Abstract
Study Design:
Controlled laboratory study.
Background and Purpose:
Anterior knee pain is one of the most common running symptoms reported in the literature. While the exact etiology is unknown, a lack of hip strength is suggested to contribute to abnormal running mechanics. The purpose of this research study was to evaluate the association between isokinetic hip strength and 3‐D running kinematics.
Methods:
33 male high school and collegiate cross country runners participated in this study. Peak isokinetic hip abductor and hip extensor strength were assessed. Each subject also completed a treadmill running protocol at a self‐selected speed (mean = 3.8 m/s). 3‐D kinematic data were collected at 240 Hz using a 10‐camera motion capture system. Pearson correlation coefficients were used to determine the relationship between hip strength and hip range of motion (ROM) during the stance phase of running (p<0.05).
Results:
Peak isokinetic hip extensor torque was inversely correlated with transverse plane hip ROM (r = −.387, p = .026) but was not significantly related to sagittal plane hip ROM or frontal plane hip ROM. Peak isokinetic hip abductor torque was inversely correlated with frontal plane hip ROM (r=−.462, p=.008) but was not significantly related to either sagittal plane hip ROM or transverse plane hip ROM. Peak isokinetic hip extensor torque and peak isokinetic hip abductor torque were not significantly related to knee kinematics in any plane.
Conclusions:
Peak isokinetic hip extensor torque and peak isokinetic hip abductor torque are associated with transverse plane and frontal plane hip kinematics, but not knee kinematics.
Levels of Evidence:
Level 3b
Keywords: cross country, hip strength, isokinetic testing, running biomechanics.
INTRODUCTION
Running continues to grow in popularity among high school and collegiate athletes. Data from the National Federation of High Schools reports that 451,601 young athletes participated in cross‐country during 2010‐2011, reflecting a 24% increase in participants since 2003‐2004.1 Concomitant with the increased participation, associated increases in running injuries are common. While injury rates vary, the annual incidence of injury among high school cross‐country runners can be as high as 17 per 1000 athletic exposures.2
Anterior knee pain is the most prevalent of all running injuries.3,4 While the exact etiology of anterior knee pain in runners is unknown, reduced hip strength is suggested to contribute to abnormal running mechanics.5‐7 An inability to stabilize the hip during the stance phase of running likely increases the dynamic Q‐angle, or dynamic lower extremity genu valgum, resulting in aberrant patellofemoral contact pressures.8 Multiple investigators have retrospectively demonstrated that a lack of adequate hip strength is associated with patellofemoral pain syndrome (PFPS), particularly in females.6,9‐16 However, the association between impaired hip strength and PFPS in males has been less extensively studied.17
Investigations that evaluated the efficacy of impro‐ved strength on functional movement patterns in injured and uninjured subjects provide equivocal evidence. While enhanced hip strength has not been demonstrated to lead to improved running mechanics in adult runners with PFPS,18,19 improved kinematics have been reported in uninjured adult runners following a hip strengthening protocol.19,20 In uninjured female subjects who underwent combined neuromuscular training and strength training, strength improvements were associated with improvements in knee biomechanics. However, due to mixed methods study design whereby subjects underwent both neuromuscular reeducation and strength training it cannot be definitively stated that improved biomechanics were due to improvements in strength alone.21‐23 In uninjured adult male subjects, both open and closed kinematic chain strengthening enhanced strength and led to kinematic and kinetic improvements in a running and cutting maneuver task.24 While the majority of these studies have been in adults, few, if any studies exist establishing the association between hip muscle strength and lower extremity function in competitive, adolescent and young adult male long‐distance runners.
The majority of literature that has documented the association between hip weakness and PFPS has utilized isometric dynamometry.10,12,13,15,16,25 A potential limitation of this method is the inability to assess muscular strength and function throughout a range of motion. This may limit the generalizability of the measure to functional tasks such as running. Recently, several authors have undertaken isokinetic testing of the hip musculature in an attempt to address these instrumentation limitations.5,6,9 Potential limitations of these methods include non‐functional test positions, limited test speeds, and reduced arcs of motion. Therefore, identification of instruments that accurately quantify hip strength in a more functional position, at higher testing speeds, and through a larger functional range of motion may provide more clinically relevant information in the assessment of risk for future running injuries.
The purpose of this research study was to evaluate the association between isokinetic hip strength and 3‐D running kinematics. The authors hypothesized that increased hip strength would be associated with decreased frontal and transverse plane hip motion during running in competitive male high school and collegiate long‐distance runners. Further, it was hypothesized that increased hip strength would be associated with reduced frontal and transverse plane motion at the knee joint.
METHODS
Participants and Setting
Running kinematics and peak concentric isokinetic hip abductor and extensor strength were assessed at 120 deg/sec on 33 uninjured male high school and collegiate cross country runners (Mean Age 18.3 +/‐ 1.9 yrs; Height: 176.9 +/‐ 6.3 cm; Mass 61.6 +/‐ 5.0 kg) using an isokinetic dynamometer (Biodex Medical Systems Inc, Shirley, NY). Testing was performed in a laboratory setting. To be considered for the study subjects needed to be male, actively participating in either high school or collegiate cross country, free from any lower extremity injury for at least 6 months prior to the study, report running at least 20 km per week, and be free from any cardiovascular or neurological condition that would preclude safe treadmill running. The Institutional Review Board at Cincinnati Children's Hospital Medical Center approved the study protocol and the rights of the subjects were protected throughout the study.
