Abstract
The goal of this investigation was to clarify the nature of spatial working memory difficulties in individuals at ultra high risk (UHR) for psychosis. We evaluated spatial working memory and intelligence in 96 individuals at UHR for psychosis, 28 patients with first episode psychosis (FEP), and 23 healthy controls. Fourteen UHR individuals developed a psychotic disorder during follow-up. Compared to controls, the UHR group was impaired in both the short-term maintenance of material and in the effective use of strategy, but not more immediate memory. These impairments were not as severe as those in the FEP group, as the UHR group performed better than the FEP group. A similar pattern of results was found for the intelligence measures. Discriminant function analyses demonstrated short-term maintenance of material significantly differentiated the UHR and healthy control groups even when accounting for full scale intelligence quotient (IQ); whereas full scale IQ significantly differentiated the UHR and FEP groups and FEP and control groups. Notably, within the UHR group, impaired spatial working memory performance was associated with lower global functioning, but not full scale IQ. The subgroup of UHR individuals who later developed psychosis was not significantly more impaired on any aspect of working memory performance than the group of UHR individuals who did not develop psychosis. Given, the relationship between spatial working memory deficits and functional outcome, these results indicate that cognitive remediation could be useful in individuals at UHR for psychosis to potentially improve functioning.
Keywords: ultra high risk, working memory, global functioning, first episode psychosis, intelligence
Working memory impairments are some of the most robust cognitive deficits in psychotic disorders and are evident in chronic, first episode, and drug-naïve patients (Forbes, Carrick, McIntosh, & Lawrie, 2009; Lee & Park, 2005), as well as individuals at ultra high risk (UHR) for psychosis (Fusar-Poli et al., 2012). Given that working memory processes are used in everyday tasks such as remembering a phone number, communicating, or doing mental arithmetic (Goldman-Rakic, 1994; Goldman-Rakic & Selemon, 1997), impairments in this domain are likely to contribute to the reductions in vocational and social function that are evident in patients with psychotic disorders and UHR individuals. The goals of the present study were to determine which components of working memory ability were impaired in individuals at UHR for psychosis, whether the severity of working memory impairments predicted the risk of the later onset of psychosis, and whether these impairments were related to lower global functioning.
Previous research has consistently demonstrated working memory deficits. In a meta-analysis, Lee and Park (2005), found an overall effect size of 0.45 for impaired working memory performance in individuals with schizophrenia compared to controls. Although this meta-analysis did not find modality-specific differences in working memory deficits, spatial working memory tasks were associated with more consistent effects than verbal working memory tasks. Additionally, similar effect sizes were found across maintenance durations, suggesting a primary encoding and/or early maintenance problem. In a more recent meta-analysis, Forbes and colleagues (2009) found effect sizes ranging from 0.51-1.41 for deficits in schizophrenia compared to controls on different working memory tasks, including phonological, visuospatial, and executive paradigms. This meta-analysis also found that the working memory deficit was not wholly attributable to differences in current intelligence between patients and controls.
Impairments in working memory have also been described in individuals at increased risk for psychotic disorders, including the healthy first degree relatives of patients and individuals with schizotypy (Giakoumaki, 2012; Snitz, Macdonald, & Carter, 2006). Similarly, a meta-analysis by Fusar-Poli and colleagues (2012) found that individuals at ultra high risk (UHR) for psychosis had impaired working memory compared to controls, with an effect size of 0.36. This meta-analysis also found that UHR individuals who later developed psychosis had a more marked impairment in working memory than UHR individuals that did not. Furthermore, this meta-analysis found that executive functioning was impaired in UHR compared to controls. Functional neuroimaging studies have shown that regional brain activation during the performance of working memory tasks is also altered in UHR subjects compared to controls, but to a lesser extent than in patients with first episode psychosis (FEP) (Allen et al., 2012; Broome et al., 2010; Broome et al., 2009).
Working memory is a multi-faceted construct. Baddeley’s model includes four components: the central executive, which controls attention and manipulates information; the phonological loop and visuospatial sketchpad, which store and rehearse information in short-term memory; and the episodic buffer, which integrates information from short-term memory with long-term memory into a unitary representation (Baddeley, 1992; 2000). Most studies of working memory in psychosis have employed tasks (such as the N-back or letter-number sequencing), which do not permit separate analyses of its different components. We sought to address this issue in the present study by using the spatial working memory task from the Cambridge Neuropsychological Test Automated Battery (CANTAB). This task focuses on two components: the short-term maintenance of material (in the visuospatial sketchpad) and the effectiveness of the task strategy (implemented by the central executive). One previous study investigating spatial working memory in 38 UHR individuals using the CANTAB task found that UHR individuals had short-term memory impairment compared to controls, but did not find significant group differences for working memory strategy (Wood et al., 2003). Another study using a similar task the Subject Ordered Pointing Task, which only measured the short-term maintenance of material, demonstrated that UHR individuals who later developed psychosis (n=44) demonstrated more memory impairment, compared with those UHR individuals who did not (n=39) (Pukrop et al., 2007).
