Abstract
Purpose
Based upon promising preclinical and phase 1 trial results, combined flavopiridol and cisplatin therapy was evaluated in patients with ovarian and primary peritoneal cancers.
Methods
A two cohort phase 2 trial of cisplatin (60 mg/m2 IV) immediately followed by flavopiridol (100 mg/m2 IV, 24 h infusion; 21 day cycles) was undertaken in patients with recurrent platin-sensitive or platin-resistant disease (progression > vs. ≤6 months following prior platin-based therapy). Measurable disease (RECIST) - or evaluable disease plus CA125 >2× post-treatment nadir - and ECOG performance ≤2 were required.
Results
Forty-five patients were enrolled between December 23, 2004 and February 25, 2010: 40 platin-resistant (Group 1), and 5 platin-sensitive (Group 2). In Group 1, the median number of treatment cycles was 3 (range 2–12). Only 10% of patients incurred grade 4 toxicities, but grade 3 toxicities were common (65%): neutropenia (17.5%); nausea (12.5%); vomiting, fatigue, thrombosis, anemia (10% each). Seven patients (17.5%) achieved a confirmed response (1 CR, 6 PR; median duration 118 days); ten additional patients (25%) attained maintained stable disease. Median time to progression was 4.3 months; overall survival was 16.1 months. Pilot translational studies assessed ascites flavopiridol level; surrogate marker studies were uninformative. In Group 2, although 4 of 5 patients responded (2 confirmed PRs with median time to progression, 10.8 months and median overall survival 20.6 months) the cohort was closed due to poor accrual.
Conclusions
The assessed flavopiridol and cisplatin regimen displayed clinical activity in platin resistant and sensitive ovarian/primary peritoneal cancers, meriting further study.
PURPOSE/INTRODUCTION
Ovarian/primary peritoneal cancer is the fifth leading cause of cancer mortality among U.S. women, accounting for >15,000 deaths annually.1 Although adjuvant platin-based chemotherapy results in early clinical remission in most women, nearly 70% are ultimately subject to tumor recurrence and succumb to their cancer.2
In recurrent platin-resistant disease, most commonly either topotecan or liposomal doxorubicin have been used as second line therapies, with reported response rates of 17% and 20%, respectively.3–5 Outcomes resulting from phase 2 trials of novel therapeutics have generally been discouraging, with <3% of patients overall responding to agents including the tubulin poison crytophycin-52 (LYY355703),6 the DNA damaging agent irofulven,7 the anti-EGFR antibody matuzamab,8 and the kinase inhibitors gefitinib9 or imatinib.10,11 Consequently, recurrent platin-resistant ovarian cancer is presently minimally responsive to therapy and incurable, with a median overall survival of only about 6 months.12
Taking a somewhat different approach, we have piloted the use of flavopiridol to heighten/restore platin sensitivity, previously demonstrating that flavopiridol interacts with DNA and disrupts transcription mediated by STAT3, a transcription factor implicated in ovarian cancer pathogenesis.13,14 We also demonstrated that i) flavopiridol effects in ovarian cancer cell lines are optimal with 24 hour exposures (accounting for the administration schedule used in this trial); ii) flavopiridol combines with cisplatin to produce heightened cytotoxic effects largely independent of sequence of administration; and that iii) flavopiridol enhances intracellular cisplatin accumulation, Pt-DNA adducts and in parallel increases the sensitivities of ovarian cancer cell lines to cisplatin.15,16 These data led to our phase I trial demonstrating tolerability of the cisplatin/flavopiridol regimen.17 Although we initially anticipated that flavopiridol/carboplatin might instead represent an attractive regimen, it unexpectedly produced greater toxicities than the flavopiridol/cisplatin combination,17 leading to the present trial.
PATIENTS AND METHODS
PATIENTS
This simultaneously accruing, multi-institution, two group phase II study was designed to assess the adverse effects and effectiveness of flavopiridol combined with cisplatin as second-line therapy in patients with advanced epithelial ovarian and primary peritoneal carcinomas. Patients who progressed during or within 6 months of primary platinum-based therapy, or patients who relapsed ≤6 months after a second challenge with their initial regimen, constituted Group 1 (“platin resistant”); patients who relapsed >6 months following completion of platinum-based therapy, and who had received only a single prior chemotherapy regimen (this could have been repeated), constituted Group 2 (“platin sensitive”). Group 2 was permanently closed on 03/10/2006 due to poor accrual, limiting the primary emphasis of this report to the platin resistant cohort (Group 1). Accrual was slow primarily due to: i) multiple competing therapeutic options, ii) low enthusiasm for cisplatin among enrolling physicians, and iii) patient disinterest in infusional therapy versus the alternative of outpatient infusions not requiring chronic intravenous access.
Eligibility required measurable disease - or evaluable disease plus CA125>2× the post-treatment nadir; ECOG performance score of ≤2; ≥18 years of age; ANC ≥1500; PLT ≥100,000; HgB ≥10 g/dL; direct bilirubin ≤1.5 times UNL; alkaline phosphatase ≤2.5 times UNL; AST ≤2.5 times UNL; and creatinine ≤1.5 times UNL.
Contraindications included: uncontrolled infection, baseline diarrhea ≥4 stools/day, grade ≥2 peripheral neuropathy, >1 prior platin-containing chemotherapy regimen, ≤3 weeks from chemotherapy and/or radiation therapy, failure to recover from reversible effects of prior therapy, CNS metastases, and/or concomitant history of malignancy within the past five years other than non-melanoma cutaneous malignancy.
Therapy consisted of 21 day cycles of 60 mg/m2 cisplatin intravenously day 1 (2 hour infusion) immediately followed by 100 mg/m2 flavopiridol infusion over 24 hours administered using ambulatory pumps. We note that flavopiridol infusion times and approaches used clinically have varied considerably, with bolus plus brief infusions used in chronic lymphocytic leukemia - and 72 h continuous infusions used in initial phase 1 trial. The 24 h infusion used in the present trial was specifically selected instead based upon preclinical data indicating optimal effects with 24 h, and not longer or shorter, exposure periods.15
There was no pre-set limitation of therapy cycles if tolerance permitted. Patients were enrolled at both academic and community medical centers with approval by all local institutional review boards and written informed consent required.