Hip Isokinetic Testing Protocol
Concentric isokinetic hip abduction strength was measured for each subject using a protocol previously described by Brent and colleagues26 (Figure 1) and torques were normalized to the subject's body mass. The subject was instructed to stand facing the dynamometer head. The subjects were secured with a strap that originated from the stationary platform on the uninvolved side and extended around the subject's waist above the iliac crest. The dynamometer head was aligned in parallel with the frontal plane of the body with the axis of rotation of the dynamometer aligned with the center of rotation of the hip. The test limb was secured to the attachment arm with a custom strap and resistance pad extending from the attachment arm positioned immediately superior to the knee. The subjects were instructed to grasp the top of the dynamometer head for support to minimize movement of the torso. The dynamometer was programmed to go through the subject's full available active hip abduction ROM, approximately 0°‐45°.
Figure 1.
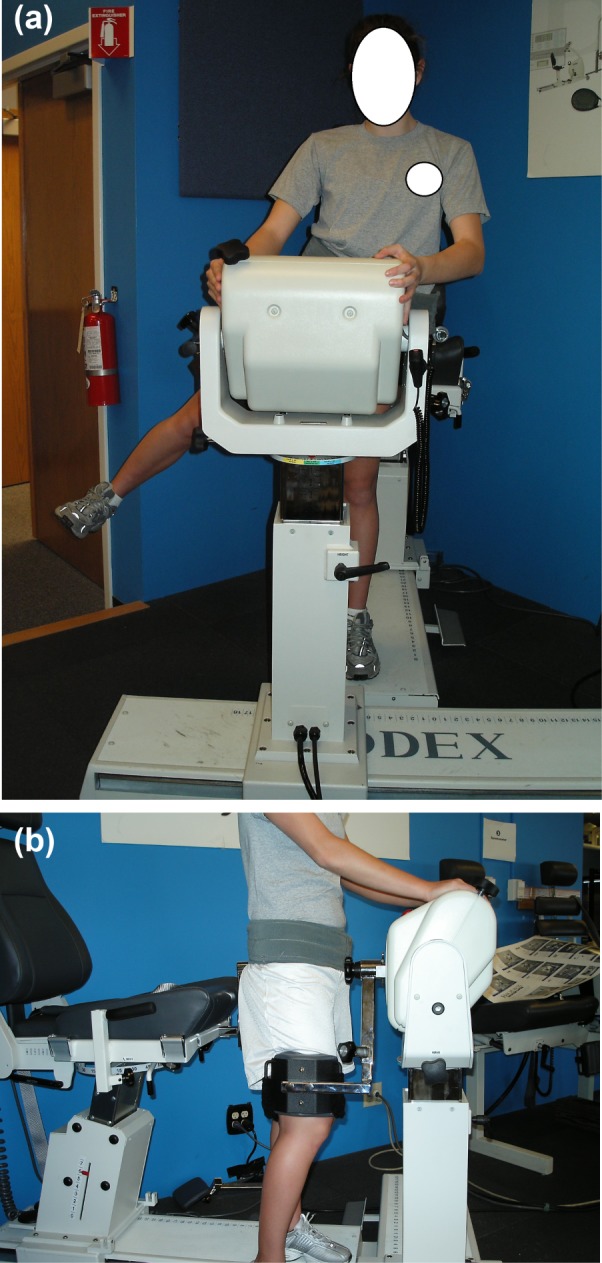
Method for Measuring Concentric Hip Abduction Isokinetic Peak Torque. (a) Anterior view of hip abduction set‐up. (b) Lateral view of hip abduction set‐up.
Concentric isokinetic hip extension strength was measured for each subject using a novel testing design (Figure 2) and torque outputs were normalized to the subject's body mass. The subjects were instructed to stand facing the chair of the isokinetic machine and to place hands on both sides of the back of the seat. The subjects' arms were positioned at approximately 90 degrees of shoulder flexion. Using a large goniometer (Patterson Medical, Bolingbrook, IL), the subject was placed in approximately 10 degrees of trunk flexion. In order for the subject to attain the trunk flexion position, a custom triangular plastic wedge (Foam ‘N More, Inc., Clawson, MI) was placed between the subject's body and the seat‐back with additional wedges used as needed for taller subjects. In order to minimize excessive trunk motion, the subject's trunk and pelvis were secured to the triangular plastic wedge using straps secured to the seat back of the dynamometer. The dynamometer head was aligned in parallel with the center of rotation of the hip at the subject's greater trochanter. The thigh pad of the moving arm was placed just superior to the popliteal space on the testing limb and was secured anteriorly around the thigh. The heel of the subject's stance limb rested on a half foam roll while the subject's tested limb was flexed approximately 90 degrees at the knee. The dynamometer was programmed to move into an arc of approximately 30 degrees of hip extension from the patient's resting hip position, which equated to an arc of motion from approximately 25° of hip flexion to approximately 5° of hip extension.
Figure 2.
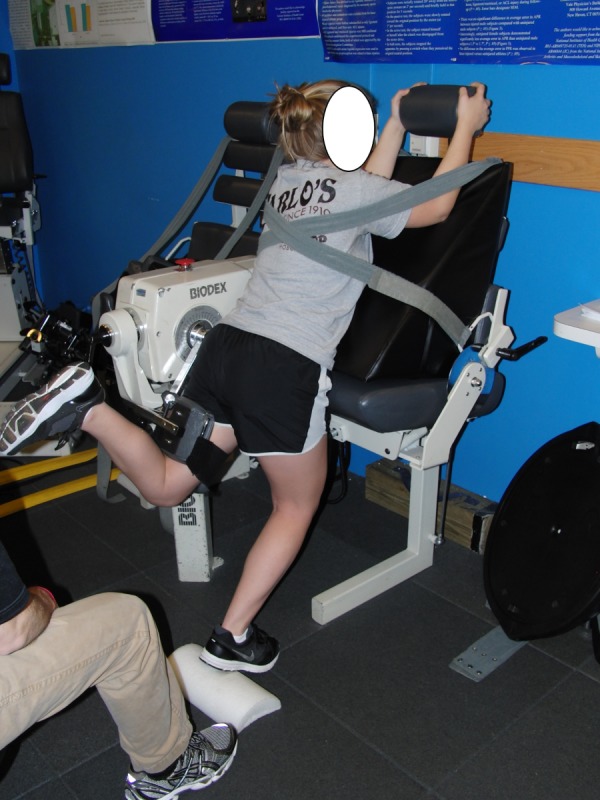
Method for Measuring Concentric Hip Extension Isokinetic Peak Torque.