Given the relevance of working memory differences to the pathophysiology of psychosis, and the limited information on how the various components of working memory compare at different stages of psychosis, the present study investigated (1) the pattern of spatial working memory deficits in individuals at UHR for psychosis compared to FEP patients and controls; (2) the relationship between these working memory deficits and overall intelligence, and the level of functioning in UHR individuals; and (3) whether spatial working memory deficits in UHR individuals who subsequently developed psychosis were more severe than in those who did not develop psychosis. Largely based on the Fusar-Poli and colleagues (2012) meta-analysis, we predicted that UHR individuals would demonstrate impaired spatial working memory compared to controls, but would be less impaired than patients with FEP. We also hypothesised that this impairment would reflect deficits in both the maintenance and strategy components of working memory, and not be wholly attributable to differences in overall intelligence. Finally, we predicted that within the UHR sample, the severity of these impairments would be related to the level of global functioning at presentation, and the risk of subsequently developing a psychotic disorder.
Materials and Methods
Participants
We studied 96 individuals at UHR for psychosis (91 completed the spatial working memory task, 92 completed intelligence testing, 88 completed both), recruited from Outreach And Support In South London (OASIS; a specialised clinical service for this group) (Broome et al., 2005), 28 patients with FEP (27 completed the spatial working memory task, 28 completed intelligence testing, 27 completed both), recruited from the Lambeth Early Onset service, and 23 healthy controls (completed all measures) with no personal or family history of psychotic disorders. All participants lived in the same geographical area (South East London). Exclusion criteria for all participants included being a non-native English speaker, history of neurological problems, head injury resulting in loss of consciousness, current substance dependence, or an intellectual disability identified before the age of 18 years. UHR participants met the Personal Assessment and Crisis Evaluation (PACE) criteria (Phillips, Yung, & McGorry, 2000) for an At Risk Mental State, based on a detailed assessment using the Comprehensive Assessment of At-Risk Mental States (CAARMS) (Yung et al., 2005), which was administered by an experienced clinician. UHR individuals were followed by OASIS at approximately monthly intervals for 2 years after initial enrolment. If an individual affiliated with OASIS made a transition to psychosis after that time and the clinic staff was aware of the transition, the database was updated. During the follow up period, 14 UHR participants developed a psychotic disorder, with a mean time to transition from the testing date of 13 months (sd=11; range= 6 days to 38 months). Transition to psychosis was defined using established criteria (Yung et al., 1998), requiring a rating of six on one or more of the three CAARMS positive items for longer than seven days. All the patients in the FEP group met criteria for a psychotic disorder, according to the ICD-10 criteria. Controls were screened for past or present psychotic symptoms and disorders using either the Structured Clinical Interview for DSM-IV Axis I Disorders (First, Spitzer, Gibbon, & Williams, 1996) or the Wisconsin Manual for the Assessment of Psychotic-Like Experiences (Kwapil, Chapman, & Chapman, 1999). Global Assessment of Functioning (GAF) was quantified at presentation for UHR individuals (APA, 2013). This investigation was carried out in accordance with the latest version of the Declaration of Helsinki, the study design was reviewed by an appropriate ethical committee, and informed consent from participants was obtained after the nature of the procedures was fully explained.
Cognitive Measures
Spatial working memory task
The spatial working memory task used was taken from the Cambridge Neuropsychological Test Automated Battery (CANTAB; Robbins et al., 1994; Sahakian & Owen, 1992) and provided a measure of spatial short-term and executive working memory ability (Owen, Downes, Sahakian, Polkey, & Robbins, 1990). Participants were required to search through a number of boxes for hidden tokens. There was a rule to guide this search, specifying that once a token had been located inside a particular box, it would not be found there again during that particular trial. It was therefore necessary for the participant to remember in which boxes the tokens had already been found as they progressed through each trial. Trials increased in difficulty according to the number of boxes (3, 4, 6 and 8).
Several performance indices were recorded: between search errors, within search errors, double errors, and strategy score. Between search errors were committed when a participant returned to search a box in which a token had already been found during a previous searching sequence. Within search errors were defined as occurring when a participant revisited a box already found to be empty during the same search sequence. Double errors were committed when a participant made an error that was categorized as both a between and within search error. Finally, a strategy score was calculated based on the notion that an efficient strategy was to follow a predetermined sequence by beginning with a specific box and then, once a token has been found, to return to that box to start a new searching sequence (Owen et al., 1990). An estimate of the use of this strategy was obtained by counting the number of times the participant began a new search with a different box to the last search; therefore, a high score indicated an inefficient strategy.