Patients underwent examinations prior to registration and upon subsequent treatment cycles, including: RECIST measurement of the indicator lesion(s) every other cycle, hematologic and chemistry groups (hemoglobin, neutrophil, platelets, WBC, AST, ALK PHOS, direct bilirubin, CA125, creatinine, albumin, calcium, potassium, magnesium, sodium and glucose), physical examination, history, weight, and PS. Chest x-ray and/or chest CT was also required prior to registration; CA125 was assessed pre-therapy and at each treatment cycle. Adverse effects were evaluated per NCI CTCAE Version 3.0, and RECIST 1.0 criteria were applied to assess tumor response/progression.
STUDY DESIGN AND ENDPOINTS
Both study groups had two stage designs, each with interim analysis to be undertaken after enrollment of 20 patients. The primary endpoint for each cohort was the proportion of confirmed RECIST responses (CR/PR on two consecutive evaluations ≥4 weeks apart). Secondary endpoints included overall survival and time to progression. Overall survival was defined as the time from registration to the date of last follow-up or death; time to progression, as time from registration to date of progression or last follow-up, whichever occurred first.
The trial was designed to have 91% power to detect a response rate of 20% versus 5%, with a 9% type I error rate for group 1. Two patient responses (PR and/or CR) in the first 20 patients were required for continued accrual beyond interim analysis. Enrollment continued while awaiting interim analysis data maturation. The final decision rule required 4 or more responses in 38 patients for the regimen to be considered attractive for further evaluation. A stopping rule was designated to halt accrual if >20% of patients incurred grade ≥4 non-hematological toxicities. Patients were enrolled from December 23, 2004 to February 25, 2010. There were no ineligible patients. Patients who withdrew from study therapy due to reasons other than disease progression were nevertheless followed for progression in intention-to-treat analyses from the standpoints of defining time to progression and overall survival (they were not censored from presented analyses).
In efforts to define the intraperitoneal levels of flavopiridol achieved in conjunction with this trial, serial sampling of ascites was pre-specified both pre-therapy and at the end of the cycle 1 24h flavopiridol infusion in patients with accessible ascites, as described in detail below. In parallel, patient tumor cells were isolated from those patient ascites samples so as to define the effects of flavopiridol on the survival/viability of those patient tumor cells at concentrations achieve in those same ascites samples. Additionally, in attempts to elucidate candidate biomarkers of flavopiridol effects in vivo, serial harvest of buccal cells was pre-specified in all Mayo Rochester trial patients pre-therapy and at the conclusion of cycle 1 24h flavopiridol infusion, again as described in detail below.
CELL CULTURE
Flavopiridol was provided by the Pharmaceuticals Resources Branch of the National Cancer Institute (Bethesda, MD); cisplatin was purchased from Sigma (St. Louis, MO) and freshly prepared prior to use. OV202 (platin sensitive) ovarian cancer cells were cultured in MEM with Earle’s salts and L-glutamine containing 20% (v/v) heat-inactivated fetal bovine serum (FBS), CaOV3 (platin sensitive) ovarian cancer cells were grown in DMEM with L-glutamine containing 10% FBS, and SKOV3 were cultured in McCoy’s 5A medium with L-glutamine containing 10% FBS. Each contained 100 units/ml penicillin G and 100 µg/ml streptomycin. Cells were passaged twice weekly, maintained at 37°C (95% air-5% CO2), while CaOV3 were maintained in 90% air-10% CO2 (v/v).
ASSESSMENT OF CELL VIABILITY
Cells were exposed to graded concentrations of flavopiridol, cisplatin, or the combination - or to equivalent volumes of diluent/DMSO for 26 hours; cisplatin was added 2 h prior to 24 h flavopiridol/cisplatin exposure - after plating cells the day prior. In some experiments, cell growth was serially assessed visually via observing confluence over time. Alternatively, rigorous mathematical synergy analyses were performed via clonogenic/colony formation assays: SKOV3 cells (400) were deposited into triplicate 35 mm plates and allowed to adhere overnight. Cells were then treated for 26 hours (cisplatin added 2 hours prior to 24 hour flavopiridol/cisplatin exposure) with graded concentrations of flavopiridol or cisplatin or the combination. After drug removal and washing twice with media, cells were allowed to proliferate 7–10 days; thereafter plates were washed, stained with Coomassie blue, and colonies counted on an imager using GeneTools software (Syngene, Frederick, MD).
ASSESSMENT OF PATIENT ASCITES
In exploratory studies of two patients, serial paired ascites samples were accessible and sampled both pre-therapy and after cycle one 24 hour flavopiridol infusion. Ascites tumor cells were isolated by sedimentation (800×G × 5 min, >80% carcinoma cells verified by microscopy), washed in PBS, and processed in two ways: i) aliquots of cells were incubated in MEM with 20% FBS along with varying concentrations of flavopiridol, with cell viability assessed after 24 h incubation using a trypan blue exclusion assay, and ii) cells were placed in lysis buffer in preparation for SDS-PAGE and immunoblotting. In parallel, the resulting supernatant ascitic fluid was analyzed for flavopiridol concentration using HPLC via established procedures.16
ASSESSMENT OF LEVELS OF CELLULAR POLYPEPTIDES IN PATIENT BUCCAL CELLS
To assess biomarkers potentially associated with therapy and/or response, paired patient buccal mucosal cells were harvested pre-therapy and immediate following cycle one 24 h flavopiridol infusion for all Mayo Rochester patients. Buccal cells were used, as: i) PBMN studies proved unrevealing in our phase 1 trial,17 ii) ex vivo flavopiridol treatment induced changes in cellular polypeptides in normal donor buccal cells and iii) buccal cells were readily non-invasively accessible. Buccal cells were obtained by gently freeing them and depositing dislodged cells into phosphate buffered saline kept at 0°C. Harvested cells were sedimented (800×G × 5 min) and lysed in RIPA buffer with protease inhibitors and 1mM DTT in preparation for SDS-PAGE and immunoblotting. Antibodies included Stat3 (Cell Signaling Technology, Danvers, MA), WT-1 and actin (Santa Cruz Biotechnology, Santa Cruz, CA). In exploratory analyses, levels of assayed polypeptides were thereafter quantified using densitometry analyses of immunoblotting film bands, so as to assess whether any consistent changes in the pre- and post-therapy levels of polypeptides might have been induced in response to flavopiridol therapy.
