Abstract
Background
Prenatal insults can program the developing fetus to develop diseases that manifest in later life. Dexamethasone is often administered to the developing fetus to accelerate pulmonary development. The purpose of the present study was to determine whether prenatal dexamethasone adversely affects renal development and predisposes rats to develop renal disease and hypertension in later life.
Methods
Pregnant rats were given either vehicle or two daily intraperitoneal injections of dexamethasone (0.2 mg/kg body weight) on gestational days: 11 and 12, 13 and 14,15 and 16,17 and 18,19 and 20, or 20 and 21. Tail cuff blood pressure, glomerular number, and inulin clearance were measured in control and prenatal dexamethasone-treated rats when the rats were 60 to 90 days of age.
Results
Prenatal dexamethasone did not affect the length of gestation, the number of animals per litter, or the total body weight or kidney weight measured at one day of age. Offspring of rats administered dexamethasone on days 15 and 16 gestation had a 30% reduction in glomerular number compared with control at 60 to 70 days of age (24,236 ± 441 vs. 30,453 ± 579, P < 0.01). Rats receiving prenatal dexamethasone on days 17 and 18 had an approximate 20% reduction in glomeruli compared with control (P < 0.01). Offspring of rats receiving dexamethasone on days 15 and 16 gestation had systolic blood pressures at 60 to 90 days of age that were higher than any other group (P < 0.05). The glomerular filtration rate was comparable in all of the groups.
Conclusions
This study shows that two daily doses of prenatal dexamethasone (0.2 mg/kg body weight) in rats do not produce intrauterine growth retardation. Adult offspring of rats that received prenatal dexamethasone during specific times of gestation have a reduced number of nephrons and hypertension.
Keywords: growth and development, neonate, intrauterine growth retardation, glucocorticoids, hypertension, glomerular number, kidney development
Antenatal glucocorticoid therapy reduces the incidence of respiratory distress syndrome and mortality of premature neonates [1–4]. However, it is possible that antenatal glucocorticoid therapy may adversely affect fetal development and have persistent consequences later in life [5–15]. Human infants born to mothers treated for infertility with prednisone (10 mg/day) throughout pregnancy had intrauterine growth retardation [5]. Similarly, rats and mice treated with dexamethasone daily throughout pregnancy had lower birth weights than vehicle-treated controls [5–7].
Normally, the fetus is protected from maternal steroids by placental 11β-hydroxysteroid dehydrogenase, which converts physiologic glucocorticoids to inactive metabolites [6, 16]. Inhibition of this enzyme with carbenoxolone during pregnancy in rats results in intrauterine growth retardation [17]. Rats fed a low-protein diet during pregnancy have lower placental 11β-hydroxysteroid dehydrogenase activity and greater exposure to maternal steroids [18]. This increased fetal exposure to glucocorticoids may be a contributing factor in the development of intrauterine growth retardation seen with dietary protein deprivation during pregnancy [18].
Recent studies have suggested that prolonged exposure to dexamethasone, which is not metabolized by placental 11β-hydroxysteroid dehydrogenase, may have an adverse effect on the developing kidney [7, 8, 12, 13]. However, in these studies, the dose or duration of administration of glucocorticoids resulted in intrauterine growth retardation. The purpose of the present study was to examine whether the administration of glucocorticoids to pregnant rats at specific times of gestation affected renal development and predisposed rats to develop renal or cardiovascular disease in later life.
METHODS
Animals
Timed pregnant Sprague-Dawley rats arrived at our institution at least two days before the initiation of the study. The males are with the females for 15 hours, and day 0 of pregnancy was confirmed the next morning by the presence of a vaginal plug. Pregnant rats received either intraperitoneal vehicle or dexamethasone (0.2 mg/kg body weight) daily on gestational days 11 and 12, 13 and 14, 15 and 16, 17 and 18, 19 and 20, or 20 and 21. After delivery, neonatal rats were cared for by their mothers until they were weaned when they were then placed on standard rat chow. Both male and female rats were studied. Prenatal dexamethasone resulted in comparable differences from controls in males and females in each of the protocols studied, and the results were therefore combined except for blood pressure. Rats from at least two different litters were studied in each protocol. The pregnant rats were used only once.
