60(3):253–64. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chrapusta SJ, Egan MF, Wyatt RJ, Weinberger DR, Lipska BK. Neonatal ventral hippocampal damage modifies serum corticosterone and dopamine release responses to acute footshock in adult Sprague-Dawley rats. Synapse. 2003;47(4):270–7. doi: 10.1002/syn.10179. [DOI] [PubMed] [Google Scholar]
- 50.Cantor-Graae E, Selten JP. Schizophrenia and migration: a meta-analysis and review. Am J Psychiatry. 2005;162(1):12–24. doi: 10.1176/appi.ajp.162.1.12. [DOI] [PubMed] [Google Scholar]
- 51.Krabbendam L, van Os J. Schizophrenia and urbanicity: a major environmental influence--conditional on genetic risk. Schizophrenia bulletin. 2005;31(4):795–9. doi: 10.1093/schbul/sbi060. [DOI] [PubMed] [Google Scholar]
- 52.Varese F, Smeets F, Drukker M, et al. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophrenia bulletin. 2012;38(4):661–71. doi: 10.1093/schbul/sbs050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lederbogen F, Kirsch P, Haddad L, et al. City living and urban upbringing affect neural social stress processing in humans. Nature. 474(7352):498–501. doi: 10.1038/nature10190. [DOI] [PubMed] [Google Scholar]
- 54.Morgan C, Charalambides M, Hutchinson G, Murray RM. Migration, ethnicity, and psychosis: toward a sociodevelopmental model. Schizophrenia bulletin. 2010;36(4):655–64. doi: 10.1093/schbul/sbq051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson JL, Pogue-Geile MF, Grace AA. Developmental pathology, dopamine, and stress: a model for the age of onset of schizophrenia symptoms. Schizophrenia bulletin. 2004;30(4):875–900. doi: 10.1093/oxfordjournals.schbul.a007139. [DOI] [PubMed] [Google Scholar]
- 56.Corcoran C, Walker E, Huot R, et al. The stress cascade and schizophrenia: etiology and onset. Schizophrenia bulletin. 2003;29(4):671–92. doi: 10.1093/oxfordjournals.schbul.a007038. [DOI] [PubMed] [Google Scholar]
- 57.Myin-Germeys I, Delespaul P, van Os J. Behavioural sensitization to daily life stress in psychosis. Psychol Med. 2005;35(5):733–41. doi: 10.1017/s0033291704004179. [DOI] [PubMed] [Google Scholar]
- 58.Walker EF, Trotman HD, Pearce BD, et al. Cortisol levels and risk for psychosis: initial findings from the north american prodrome longitudinal study. Biological psychiatry. 2013;74(6):410–7. doi: 10.1016/j.biopsych.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lapiz MD, Fulford A, Muchimapura S, Mason R, Parker T, Marsden CA. Influence of postweaning social isolation in the rat on brain development, conditioned behavior, and neurotransmission. Neurosci Behav Physiol. 2003;33(1):13–29. doi: 10.1023/a:1021171129766. [DOI] [PubMed] [Google Scholar]
- 60.Grant KA, Shively CA, Nader MA, et al. Effect of social status on striatal dopamine D2 receptor binding characteristics in cynomolgus monkeys assessed with positron emission tomography. Synapse. 1998;29(1):80–3. doi: 10.1002/(SICI)1098-2396(199805)29:1<80::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 61.Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52(5):1655–8. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- 62.Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le Moal M, Piazza PV. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur J Neurosci. 2000;12(3):973–9. doi: 10.1046/j.1460-9568.2000.00996.x. [DOI] [PubMed] [Google Scholar]
- 63.Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721(1-2):140–9. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- 64.Anstrom KK, Miczek KA, Budygin EA. Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience. 2009;161(1):3–12. doi: 10.1016/j.neuroscience.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trainor BC. Stress responses and the mesolimbic dopamine system: social contexts and sex differences. Horm Behav. 2011;60(5):457–69. doi: 10.1016/j.yhbeh.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wand GS, Oswald LM, McCaul ME, et al. Association of amphetamine-induced striatal dopamine release and cortisol responses to psychological stress. Neuropsychopharmacology. 2007;32(11):2310–20. doi: 10.1038/sj.npp.1301373. [DOI] [PubMed] [Google Scholar]