RESULTS
PRECLINICAL STUDIES
In developing preclinical rationale for the present trial, OV202 and CaOV3 studies confirmed those previously reported in the A459 lung carcinoma model18 to indicate that flavopiridol + cisplatin produced superior anti-proliferative and cytotoxic effects in comparison to either single agent (Figure 1 A and B). Combined effects were also rigorously evaluated using the formal synergy analyses of Chou and Talalay19 - as well as the response surface model of Greco20 using the SYNERGY software.21 Full response surface results for the SKOV3 ovarian cancer cell line are shown in Figure 1C, with Greco Model parameters indicated in the adjacent table. Although synergy was demonstrated using all models, it consistently attained statistical significance using the Greco model only in the SKOV3 cell line; the alpha parameter of 0.74 indicates significant Loewe synergy across the measured range of cisplatin and flavopiridol concentrations.
Figure 1.
In vitro effects of flavopiridol and cisplatin alone or in combination on viability and proliferation of OV202 (A) or CaOV3 (B) human ovarian cancer cells. Exposures of cells to all agents/combinations were of 26 hour duration (cisplatin applied 2 hours prior to combined flavopiridol/cisplatin exposure for 24 hours); cell growth assessed by observing confluence. (C) Predicted response surface for flavopiridol/cisplatin synergy model from SKOV3 human ovarian cancer cells, with Greco model parameters indicated in the adjacent table.
PATIENT RESPONSE
Forty-five patients were enrolled between December 23, 2004 and February 25, 2010: 40 patients to Group 1 (platin resistant; 14 evaluable, 26 measurable) and 5 patients to Group 2 (platin sensitive; 1 evaluable, 4 measurable). Group 2 was closed on 03/10/2006 due to poor accrual for reasons articulated in the Patients and Methods section above. Patient characteristics are presented by group in Table 1. All information contained in further tables and figures include Group 1 patients only.
Table 1.
Patient Characteristics at Entry (Both Groups)
| Characteristic | Group 1 | Group 2 | |||
|---|---|---|---|---|---|
| N=40 | % | N=5 | % | ||
| Median Age (range) | 59 (29–79) | 61 (41–73) | |||
| Age Group: | 18–49 | 8 | 20 | 1 | 20 |
| 50–59 | 12 | 30 | 1 | 20 | |
| 60–69 | 12 | 30 | 1 | 20 | |
| >= 70 | 8 | 20 | 2 | 40 | |
| Performance Status: | 0 | 24 | 60 | 5 | 100 |
| 1 | 16 | 40 | 0 | 0 | |
| 2 | 0 | 0 | 0 | 0 | |
| Site: | ovarian | 26 | 65 | 5 | 100 |
| primary peritoneal | 14 | 35 | 0 | 0 | |
| Race: | Unk/not reported | 2 | 5 | 0 | 0 |
| Native American | 1 | 2.5 | 0 | 0 | |
| White | 37 | 92.5 | 5 | 100 | |
Group 1 patients underwent a median of 3 cycles of treatment (range 2–12); all 40 eligible patients have since discontinued study treatment due to: disease progression (24 pts, 60.0%), patient refusal (8 pts, 20%; primarily over concerns about incrementing peripheral neuropathy), adverse events (3 pts, 7.5%), alternate treatments (3 pts, 7.5%) and otherwise (2 pts, 5%).