Measurement of glomerular filtration rate
Control and rats that received prenatal dexamethasone were allowed free access to food and water until they were studied at 60 to 70 days of age. Rats were anesthetized with intraperitoneal Inactin (100 mg/kg) and placed on a servo-controlled heated table set to maintain a constant body temperature of 38°C. Catheters were placed in the internal jugular vein and carotid artery, and a tracheostomy was performed. A bladder catheter was placed to ensure free flow of urine. Normal saline was infused at 0.6 cc/kg per hour. Inulin clearance was performed in a similar fashion to that previously described [19]. Briefly, a prime of 6 µCi 3H-methoxy inulin was infused followed by a maintenance intravenous infusion of 16 µCi per hour. After one hour of equilibration, at least three 30-minute urine and midpoint blood samples were collected for analysis of [methoxy-3H] inulin using liquid scintillation counting. The mean of the clearance periods was used as the measurement of glomerular filtration rate. There was no difference in the glomerular filtration rate in the three periods. The glomerular filtration rates were 2.27 ± 0.07, 2.35 ± 0.07, and 2.45 ± 0.09 mL/min in the first three periods, respectively.
Measurement of blood pressure
Blood pressure was measured in 60- to 90-day-old rats using an IITC Model 179 blood pressure analyzer (Landing, NJ, USA). Animals were placed in a Lucite tube, and a tail cuff blood pressure cuff was inflated several times daily on the three days prior to the actual measurement of blood pressure. The rat was then returned to the animal care facility. On the day of the actual measurement, blood pressure was measured four times, and the mean of these values was used as the blood pressure for that rat.
Glomerular number
Glomerular number was determined using a variation of the technique of Damadian, Shwayri, and Bricker with significant modifications [20–22]. Alcian blue is superior to India ink to stain glomeruli [20, 22]. After measurement of glomerular filtration rate, the rats received an intra-arterial infusion of 0.2 cc per 100 g body weight of 5% alcian blue (Aldrich Chemical Company, Milwaukee, WI, USA) in normal saline over one minute using a femoral arterial catheter placed above the renal arteries. A second 0.2 cc per 100 g body weight bolus of alcian blue was then administered five to seven minutes after the first infusion. The kidneys were then removed five minutes after the second infusion, sliced in half, and incubated in 5 cc of 27% ammonia for two hours at room temperature. The kidney was then incubated in 5 cc of 8 N HCl at 50°C for one hour. The suspension was diluted to a final volume of 20 cc with water and incubated overnight at 4°C. The number of glomeruli in 10 30 µL samples was counted using a light microscope and averaged to determine the number of glomeruli per kidney.
DNA and protein content
Kidneys were removed from 1- and 60-day-old adult rats that received prenatal dexamethasone. The organs were blotted to remove any blood and were weighed and frozen in liquid nitrogen. The organs were stored at −80°C until study. Organs were homogenized in phosphate-buffered saline (PBS; pH = 7.4) with a Teflon-glass homogenizer at 4°C, and an aliquot was removed for measurement of protein and DNA content. Protein was measured using the bicinchoninic acid assay (BCA; Pierce Chemical Company, Rockford, IL, USA) using bovine serum albumin as the standard. DNA was assayed using the technique of Labarca and Paigen [23].
Histology and glomerular size
Kidneys from four 60- to 70-day-old control rats and four rats that received prenatal dexamethasone on days 15 and 16 gestation were fixed in 10% phosphate-buffered formalin, embedded in paraffin, sectioned at 4 microns, and stained with periodic acid-Schiff stain. One hundred glomeruli from each animal were examined for evidence of glomerulosclerosis. For estimation of glomerular size, slides were evaluated at ×40 magnification with a Zeiss microscope on an Autocyte Pathology Workstation (Tripath Imaging, Burlington, NC, USA). The perimeters of 100 glomeruli from each animal were outlined, and the glomerular area was determined using Autocyte Quic DNA software (Tripath Imaging, Burlington, NC, USA). Measurement was conducted without knowledge of the whether the animal was a control or a rat that received prenatal dexamethasone.
Statistical analysis
Values are expressed as mean ± SEM. Analysis of variance was used to determine statistical significance except for studies examining number of sclerotic glomeruli, where chi-square analysis was used.
RESULTS
Effect of prenatal dexamethasone on litter size and gestation
There was an average of 9.8 ± 0.9 rats per litter in the control group, which was not different from any of the prenatal dexamethasone-treated groups. The number of animals per litter in the groups that received prenatal dexamethasone on days 11 and 12, 13 and 14, 15 and 16,17 and 18,19 and 20, and 20 and 21 were 10.5 ± 1.1,10.8 ± 0.9, 9.8 ± 0.9, 8.8 ± 0.5, 9.6 ± 0.8, and 10.5 ± 0.4, respectively. The mean gestation in the control group was 21.7 ± 0.1 days and was not different than any dexamethasone-treated group.