Prior to testing, all subjects were provided detailed instructions of each testing protocol. Each subject was provided 5 submaximal practice repetitions on each limb for each test condition. Five maximal repetitions for hip extension and hip abduction were collected concentrically for each strength test. The authors chose 120 deg/sec in an attempt to better approximate the joint torque that the hip joint experiences during the running motion.27 While muscles have been demonstrated to produce greater concentric force at decreased isokinetic testing velocities28, the authors felt 120 deg/sec would adequately capture the torque producing qualities of the muscle groups in question. A pilot study assessed intertester and intratester reliability for the described method of hip extension isokinetic testing through an intraclass correlation coefficent (3,1) (ICC)29. An ICC value greater than 0.81 was considered excellent.30 The hip abduction testing protocol was performed by one laboratory assistant who has previously established excellent intratester reliability.31
Treadmill Running Protocol
Each subject also completed a running protocol, as previously described in detail by Ford and colleagues,32 wearing standardized neutral‐cushioned footwear (Adidas Supernova Glide, Adidas, Inc.) on a custom high‐speed treadmill at a self‐selected speed (SS) (mean=3.8 m/s). Three‐dimensional kinematic data were collected at 240 Hz using a 10 camera motion capture system (Motion Analysis Corporation, Santa Rosa, CA) utilizing a previously established marker set with a minimum of 3 retroreflective markers attached to the pelvis, thorax, and each lower extremity segment (foot, shank, and thigh) (Figure 3).32,33 Thirty consecutive steps were captured bilaterally at each speed, and the first twenty steps during the SS run were used for analysis (Figure 3). Each trial was visually inspected to ensure proper identification of the stance phase, defined as the period between initial foot strike to toe‐off. The motion analysis system was calibrated based on manufacturer's recommendations. Marker trajectories were filtered at a cutoff frequency of 12 Hz (low‐pass further order Butterworth filter) prior to calculating knee and hip angles (Visual3d, C‐Motion, Inc.).
Figure 3.
Marker Set‐Up and Treadmill Protocol.
STATISTICAL METHODS
Pearson correlation coefficients were used to determine the relationship between hip strength and hip and knee range of motion (ROM) during the stance phase of running. Correlations were determined to be statistically significant at p < 0.05.
An exploratory factor analysis using principal axis factoring extraction with a direct oblmin rotation was conducted using sagittal, frontal, and transverse plane angles of the hip and knee joint during stance. Specifically, six variables were entered into the factor analysis: hip internal/external, hip abduction/adduction, hip flexion/extension, knee internal/external, knee abduction/adduction and knee flexion/extension. If any variable had a value <0.5 on the diagonal of its anti‐image correlation matrix, that with the lowest value was removed from the analysis in an iterative process until all diagonal values were >0.5. The diagonal of the anti‐image correlation matrix was >0.5 for all six variables, and therefore all were included in the analysis. Once a final model was developed, parallel analysis using permutations of the raw data set was used to determine the number of factors to be retained in the final model. The final model, detailed in the results, contained three factors. Variables with scores >0.5 in the pattern matrix were considered to be key contributors to each factor. Anderson‐Rubin factor loading scores were then saved for each subject. Pearson correlation coefficients were used to determine the relationship between the extracted factors to hip strength.
RESULTS
The hip extension isokinetic testing method demonstrated excellent ICC reliability. The mean ICC values for peak torque intra‐rater reliability were greater than 0.89 and for inter‐rater reliability were greater than 0.85 (Table 1 & 2).
Table 1.
ICC Reliability for Hip Extension Isokinetic Testing Method
| Day 1 | Day 2 | |||
|---|---|---|---|---|
| Tester 1 | Tester 2 | Tester 1 | Tester 2 | |
| Right Hip | ||||
| Subject 1 | 229.7 | 185.1 | 213.6 | 189.2 |
| Subject 2 | 147.6 | 139.5 | 175.9 | 178.1 |
| Subject 3 | 90.9 | 89.3 | 76.3 | 82.7 |
| Subject 4 | 107.7 | 106.7 | 105.5 | 101.5 |
| Subject 5 | 83.5 | 98.5 | 98 | 98.3 |
| Subject 6 | 175.2 | 184 | 202 | 153.8 |
| Subject 7 | 202.8 | 162 | 180.5 | 146.3 |
| Subject 8 | 82.8 | 92 | 94.5 | 78.5 |
| Subject 9 | 98.1 | 87.3 | 79.8 | 84.5 |
| Subject 10 | 147.5 | 128 | 183.3 | 156.9 |
| Left Hip | ||||
| Subject 1 | 180.7 | 206 | 200.1 | 212.9 |
| Subject 2 | 141 | 154.8 | 125.3 | 184.7 |
| Subject 3 | 74.5 | 96.2 | 73.1 | 85.3 |
| Subject 4 | 98.6 | 124.9 | 103.4 | 112.5 |
| Subject 5 | 96.1 | 90.8 | 81.6 | 102.4 |
| Subject 6 | 136.8 | 217.5 | 160.3 | 157.3 |
| Subject 7 | 160.4 | 145 | 150.1 | 165.2 |
| Subject 8 | 61.1 | 58.5 | 77.8 | 67.8 |
| Subject 9 | 102.7 | 85 | 81.4 | 92.3 |
| Subject 10 | 117.7 | 151.7 | 100.8 | 133.5 |
Hip isokinetic test data used to calculate intra‐tester and inter‐tester reliability. Values reported inft*lbs.