The trial was designed such that ≥4 confirmed responses in Group 1 would be required to recommended further testing in this patient population. Seven of 40 total enrolled Group 1 patients (17.5%) achieved a confirmed response (1 CR, 6 PR, verified in a CTEP audit), surpassing the pre-specified endpoint required to consider the regimen attractive for further study; among the 26 Group 1 patients with measurable disease, 6 responded (1 CR, 5 PR; 23%). Median response duration (PR/CR) was 118 days (range 84–212). Ten additional patients (25%) achieved maintained stable disease (median duration 80 days), for a “disease control” rate of 42.5%.
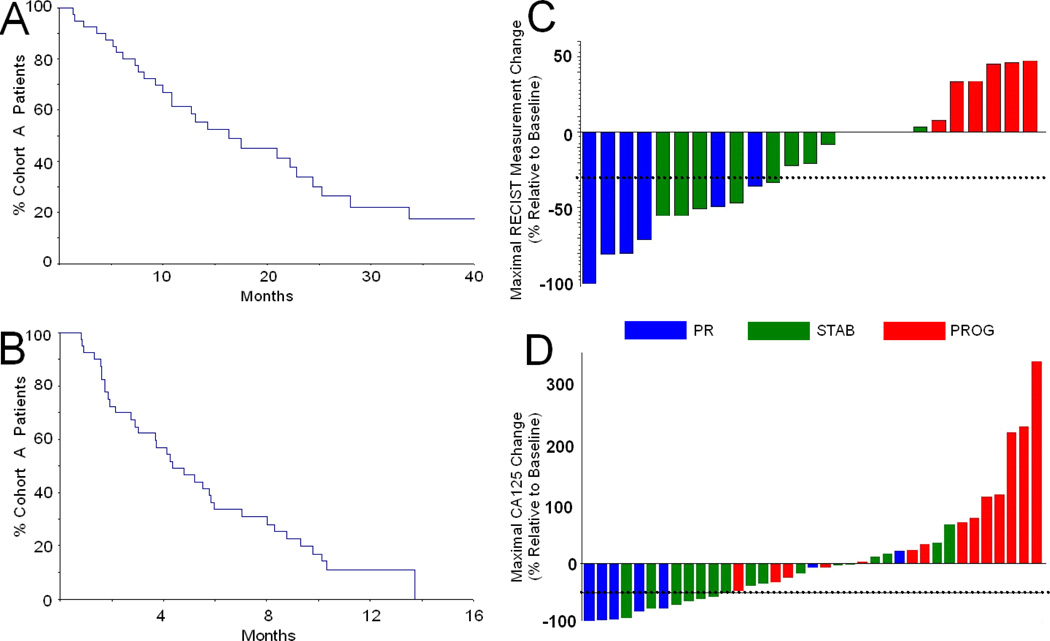
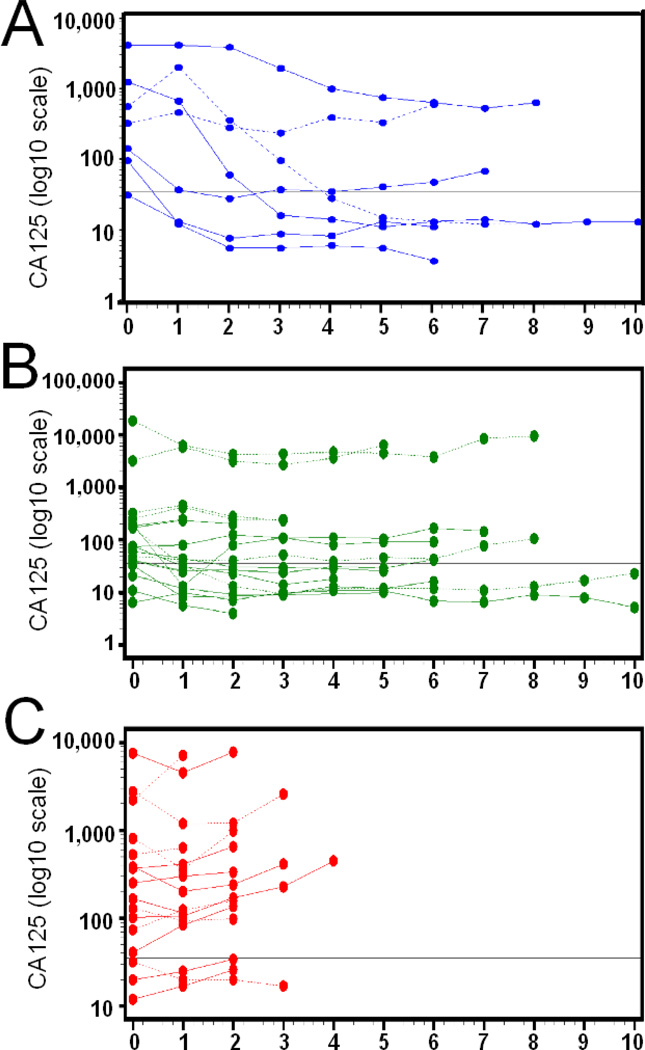
At last contact, 13 (32.5%) Group 1 patients were still alive; however, disease progression occurred in 35 (87.5%). Despite encouraging response data, median time to progression was only 4.3 months; median overall survival was more encouraging at 16.1 months (Kaplan-Meier plots shown in Figure 2A and B). Maximal effects on RECIST tumor measurements or CA125 relative to baseline are shown in Figure 2C (data from only the 26 measurable patients are shown) and D respectively (data from all 40 study patients are shown), while the time courses of CA125 changes for patient subgroups are shown in Figure 3 - as some patients had evaluable disease, with RECIST measurements unavailable.
Figure 2.
Kaplan-Meier plots of overall survival (A) or time to progression (B) for Group 1 platin-resistant patients. Waterfall plots showing best RECIST response (C, only data from the 26 measurable patients shown) or CA125 response (D, data from all 40 study patients shown); values are presented as % baseline pre-therapy measurements. Blue indicates patients attaining partial or complete responses (CR or PR), green indicates those with stable disease (STAB) and red indicate patients with progressive disease (PROG).
Figure 3.
Time courses of patient CA125 values in patients attaining partial or complete responses (PR or CR, A), stable disease (STAB, B) or progressive disease (PROG, C). Values are presented as % baseline pre-therapy measurements. Solid lines indicate measurable disease while dashed lines indicate evaluable disease.
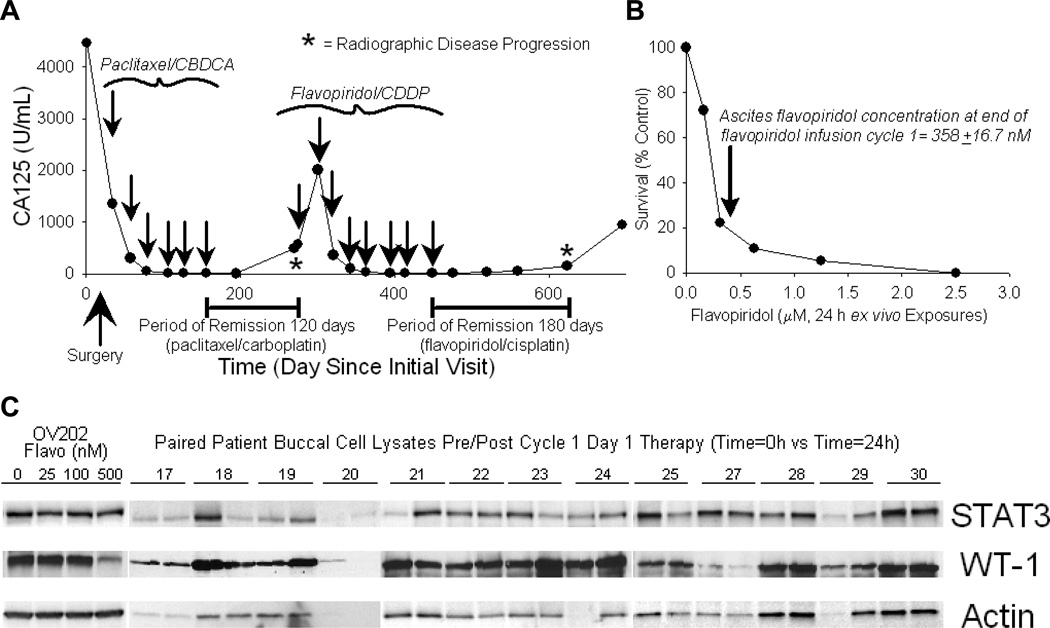
Response to flavopiridol/cisplatin was impressively superior to that attained from paclitaxel/cisplatin adjuvant therapy in some platin-resistant patients. For example, one patient (stage 4 at diagnosis) achieved a CR with remission lasting 50% longer than that previously attained after primary adjuvant paclitaxel/carboplatin therapy (180 days vs. 120 days, Figure 4A). In this patient, flavopiridol ascites concentration was 358+16.7 nM - exceeding that necessary to kill >80% of the patient’s harvested pre-therapy tumor cells after 24 h ex vivo flavopiridol exposure (Figure 4B), concordant with the observed patient response.
Figure 4.