Effect of prenatal glucocorticoids on postnatal body and kidney weight and kidney DNA and protein content
The effect of prenatal dexamethasone on postnatal body weight is shown in Table 1. It is clear from this table that maternal administration of dexamethasone at any of the time points does not produce intrauterine growth retardation. Only the group of animals born to mothers receiving dexamethasone on days 19 and 20 were somewhat smaller than those receiving dexamethasone on days 11 and 12 and 13 and 14, but they were not smaller than controls. The kidneys (left kidney) of animals that received dexamethasone on days 17 and 18 and 20 and 21 were slightly smaller than controls as well as the dexamethasone days 11 and 12 and 15 and 16 groups. As is shown in Table 1, the DNA content (left kidney) was only different in the 17- and 18-day dexamethasone group and was actually greater than the control and the dexamethasone 11- and 12-day and 15- and 16-day groups. The renal protein content (left kidney) was also comparable in all groups except in the 15- and 16-day group, where the content was greater than controls, as well as the dexamethasone days 11 and 12, 13 and 14, 19 and 20 groups.
Table 1.
Effect of prenatal dexamethasone (Dex) on rat body and kidney weight and kidney DNA and protein content at one day of age
| Dexamethasone, days |
||||||||
|---|---|---|---|---|---|---|---|---|
| Control | 11 and 12 | 13 and 14 | 15 and 16 | 17 and 18 | 19 and 20 | 20 and 21 | ||
| Body g | 5.95±0.11 (70) |
6.37±0.14 (33) |
6.27±0.16 (33) |
6.06±0.09 (72) |
5.87±0.07 (50) |
5.65±0.09a (40) |
6.07±0.11 (50) |
|
| Kidney g | 0.034±0.001 (60) |
0.035±0.001 (31) |
0.032±0.001 (26) |
0.034±0.001 (70) |
0.031±0.001b (50) |
0.032±0.001 (44) |
0.032±0.001b (68) |
|
| DNA µg/kidney | 80.9±5.1 (14) |
81.2±6.0 (12) |
99.9±4.0 (15) |
83.6±2.4 (18) |
104.4±7.2c (14) |
93.6±6.2 (9) |
85.9±5.3 (14) |
|
| DNA mg/g tissue | 2.5±0.2 (14) |
2.8±0.3 (12) |
3.1±0.2 (15) |
2.6±0.1 (18) |
3.5±0.2d (14) |
3.1±0.2 (9) |
2.9±0.1 (14) |
|
| Protein mg/kidney | 2.9±0.2 (24) |
3.0±0.2 (19) |
3.1±0.1 (24) |
3.8±0.2e (24) |
3.5±0.2 (21) |
2.9±0.2 (14) |
3.3±0.2 (20) |
|
| Protein mg/g tissue | 94.5±6.1 (24) |
92.8±5.8 (18) |
89.5±4.3 (24) |
108.7±5.9 (23) |
109.4±5.9 (21) |
93.2±6.8 (14) |
96.5±5.5 (22) |
|
The number or determinations is in parentheses.
Less than 11 & 12, 13 & 14 at P <0.05
Less than control, 11 & 12, 15 & 16 at P <0.05
Greater than control, 11 & 12, 15 and 16 at P <0.05
Greater than control and 15 & 16 at P < 0.05
Greater than control, 11 & 12, 13 & 14, and 19 & 20 at P <0.05
Table 2 shows the rat body and kidney weight as well as the DNA and protein content at 60 days of age. Only the DNA content in the 15 and 16 dexamethasone group was different from that of the control. There was less protein per gram of kidney in the 11- and 12-day dexamethasone group compared with dexamethasone days 13 and 14,15 and 16, and 20 and 21 groups, but this was not significantly different than that in the controls.
Table 2.
