Abstract
Background:
Several glenohumeral joint (GHJ) positions have been recommended for assessing and correcting posterior shoulder tightness (PST) however, there is no agreement on which position is better for differentiating posterior muscle tightness from posterior capsular tightness. The purpose of this study was to compare the range of motion change before and after an external humeral rotator muscle fatigue protocol in order to identify a position that shows maximum range of motion change.
Methods:
ROM changes across four PST measurements were compared before, immediately after, at 24 hours after, and 48 hours after an external rotator fatigue protocol. Muscle stiffness of the infraspinatus and the teres minor (using a myotonometer) and external rotation force production (using hand‐held dynamometry) were measured to verify muscle fatigue.
Results:
There was a statistically significant interaction between measurement and condition (F = 2.47, p = 0.02). The planned one factor repeated measure ANOVA for each condition revealed that ROM change was statistically significant between PST measurements for all conditions. Post hoc comparisons indicated statistically significant greater overall ROM changes in a measurement combining GHJ extension and internal rotation compared to other tested measurements. There was also a main effect of time on infraspinatus muscle stiffness (F = 10.5, p < 0.0001). Post hoc comparison indicated a statistically significant increase in infraspinatus stiffness immediately after the fatigue protocol (p < 0.05).
Conclusion:
Immediate ROM reduction was observed across all the measurements except horizontal adduction (HAD). Maximum ROM reduction after an external rotation fatigue protocol was measured in a position of GHJ extension.
Clinical Relevance:
Posterior muscle tightness may influence the internal rotation range of motion to a greater extent when measured in glenohumeral joint extension.
Levels of Evidence:
II‐B
Keywords: Glenohumeral joint, muscle stiffness, clinical measurement, horizontal adduction, internal rotation
INTRODUCTION
Loss of internal rotation range of motion (ROM) of the dominant glenohumeral joint of overhead‐throwing athletes is well documented.1‐13 This internal rotation (IR) loss is attributed to osseous and soft tissue adaptation and is referred to as posterior shoulder tightness (PST) and develops in response to prolonged exposure to high levels of repeated overloading.1,8,14 Although several positions for assessing and correcting PST have been recommended, there is no consensus on which position is better for differentiating posterior muscle tightness from posterior capsular tightness. Positions for assessing and stretching posterior shoulder tissues usually involve either adducting the humerus across the body or a combination of humeral flexion and IR.14‐18 Tyler et al. described a measurement in which the subject is side lying, and the humerus is flexed to 90° and is horizontally adducted (HAD). Both Laudner and Myers described another measurement of PST similar to the horizontal adduction position, but performed in supine lying.17,19 A disadvantage of both of these measurements is that it is not known which structure from the posterior shoulder limits the motion.16 Laudner discusses that posterior deltoid, infraspinatus, teres minor and, latissimus dorsi may impact the magnitude of glenohumeral joint (GHJ) motion in horizontal adduction.16 Others have also noted that external rotator (ER) muscles extensibility possibly influnces the PST measurement in both the supine and HAD postions.20
Another method to assess PST is by using the Sleeper stretch position. In this position the humerus is elevated to 90° and internally rotated with patient in side lying.8,18,21 The strains on the posterior shoulder tissues in a position simulating Sleeper stretch were tested by Borstad and Dashottar using cadaver shoulders.22 They also included a modified version of the Sleeper's stretch where the humerus was only elevated to 60° (named “low flexion”) in order to avoid impinging the rotator cuff muscles during the measurment. Higher strains on the posterior capsule but not on the posterior muscles were reported in both of these positions suggesting that these positions may be better for assessing posterior capsule but may not be optimal for assessing the posterior muscles. In the same study, higher strains on the posterior muscles were reported in two measurements; 1) simulating HAD and, 2) simulating scapular plane humeral abduction to 60° (SAB) with internal rotation. In a similar study, Muraki et al aimed to identify the most effective stretching position for rotator cuff muscles by comparing the strains on the posterior shoulder muscles across several GHJ positions.23 Their assumption was that positions that lead to higher strains on the muscles are better for stretching. They reported higher strains on the external humeral rotators in a postion of humeral extension and internal rotation (EIR) but not in a position simulating horizontal adduction.23 A limitation of these studies is that strain, which is a valid outcome measure for assessing muscle length change cannot be easily quantified in the clinical setting. Therefore, it is important to assess a clinically quantifiable variable across these positions, such as ROM, before one position can be recommended.22
To study the GHJ ROM changes surgical alteration of the GHJ posterior capsule in a cadaveric model is commonly used. A benefit of using a cadaveric model is that it allows the researchers to alter the length and mechanical properties by plicating24,25 or thermally treating the capsule22,26 and observing the direct effects of capsular alteration on the GHJ ROM. Any such direct manipulation of muscle length or mechanical properties cannot be done on human subjects for testing the ROM changes, however, immediate ROM changes can be induced by acute bouts of repeated eccentric exercises.27 These acute ROM changes are attributed to muscle microstructural damage, edema accumulation,28 and increase in muscle passive tension and stiffness.29 The authors of the current study decided to use this known muscular response to acute eccentric exercise in order to induce ROM changes and compare the effect across four positions recommended for assessing and correcting PST.