A. Effects of the flavopiridol/cisplatin regimen relative to initial therapy with paclitaxel/carboplatin for a patient with platin-resistant primary peritoneal carcinoma who attained a CR in conjunction with the trial. CA125 levels are plotted vs. time since diagnosis; arrows indicate times of chemotherapy administration and asterisks indicate times of radiographic disease progression. Note that the period of disease control in response to flavopiridol/cisplatin was greater than that attained from initial adjuvant therapy. B. Effects of flavopiridol alone on the ex vivo survival of tumor cells isolated from the ascites of the patient shown in A immediately prior to flavopiridol/cisplatin therapy, indicating sensitivity of her tumor cells to flavopiridol in response to achieved ascetic flavopiridol concentrations. C. Immunoblotting results comparing relative levels of selected polypeptides in paired patient study samples pre/post day 1, cycle 1, therapy (SDS-PAGE, immunoblotting; actin loading controls; samples were loaded to have equivalent pre/post protein levels; 13 representative paired patient samples are shown).
In additional studies, selected polypeptides in patient buccal cells were also examined. Selection of assessed polypeptides was based upon effects of flavopiridol on each polypeptide in in vitro-treated tumor cells - and also upon recently published demonstrations of the effect of flavopiridol on STAT322 and WT-1.23 Consequently, Mcl-1, Bcl-2, p53, RNA polymerase II, p65, PARP, WT-1, STAT3 and HtrA1 were probed in lysates of paired patient buccal and/or ascites cells using SDS-PAGE and immunoblotting, with actin loading controls. Unfortunately, only STAT3, WT-1 and actin were consistently detectable in patient buccal cells, with no interpretable pattern of alteration found in response to therapy (Figure 4C).
Due to the early group 2 closure, the cohort was not statistically evaluated; however, some response was seen in 4 of 5 patients (80%); 2 confirmed PRs, 1 unconfirmed PR and 1 unconfirmed CR; median time to progression was 10.8 months, while median survival was 20.6 months.
We separately assessed responses among patients aged 18–49 years so as to provide hypothesis-generating results as to whether response to flavopiridol/cisplatin therapy may vary by age. Among platin-resistant study patients aged 18–49 years (Group 1, n=8), one patient attained a PR/CR, with the remaining 7 patients attaining SD; the single platin-sensitive patient aged 18–49 years (Group 2) attained an unconfirmed PR, with disease progression noted by the time of confirmation visit.
ADVERSE EVENTS
Adverse event data were available on all 40 Group 1 patients, summarized in Table 2 by grade and attribution24. No deaths resulted from study therapy. While only 10% of patients incurred grade 4 toxicities, grade 3 toxicities were seen in the majority (65%). The most frequent grade 3–4 toxicities were neutropenia (all grade 3, 17.5%); nausea (12.5%); vomiting, fatigue, thrombosis, anemia (10% each). Sensory neuropathy was a significant issue also, observed in 75% of patients. Grade 3 and 4 neuropathy was not observed, however, due to pre-specified aggressive cisplatin dose reduction.
Table 2.
Adverse Events (Regardless of Attribution)*
| N | % | ||
|---|---|---|---|
| Patients with at least one: | Arm | 26 (23) | 65.0 (57.5) |
| Grade 3+ Adverse Event | A | ||
| Grade 4+ Adverse Event | A | 4 (2) | 10.0 (5.0) |
| Grade 3+ Hem Adverse Event | A | 12 (11) | 30.0 (27.5) |
| Grade 3+ Non-Hem Adverse Event | A | 22 (19) | 55.0 (47.5) |
| Grade 4+ Non-Hem Adverse Event | A | 4 (2) | 10.0 (5.0) |
| Adverse Event | Grade | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||||
| N | % | N | % | N | % | N | % | ||
| Type | Arm | 11 | 27.5 | 24 | 60.0 | 4 | 10.0 | ||
| FATIGUE | A | ||||||||
| ANEMIA | A | 16 | 40.0 | 17 | 42.5 | 4 | 10.0 | ||
| NAUSEA | A | 14 | 35.0 | 18 | 45.0 | 5 | 12.5 | ||
| PERIPHERAL SENSORY NEUROPATHY | A | 29 | 72.5 | 2 | 5.0 | ||||
| DIARRHEA-NO COLOSTOM | A | 21 | 52.5 | 5 | 12.5 | 3 | 7.5 | ||
| NEUTROPENIA | A | 6 | 15.0 | 15 | 37.5 | 7 | 17.5 | ||
| VOMITING | A | 12 | 30.0 | 12 | 30.0 | 4 | 10.0 | ||
| HYPERGLYCEMIA | A | 24 | 60.0 | 1 | 2.5 | 2 | 5.0 | ||
| PLATELET COUNT DECREASED | A | 19 | 47.5 | 5 | 12.5 | 1 | 2.5 | ||
| HYPOMAGNESEMIA | A | 17 | 42.5 | 3 | 7.5 | 1 | 2.5 | 1 | 2.5 |
| LEUKOPENIA | A | 2 | 5.0 | 10 | 25.0 | 1 | 2.5 | ||
| ALK PHOSPHATASE | A | 10 | 25.0 | 1 | 2.5 | ||||
| ANOREXIA | A | 9 | 22.5 | 2 | 5.0 | ||||
| CREATININE | A | 7 | 17.5 | 3 | 7.5 | 1 | 2.5 | ||
| HYPOKALEMIA | A | 8 | 20.0 | 1 | 2.5 | 1 | 2.5 | ||
| HYPONATREMIA | A | 7 | 17.5 | 2 | 5.0 | ||||
| ALOPECIA | A | 7 | 17.5 | 1 | 2.5 | ||||
| ASPARTATE AMINOTRANSFERASE INCRE | A | 5 | 12.5 | 1 | 2.5 | ||||
| DEHYDRATION | A | 1 | 2.5 | 2 | 5.0 | 3 | 7.5 | ||
| HYPOCALCEMIA | A | 4 | 10.0 | 2 | 5.0 | ||||
| HYPOALBUMINEMIA | A | 3 | 7.5 | 1 | 2.5 | 1 | 2.5 | ||
| ORAL CAV MS FS | A | 5 | 12.5 | ||||||
| THROMBOSIS | A | 1 | 2.5 | 4 | 10.0 | ||||
| TINNITUS | A | 4 | 10.0 | ||||||
| CONSTIPATION | A | 3 | 7.5 | ||||||