Effect of prenatal dexamethasone on rat body and kidney weight and kidney DNA and protein content at 60 days of age
| Dexamethasone, days |
|||||||
|---|---|---|---|---|---|---|---|
| Control | 11 and 12 | 13 and 14 | 15 and 16 | 17 and 18 | 19 and 20 | 20 and 21 | |
| Body g | 259.2±9.4 (34) |
285.7±7.4 (26) |
269.8±12.0 (18) |
264.0±6.9 (35) |
245.5±8.1 (31) |
248.3±7.4 (21) |
264.3±9.3 (29) |
| Kidney g | 1.08±0.03 (38) |
1.12±0.04 (26) |
1.05±0.06 (18) |
1.13±0.03 (35) |
1.03±0.06 (24) |
1.14±0.03 (20) |
0.98±0.04 (31) |
| DNA mg/kidney | 4.7±0.3 (27) |
5.4±0.4 (22) |
5.2±0.5 (12) |
6.2±0.3a (30) |
4.7±0.3 (20) |
4.6±0.4 (21) |
5.0±0.3 (18) |
| DNA mg/g tissue | 4.6±0.3 (27) |
4.8±0.3 (22) |
5.2±0.5 (12) |
5.7±0.2 (30) |
5.4±0.4 (19) |
4.3±0.3 (22) |
5.2±0.3 (18) |
| Protein mg/kidney | 130.6±7.7 (32) |
129.1±11.5 (20) |
149.8±9.7 (16) |
158.9±6.8 (28) |
125.5±8.9 (11) |
143.6±6.6 (17) |
137.8±8.8 (20) |
| Protein mg/g tissue | 136.2±8.5 (32) |
116.1±7.6b (20) |
149.4±8.4 (16) |
146.7±2.8 (29) |
131.6±4.8 (11) |
125.9±4.2 (17) |
144.9±6.4 (20) |
The number of determinations is in parentheses.
Greater than control, 17 & 18, and 19 & 20 at P <0.05
Less than 13 & 14, 15 & 16, 20 & 21
Effect of prenatal glucocorticoids on number of glomeruli and glomerular filtration rate at two months of age
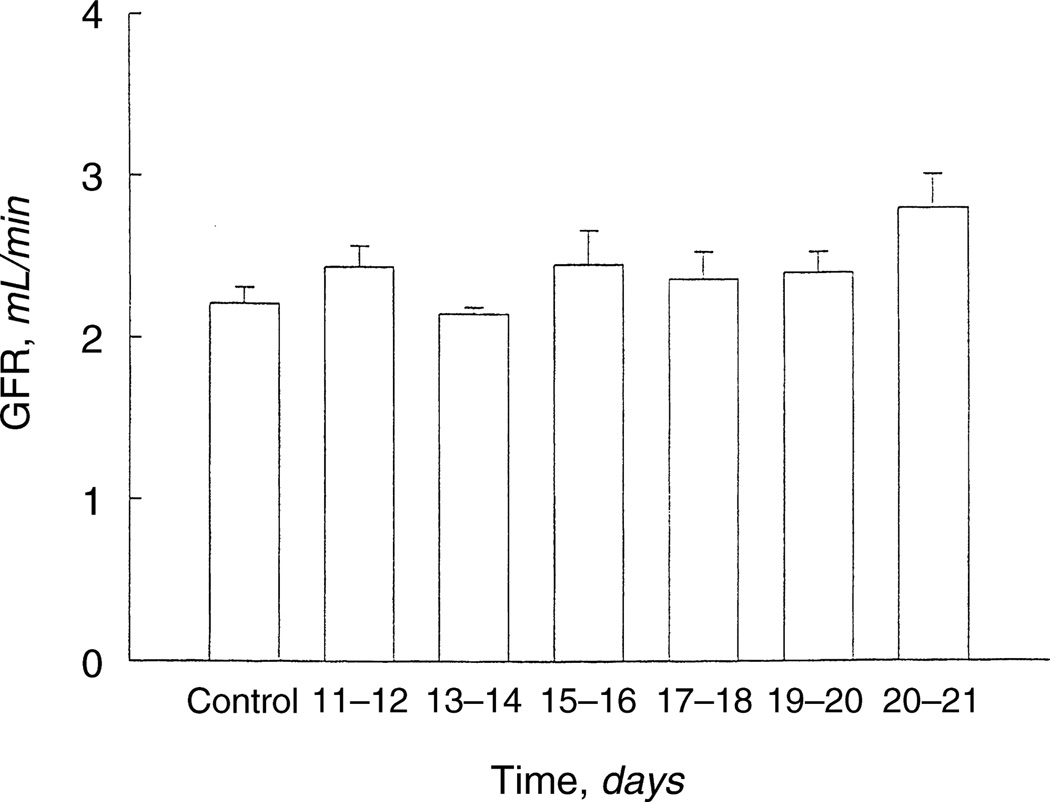
The effect of prenatal dexamethasone on glomerular number was determined in rats at 60 to 70 days of age. As shown in Figure 1, prenatal administration of glucocorticoids on days 15 and 16 of gestation resulted in a 30% reduction in glomeruli compared with control and fewer glomeruli than any of the other groups (P < 0.001). The rats that received dexamethasone on days 17 and 18 of gestation also had a reduction in the number of glomeruli compared with all other groups except the group that received dexamethasone on days 15 and 16 gestation (P < 0.01). As shown in Figure 2, despite the reduction in glomerular number, there was no difference in the glomerular filtration rate in any group.