In this study, muscle stiffness was used as a way to substantiate muscle fatigue after repeated exercises aimed at the external rotator muscles. Muscle stiffness is quantified by measuring the magnitude of resistance when muscle is compressed perpendicular to its length.30 A myotonometer (Neurogenic Technologies Inc., Missoula, MT, USA) was used to objectively quantify the muscle stiffness in this study. The purpose of this study was to compare the ROM changes before and after external humeral rotator muscle fatigue to identify a position with maximum ROM change. The hypothesis was that following external rotator fatigue, the magnitude of ROM change will be greater in a measurement combining humeral extension and internal rotation (EIR) of all the measurements tested. This hypothesis was formulated on the basis of maximum strains reported in this position in previous studies.22,23
METHODS
Subjects
Twenty‐seven participants (18 females and 9 males) between the ages of 18‐40 years (Mean age 27 ± 4.3 years) without shoulder pain were recruited for this study. All but one was right hand dominant. Using G power software (Heinrich‐Heine‐Universität Düsseldorf), with the power set at 0.8 and α at 0.05, the sample size for a small effect size (0.25) was calculated to be 24. The Ohio State University Institutional Review Board approved this study. All volunteers were informed about the study procedures and provided informed consent to participate.
Procedure
Primary Study
The experiment began with baseline recordings for muscle stiffness and ROM. After these baseline measurements, external rotation force was measured using a hand held dynamometer and subjects were instructed to initiate the fatigue protocol. When the criterion for fatigue (40% reduction) was achieved (determined by external rotation force testing), the muscle stiffness and ROM measurements were repeated. Two examiners performed the measurements; examiner 1 oriented the shoulder joint for measurements and was blinded to GHJ ROM values. In addition, examiner 1 also measured external rotation force and muscle stiffness. Examiner 2 measured and recorded the GHJ ROM values and was blinded to the hypothesis of the study. All measurements were repeated 3 times and the means were used for statistical analysis.
Range of Motion Measurement
Repeated measures of GHJ ROM across four PST measurements were recorded before, immediately after, at 24 hours, and at 48 hours after a fatigue protocol of the non‐dominant GHJ external rotators. The order of the PST measurements was randomized using a computer‐generated sequence. All measurements were taken at the end of the passive ROM with no overpressure. The effect of gravity on the arm was allowed to move the joint to the end of passive range for SAB and Low Flexion (LF), while the examiner moved the joint to the end of passive range for both HAD and EIR (details in Table 1).
Table 1.
Tested positions. All the measurements were performed passively and subjects were instructed to relax their shoulder and arm. Note: No overpressure was applied in scapular plane abduction and low flexion, rather; only gravity was allowed to pull the forearm to the end range. In extension with internal rotation and horizontal adduction tests, scapular movement marked the end of passive range.
| Position | Experimental Position and Procedure | Figure of the Position |
|---|---|---|
| Extension with internal rotation (EIR) | In sitting, shoulder joint abducted to 60°*in the plane of scapula (POS) and then horizontally abducted 90° with the elbow maintained in 90° flexion; add GH internal rotation. | 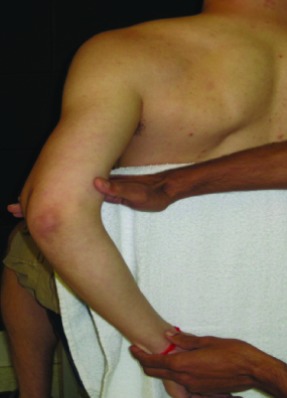 |
| Scapular Plane Abduction (SAB) | In standing, shoulder joint abducted 60° in the POS with the neutral GH IR/ ER rotation; add GH internal rotation. | 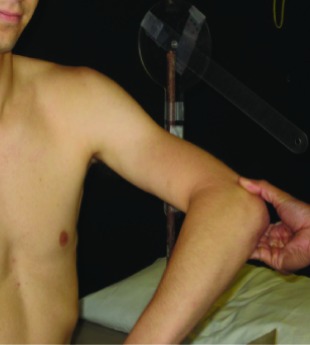 |
| Low Flexion (LF) | In standing, shoulder joint flexed to 60°; add internal rotation | 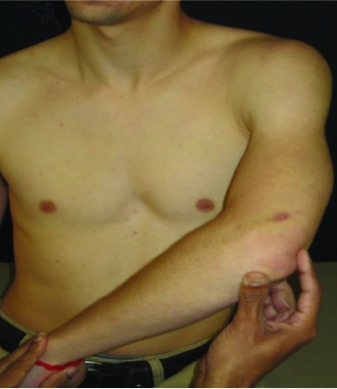 |
| Horizontal adduction (HAD) | In supine lying, shoulder joint flexed to 90°; adducted across the body. A wedge angled 30° was placed under the scapula to maintain the scapular plane | 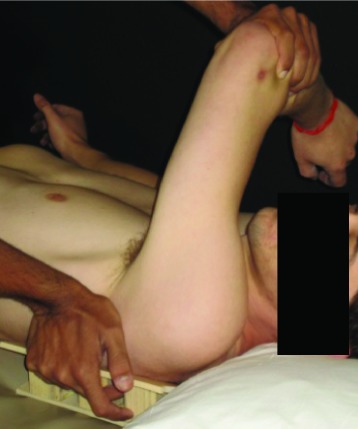 |
Goniometers fixed at 60° and 90° were used to orient the shoulder joint to the starting position of all the measurements.