Estimated Intelligence Quotient
Full scale IQ was estimated using four subtests of the Wechsler Adult Intelligence Scale – Third Edition (WAIS-III; Wechsler, 1997) - two providing indices of verbal IQ (Vocabulary, Similarities), and two providing indices of performance IQ (Block Design, Matrix Reasoning).
Statistical Analysis
IBM SPSS Statistics 20 was used to analyze the data. The normality of the cognitive data was tested using the one-sample Kolmogrov-Smirnov test. For the spatial working memory data, the within search and double errors were skewed (p’s<0.001), whereas the between search errors and strategy data were normally distributed (p’s>0.08) in all participants. The results were similar within the three groups. The within search and double errors analyses were conducted with both the raw and Blom’s transformed data. As both analyses revealed similar results, the raw data are presented in the manuscript. All the intelligence measures were normally distributed (p’s>0.10).
Univariate analyses of variance (ANOVAs) were used to test for overall group differences for the spatial working memory and intelligence measures, and to control for multiple comparisons. Only ANOVAs that demonstrated group differences for the spatial working memory and intelligence tests were followed up with least significant difference (LSD) post-hoc analyses. Repeated measures ANOVAs investigated the effect of working memory load for conditions that demonstrated significant overall group effects. Greenhouse-Geisser corrected statistics are reported for the repeated measures ANOVAs. Stepwise and blocked entry discriminant function analyses investigated whether spatial working memory measures and/or full scale IQ better differentiated the groups. ANOVAs were used to compare the cognitive measures in UHR individuals who later developed psychosis and in those who did not. Pearson’s correlations investigated whether quicker time to transition was related to worse spatial working memory performance. Intelligence quotient was not entered as a covariate in the analyses as there were medium to large correlations between full scale IQ and between errors and strategy scores in all three groups. As IQ variations between groups are likely non-random, these variables may not be appropriate as a covariate (Miller & Chapman, 2001). Given the gender differences between groups, we investigated whether gender influenced the dependent measures. No effect of gender was found on the four spatial working memory measures (F’s=0.40-3.26, p’s=0.07-0.53) or three IQ measures (F’s=0.12-1.09, p’s=0.30-0.73) and therefore gender was not included in the models. Cohen’s d and partial-eta squared effect size statistics were reported. The relationship between spatial working memory measures, full scale intelligence, and global functioning was assessed using linear regression in UHR individuals.
Results
Participants
There was a trend towards a group difference in age (F(2, 142)=2.25, p=0.11), with controls being older than FEP patients (p=0.05) and tending to be older than UHR individuals (p=0.07; see Table 1). The groups also differed in gender distribution (X2(2)=6.67, p=0.04); the FEP group had fewer females than the control group (X2(1)=6.65, p=0.01), and there was a trend in this direction relative to the UHR group (X2(1)=3.46, p=0.06). The groups differed in level of education completed (F(2, 125)=5.52, p=0.005), with controls having completed more years of education than both UHR (p=0.009) and FEP (p=0.002) groups.
Table 1.
Participant Characteristics
| Ultra High Risk | First episode Psychosis | Control | |
|---|---|---|---|
| N | 96 | 28 | 23 |
| Age: Range | 23.2 (4.4): 16-35 | 22.6 (4.4): 17-35 | 25.2 (5.4): 19-37 |
| Gender (% female) | 21 | 41 | 57 |
| Education (Years Completed) | 13.9 (2.9) | 12.9 (2.0) | 15.6 (2.8) |