Fig. 1. Effect of prenatal dexamethasone on the number of glomeruli in adult rats.
Pregnant rats were administered either vehicle (Control) or dexamethasone (0.2 mg/kg body wt) on gestational days 11 and 12, 13 and 14, 15 and 16, 17 and 18,19 and 20, 20 and 21. At 60 to 70 days of age, the number of glomeruli was counted using alcian blue to stain the glomeruli followed by acid digestion. The number of glomeruli were reduced in animals that received dexamethasone either at 15 and 16 or 17 and 18 days of gestation. There were at least 20 rats in each group. Symbols are: (+) less than other groups at P < 0.01; (+ +) less than all other groups except for gestational days 15–16 at P < 0.001.
Fig. 2. Effect of prenatal dexamethasone on glomerular filtration rate (GFR) at 60 days.
Glomerular filtration rate was measured in anesthetized 60- to 70-day-old rats that received either prenatal dexamethasone or vehicle (Control). GFR was measured using [methoxy-3H] inulin. There was no difference in GFR in any of the groups. There were at least 10 rats in each group.
Effect of prenatal glucocorticoids on blood pressure at 60 to 90 days of age
We also assessed whether prenatal dexamethasone would result in an elevation in systolic blood pressure when the rats reached 60 to 90 days of age. Control males tended to have somewhat higher blood pressures than females, and the results were divided by gender. Control male systolic blood pressure was 143 ± 3 compared with 135 ± 4 in females (P = 0.11). The results are shown in Figure 3. Prenatal dexamethasone administered on days 15 and 16 of gestation resulted in an increase in systolic blood pressure in both males and female rats when studied at 60 to 90 days of age compared with all other groups. The males that received prenatal dexamethasone on days 17 and 18 of gestation had a higher systolic blood pressure than that of the other groups except the 15- and 16-day group (P < 0.05).
Fig. 3. Systolic blood pressure in 60- to 90-day-old rats that received prenatal dexamethasone or vehicle.
Blood pressure was taken in trained rats using a tail cuff. Male (A) and female (B) rats that received prenatal dexamethasone on days 15 and 16 of gestation developed hypertension, as did male rats that received dexamethasone on days 17 and 18 of gestation. There are at least eight rats in each group except days 20–21, where there were five females and four males. *Greater than all other groups at P < 0.05; +greater than all groups except the dexamethasone on gestational days 15–16 group.
Histology and glomerular size
Histology of kidneys from 60-day-old control rats and rats that received prenatal dexamethasone on days 15 and 16 gestation is shown in Figure 4. As can be seen, there was no significant difference in the histology between the two groups. There was one sclerotic glomerulus in 400 glomeruli examined from four control animals, and three of 400 glomeruli had focal sclerosis from four offspring of animals that received prenatal dexamethasone on days 15 and 16 of gestation (P = NS). We also examined whether there was evidence of glomerular hypertrophy in the offspring of rats that received dexamethasone on days 15 and 16 of gestation. Glomerular area was 8149 ± 165 square microns in the dexamethasone-treated group and 7755 ± 151 in the control group. This 5% difference did not reach statistical significance (0.05 < P < 0.01).
Fig. 4. Low-power view (×100) of typical renal cortical field from a control (A) and dexamethasone-treated animal (B) using periodic acid-Schiff stain.
The histologic features are similar in both animals. No interstitial fibrosis or glomerular lesions are present.
DISCUSSION
The present study examined whether the administration of dexamethasone to pregnant rats at different times during gestation has adverse effects on the kidney. Our data show that the dosing regimens of dexamethasone used in this study did not produce intrauterine growth retardation. However, the administration of dexamethasone at specific times during fetal development produced a reduction of nephron number and resulted in hypertension when the animals were studied as adults.
Normally, the fetus is protected from maternal glucocorticoids by placental 11β-hydroxysteroid dehydrogenase. Pharmacologic inhibition of placental 11β-hydroxysteroid dehydrogenase results in hypertension when treated rats become adults [17]. Pregnant rats fed a low-protein diet have reduced placental 11β-hydroxysteroid dehydrogenase activity, and the offspring develop systolic hypertension as adults [18]. Furthermore, inhibition of glucocorticoid synthesis with metyrapone during the first 14 days of gestation prevents the development of hypertension in rats born to mothers fed a low-protein diet during gestation [24].