| HYPERSENSITIVITY | A | 1 | 2.5 | 2 | 5.0 | ||||
| PAIN-ABDOMINAL | A | 1 | 2.5 | 2 | 5.0 | ||||
| PERIPHERAL MOTOR NEUROPATHY | A | 3 | 7.5 | ||||||
| SM INTESTN OBSTRUCT | A | 2 | 5.0 | 1 | 2.5 | ||||
| WEIGHT LOSS | A | 3 | 7.5 | ||||||
| ACNE NOS | A | 2 | 5.0 | ||||||
| DYSGEUSIA | A | 1 | 2.5 | 1 | 2.5 | ||||
| DYSPNEA | A | 1 | 2.5 | 1 | 2.5 | ||||
| FEVER-NO ANC | A | 1 | 2.5 | 1 | 2.5 | ||||
| HEADACHE | A | 2 | 5.0 | ||||||
| HEARING IMPAIRED | A | 2 | 5.0 | ||||||
| PAIN | A | 1 | 2.5 | 1 | 2.5 | ||||
| SYNCOPE | A | 2 | 5.0 | ||||||
| ANXIETY | A | 1 | 2.5 | ||||||
| ARRYTHMIA | A | 1 | 2.5 | ||||||
| ATAXIA | A | 1 | 2.5 | ||||||
| ATRIAL FIBRILLATION | A | 1 | 2.5 | ||||||
| BACK PAIN | A | 1 | 2.5 | ||||||
| BLOOD BILIRUBIN INCREASED | A | 1 | 2.5 | ||||||
| COLITIS | A | 1 | 2.5 | ||||||
| COUGH | A | 1 | 2.5 | ||||||
| DIZZINESS | A | 1 | 2.5 | ||||||
| DRY MOUTH | A | 1 | 2.5 | ||||||
| DYSPEPSIA | A | 1 | 2.5 | ||||||
| EXTRAPYRAMIDAL DISORDER | A | 1 | 2.5 | ||||||
| GALLBLADDER OBSTRUCTION | A | 1 | 2.5 | ||||||
| GASTRITIS | A | 1 | 2.5 | ||||||
| HEARING DISABILITY | A | 1 | 2.5 | ||||||
| HICCUPS | A | 1 | 2.5 | ||||||
| HYPERCALCEMIA | A | 1 | 2.5 | ||||||
| HYPERMAGNESEMIA | A | 1 | 2.5 | ||||||
| HYPOPHOSPHATEMIA | A | 1 | 2.5 | ||||||
| MYALGIA | A | 1 | 2.5 | ||||||
| NECK PAIN | A | 1 | 2.5 | ||||||
| Oral cavity MS CE | A | 1 | 2.5 | ||||||
| PAIN IN EXTREMITY | A | 1 | 2.5 | ||||||
| PAIN-CHEST | A | 1 | 2.5 | ||||||
| PALPITATIONS | A | 1 | 2.5 | ||||||
| PNEUMONIA GR 3–4 ANC | A | 1 | 2.5 | ||||||
| SKIN IRRITATION | A | 1 | 2.5 | ||||||
| SKIN ODOR ABN | A | 1 | 2.5 | ||||||
| SWEATING | A | 1 | 2.5 | ||||||
| VASC ACCESS COMPLIC | A | 1 | 2.5 | ||||||
Numbers in parentheses refers to Adverse events with attribution of possible, probable or definite – realizing that there is strong evidence that AE attribution is difficult to determine, unreliable, and of uncertain value in interpreting AE data.24
One patient experienced a cisplatin hypersensitivity reaction and was therefore treated subsequently with flavopiridol alone, interestingly attaining prolonged stable disease thereafter. The majority of other patients, however, received both study agents as intended, with >86.5% receiving full doses of each drug cycles 1–6.
DISCUSSION
To our knowledge, this is the first report demonstrating that flavopiridol used either alone or in combination has appreciable clinical activity in ovarian and primary peritoneal cancers. Treatment with the utilized flavopiridol/cisplatin regimen yielded a 17.5% confirmed CR+PR rate in platin-resistant disease – remarkably indicating that flavopiridol can attenuate platin resistance in this context.
Outcome data for patients enrolled in the present trial compare favorably with those reported in previous clinical trials in platin-resistant disease even in first relapse (reviewed in Ushijima, et al),25 suggesting that the flavopiridol/cisplatin regimen has promise and might reasonably be considered for further clinical study. Median overall survival attained from flavopiridol/cisplatin therapy (16.1 months) was greater than reported in single agent phase 2 clinical trials of gemcitabine (14–15 months),25,26 liposomal doxorubicin (9 months),3,4 pemetrexed (11 months)26 or topotecan (12 months)5 in patients similarly treated at first relapse. Hence, in this comparison, the flavopiridol/cisplatin regimen appears potentially competitive or superior to “standard” salvage therapy in this context, realizing that multi-arm randomized trials examining comparative efficacies of various regimens in platin-resistant disease are necessary.
Median overall survival in patients treated with flavopiridol/cisplatin was also greater than that attained from gemcitabine+liposomal doxorubicin (13 months),25 gemcitabine+paclitaxel (13 months),25 topotecan+liposomal doxorubicin (10 months),25 gemcitabine plus pertuzumab (13 months),28 dose dense-paclitaxel+carboplatin (13 months)29 or single agent ixabepilone (14.8 months).30 Presented flavopiridol+cisplatin outcomes were moreover competitive with results attained from the bevacizumab and erlotinib combination (23.1% CR+PR; median time to progression, 4 months).31 Hence, flavopiridol/cisplatin as administered appears competitive with “standard” and investigational salvage alternatives alike, again with the caveat that randomized multi-arm comparison trial of available regimens need to be undertaken.
Although the RECIST PR+CR rate from flavopiridol+cisplatin (17.5%) was also competitive with those attained from single agent gemcitabine (5–16%),22 liposomal doxorubicin (18%)18 or topotecan (12%)5 - as well as from the combination of gemcitabine plus pertuzumab (14%)24 - it was nevertheless inferior to that reported from gemcitabine+liposomal doxorubicin (22%),22 bevacizumab and erlotinib (23.1%),31 gemcitabine+paclitaxel (40%),21 topotecan+liposomal doxorubicin (28%),22 or dose-dense paclitaxel+carboplatin (60%). However, as enumerated above, these higher response rates did not translate into improved overall survival.