Table 2.
Intra‐tester and Inter‐tester ICC reliability for left, right, and combined sides calculated from peak hip isokinetic strength data.
| Intra‐tester ICC (C‐1) | Inter‐tester ICC (C‐1) | |||||
|---|---|---|---|---|---|---|
| Side | Tester 1 | Tester 2 | Mean | Day 1 | Day 2 | Mean |
| Left | 0.91 | 0.87 | 0.89 | 0.79 | 0.91 | 0.85 |
| Right | 0.92 | 0.87 | 0.90 | 0.90 | 0.93 | 0.91 |
| Combined | 0.96 | 0.90 | 0.93 | 0.91 | 0.94 | 0.93 |
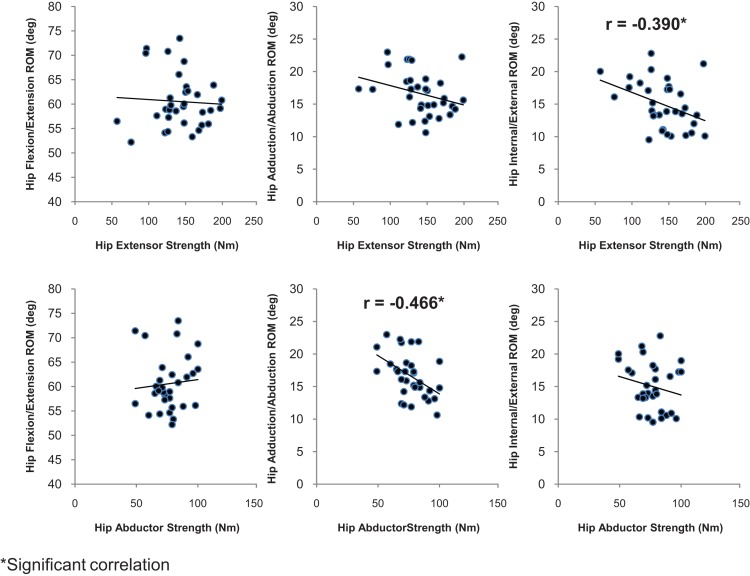
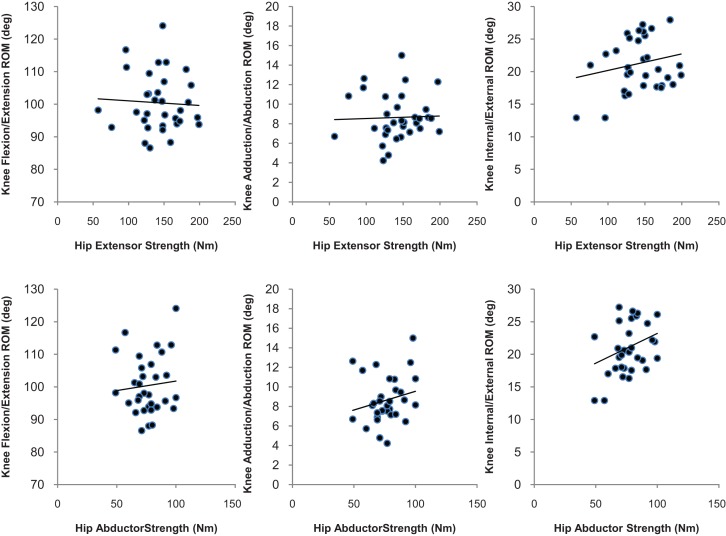
Peak isokinetic hip extensor torque demonstrated a moderate negative relationship with transverse plane hip ROM (r = −0.390, p = 0.012) but was not significantly related to sagittal plane hip ROM (r = −0.057 p = 0.752) and frontal plane hip ROM (r = −0.294, p = 0.097) (Figure 4) in subjects running at a self‐selected speed. Peak isokinetic hip abductor torque showed a strong negative relationship with frontal plane hip ROM (r = −0.462, p =.008) but was not significantly related to either sagittal plane hip ROM (r = 0.089, p = 0.630) or transverse plane hip ROM (r = −0.210, p = 0.248) (Figure 4). Peak isokinetic hip extensor torque was not significantly related to transverse plane knee ROM (r = 0.191, p = 0.287) (See Figure 5), frontal plane knee ROM (r = 0.036, p = 0.842), or sagittal plane knee ROM (r = −0.052, p = 0.775). Peak isokinetic hip abductor torque was not significantly related to frontal plane knee ROM (r = 0.206, p = 0.258) (See Figure 5), transverse plane knee ROM (r = 0.258, p = 0.114), or sagittal plane knee ROM (r = 0.089, p = 0.630).
Figure 4.
Hip Strength Associated with Hip Range of Motion.
Figure 5.
Hip Strength Associated with Knee Range of Motion.