Prenatal administration of pharmacologic doses of dexamethasone, which is not metabolized by 11β-hydroxy-steroid dehydrogenase, has previously been shown to produce altered fetal renal development. The administration of betamethasone to pregnant rhesus monkeys was shown 25 years ago to result in the narrowing of the nephrogenic zone. This was interpreted as glucocorticoid induced acceleration in renal development [8]. However, other studies have shown that the administration of glucocorticoids to pregnant rats results in a decrease in neonatal weight, in total DNA content and a decrease in 3H-thymidine incorporation in the kidney as well as other organs [12, 13]. In these previously mentioned studies, either dexamethasone was administered at significantly higher doses per kilogram body weight or for more days than that used in the present study and resulted in intrauterine growth retardation. While prenatal dexamethasone can affect intrauterine DNA synthesis [12, 13], it is clear that the doses and regimen of dexamethasone used in this study did not result in significant reductions in renal DNA or protein content compared with controls when measured in the first day of life or at 60 days of age. It is of interest that the renal DNA content at one day of age in the 15 and 16 dexamethasone group, the group that developed the greatest reduction in nephron number was actually greater than that of controls. It is clear that gross measurement of DNA, protein content, and renal weight are not adequate parameters to demonstrate important changes that can occur in nephron number.
The administration of dexamethasone to pregnant rats (0.1 mg/kg/day) throughout gestation resulted in an increase in mean blood pressure when the offspring were studied at 60 days of age [6, 7]. There was over a 50% reduction in the number of glomeruli when assessed at 20 days of age, and unlike the present study, there was a 30% reduction in glomerular filtration rate when measured at 60 days of age. The one-day-old rats treated with dexamethasone throughout gestation weighed 5.1 ± 0.1 g, which was significantly less than the 7.1 ± 0.1 g of control rats. These studies show that the administration of dexamethasone throughout gestation produces hypertension when the animals are studied as adults. However, daily administration of dexamethasone throughout pregnancy produced intrauterine growth retardation, which itself is a predisposing factor for the development of hypertension [25–28] and renal disease in later life [25, 29].
The present study demonstrates that the administration of two doses of dexamethasone (0.2 mg/kg body wt) to pregnant rats does not lead to intrauterine growth retardation. However, prenatal dexamethasone results in a paucity of glomeruli and hypertension when the rats were studied at 60 to 90 days of age. In addition, there appears to be a specific time during renal development when the kidney is susceptible to the adverse effects of glucocorticoids. Nephrogenesis commences in the rat at 12 to 13 days of gestation and continues until approximately one week after birth [22, 30, 31]. It is unclear why glucocorticoids produced adverse effects only when administered between 15 and 18 days of gestation. It is possible that there are also critical times during human renal development when the administration of glucocorticoids may adversely affect renal development and predispose the infant to develop hypertension in adulthood.
There is increasing evidence that a paucity of nephrons at birth is an important factor that predisposes to the development of hypertension in later life [26, 32]. Brenner et al have proposed that intrauterine growth retardation results in congenital oligonephropathy [25, 26, 32]. In support of this hypothesis, human neonates with intrauterine growth retardation and birth weights below the 10th percentile had a 35% reduction in nephron number [33]. In addition, infants with intrauterine growth retardation are predisposed to developing hypertension and renal disease as adults [25–29].
The mechanism whereby a reduction in nephron number results in hypertension is not clear at present. It has been proposed that a reduction in nephron number impairs renal sodium excretion, which results in the development of hypertension [25, 26, 32]. In the present study, the rats with the fewest nephrons developed hypertension as adults. However, there was no change in glomerular filtration rate, and it is hard to conceive that a 30% reduction in nephron number was the sole variable producing hypertension. There was also a 5% increase in glomerular surface area in the offspring of rats that received dexamethasone on days 15 and 16 of gestation consistent with compensatory hypertrophy, but this small increase was not statistically significant. It is possible that prenatal glucocorticoids produced other abnormalities in addition to the reduction in nephron number that predisposes these animals to develop hypertension. It is unclear what this factor may be at present, but prenatal administration of glucocorticoids may also alter vascular development resulting in an increased vascular resistance when the rats became adults. It is also possible that prenatal dexamethasone programs the nephrons to develop premature glomerulosclerosis. However, we found no evidence for a difference glomerulosclerosis or interstitial fibrosis in the histologic evaluation of kidneys from offspring of control rats and offspring of rats that received dexamethasone on days 15 and 16 of gestation.