- 67.Brunelin J, d’Amato T, van Os J, Cochet A, Suaud-Chagny MF, Saoud M. Effects of acute metabolic stress on the dopaminergic and pituitary-adrenal axis activity in patients with schizophrenia, their unaffected siblings and controls. Schizophr Res. 2008;100(1-3):206–11. doi: 10.1016/j.schres.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 68.Montgomery AJ, Mehta MA, Grasby PM. Is psychological stress in man associated with increased striatal dopamine levels?: A [11C]raclopride PET study. Synapse. 2006;60(2):124–31. doi: 10.1002/syn.20282. [DOI] [PubMed] [Google Scholar]
- 69.Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci. 2004;24(11):2825–31. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mizrahi R, Addington J, Rusjan PM, et al. Increased stress-induced dopamine release in psychosis. Biological psychiatry. 2012;71(6):561–7. doi: 10.1016/j.biopsych.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 71.Slotkin TA, Kreider ML, Tate CA, Seidler FJ. Critical prenatal and postnatal periods for persistent effects of dexamethasone on serotonergic and dopaminergic systems. Neuropsychopharmacology. 2006;31(5):904–11. doi: 10.1038/sj.npp.1300892. [DOI] [PubMed] [Google Scholar]
- 72.Marsden CA, King MV, Fone KC. Influence of social isolation in the rat on serotonergic function and memory--relevance to models of schizophrenia and the role of 5-HT(6) receptors. Neuropharmacology. 2011;61(3):400–7. doi: 10.1016/j.neuropharm.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 73.Egerton A, Mehta MA, Montgomery AJ, et al. The dopaminergic basis of human behaviors: A review of molecular imaging studies. Neurosci Biobehav Rev. 2009;33(7):1109–32. doi: 10.1016/j.neubiorev.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boileau I, Dagher A, Leyton M, et al. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry. 2006;63(12):1386–95. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- 75.Jezierski G, Zehle S, Bock J, Braun K, Gruss M. Early stress and chronic methylphenidate cross-sensitize dopaminergic responses in the adolescent medial prefrontal cortex and nucleus accumbens. J Neurochem. 2007;103(6):2234–44. doi: 10.1111/j.1471-4159.2007.04927.x. [DOI] [PubMed] [Google Scholar]
- 76.Prasad BM, Sorg BA, Ulibarri C, Kalivas PW. Sensitization to stress and psychostimulants. Involvement of dopamine transmission versus the HPA axis. Ann N Y Acad Sci. 1995;771:617–25. doi: 10.1111/j.1749-6632.1995.tb44714.x. [DOI] [PubMed] [Google Scholar]
- 77.de Jong JG, Wasilewski M, van der Vegt BJ, Buwalda B, Koolhaas JM. A single social defeat induces short-lasting behavioral sensitization to amphetamine. Physiol Behav. 2005;83(5):805–11. doi: 10.1016/j.physbeh.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 78.Kosten TA, Zhang XY, Kehoe P. Chronic neonatal isolation stress enhances cocaine-induced increases in ventral striatal dopamine levels in rat pups. Brain Res Dev Brain Res. 2003;141(1-2):109–16. doi: 10.1016/s0165-3806(03)00003-8. [DOI] [PubMed] [Google Scholar]
- 79.Boileau I, Dagher A, Leyton M, et al. Conditioned dopamine release in humans: a positron emission tomography [11C]raclopride study with amphetamine. J Neurosci. 2007;27(15):3998–4003. doi: 10.1523/JNEUROSCI.4370-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’Daly OG, Joyce D, Stephan KE, Murray RM, Shergill SS. Functional magnetic resonance imaging investigation of the amphetamine sensitization model of schizophrenia in healthy male volunteers. Archives of general psychiatry. 2011;68(6):545–54. doi: 10.1001/archgenpsychiatry.2011.3. [DOI] [PubMed] [Google Scholar]