Factor Analysis
The Keiser‐Meyer‐Olkin measure of sampling adequacy was 0.610, which supported the appropriateness of factor analysis for this data set. The diagonal of the anti‐image correlation matrix was >0.5 for all six variables, and therefore all were included in the analysis. Parallel analysis indicated three factors could be retained in the model. Factor 1 accounted for strong loading scores for hip flexion (0.774) and knee flexion (0.842) range of motion. Factor 2 had strong loading scores for hip adduction (0.779) range of motion. Factor 3 had strong loading scores for hip rotation (0.582) and knee abduction (0.661) range of motion. The factor correlation matrix revealed weak correlations between each of the three factors (range: −0.83 < r < 0.304).
There was a significant correlation between hip ab‐duction strength and Factor 2 (r = −0.533, p = 0.002). Hip abduction strength and extension strength were not significantly correlated to any other factors.
DISCUSSION
The results of the current study demonstrated that peak isokinetic hip extensor torque and peak isokinetic hip abductor torque are associated with transverse plane and frontal plane hip kinematics, respectively, in healthy, adolescent and young adult male long‐distance runners. As the authors hypothesized, runners with lower hip abductor and hip extensor strength exhibited greater frontal and transverse plane hip motion. Hip motion represents femur movement relative to the pelvis. Therefore, this motion may relate to pelvic, femoral, or a combination of motions. However, contrary to the authors stated hypothesis, peak isokinetic hip extensor torque and peak isokinetic hip abductor torque were not associated with transverse plane and frontal plane knee mechanics. Furthermore, a factor analysis was utilized to help describe the variability among related biomechanical parameters during running. Interestingly, the results of the factor analysis identified three unique factors that relate to a sagittal plane pattern (Factor 1), hip adduction pattern (Factor 2) and combined hip rotation/knee abduction pattern (Factor 3). Increased hip adduction range of motion was heavily weighted in factor 2 which was significantly related to hip abduction isokinetic strength. This further indicates that decreased abduction concentric strength may increase the hip adduction motion during stance phase of running.
Decreased hip strength may lead to altered hip mechanics in a young, competitive running population. Altered hip strength has been linked to a variety of lower extremity injuries such as iliotibial band syndrome,34‐36 patellofemoral pain syndrome (PFPS),6,9,10,12,15,16,37,38 and tibial stress fracture.39 The association of decreased hip extensor strength and increased hip internal rotation found in this study is in agreement with prior reports indicating this relationship in runners diagnosed with PFPS.5,9 Souza and Powers reported that adult females with decreased hip extension and hip abduction strength demonstrated increased femoral internal rotation during running, a drop jump, and a step down, despite increased gluteus maximus activation.5 The current findings are in partial agreement with a prior report that noted a negative correlation between hip abductor strength and hip adduction angle toward the end of a prolonged run, but not at the beginning of the run, in adult females with PFPS.40
A lack of hip abductor and hip extensor strength appears to be associated with increased hip adduction and hip internal rotation, respectively. Increased hip adduction has previously been associated with iliotibial band syndrome,36 patellofemoral pain syndrome,41,42 and tibial stress fracture,43,44 in adult female long‐distance runners. Increased hip internal rotation has previously been associated with iliotibial band syndrome in adult male long‐distance runners45 and patellofemoral pain syndrome in adult female long‐distance runners.9,46,47 To the authors' knowledge, this is the first study that has demonstrated these atypical hip kinematics in a cohort of young, healthy, and competitive male long‐distance runners. This indicates that interventions which reduce excessive transverse and frontal plane movements at the hip during running may be clinically relevant.
The findings from this study, that hip strength is not associated with frontal or transverse plane knee kinematics, are in agreement with the findings of previous reports investigating the this association in adult females with PFPS19,48 and healthy adult females.18 Both Ferber et al and Earl et al found that proximal strengthening programs focusing on improving hip abductor strength19,48 and hip external rotator strength48 led to pain reductions but did not lead to changes in knee kinematics. Similarly, Willy and Davis demonstrated that improving hip abductor and hip extensor strength did not alter running kinematics.18 In contrast, the current findings differ from Heinert et al, who reported that uninjured collegiate female recreational athletes with reduced hip abductor strength demonstrated significantly increased knee abduction angle during the stance phase of running as compared to a stronger cohort.7
Several potential factors exist to explain the differences noted in the current study relative to prior reports. First, the subject population consisted of healthy, adolescent and young adult males. Prior investigations have noted differences in running kinematics between adult males and adult female runners,49 and thus it is possible that gender and age may play a role in contrasting running mechanics. Second, as our subject population was uninjured, it is possible that pain may play a role in mediating running mechanics. While knee pain inhibits quadriceps activity,50 it is not clearly understood the effect that knee pain may have in altering hip muscle activity or compensatory movement patterns. While adult female runners with PFPS demonstrated delayed and shorter gluteus medius muscle activation than pain‐free subjects,51 it was unknown if these differences were due to pain or were present prior to the onset of their condition. Therefore, the differences in study design and study populations may underlie the divergence of the current results from prior reports. Finally, dynamic knee valgus is the result of several movements, particularly femoral internal rotation, femoral adduction, knee abduction, and knee external rotation. Current evidence suggests a position of dynamic knee valgus, particularly femoral internal rotation, results in altered patellofemoral joint kinematics, which places stress on the patellofemoral joint.52 This study demonstrates that weak hip abductors and hip extensors are associated with increased hip adduction and hip internal rotation. Therefore, this study may demonstrate a link between altered hip strength and high‐risk lower extremity kinematics which may predispose the cohort with weaker hip strength to future injury.