| Global Assessment of Functioning | 57.8 | - | - |
Cognitive Measures
Group differences in spatial working memory
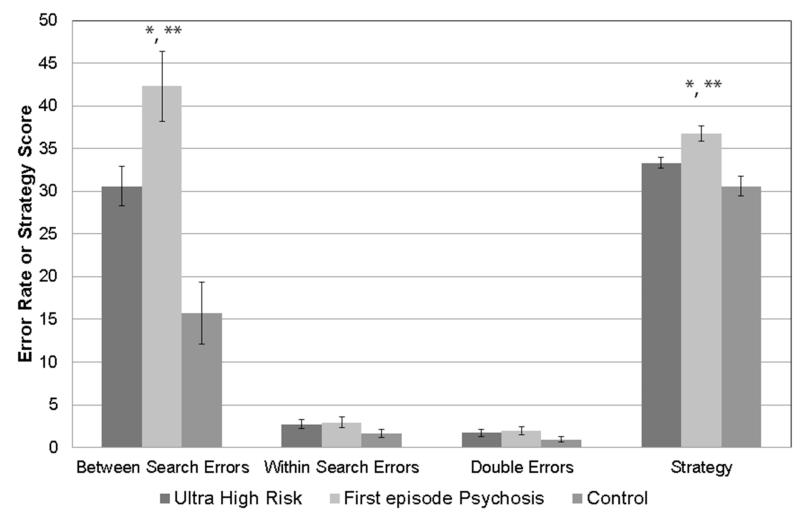
The overall ANOVA demonstrated group differences for between search errors (F(2, 138)=9.81, p=0.001), with the controls demonstrating fewer between search errors than both the UHR (p=0.003, Cohen’s d=0.75) and FEP (p<0.001, Cohen’s d=1.37) groups (see Table 2 and Figure 1). UHR individuals also made fewer between search errors than the FEP patients (p=0.01, Cohen’s d=0.54). Given, the significant overall group result, we followed-up to investigate whether there was a significant effect of load on between search errors. A 3 load (4, 6, 8 boxes) by 3 group repeated measures ANOVA demonstrated a significant effect of load (F(1, 190)=161.32, p<0.001). Furthermore, there was a load by group interaction (F(3, 190)=3.41, p=0.02, partial eta-squared=0.05). This load by group interaction was due to the increase in load from 4 to 6 boxes (F(2, 138)=11.11, p<0.001, partial eta-squared=0.14) and not 6 to 8 boxes (F(2, 138)=0.04, p=0.96, partial eta-squared=0.001). Controls demonstrated less of an increase in errors from 4 to 6 boxes than UHR individuals (F(1, 112)=11.08, p=0.001, partial eta-squared=0.09) and FEP patients (F(1, 48)=33.15, p<0.001, partial eta-squared=0.41). UHR individuals also demonstrated less of an increase in errors from 4 to 6 boxes than FEP patients (F(1, 116)=5.66, p=0.02, partial eta-squared=0.05).
Table 2.
Neurocognition Data
| Ultra high risk | First episode Psychosis | Control | |
|---|---|---|---|
| Spatial Working Memory | |||
| Between Errors | 30.6 (22.0) | 42.3 (21.3) | 15.7 (17.4) |
| Within Errors | 2.7 (5.2) | 2.9 (3.3) | 1.7 (2.2) |
| Double Errors | 1.7 (4.2) | 1.96 (2.5) | 0.9 (1.5) |
| Strategy | 33.3 (5.7) | 36.7 (4.5) | 30.6 (5.5) |
| Estimated Intelligence Quotient | |||
| Full Scale IQ | 100.8 (19.5) | 84.0 (16.9) | 113.7 (19.1) |
| Verbal IQ | 98.6 (19.2) | 84.1 (16.9) | 110.2 (17.0) |
| Performance IQ | 103.8 (21.1) | 87.3 (16.6) | 115.7 (22.8) |
Figure 1.
Spatial working memory performance in individuals at ultra high risk for psychosis, first episode psychosis patients, and controls.
* Controls demonstrated better performance that ultra high risk individuals and first episode psychosis patients
** High risk individuals demonstrated better performance than first episode psychosis patients
There were no overall group differences for within search errors (F(2, 138)=0.62, p=0.54, Cohen’s d=0.04-0.05) or double errors (F(2, 138)=7.82, p=0.54, Cohen’s d=0.08-0.51); therefore, no follow-up testing was conducted.
The overall ANOVA demonstrated group differences in strategy (F(2, 138)=8.12, p<0.001), with controls demonstrating a better strategy than UHR (p=0.03, Cohen’s d=0.48) and FEP patients (p<0.001, Cohen’s d=1.21). UHR individuals had a better strategy than FEP patients (p=0.005, Cohen’s d=0.66). Last, we assessed whether between search errors were predictive of the strategy score. The linear regression demonstrated that between search errors was predictive of strategy scores in all participants (R2=0.54, Beta=0.19, p<0.001).
Estimated IQ
The overall ANOVA demonstrated a group difference in full scale IQ (F(2, 139)=15.57, p<0.001), with controls having a higher full scale IQ than both UHR (p=0.004, Cohen’s d=0.67) and FEP patients (p<0.001, Cohen’s d=1.65). UHR individuals had higher IQ than FEP patients (p’s<0.001, Cohen’s d=0.92). The groups also differed in both verbal and performance IQ (F’s=12.22-13.01, p’s<0.001), with controls having higher estimated verbal and performance IQ than both UHR (p’s=0.008-0.02, Cohen’s d=0.54-0.64) and FEP patients (p’s<0.001, Cohen’s d=1.42-1.54). UHR individuals had higher estimated verbal and performance IQ than FEP patients (p’s<0.001, Cohen’s d=0.80-0.87).