In summary, this study examined whether prenatal dexamethasone programmed rats to develop renal abnormalities and hypertension when they became adults. These data show that there are specific times during development when prenatal dexamethasone resulted in a reduction in nephron number and hypertension when the rats were studied as adults. The effects of prenatal dexamethasone were not associated with intrauterine growth retardation. The mechanism whereby prenatal dexamethasone produces a reduction in nephron number and hypertension is unclear at present but is likely due to an insult at a critical point in renal and/or vascular development.
ACKNOWLEDGMENT
This work was supported by National Institute of Diabetes and Digestive and Kidney Disease Grant DK-41612 (M. Baum).
REFERENCES
- 1.Ballard P, Granberg LP, Ballard RA. Glucocorticoid levels in maternal and cord serum after prenatal betamethasone therapy to prevent respiratory distress syndrome. J Clin Invest. 1975;56:1548–1554. doi: 10.1172/JCI108236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crowley P. Promoting pulmonary maturity. In: Chalmers IM, Keirse KJNC, editors. Effective Care in Pregnancy. Oxford: Oxford University Press; 1989. pp. 746–764. [Google Scholar]
- 3.NIH Consensus Development Panel on the Effect of Corticosteroids foe Fetal Maturation on Perinatal Outcomes. Effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA. 1995;273:413–418. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 4.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature rats. Pediatrics. 1972;50:515–525. [PubMed] [Google Scholar]
- 5.Reinisch JM, Simon NG, Karow WG, Gandelman R. Prenatal exposure to prednisone in humans and animals retards intrauterine growth. Science. 1978;202:436–438. doi: 10.1126/science.705336. [DOI] [PubMed] [Google Scholar]
- 6.Benediktsson R, Lindsay RS, Noble J, et al. Glucocorticoid exposure in utero: New model for adult hypertension. Lancet. 1993;341:339–341. doi: 10.1016/0140-6736(93)90138-7. [DOI] [PubMed] [Google Scholar]
- 7.Celci G, Kistner A, Aizman R, et al. Prenatal dexamethasone causes oligonephronia, sodium retention and a higher blood pressure in the offspring. Pediatr Res. 1998;44:317–322. doi: 10.1203/00006450-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Epstein MF, Farrell PM, Sparks JW, et al. Maternal betamethasone and fetal growth and development in the monkey. Am J Obstet Gynecol. 1977;127:261–263. doi: 10.1016/0002-9378(77)90465-3. [DOI] [PubMed] [Google Scholar]
- 9.Massoud EAS, Sekhon HS, Rotschild A, Thurlbeck WM. The in vitro effect of triamcinolone acetonide on branching morphogenesis in the fetal rat lung. Pediatr Pulmonol. 1992;14:28–36. doi: 10.1002/ppul.1950140107. [DOI] [PubMed] [Google Scholar]
- 10.Nyirenda MJ, Lindsay RS, Kenyon CJ, et al. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clin Invest. 1998;101:2174–2181. doi: 10.1172/JCI1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudolph AM, Roman C, Gournay V. Perinatal myocardial DNA and protein changes in the lamb: Effect of cortisol in the fetus. Pediatr Res. 1999;46:141–146. doi: 10.1203/00006450-199908000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Sanfacon R, Possmayer F, Harding PGR. Dexamethasone treatment of the guinea pig fetus: Its effects on the incorporation of 3H-thymidine into deoxyribonucleic acid. Am J Obstet Gynecol. 1977;127:745–752. doi: 10.1016/0002-9378(77)90250-2. [DOI] [PubMed] [Google Scholar]
- 13.Slotkin TA, Seidler FJ, Kavlock RJ, Bartolome JV. Fetal dexamethasone exposure impairs cellular development in neonatal rat heart and kidney: Effects on DNA and protein in whole tissues. Teratology. 1991;43:301–306. doi: 10.1002/tera.1420430404. [DOI] [PubMed] [Google Scholar]
- 14.Bunton TE, Plopper CG. Triamcinolone-induced structural alterations in the development of the lung of the fetal rhesus macaque. Am J Obstet Gynecol. 1984;148:203–215. doi: 10.1016/s0002-9378(84)80177-5. [DOI] [PubMed] [Google Scholar]