Previous studies that have quantified hip muscle deficits in patients with PFPS have primarily used isometric dynamometry in order to quantify hip strength.10,12,13,15,16,25 Isometric dynamometry allows clinicians to examine the strength of isolated joints at fixed rotational positions. The static condition of isometric dynamometry may allow patients to produce larger peak torques than isokinetic dynamometry at a respective position.53 During the stance phase of running, muscles around the hip joint primarily are contracting eccentrically and concentrically,54 thus isometric dynamometry may lack construct validity. Conversely, isokinetic tests allow patients to progress through a range of motion in a single degree of freedom and can provide an assessment of both concentric and eccentric muscle strength which, in turn, may better measure the construct of hip muscle strength. Disadvantages to isokinetic testing include cost, prolonged set‐up time, and access for both clinicians and researchers. While isokinetic testing improves upon measuring the construct of hip strength, at this time it is not possible to measure hip strength in the exact position, joint speed, and contraction type that the muscles are utilized during the running gait cycle.
Recently, various methods of hip isokinetic dynamometry have been utilized in an attempt to address the perceived deficits of isometric dynamometry testing.5,6,9 Souza and Powers5,9 utilized an isokinetic hip extension strength assessment protocol similar to this study with respect to positioning and ROM of the testing limb. Their test was performed in prone, which is consistent with manual muscle testing procedures that are conducted against gravity. The novel approach utilized in this study was chosen to simulate the position of running with an upright stance. Souza utilized isometric peak hip extension torque at 30 degrees of hip flexion,9 and isometric, isotonic and isokinetic dynamometry measures were utilized with isokinetic testing performed at 10 deg/sec.5 Boling et al6 also utilized the prone method, however their range of motion was 30 degrees of hip extension from a starting position of 90 degrees of hip flexion and the testing speed for concentric and eccentric contractions was set at 60 deg/sec based on previous research6. Boling reported ICC's of 0.79 for their intrasession reliability of peak concentric strength which is below the ICC achieved in the current study. The higher testing speed of 120 deg/sec for the current study was chosen to capture the construct of strength at a faster speed, while still maintaining the ability to reliably evaluate peak torque.
While the method used for measuring peak hip extension torque is novel, the ICC data demonstrated that the methods outlined in this paper have good reliability. ICC values indicated that within‐rater and between‐rater reliability were both almost perfect. Inter‐tester reliability was slightly lower than intra tester reliability. Reliability studies have been previously performed on alternative protocols that examine hip extension isokinetic strength. Previous authors have found that hip extension ICC was 0.84 at 90 deg/sec in young boys and 0.96 at deg/sec at 60 deg/sec.55,56 The current data suggests that the reliability of the novel hip isokinetic testing protocol used in the current study is comparable to these previously tested methods and are considered almost perfect based on ICC values.
Limitations to the current study include that kinetic data was not collected during treadmill running which limits interpretation of kinematic data. Additionally, the authors did not measure gluteal muscle activation using EMG. Recent reports demonstrate that alterations in running kinematics in adult female runners with PFPS may be due, in part, to alterations in gluteus medius and gluteus maximus activation, respectively.9,51 The underlying factor of motor control is difficult to evaluate since it encompasses not only the strength of the musculature, but also the timing and efficiency with which it is able to control movement. Future research efforts should be directed at assessing the roles that muscle activation and maturation play, if any, in affecting lower extremity kinematics in healthy male runners. Finally, our results should be viewed with caution due to the relative low coefficient of determination (r2) values of our findings (gluteus maximus r2 = 0.152 and gluteus medius r2 = 0.213) as it is likely other variables, in addition to hip strength, also explain the studied hip motions.
CONCLUSION
Peak isokinetic hip extensor torque and peak isokinetic hip abductor torque are negatively associated with transverse plane and frontal plane hip motion, but not knee kinematics in male adolescent and young adult long‐distance runners. Three unique factors were identified to explain three‐dimensional range of motion occurring at the hip and knee during running. The factor with strong loading scores for hip adduction range of motion was significantly correlated to hip abduction strength. This indicates that a potential underlying mechanism for this unique description of running may relate to hip abduction strength in males. However, the factors with strong loading scores for sagittal plane range of motion (hip and knee) and hip rotation/knee abduction were not significantly correlated with hip strength.
This study uniquely identifies the relationship between peak isokinetic hip strength and 3‐D hip mechanics, which may be associated with pathomechanics that have been shown to increase the risk of anterior knee pain in runners. Utilization of isokinetic testing to assess peak hip extensor and hip abductor torque may aid in the identification of runners who may be susceptible for future running injuries. Future prospective studies are warranted to assess the effect that alterations in hip strength, muscle activation, running kinematics, and running kinetics have on both injury occurrence and type of injury in at‐risk populations.
Acknowledgments
This work was supported by Cincinnati Children's Hospital Research Foundation. The authors would also like to thank James Smoliga, DVM, PhD for his help with the statistical design of the study.