- 81.Talkowski ME, Kirov G, Bamne M, et al. A network of dopaminergic gene variations implicated as risk factors for schizophrenia. Hum Mol Genet. 2008;17(5):747–58. doi: 10.1093/hmg/ddm347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Allen NC, Bagade S, McQueen MB, et al. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. 2008;40(7):827–34. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- 83.Stokes PR, Shotbolt P, Mehta MA, et al. Nature or Nurture? Determining the Heritability of Human Striatal Dopamine Function: an [18F]-DOPA PET Study. Neuropsychopharmacology. 2013;38(3):485–91. doi: 10.1038/npp.2012.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jones P, Murray RM. The genetics of schizophrenia is the genetics of neurodevelopment. The British journal of psychiatry. 1991;158:615–23. doi: 10.1192/bjp.158.5.615. [DOI] [PubMed] [Google Scholar]
- 85.Sun J, Jia P, Fanous AH, et al. Schizophrenia gene networks and pathways and their applications for novel candidate gene selection. PLoS One. 2010;5(6):e11351. doi: 10.1371/journal.pone.0011351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ayalew M, Le-Niculescu H, Levey DF, et al. Convergent functional genomics of schizophrenia: from comprehensive understanding to genetic risk prediction. Molecular psychiatry. 2012 doi: 10.1038/mp.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stefansson H, Ophoff RA, Steinberg S, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460(7256):744–7. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Molecular psychiatry. 2012 doi: 10.1038/mp.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends Neurosci. 2009;32(9):485–95. doi: 10.1016/j.tins.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schmidt-Kastner R, van Os J, H WMS, Schmitz C. Gene regulation by hypoxia and the neurodevelopmental origin of schizophrenia. Schizophr Res. 2006;84(2-3):253–71. doi: 10.1016/j.schres.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 91.Grayton HM, Rujescu D, Collier DA. Copy number variations in neurodevelopmental disorders. Progress in neurobiology. 2012 doi: 10.1016/j.pneurobio.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 92.Weiss LA. Autism genetics: emerging data from genome-wide copy-number and single nucleotide polymorphism scans. Expert review of molecular diagnostics. 2009;9(8):795–803. doi: 10.1586/erm.09.59. [DOI] [PubMed] [Google Scholar]
- 93.Mefford HC, Muhle H, Ostertag P, et al. Genome-wide copy number variation in epilepsy: novel susceptibility loci in idiopathic generalized and focal epilepsies. PLoS Genet. 2010;6(5):e1000962. doi: 10.1371/journal.pgen.1000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Girirajan S, Brkanac Z, Coe BP, et al. Relative burden of large CNVs on a range of neurodevelopmental phenotypes. PLoS Genet. 2011;7(11):e1002334. doi: 10.1371/journal.pgen.1002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Owen MJ, O’Donovan MC, Thapar A, Craddock N. Neurodevelopmental hypothesis of schizophrenia. The British journal of psychiatry. 2011;198(3):173–5. doi: 10.1192/bjp.bp.110.084384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Niwa M, Kamiya A, Murai R, et al. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron. 2010;65(4):480–9. doi: 10.1016/j.neuron.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kato T, Abe Y, Sotoyama H, et al. Transient exposure of neonatal mice to neuregulin-1 results in hyperdopaminergic states in adulthood: implication in neurodevelopmental hypothesis for schizophrenia. Mol Psychiatry. 2011;16(3):307–20. doi: 10.1038/mp.2010.10. [DOI] [PubMed] [Google Scholar]
- 98.Papaleo F, Yang F, Garcia S, et al. Dysbindin-1 modulates prefrontal cortical activity and schizophrenia-like behaviors via dopamine/D2 pathways. Mol Psychiatry. 2012;17(1):85–98. doi: 10.1038/mp.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yoon SO, Chikaraishi DM. Isolation of two E-box binding factors that interact with the rat tyrosine hydroxylase enhancer. J Biol Chem. 1994;269(28):18453–62. [PubMed] [Google Scholar]