We evaluated the contribution of full scale IQ to spatial short-term maintenance and strategy in UHR individuals. In UHR individuals full scale IQ was predictive of working memory between search errors (R2=0.20, Beta=-0.45, p<0.001) and strategy scores (R2=0.21, Beta=-0.46, p<0.001).
Discriminant function analysis
Stepwise discriminant function analysis was used on a set of potential variables: full scale IQ, between search errors, and strategy score to determine which predictors best discriminated groups. Follow-up blocked entry discriminant function analysis was conducted in the case of significant spatial working memory variables to determine whether spatial working memory variables predicted group status above and beyond full scale IQ. For the stepwise analysis of the UHR group compared to control group, only the between search errors statistically significant discriminated these two groups. The function was significant (Wilks’ λ=0.92, χ2(1)=8.85, p=0.003; canonical discriminant coefficients: between search errors=0.05, constant=-1.31; functions at group centroids: UHR=0.15, controls = −0.57). Sixty percent of individuals were correctly classified as either UHR or control. Furthermore, the blocked entry analysis demonstrated that when accounting for full scale IQ, spatial working memory between search errors was still a significant discriminator of group (F(1,108)=4.14, p=0.04).
For the UHR group compared to FEP group, only the full scale IQ measure statistically significant discriminated these two groups. The function was significant (Wilks’ λ=0.87, χ2(1)=15.52, p<0.001; canonical discriminant coefficient: Full scale IQ=0.05, constant=-5.06; functions at group centroids: UHR=0.21; FEP = −0.70). Sixty-eight percent of individuals were correctly classified as either UHR or FEP.
For the FEP group compared to control group, again only the full scale IQ statistically significant discriminated these two groups. The function was significant (Wilks’ λ=0.59, χ2(1)=24.82, p<0.001; canonical discriminant coefficient: Full scale IQ=0.06, constant=-5.39; functions at group centroids: FEP= −0.77; controls=0.88). Seventy-four percent of individuals were correctly classified as either FEP or control.
Association between spatial working memory with global functioning in UHR individuals
We investigated whether spatial working memory measures that demonstrated significant differences between controls and UHR individuals were predictive of global functioning in UHR individuals at baseline using stepwise linear regression including full scale IQ in the models. Both between search errors (R2=0.07, Beta=-0.13, p=0.02) and strategy score (R2=0.06, Beta=-0.51, p=0.03) were significant predictors of global functioning, whereas full scale IQ was not.
Spatial working memory and transition to psychosis
We compared UHR individuals who subsequently transitioned to psychosis (n=14) with those who did not (n=78) on the spatial working memory and IQ measures that demonstrated differences between groups. No differences were found between individuals who transitioned versus those who did not for the spatial working memory between search errors and strategy measures (F’s=0.14-1.01, p’s=0.32-0.71, Cohen’s d=0.12-0.28), or on the IQ measures (F’s=0.003-0.17, p’s=0.68-0.96, Cohen’s d=0.02-0.13). Last, we evaluated whether ultra high risk individuals who became psychotic sooner in our sample had more severe working memory deficits by conducting correlations between time to transition and our between errors and strategy scores. We found no significant associations between the variables (r’s=−0.01- −0.05, p’s=0.87-0.96).
Discussion
Consistent with our hypotheses, we found that UHR individuals had working memory impairments in both the short-term maintenance of material and in the effective use of strategy compared to controls. Furthermore, short-term maintenance of material better differentiated UHR individuals from controls than did full scale IQ and strategy scores; however, full scale IQ better differentiated UHR individuals from FEP patients and FEP patients from controls than did short-term maintenance and strategy. Also as predicted, these working memory impairments were not as severe as those in the FEP patients. Of particular relevance was that better performance on spatial working memory measures was associated with a lower level of global functioning, whereas IQ was not. Contrary to our hypothesis, UHR individuals who subsequently developed psychosis did not have more marked impairments in working memory than those who did not.
One of our findings was that UHR individuals made more between search errors on the spatial working memory task than controls, but fewer errors than FEP patients. Furthermore, the error rates increased with maintenance load, with the greatest impairments occurring with the initial increase from 4 to 6 boxes. These observations are consistent with previous studies in UHR individuals, which have found that performance on working memory (Fusar-Poli et al., 2012), and other cognitive tests is at a level intermediate between that in controls and FEP patients (Kim, Park, Song, Koo, & An, 2011; Valli, Tognin, Fusar-Poli, & Mechelli, 2012). In contrast to the impairment for between search errors, UHR and FEP patients did not make more within search errors than controls. This suggests that UHR and FEP patients have a deficit in short-term, but not more immediate memory, as avoiding between search errors requires the maintenance of information from a previous search, whereas avoiding within search errors requires its maintenance during the same search (van Asselen, Kessels, Wester, & Postma, 2005).