REFERENCES
- 1.National Federation of High Schools survey of participation. Available at: http://www.nfhs.org/Participation/ Accessed July 3
- 2.Rauh MJ Koepsell TD Rivara FP et al. Epidemiology of musculoskeletal injuries among high school cross‐country runners. Am J Epidemiol. 2006;163:151‐9 [DOI] [PubMed] [Google Scholar]
- 3.Taunton JE Ryan MB Clement DB et al. A retrospective case‐control analysis of 2002 running injuries. Br J Sports Med. 2002;36:95‐101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taunton JE Ryan MB Clement DB et al. A prospective study of running injuries: the Vancouver Sun Run “In Training” clinics. Br J Sports Med. 2003;37:239‐44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Souza RB Powers CM Predictors of hip internal rotation during running: an evaluation of hip strength and femoral structure in women with and without patellofemoral pain. Am J Sports Med. 2009;37:579‐87 [DOI] [PubMed] [Google Scholar]
- 6.Boling MC Padua DA Alexander Creighton R Concentric and eccentric torque of the hip musculature in individuals with and without patellofemoral pain. J Athl Train. 2009;44:7‐13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinert BL Kernozek TW Greany JF et al. Hip Abductor Weakness and Lower Extremity Kinematics During Running. Journal of Sport Rehabilitation. 2008;17:243‐56 [DOI] [PubMed] [Google Scholar]
- 8.Powers CM The influence of altered lower‐extremity kinematics on patellofemoral joint dysfunction: a theoretical perspective. J Orthop Sports Phys Ther. 2003;33:639‐46 [DOI] [PubMed] [Google Scholar]
- 9.Souza RB Powers CM Differences in hip kinematics, muscle strength, and muscle activation between subjects with and without patellofemoral pain. J Orthop Sports Phys Ther. 2009;39:12‐9 [DOI] [PubMed] [Google Scholar]
- 10.Bolgla LA Malone TR Umberger BR et al. Hip strength and hip and knee kinematics during stair descent in females with and without patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2008;38:12‐8 [DOI] [PubMed] [Google Scholar]
- 11.Baldon Rde M Nakagawa TH Muniz TB et al. Eccentric hip muscle function in females with and without patellofemoral pain syndrome. J Athl Train. 2009;44:490‐6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cichanowski HR Schmitt JS Johnson RJ et al. Hip strength in collegiate female athletes with patellofemoral pain. Med Sci Sports Exerc. 2007;39:1227‐32 [DOI] [PubMed] [Google Scholar]
- 13.Cowan SM Crossley KM Bennell KL Altered hip and trunk muscle function in individuals with patellofemoral pain. Br J Sports Med. 2009;43:584‐8 [DOI] [PubMed] [Google Scholar]
- 14.Prins MR van der Wurff P Females with patellofemoral pain syndrome have weak hip muscles: a systematic review. Aust J Physiother. 2009;55:9‐15 [DOI] [PubMed] [Google Scholar]
- 15.Robinson RL Nee RJ Analysis of hip strength in females seeking physical therapy treatment for unilateral patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2007;37:232‐8 [DOI] [PubMed] [Google Scholar]
- 16.Ireland ML Willson JD Ballantyne BT et al. Hip Strength in Females with and without Patellofemoral Pain. J Orthop Sports Phys Ther. 2003;33:671‐6 [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa TH Moriya ET Maciel CD et al. Frontal plane biomechanics in males and females with and without patellofemoral pain. Med Sci Sports Exerc. 2012;44:1747‐55 [DOI] [PubMed] [Google Scholar]
- 18.Willy RW Davis IS The effect of a hip‐strengthening program on mechanics during running and during a single‐leg squat. J Orthop Sports Phys Ther. 2011;41:625‐32 [DOI] [PubMed] [Google Scholar]
- 19.Ferber R Kendall KD Farr L Changes in knee biomechanics after a hip‐abductor strengthening protocol for runners with patellofemoral pain syndrome. J Athl Train. 2011;46:142‐9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snyder KR Earl JE O'Connor KM et al. Resistance training is accompanied by increases in hip strength and changes in lower extremity biomechanics during running. Clin Biomech (Bristol, Avon). 2009;24:26‐34 [DOI] [PubMed] [Google Scholar]
- 21.Myer GD Brent JL Ford KR et al. A pilot study to determine the effect of trunk and hip focused neuromuscular training on hip and knee isokinetic strength. Br J Sports Med. 2008;42:614‐9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim BO Lee YS Kim JG et al. Effects of sports injury prevention training on the biomechanical risk factors of anterior cruciate ligament injury in high school female basketball players. Am J Sports Med. 2009;37:1728‐34 [DOI] [PubMed] [Google Scholar]
- 23.Greska EK Cortes N Van Lunen BL et al. A feedback inclusive neuromuscular training program alters frontal plane kinematics. J Strength Cond Res. 2012;26:1609‐19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cochrane JL Lloyd DG Besier TF et al. Training affects knee kinematics and kinetics in cutting maneuvers in sport. Med Sci Sports Exerc. 2010;42:1535‐44 [DOI] [PubMed] [Google Scholar]
- 25.Boling MC Bolgla LA Mattacola CG et al. Outcomes of a weight‐bearing rehabilitation program for patients diagnosed with patellofemoral pain syndrome. Arch Phys Med Rehabil. 2006;87:1428‐35 [DOI] [PubMed] [Google Scholar]
- 26.Brent J Myer GD Ford KR et al. The Effect of Sex and Age on Isokinetic Hip Abduction Torques. J Sport Rehabil. 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunter JP Marshall RN McNair PJ Segment‐interaction analysis of the stance limb in sprint running. Journal of biomechanics. 2004;37:1439‐46 [DOI] [PubMed] [Google Scholar]
- 28.Perrin DH Isokinetic exercise and assessment: Human Kinetics Publishers; 1993 [Google Scholar]