- 100.Thompson JL, Urban N, Slifstein M, et al. Striatal dopamine release in schizophrenia comorbid with substance dependence. Molecular psychiatry. 2012 doi: 10.1038/mp.2012.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol. 1999;13(4):358–71. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- 102.Emamian ES. AKT/GSK3 signaling pathway and schizophrenia. Front Mol Neurosci. 2012;5:33. doi: 10.3389/fnmol.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kunii Y, Ikemoto K, Wada A, et al. Detailed DARPP-32 expression profiles in postmortem brains from patients with schizophrenia: an immunohistochemical study. Med Mol Morphol. 2011;44(4):190–9. doi: 10.1007/s00795-010-0524-1. [DOI] [PubMed] [Google Scholar]
- 104.Albert KA, Hemmings HC, Jr., Adamo AI, et al. Evidence for decreased DARPP-32 in the prefrontal cortex of patients with schizophrenia. Archives of general psychiatry. 2002;59(8):705–12. doi: 10.1001/archpsyc.59.8.705. [DOI] [PubMed] [Google Scholar]
- 105.Gould TD, O’Donnell KC, Picchini AM, Manji HK. Strain differences in lithium attenuation of d-amphetamine-induced hyperlocomotion: a mouse model for the genetics of clinical response to lithium. Neuropsychopharmacology. 2007;32(6):1321–33. doi: 10.1038/sj.npp.1301254. [DOI] [PubMed] [Google Scholar]
- 106.Beaulieu JM, Sotnikova TD, Yao WD, et al. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(14):5099–104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hiroi N, Fienberg AA, Haile CN, et al. Neuronal and behavioural abnormalities in striatal function in DARPP-32-mutant mice. Eur J Neurosci. 1999;11(3):1114–8. doi: 10.1046/j.1460-9568.1999.00570.x. [DOI] [PubMed] [Google Scholar]
- 108.Fienberg AA, Hiroi N, Mermelstein PG, et al. DARPP-32: regulator of the efficacy of dopaminergic neurotransmission. Science. 1998;281(5378):838–42. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- 109.Chambers RA, Sentir AM, Engleman EA. Ventral and dorsal striatal dopamine efflux and behavior in rats with simple vs. co-morbid histories of cocaine sensitization and neonatal ventral hippocampal lesions. Psychopharmacology. 2010;212(1):73–83. doi: 10.1007/s00213-010-1929-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nicodemus KK, Marenco S, Batten AJ, et al. Serious obstetric complications interact with hypoxia-regulated/vascular-expression genes to influence schizophrenia risk. Molecular psychiatry. 2008;13(9):873–7. doi: 10.1038/sj.mp.4002153. [DOI] [PubMed] [Google Scholar]
- 111.Di Forti M, Iyegbe C, Sallis H, et al. Confirmation that the AKT1 (rs2494732) Genotype Influences the Risk of Psychosis in Cannabis Users. Biological psychiatry. 2012 doi: 10.1016/j.biopsych.2012.06.020. EPub:07/27. [DOI] [PubMed] [Google Scholar]
- 112.van Winkel R. Family-based analysis of genetic variation underlying psychosis-inducing effects of cannabis: sibling analysis and proband follow-up. Archives of general psychiatry. 2011;68(2):148–57. doi: 10.1001/archgenpsychiatry.2010.152. [DOI] [PubMed] [Google Scholar]
- 113.Bentall RP, Rowse G, Shryane N, et al. The cognitive and affective structure of paranoid delusions: a transdiagnostic investigation of patients with schizophrenia spectrum disorders and depression. Arch Gen Psychiatry. 2009;66(3):236–47. doi: 10.1001/archgenpsychiatry.2009.1. [DOI] [PubMed] [Google Scholar]
- 114.Garety PA, Bebbington P, Fowler D, Freeman D, Kuipers E. Implications for neurobiological research of cognitive models of psychosis: a theoretical paper. Psychol Med. 2007:1–15. doi: 10.1017/S003329170700013X. [DOI] [PubMed] [Google Scholar]