Also remarkable is that the flavopiridol/cisplatin regimen not only produced PRs, but occasional durable CRs even in platin-resistant disease - something rarely encountered in this patient population. Surprisingly, some patients attained more durable responses to salvage flavopiridol/cisplatin than to primary adjuvant paclitaxel/carboplatin therapy (e.g. Figure 4A). Collectively, flavopiridol/cisplatin therapy outcomes provide support for further study of the combination; nevertheless, several important questions remain.
In particular, as indicated above, it will be critical to directly compare flavopiridol/cisplatin to alternative regimens in randomized trials. Also, as flavopiridol/cisplatin moreover has activity in platin-sensitive disease, the regimen may additionally have promise in the platin-sensitive patient population.
Importantly, adverse events resulting from the flavopiridol/cisplatin regimen were also manageable. Platin-related peripheral neuropathy was an issue for many patients remaining on the regimen for longer time periods, however, in part aggravated by prior extensive treatment with carboplatin administered in conjunction with preceding adjuvant therapy.
Presented preclinical and correlative translational work are also deserving of comment. First, in a sort of “proof of principle”, promising in vitro synergy results (Figure 1) were successfully translated to the clinic - with activity also noted in patients. Moreover, in a “bench-to-bedside-to bench” proof of concept, anecdotally assessed flavopiridol concentration in a study patient’s ascites was cytotoxic to the same patient’s ovarian tumor cells (Figure 4B), concordant with patient response to therapy. Unfortunately, exploratory biomarker studies proved unrevealing - we suspect perhaps in part because of the use of surrogate (buccal) cells rather than tumor cells; the same issues arose in conjunction with the preceeding phase I trial, in that surrogate PBMNCs also showed no promising biomarker effects resulting from flavopiridol/cisplatin therapy.17 We note, however, that STAT3 was found by others (Karp et al) to represent a candidate biomarker in studies of the in vivo effects of flavopiridol in patient AML cells,22 highlighting the importance of examing effects in tumor, rather than in surrogate, tissues/cells.
In summary, flavopiridol as combined with cisplatin within the context of this phase 2 trial has clinical activity in both platin-sensitive and in platin-resistant ovarian and primary peritoneal cancers. We believe that additional study of the combination is merited so as to more precisely clarify the relative efficacy of this regimen with respect to alternative approaches.
Highlights.
Flavopiridol and cisplatin can be combined with acceptable toxicities in recurrent ovarian and primary peritoneal cancer
Combined flavopiridol and cisplatin therapy has activity in platinresistant and platin-sensitive ovarian cancer
ACKNOWLEDGEMENTS
We are indebted to patients participating in this trial and to the enrolling investigators for their interest in and dedication to this study. We also thank Dr. Lynn C. Hartmann and the Women’s Cancer Program of the Mayo Clinic Cancer Center for commitment to the completion of this trial and Ms. Candy Kostelec for administrative assistance.
FUNDING
This work was supported by National Cancer Institute CM17104, CA097129, CA15083 and CM62205; Clinicaltrials.gov identifier: NCT00083122.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST: None
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Muggia FM. Recent updates in the clinical use of platinum compounds for the treatment of gynecologic cancers. Semin Oncol. 2004;31:17–24. doi: 10.1053/j.seminoncol.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. 2001;19:3312–3322. doi: 10.1200/JCO.2001.19.14.3312. [DOI] [PubMed] [Google Scholar]
- 4.Stebbing J, Gaya A. Pegylated liposomal doxorubicin (Caelyx) in recurrent ovarian cancer. Cancer Treat Rev. 2002;28:121–125. doi: 10.1053/ctrv.2002.0262. [DOI] [PubMed] [Google Scholar]
- 5.Herzog TJ. Clinical experience with topotecan in relapsed ovarian cancer. Gynecol Oncol. 2003;90:S3–S7. doi: 10.1016/s0090-8258(03)00467-0. [DOI] [PubMed] [Google Scholar]
- 6.D'Agostino G, del Campo J, Mellado B, Izquierdo MA, Minarik T, Cirri L, et al. A multicenter phase II study of the cryptophycin analog LY355703 in patients with platinum-resistant ovarian cancer. Int J Gynecol Cancer. 2006;16:71–76. doi: 10.1111/j.1525-1438.2006.00276.x. [DOI] [PubMed] [Google Scholar]
- 7.Seiden MV, Gordon AN, Bodurka DC, Matulonia UA, Person RT, Reed E, et al. 3rd A phase II study of irofulven in women with recurrent and heavily pretreated ovarian cancer. Gynecol Oncol. 2006;101:55–61. doi: 10.1016/j.ygyno.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 8.Seiden MV, Burris HA, Matulonis U, Hall JB, Armstrong DK, Speyer J, et al. A phase II trial of EMD72000 (matuzumab), a humanized anti-EGFR monoclonal antibody, in patients with platinum-resistant ovarian and primary peritoneal malignancies. Gynecol Oncol. 2007;104:727–731. doi: 10.1016/j.ygyno.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 9.Wagner U, du Bois A, Pfisterer J, Huober J, Loibl S, Lück HJ, et al. Gefitinib in combination with tamoxifen in patients with ovarian cancer refractory or resistant to platinum-taxane based therapy--a phase II trial of the AGO Ovarian Cancer Study Group (AGO-OVAR 2.6) Gynecol Oncol. 2007;105:132–137. doi: 10.1016/j.ygyno.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 10.Alberts DS, Liu PY, Wilczynski SP, Jang A, Moon J, Ward JH, et al. Phase II trial of imatinib mesylate in recurrent, biomarker positive, ovarian cancer (Southwest Oncology Group Protocol S0211) Int J Gynecol Cancer. 2007;17:784–788. doi: 10.1111/j.1525-1438.2007.00882.x. [DOI] [PubMed] [Google Scholar]