- 29.McGraw KOaW, S.P Forming inferences about some intraclass correlation coefficients. Psychological Methods. 1996;1:30‐46 [Google Scholar]
- 30.Landis JR Koch GG The measurement of observer agreement for categorical data. Biometrics. 1977;33:159‐74 [PubMed] [Google Scholar]
- 31.Brent JL Myer GD Ford KR et al. The effect of sex and age on isokinetic hip‐abduction torques. J Sport Rehabil. 2013;22:41‐6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ford KR Taylor‐Haas JA Genthe K et al. Relationship between hip strength and trunk motion in college cross‐country runners. Med Sci Sports Exerc. 2013;45:1125‐30 [DOI] [PubMed] [Google Scholar]
- 33.Bates NA Ford KR Myer GD et al. Impact differences in ground reaction force and center of mass between the first and second landing phases of a drop vertical jump and their implications for injury risk assessment. J Biomech. 2013;46:1237‐41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferber R Noehren B Hamill J et al. Competitive female runners with a history of iliotibial band syndrome demonstrate atypical hip and knee kinematics. J Orthop Sports Phys Ther. 2010;40:52‐8 [DOI] [PubMed] [Google Scholar]
- 35.Fredericson M Cookingham CL Chaudhari AM et al. Hip abductor weakness in distance runners with iliotibial band syndrome. Clin J Sport Med. 2000;10:169‐75 [DOI] [PubMed] [Google Scholar]
- 36.Noehren B Davis I Hamill J ASB clinical biomechanics award winner 2006 prospective study of the biomechanical factors associated with iliotibial band syndrome. Clin Biomech (Bristol, Avon). 2007;22:951‐6 [DOI] [PubMed] [Google Scholar]
- 37.Bolgla LA Malone TR Umberger BR et al. Comparison of hip and knee strength and neuromuscular activity in subjects with and without patellofemoral pain syndrome. International journal of sports physical therapy. 2011;6:285‐96 [PMC free article] [PubMed] [Google Scholar]
- 38.Tyler TF Nicholas SJ Mullaney MJ et al. The role of hip muscle function in the treatment of patellofemoral pain syndrome. Am J Sports Med. 2006;34:630‐6 [DOI] [PubMed] [Google Scholar]
- 39.Niemuth PE Johnson RJ Myers MJ et al. Hip muscle weakness and overuse injuries in recreational runners. Clin J Sport Med. 2005;15:14‐21 [DOI] [PubMed] [Google Scholar]
- 40.Dierks TA Manal KT Hamill J et al. Proximal and distal influences on hip and knee kinematics in runners with patellofemoral pain during a prolonged run. J Orthop Sports Phys Ther. 2008;38:448‐56 [DOI] [PubMed] [Google Scholar]
- 41.Willson JD Davis IS Lower extremity mechanics of females with and without patellofemoral pain across activities with progressively greater task demands. Clin Biomech. 2008;23:203‐11 [DOI] [PubMed] [Google Scholar]
- 42.Noehren B Hamill J Davis I Prospective evidence for a hip etiology in patellofemoral pain. Med Sci Sports Exerc. 2013;45:1120‐4 [DOI] [PubMed] [Google Scholar]
- 43.Milner CE Hamill J Davis IS Distinct hip and rearfoot kinematics in female runners with a history of tibial stress fracture. J Orthop Sports Phys Ther. 2010;40:59‐66 [DOI] [PubMed] [Google Scholar]
- 44.Pohl MB Mullineaux DR Milner CE et al. Biomechanical predictors of retrospective tibial stress fractures in runners. J Biomech. 2008;41:1160‐5 [DOI] [PubMed] [Google Scholar]
- 45.Noehren B Schmitz A Hempel R et al. Assessment of strength, flexibility, and running mechanics in men with iliotibial band syndrome. J Orthop Sports Phys Ther. 2014;44:217‐22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wirtz AD Willson JD Kernozek TW et al. Patellofemoral joint stress during running in females with and without patellofemoral pain. Knee. 2012;19:703‐8 [DOI] [PubMed] [Google Scholar]
- 47.Noehren B Pohl MB Sanchez Z et al. Proximal and distal kinematics in female runners with patellofemoral pain. Clin Biomech (Bristol, Avon). 2012;27:366‐71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Earl JE Hoch AZ A proximal strengthening program improves pain, function, and biomechanics in women with patellofemoral pain syndrome. American Journal of Sports Medicine. 2011;39:154‐63 [DOI] [PubMed] [Google Scholar]
- 49.Ferber R Davis IM Williams DS 3rd Gender differences in lower extremity mechanics during running. Clin Biomech. 2003;18:350‐7 [DOI] [PubMed] [Google Scholar]
- 50.Hodges PW Mellor R Crossley K et al. Pain induced by injection of hypertonic saline into the infrapatellar fat pad and effect on coordination of the quadriceps muscles. Arthritis Rheum. 2009;61:70‐7 [DOI] [PubMed] [Google Scholar]
- 51.Willson JD Kernozek TW Arndt RL et al. Gluteal muscle activation during running in females with and without patellofemoral pain syndrome. Clin Biomech. 2011;26:735‐40 [DOI] [PubMed] [Google Scholar]
- 52.Powers CM Ward SR Fredericson M et al. Patellofemoral kinematics during weight‐bearing and non‐weight‐bearing knee extension in persons with lateral subluxation of the patella: a preliminary study. J Orthop Sports Phys Ther. 2003;33:677‐85 [DOI] [PubMed] [Google Scholar]
- 53.Prentice WE Arnheim's Principles of Athletic Training: A Competency‐Based Approach, ed 12 2006:89‐128 [Google Scholar]
- 54.McClay I Manal K Three‐dimensional kinetic analysis of running: significance of secondary planes of motion. Med Sci Sports Exerc. 1999;31:1629‐37 [DOI] [PubMed] [Google Scholar]
- 55.Eng JJ Kim CM Macintyre DL Reliability of lower extremity strength measures in persons with chronic stroke. Arch Phys Med Rehabil. 2002;83:322‐8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burnett CN Betts EF King WM Reliability of isokinetic measurements of hip muscle torque in young boys. Phys Ther. 1990;70:244‐9 [DOI] [PubMed] [Google Scholar]