- 115.Heinz A, Schlagenhauf F. Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr Bull. 2010;36(3):472–85. doi: 10.1093/schbul/sbq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Murray GK. The emerging biology of delusions. Psychol Med. 2011;41(1):7–13. doi: 10.1017/S0033291710000413. [DOI] [PubMed] [Google Scholar]
- 117.de Lafuente V, Romo R. Dopamine neurons code subjective sensory experience and uncertainty of perceptual decisions. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(49):19767–71. doi: 10.1073/pnas.1117636108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.de Lafuente V, Romo R. Dopaminergic activity coincides with stimulus detection by the frontal lobe. Neuroscience. 2012;218:181–4. doi: 10.1016/j.neuroscience.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 119.Rabinowicz EF, Silipo G, Goldman R, Javitt DC. Auditory sensory dysfunction in schizophrenia: imprecision or distractibility? Archives of general psychiatry. 2000;57(12):1149–55. doi: 10.1001/archpsyc.57.12.1149. [DOI] [PubMed] [Google Scholar]
- 120.de la Fuente-Sandoval C, Favila R, Gomez-Martin D, Pellicer F, Graff-Guerrero A. Functional magnetic resonance imaging response to experimental pain in drug-free patients with schizophrenia. Psychiatry research. 2010;183(2):99–104. doi: 10.1016/j.pscychresns.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 121.Valkonen-Korhonen M, Purhonen M, Tarkka IM, et al. Altered auditory processing in acutely psychotic never-medicated first-episode patients. Brain Res Cogn Brain Res. 2003;17(3):747–58. doi: 10.1016/s0926-6410(03)00199-x. [DOI] [PubMed] [Google Scholar]
- 122.Shergill SS, Samson G, Bays PM, Frith CD, Wolpert DM. Evidence for sensory prediction deficits in schizophrenia. The American journal of psychiatry. 2005;162(12):2384–6. doi: 10.1176/appi.ajp.162.12.2384. [DOI] [PubMed] [Google Scholar]
- 123.Fletcher PC, Frith CD. Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nature reviews Neuroscience. 2009;10(1):48–58. doi: 10.1038/nrn2536. [DOI] [PubMed] [Google Scholar]
- 124.Simpson EH, Waltz JA, Kellendonk C, Balsam PD. Schizophrenia in translation: dissecting motivation in schizophrenia and rodents. Schizophrenia bulletin. 2012;38(6):1111–7. doi: 10.1093/schbul/sbs114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Alvarez-Jimenez M, Priede A, Hetrick SE, et al. Risk factors for relapse following treatment for first episode psychosis: A systematic review and meta-analysis of longitudinal studies. Schizophr Res. 2012;139(1):116–28. doi: 10.1016/j.schres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 126.Simpson EH, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65(5):585–96. doi: 10.1016/j.neuron.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lisman JE, Coyle JT, Green RW, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31(5):234–42. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Marcelis M, Suckling J, Hofman P, Woodruff P, Bullmore E, van Os J. Evidence that brain tissue volumes are associated with HVA reactivity to metabolic stress in schizophrenia. Schizophrenia research. 2006;86(1-3):45–53. doi: 10.1016/j.schres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 129.Robinson DG, Woerner MG, Alvir JM, et al. Predictors of treatment response from a first episode of schizophrenia or schizoaffective disorder. The American journal of psychiatry. 1999;156(4):544–9. doi: 10.1176/ajp.156.4.544. [DOI] [PubMed] [Google Scholar]