- 15.Oshika E, Liu S, Ung LP, et al. Glucocorticoid-induced effects on pattern formation and epithelial cell differentiation in early embryonic rat lungs. Pediatr Res. 1998;43:305–314. doi: 10.1203/00006450-199803000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Edwards CRW, Benediktsson R, Lindsay RS, et al. Dysfunction of placental glucocorticoid barrier: Link between fetal environment and adult hypertension? Lancet. 1993;341:355–357. doi: 10.1016/0140-6736(93)90148-a. [DOI] [PubMed] [Google Scholar]
- 17.Lindsay RS, Lindsay RM, Edwards CRW, Seckl JR. Inhibition of 11β-hydroxysteroid dehydrogenase in pregnant rats and the programming of blood pressure in the offspring. Hypertension. 1996;27:1200–1204. doi: 10.1161/01.hyp.27.6.1200. [DOI] [PubMed] [Google Scholar]
- 18.Langley-Evans SC, Phillips GJ, Benediktsson R, et al. Protein intake in pregnancy, placental glucocorticoid metabolism and the programming of hypertension in the rat. Placenta. 1996;17:169–172. doi: 10.1016/s0143-4004(96)80010-5. [DOI] [PubMed] [Google Scholar]
- 19.Cogan MG, Liu F-Y. Metabolic alkalosis in the rat: Evidence that reduced glomerular filtration rather than enhanced tubular bicarbonate reabsorption is responsible for maintaining the alkalotic state. J Clin Invest. 1983;71:1141–1160. doi: 10.1172/JCI110864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bankir L, Hollenberg NK. In vivo staining of the kidney with Alcian blue: An adjunct to morphological and physiological studies. Renal Physiol. 1983;6:151–155. doi: 10.1159/000172894. [DOI] [PubMed] [Google Scholar]
- 21.Damadian RV, Shwayri E, Bricker NS. On the existence of non-urine forming nephrons in the diseased kidney of the dog. J Lab Clin Med. 1965;65:26–39. [PubMed] [Google Scholar]
- 22.Gilbert T, Lelievre-Pegorier M, Malienou R, et al. Effects of prenatal and postnatal exposure to gentamicin on renal differentiation in the rat. Toxicology. 1987;43:301–313. doi: 10.1016/0300-483x(87)90089-8. [DOI] [PubMed] [Google Scholar]
- 23.Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980;102:344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- 24.Langley-Evans SC. Hypertension induced by foetal exposure to a maternal low-protein diet, in the rat, is prevented by pharmacological blockade of maternal glucocorticoid synthesis. J Hypertens. 1997;15:537–544. doi: 10.1097/00004872-199715050-00010. [DOI] [PubMed] [Google Scholar]
- 25.Brenner BM, Chertow GM. Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. Am J Kidney Dis. 1994;23:171–175. [PubMed] [Google Scholar]
- 26.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure: Less of one, more the other? Am J Hypertens. 1988;1:335–347. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- 27.Barker DJP, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. Br Med J. 1990;301:259–262. doi: 10.1136/bmj.301.6746.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barker DJP, Gluckman PD, Godfrey KM, et al. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 29.Rossing P, Tarnow L, Nielsen FS, et al. Low birth weight: A risk factor for development of diabetic nephropathy? Diabetes. 1995;44:1405–1407. doi: 10.2337/diab.44.12.1405. [DOI] [PubMed] [Google Scholar]
- 30.Merlet-Bénichou C, Gilbert T, Muffat-Joly M, et al. Intrauterine growth retardation leads to a permanent nephron deficit in the rat. Pediatr Nephrol. 1994;8:175–180. doi: 10.1007/BF00865473. [DOI] [PubMed] [Google Scholar]
- 31.Neiss WF, Klehn KL. The postnatal development of the rat kidney with special reference to the chemodifferentiation of the proximal tubule. Histochemistry. 1981;73:251–268. doi: 10.1007/BF00493025. [DOI] [PubMed] [Google Scholar]
- 32.Mackenzie HS, Lawler EV, Brenner BM. Congenital oligonephropathy: The fetal flaw in essential hypertension? Kidney Int. 1996;49(Suppl 55):S30–S34. [PubMed] [Google Scholar]
- 33.Hinchliffe SA, Lynch MRJ, Sargent PH, et al. The effect of intrauterine growth retardation on the development of renal nephrons. Br J Obstet Gynaecol. 1992;99:296–301. doi: 10.1111/j.1471-0528.1992.tb13726.x. [DOI] [PubMed] [Google Scholar]