We also found that UHR subjects were less likely to use an efficient strategy than controls, but were more likely to do so than FEP patients. Strategy use during the CANTAB spatial working memory task is a measure of executive working memory, and is thought to reflect planning ability and the selection of efficient response sequences to integrate information (Owen et al., 1990; van Asselen et al., 2005; Wood et al., 2003). Wood and colleagues (2003), also using the CANTAB spatial working memory task, found that UHR individuals made more between search errors than controls, which also increased with load, but did not find significant group differences for working memory strategy. The difference in the findings from that and the present study might be related to differences in statistical power, as the UHR sample sizes were n= 38 and n= 96, respectively. This would be in line with evidence from a meta-analysis of executive functioning (in general) in UHR individuals, which found that this was impaired relative to that in controls (Fusar-Poli et al., 2012). The working memory deficits in this sample may be associated with striatal dopamine dysfunction that has been found UHR individuals recruited from the same clinical service (Howes et al., 2011; Howes et al., 2009), as striatal dysfunction has been associated greater prodromal symptomology and worse cognitive functioning (Howes et al., 2009).
We also found significant differences in estimated intelligence between UHR individuals and controls. However, in the samples we studied, the mean estimated IQ in the UHR individuals was consistent with the mean in the general population, whereas our controls had IQ values higher than those in the general population. Thus, although we recruited controls from the same geographic location as the UHR sample, they may have had an IQ that was unrepresentative of that in the local community. However, it is also possible that UHR individuals have a lower IQ than controls. Previous studies have reported this, and have also noted that UHR individuals have IQs close to the standard mean, as we found (Brewer et al., 2005; Woodberry et al., 2010). Regardless, one limitation of this study is the large IQ difference between the three groups, which makes an unequivocal interpretation of working memory differences more difficult.
In this study, IQ was not entered as a covariate as there were moderate to large correlations between IQ and spatial short-term memory and strategy. However, analyses demonstrated that in the UHR group full scale IQ accounted for approximately 20% of the variance in working memory, suggesting that although IQ was a significant contributor, it was not explaining the majority of working memory performance. This finding is similar to a meta-analysis in schizophrenia patients and controls which demonstrated that working memory deficits were not simply a result of discrepancies in IQ (Forbes et al., 2009).
Furthermore, we conducted discriminant function analyses to determine which of the spatial working memory measures and/or full scale IQ measure alone or in combination best differentiated groups. We found that short-term maintenance did significantly differentiate the UHR and control groups, even when accounting for IQ; however, this was not the case for all groups. For the FEP and control groups and UHR and FEP groups, full scale IQ better differentiated the groups. This suggests that different cognitive measures might be useful in differentiating different groups with different cognitive profiles. Moreover, these results suggest that the short-term maintenance of information is useful in understanding cognition in UHR individuals beyond IQ. However, it is important to note that the poorest classification occurred between UHR individuals and controls, with only 60% being successfully classified, suggesting there is the largest cognitive overlap between these two groups. These findings merit further exploration in large samples of UHR, FEP, and chronic psychosis individuals, as well as controls.
Our hypothesis that working memory ability would be particularly impaired in UHR individuals who later transitioned to psychosis was not confirmed. A recent meta-analysis found that UHR individuals who subsequently developed psychosis had more severe working memory impairments than those who did not (Fusar-Poli et al., 2012). The reason for this discrepancy could be a difference in statistical power, as in the present study the sample that later developed psychosis comprised of 14 subjects, compared to a sample of 233 individuals in the meta-analysis. Additionally, the meta-analysis demonstrated that the effect size for differences is small (Hedge’s g approximately 0.35) (Fusar-Poli et al., 2012).
One of the most important findings of this study was the relationship between spatial working memory measures and the level of global functioning, which was not found for IQ. This finding is consistent with a large body of evidence that suggests cognitive impairments are associated with reduced levels of functioning in patients with psychotic disorders (Green, 1996; Green, Kern, Braff, & Mintz, 2000), and in UHR individuals (Carrion et al., 2011; Lin et al., 2011). This is an important finding as it raises the possibility that if cognitive function could be improved in UHR individuals, this might increase the level of global functioning. Interventions such as cognitive remediation have shown promise for improving cognitive ability in schizophrenia, and could also be helpful in UHR individuals (Wykes, Huddy, Cellard, McGurk, & Czobor, 2011; Wykes & Spaulding, 2011). Additionally, given the significant association between cognitive ability and functional outcome, but not necessarily transition, focus on need for care maybe more useful clinically than the somewhat arbitrary distinction of transitioning to psychosis.