- 130.Joyce EM, Roiser JP. Cognitive heterogeneity in schizophrenia. Current opinion in psychiatry. 2007;20(3):268–72. doi: 10.1097/YCO.0b013e3280ba4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Perry W, Minassian A, Lopez B, Maron L, Lincoln A. Sensorimotor gating deficits in adults with autism. Biol Psychiatry. 2007;61(4):482–6. doi: 10.1016/j.biopsych.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 132.Cheung C, Yu K, Fung G, et al. Autistic disorders and schizophrenia: related or remote? An anatomical likelihood estimation. PLoS One. 2010;5(8):e12233. doi: 10.1371/journal.pone.0012233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Holstein DH, Vollenweider FX, Geyer MA, Csomor PA, Belser N, Eich D. Sensory and sensorimotor gating in adult attention-deficit/hyperactivity disorder (ADHD) Psychiatry research. 2013;205(1-2):117–26. doi: 10.1016/j.psychres.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 134.Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Archives of general psychiatry. 2011;68(2):128–37. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zipursky RB, Reilly TJ, Murray RM. The Myth of Schizophrenia as a Progressive Brain Disease. Schizophr Bull. 2012 doi: 10.1093/schbul/sbs135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Marsman A, van den Heuvel MP, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE. Glutamate in schizophrenia: a focused review and meta-analysis of (1)H-MRS studies. Schizophr Bull. 2013;39(1):120–9. doi: 10.1093/schbul/sbr069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kegeles LS, Abi-Dargham A, Zea-Ponce Y, et al. Modulation of amphetamine-induced striatal dopamine release by ketamine in humans: implications for schizophrenia. Biol Psychiatry. 2000;48(7):627–40. doi: 10.1016/s0006-3223(00)00976-8. [DOI] [PubMed] [Google Scholar]
- 138.Disshon KA, Dluzen DE. Estrogen reduces acute striatal dopamine responses in vivo to the neurotoxin MPP+ in female, but not male rats. Brain research. 2000;868(1):95–104. doi: 10.1016/s0006-8993(00)02329-5. [DOI] [PubMed] [Google Scholar]
- 139.Bloomfield MA, Morgan CJ, Egerton A, Kapur S, Curran HV, Howes OD. Dopaminergic Function in Cannabis Users and Its Relationship to Cannabis-Induced Psychotic Symptoms. Biological psychiatry. 2013 doi: 10.1016/j.biopsych.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 140.Howes OD, McDonald C, Cannon M, Arseneault L, Boydell J, Murray RM. Pathways to schizophrenia: the impact of environmental factors. Int J Neuropsychopharmacol. 2004;7(Suppl 1):S7–S13. doi: 10.1017/S1461145704004122. [DOI] [PubMed] [Google Scholar]
- 141.Murray RM, Lappin J, Di FM. Schizophrenia: from developmental deviance to dopamine dysregulation. Eur Neuropsychopharmacol. 2008;18(Suppl 3):S129–S34. doi: 10.1016/j.euroneuro.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 142.Selten JP, Cantor-Graae E. Hypothesis: social defeat is a risk factor for schizophrenia? Br J Psychiatry Suppl. 2007;51:s9–12. doi: 10.1192/bjp.191.51.s9. [DOI] [PubMed] [Google Scholar]
- 143.Eyles D, Feldon J, Meyer U. Schizophrenia: do all roads lead to dopamine or is this where they start? Evidence from two epidemiologically informed developmental rodent models. Transl Psychiatry. 2012;2:e81. doi: 10.1038/tp.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Du Y, Grace AA. Peripubertal Diazepam Administration Prevents the Emergence of Dopamine System Hyperresponsivity in the MAM Developmental Disruption Model of Schizophrenia. Neuropsychopharmacology. 2013;38(10):1881–8. doi: 10.1038/npp.2013.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McGuire P, Howes OD, Stone J, Fusar-Poli P. Functional neuroimaging in schizophrenia: diagnosis and drug discovery. Trends Pharmacol Sci. 2008;29(2):91–8. doi: 10.1016/j.tips.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 146.Gill KM, Lodge DJ, Cook JM, Aras S, Grace AA. A novel alpha5GABA(A)R-positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia. Neuropsychopharmacology. 2011;36(9):1903–11. doi: 10.1038/npp.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Buchanan RW, Freedman R, Javitt DC, Abi-Dargham A, Lieberman JA. Recent advances in the development of novel pharmacological agents for the treatment of cognitive impairments in schizophrenia. Schizophrenia bulletin. 2007;33(5):1120–30. doi: 10.1093/schbul/sbm083. [DOI] [PMC free article] [PubMed] [Google Scholar]