- 11.Coleman RL, Broaddus RR, Bodurka DC, Wolf JK, Burke TW, Kavanagh JJ, et al. Phase II trial of imatinib mesylate in patients with recurrent platinumand taxane-resistant epithelial ovarian and primary peritoneal cancers. Gynecol Oncol. 2006;101:126–131. doi: 10.1016/j.ygyno.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 12.Markman M, Webster K, Zanotti K, Peterson G, Kulp B, Belinson J. Survival following the documentation of platinum and taxane resistance in ovarian cancer: a single institution experience involving multiple phase 2 clinical trials. Gynecol Oncol. 2004;93:699–701. doi: 10.1016/j.ygyno.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 13.Bible KC, Bible RH, Jr, Kottke TJ, Svingen PA, Xu K, Pang YP, et al. Flavopiridol binds to Duplex DNA. Cancer Research. 2000;60:2419–2428. [PubMed] [Google Scholar]
- 14.Lee YK, Isham CR, Kaufmann SH, Bible KC. Flavopiridol Disrupts STAT3/DNA Interactions, Attenuates STAT3-directed Transcription and Combines with the Jak Kinase Inhibitor AG490 to Achieve Cytotoxic Synergy. Mol Cancer Ther. 2006;5:138–148. doi: 10.1158/1535-7163.MCT-05-0235. [DOI] [PubMed] [Google Scholar]
- 15.Bible KC, Kaufmann SH. Flavopiridol (NSC 649890, L86-8275): A cytotoxic flavone that induces death in non-cycling A549 human lung carcinoma cells. Cancer Res. 1996;56:4856–4861. [PubMed] [Google Scholar]
- 16.Bible KC, Boerner SA, Kirkland K, Anderl KL, Bartlet D, Jr, Svingen PA, et al. Characterization of an ovarian carcinoma cell line resistant to cisplatin and flavopiridol. Clin Cancer Res. 2000;6:661–670. [PubMed] [Google Scholar]
- 17.Bible KC, Lensing JL, Nelson SA, Lee YK, Reid JM, Ames MM, et al. A phase 1 trial of flavopiridol combined with cisplatin or carboplatin in patients with advanced malignancies with the assessment of pharmacokinetic and pharmacodynamic endpoints. Clin Cancer Res. 2005;11:5935–5941. doi: 10.1158/1078-0432.CCR-04-2566. [DOI] [PubMed] [Google Scholar]
- 18.Bible KC, Kaufmann SH. Cytotoxic synergy between flavopiridol (NSC 649890, L86-8275) and various antineoplastic agents: The importance of sequence of administration. Cancer Res. 1997;57:3375–3380. [PubMed] [Google Scholar]
- 19.Chou TC, Talalay P. Analyses of combined drug effects: a new look at a very old problem. Trends Pharmacol Sci. 1983;4:450–454. [Google Scholar]
- 20.Greco WR, Bravo G, Parsons JC. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev. 1995;47:331–385. [PubMed] [Google Scholar]
- 21.Lee JJ, Kong M, Ayers GD, Lotan R. Interaction Index and Different Methods For Determining Drug Interaction In Combination Therapy. J Biopharm Stat. 2007;17:461–480. doi: 10.1080/10543400701199593. [DOI] [PubMed] [Google Scholar]
- 22.Nelson DM, Joseph B, Hillion J, Resar LS, Karp J. Pharmacodynamic effects of bolus infusion flavopiridol treatment on gene expression in adult patients with relapsed and refractory acute myeloid leukemia. Proc Am Assoc Cancer Res. 2011 Abstract #4713. [Google Scholar]
- 23.Ohta T, Khurana A, Maguire J, He X, Chien J, Bible K, Shridhar V. Flavopiridol-induced upregulation of HtrA1 is associated with suppression of its negative transcriptional regulator WT-1 and with enhanced chemosensitivity. Proc Am Assoc Cancer Res. 2011 Abstract #4015. [Google Scholar]
- 24.Hillman SL, Mandrekar SJ, Bot B, DeMatteo RP, Perez EA, Ballman KV, Nelson H, Buckner JC, Sargent DJ. Evaluation of the value of attribution in the interpretation of adverse event data: a North Central Cancer Treatment Group and American College of Surgeons Oncology Group investigation. J Clin Oncol. 2010;28:3002–3007. doi: 10.1200/JCO.2009.27.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ushijima K. Treatment for recurrent ovarian cancer-at first relapse. J Oncol. 2010:497429. doi: 10.1155/2010/497429. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ojeda GB, Gonzalez MA, Bover BI, Fabugati MX, Mellado B, Rubio Perez MJ, et al. A phase II trial of fixed-dosed rate gemcitabine in platinumresistant ovarian cancer: a GEICO (Grupo Español de Investigación en Cáncer de Ovario) Trial. Am J Clin Oncol. 2008;31:481–487. doi: 10.1097/COC.0b013e31816d1c7b. [DOI] [PubMed] [Google Scholar]
- 27.Miller DS, Blessing JA, Krasner CN, Mannel RS, Hanjani P, Pearl ML, et al. Phase II evaluation of pemetrexed in the treatment of recurrent or persistent platinum-resistant ovarian or primary peritoneal carcinoma: a study of the Gynecologic Oncology Group. J Clin Oncol. 2009;27:2686–2691. doi: 10.1200/JCO.2008.19.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makhija S, Amler LC, Glenn D, Ueland FR, Gold MA, Dizon DS, et al. Clinical activity of gemcitabine plus pertuzumab in platinum-resistant ovarian cancer, fallopian tube cancer, or primary peritoneal cancer. J Clin Oncol. 2010;28:1215–1223. doi: 10.1200/JCO.2009.22.3354. [DOI] [PubMed] [Google Scholar]
- 29.Sharma R, Graham J, Mitchell H, Brooks A, Blagden S, Gabra H. Extended weekly dose-dense paclitaxel/carboplatin is feasible and active in heavily pre-treated platinum-resistant recurrent ovarian cancer. Br J Cancer. 2009;100:707–712. doi: 10.1038/sj.bjc.6604914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Geest K, Blessing JA, Morris RT, Yamada SD, Monk BJ, Zweizig SL, Matei D, Muller CY, Richards WE. Phase II clinical trial of ixabepilone in patients with recurrent or persistent platinum- and taxane-resistant ovarian or primary peritoneal cancer: a gynecologic oncology group study. J Clin Oncol. 2010;28:149–153. doi: 10.1200/JCO.2009.24.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chambers SK, Clouser MC, Baker AF, Roe DJ, Cui H, Brewer MA, et al. Overexpression of tumor vascular endothelial growth factor A may portend an increased likelihood of progression in a phase II trial of bevacizumab and erlotinib in resistant ovarian cancer. Clin Cancer Res. 2010;16:5320–5328. doi: 10.1158/1078-0432.CCR-10-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]