Conclusions
Spatial working memory was altered in people at UHR for psychosis, and both short-term maintenance and the use of strategy were impaired, whereas more immediate memory was spared compared to controls. Impairments were less severe than in FEP patients. This suggests the importance of investigating different facets of working memory in psychosis. Importantly, these spatial working memory impairments have real world significance as these deficits contribute to a reduction in overall functioning in UHR individuals and this relationship was not found for IQ. Furthermore, these spatial working memory deficits found between groups are not wholly attributable to a reduction in IQ; however, the relationship between working memory and IQ at different stages of illness of psychosis needs to be clarified in future studies. Last, in this sample, there was no evidence that spatial working memory predicted the later onset of psychosis in UHR individuals, but the study had limited statistical power for this analysis. Remediation of working memory deficits in this population is warranted.
References
- Allen P, Chaddock CA, Howes OD, Egerton A, Seal ML, Fusar-Poli P, Valli I, Day F, McGuire PK. Abnormal Relationship Between Medial Temporal Lobe and Subcortical Dopamine Function in People With an Ultra High Risk for Psychosis. Schizophrenia Bulletin. 2012;38:1040–1049. doi: 10.1093/schbul/sbr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA . Diagnostic and Statistical Manual of Mental Disorders : DSM-V. 5th ed American Psychiatric Publishing; Washington, DC: 2013. [Google Scholar]
- Baddeley A. Working Memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Baddeley A. The episodic buffer: a new component of working memory? Trends in Cognitive Science. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Brewer WJ, Francey SM, Wood SJ, Jackson HJ, Pantelis C, Phillips LJ, Yung AR, Anderson VA, McGorry PD. Memory impairments identified in people at ultra-high risk for psychosis who later develop first-episode psychosis. American Journal of Psychiatry. 2005;162:71–78. doi: 10.1176/appi.ajp.162.1.71. [DOI] [PubMed] [Google Scholar]
- Broome MR, Fusar-Poli P, Matthiasson P, Woolley JB, Valmaggia L, Johns LC, Tabraham P, Bramon E, Williams SCR, Brammer MJ, Chitnis X, Zelaya F, McGuire PK. Neural correlates of visuospatial working memory in the ‘at-risk mental state’. Psychological Medicine. 2010;40:1987–1999. doi: 10.1017/S0033291710000280. [DOI] [PubMed] [Google Scholar]
- Broome MR, Matthiasson P, Fusar-Poli P, Woolley JB, Johns LC, Tabraham P, Bramon E, Valmaggia L, Williams SC, Brammer MJ, Chitnis X, McGuire PK. Neural correlates of executive function and working memory in the ‘at-risk mental state’. British Journal of Psychiatry. 2009;194:25–33. doi: 10.1192/bjp.bp.107.046789. [DOI] [PubMed] [Google Scholar]
- Broome MR, Woolley JB, Johns LC, Valmaggia LR, Tabraham P, Gafoor R, Bramon E, McGuire PK. Outreach and support in south London (OASIS): implementation of a clinical service for prodromal psychosis and the at risk mental state. European Psychiatry. 2005;20:372–378. doi: 10.1016/j.eurpsy.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Carrion RE, Goldberg TE, McLaughlin D, Auther AM, Correll CU, Cornblatt BA. Impact of Neurocognition on Social and Role Functioning in Individuals at Clinical High Risk for Psychosis. American Journal of Psychiatry. 2011;168:806–813. doi: 10.1176/appi.ajp.2011.10081209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, editors. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) American Psychiatric Press; Washington, D.C.: 1996. [Google Scholar]
- Forbes NF, Carrick LA, McIntosh AM, Lawrie SM. Working memory in schizophrenia: a meta-analysis. Psychological Medicine. 2009;39:889–905. doi: 10.1017/S0033291708004558. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Deste G, Smieskova R, Barlati S, Yung AR, Howes O, Stieglitz RD, Vita A, McGuire P, Borgwardt S. Cognitive functioning in prodromal psychosis: a meta-analysis. Archives of General Psychiatry. 2012;69:562–571. doi: 10.1001/archgenpsychiatry.2011.1592. [DOI] [PubMed] [Google Scholar]
- Giakoumaki SG. Cognitive and prepulse inhibition deficits in psychometrically high schizotypal subjects in the general population: relevance to schizophrenia research. Journal of the International Neuropsychological Society. 2012;18:643–656. doi: 10.1017/S135561771200029X. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory dysfunction in schizophrenia. The Journal of Neuropsychiatry and Clinical Neurosciences. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD. Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophrenia Bulletin. 1997;23:437–458. doi: 10.1093/schbul/23.3.437. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? American Journal of Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophrenia Bulletin. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Howes O, Bose S, Turkheimer F, Valli I, Egerton A, Stahl D, Valmaggia L, Allen P, Murray R, McGuire P. Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: a PET study. Molecular Psychiatry. 2011;16:885–886. doi: 10.1038/mp.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, Bramon-Bosch E, Valmaggia L, Johns L, Broome M, McGuire PK, Grasby PM. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Archives of General Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- Kim KR, Park JY, Song DH, Koo HK, An SK. Neurocognitive performance in subjects at ultrahigh risk for schizophrenia: a comparison with first-episode schizophrenia. Comprehensive Psychiatry. 2011;52:33–40. doi: 10.1016/j.comppsych.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Kwapil TR, Chapman LJ, Chapman J. Validity and usefulness of the Wisconsin Manual for Assessing Psychotic-like Experiences. Schizophrenia Bulletin. 1999;25:363–375. doi: 10.1093/oxfordjournals.schbul.a033384. [DOI] [PubMed] [Google Scholar]
- Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. Journal of Abnormal Psychology. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Lin A, Wood SJ, Nelson B, Brewer WJ, Spiliotacopoulos D, Bruxner A, Broussard C, Pantelis C, Yung AR. Neurocognitive predictors of functional outcome two to 13 years after identification as ultra-high risk for psychosis. Schizophrenia Research. 2011;132:1–7. doi: 10.1016/j.schres.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28:1021–1034. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- Phillips LJ, Yung AR, McGorry PD. Identification of young people at risk of psychosis: validation of Personal Assessment and Crisis Evaluation Clinic intake criteria. Australian & New Zealand Journal of Psychiatry. 2000;34(Suppl):S164–169. doi: 10.1080/000486700239. [DOI] [PubMed] [Google Scholar]
- Pukrop R, Ruhrmann S, Schultze-Lutter F, Bechdolf A, Brockhaus-Dumke A, Klosterkotter J. Neurocognitive indicators for a conversion to psychosis: Comparison of patients in a potentially initial prodromal state who did or did not convert to a psychosis. Schizophrenia Research. 2007;92:116–125. doi: 10.1016/j.schres.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5:266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Owen AM. Computerized Assessment in Neuropsychiatry Using Cantab - Discussion Paper. Journal of the Royal Society of Medicine. 1992;85:399–402. [PMC free article] [PubMed] [Google Scholar]
- Snitz BE, Macdonald AW, 3rd, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophrenia Bulletin. 2006;32:179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valli I, Tognin S, Fusar-Poli P, Mechelli A. Episodic Memory Dysfunction in Individuals at High-Risk of Psychosis: A Systematic Review of Neuropsychological and Neurofunctional Studies. Current Pharmaceutical Design. 2012;18:443–458. doi: 10.2174/138161212799316271. [DOI] [PubMed] [Google Scholar]
- van Asselen M, Kessels RP, Wester AJ, Postma A. Spatial working memory and contextual cueing in patients with Korsakoff amnesia. Journal of Clinical and Experimental Neuropsychology. 2005;27:645–655. doi: 10.1081/13803390490919281. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3rd Edition Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Wood SJ, Pantelis C, Proffitt T, Phillips LJ, Stuart GW, Buchanan JA, Mahony K, Brewer W, Smith DJ, McGorry PD. Spatial working memory ability is a marker of risk-for-psychosis. Psychological Medicine. 2003;33:1239–1247. doi: 10.1017/s0033291703008067. [DOI] [PubMed] [Google Scholar]
- Woodberry KA, Seidman LJ, Giuliano AJ, Verdi MB, Cook WL, McFarlane WR. Neuropsychological profiles in individuals at clinical high risk for psychosis: Relationship to psychosis and intelligence. Schizophrenia Research. 2010;123:188–198. doi: 10.1016/j.schres.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. American Journal of Psychiatry. 2011;168:472–485. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- Wykes T, Spaulding WD. Thinking about the future cognitive remediation therapy--what works and could we do better? Schizophrenia Bulletin. 2011;37(Suppl 2):S80–90. doi: 10.1093/schbul/sbr064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung AR, Phillips LJ, McGorry PD, McFarlane CA, Francey S, Harrigan S, Patton GC, Jackson HJ. Prediction of psychosis. A step towards indicated prevention of schizophrenia. British Journal of Psychiatry. 1998;172:14–20. [PubMed] [Google Scholar]
- Yung AR, Yuen HP, McGorry PD, Phillips LJ, Kelly D, Dell’Olio M, Francey SM, Cosgrave EM, Killackey E, Stanford C, Godfrey K, Buckby J. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Australian & New Zealand Journal of Psychiatry. 2005;39:964–971. doi: 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]