A goniometer (Baseline evaluation instruments, Fabrication Enterprises Inc. White Plains, NY, USA) was used to control the starting position for each measurement. For EIR, SAB and LF a digital inclinometer (Baseline evaluation instruments, Fabrication enterprises Inc. White Plains, NY, USA) was aligned with the radial styloids. For HAD the inclinometer was placed on the posterior arm. All the angles were controlled by aligning the arm with a 360° goniometer fixed at 60° and kept in direct line of sight of examiner 1.
Muscle stiffness
Infraspinatus and teres minor muscle stiffness were measured using a myotonometer. The myotonometer is considered a reliable tool for measuring muscle stiffness and, has been used in the past to assess muscle stiffness.30‐34 Briefly, the myotonometer measures muscle stiffness by quantifying the amount of tissue displacement as the probe is pushed down perpendicular to the muscle belly. The magnitude of tissue displacement is recorded at eight 0.25 N force increments up to a maximum force of 2 N. Subsequently, manufacturer provided software generates a force‐displacement graph and calculates the area under the curve (AUC) of the graph expressed in N.mm.31 Less tissue displacement under the same force results in smaller AUC, indicating a harder tissue. To measure the muscle stiffness each subject was in a sitting position with arms at side. To ensure reproducibility, a standardized measurement location was marked on each subject (Table 2) and, examiner 1 did all the measurements. To verify repeatability of Myotonometer measurements, pre‐fatigue muscle stiffness was measured twice in 15 participants. These repeated pre fatigue muscle stiffness measurements were done at an interval of approximately 20 minutes, based on the time it took for participants to finish the experimental protocol.
Table 2.
Placement of the Myotonometer, in all the measurements participants were sitting with the, arms at the side, forearm pronated and palm resting on thighs.
| Muscle | Placement of the Myotonometer |
|---|---|
| Infraspinatus | 2.5 cm inferior from the midpoint of the spine of scapula |
| Teres Minor | 1/3rd of the way on a straight line between the posterior‐lateral angle of acromion and the inferior angle of the scapula along its lateral border |
External rotation force
External rotation force was measured to objectively mark the end of the fatigue protocol. External rotation force was measured using a hand‐held dynamometer (Lafayette instruments Co., IN, USA) with participants positioned in side lying on their dominant side. The non‐dominant arm was kept adducted and in neutral rotation with the elbow flexed to 90° while examiner 1 performed an internal rotation break test. The hand held dynamometer was placed on the posterior aspect of the forearm between the ulnar and radial styloids. The external rotation force was recorded prior to and immediately following the fatigue protocol. A force reduction of 40% from the pre‐fatigue value was used to objectively define the end of fatigue protocol. A 25% force reduction has also been used to indicate fatigue35 however, in pilot testing the authors’ found that most participants retained the ability to lower the weight under control at 25% force reduction, but lost that ability when they reached about 40% force reduction. Therefore, 40% force reduction was used to define the end of the fatigue protocol.
Each participant performed the fatigue protocol in side lying by repeatedly raising and lowering the forearm from a position of maximum internal rotation while holding a dumbbell equivalent to approximately 5% of their body weight.36 Oral cues were given to participants to maintain proper form during the protocol. The concentric (external rotation) phase of the protocol was assisted when the participants were unable to externally rotate the arm actively. To emphasize the eccentric contraction, no assistance to the eccentric (internal rotation) phase was given and participants were instructed to lower the dumbbell under control. The movement was considered controlled if the lowering phase lasted at least 2 seconds. Inability to lower the dumbbell under control in two consecutive attempts was used to mark the subjective end of the fatigue protocol, after which external rotation force was immediately measured. If the objective force decrease did not meet the criteria participants resumed the fatigue protocol.
Secondary Study
Forearm Rest Angle: A secondary study was conducted to objectively assess the change in the ROM measured in EIR position with out relying on the examiners subjective determination of end range passive motion. For this we measured the angle that the forearm makes with the vertical in the starting position of Extension with IR. Briefly, after abducting the arm to 60° in the plane of scapula, 90° of horizontal abduction was added with the elbow maintained in 90° flexion (Figure 1). These measurements were recorded in 20 participants before, immediately after the fatigue protocol, and at 24 and 48 hours post fatigue.
Figure 1.
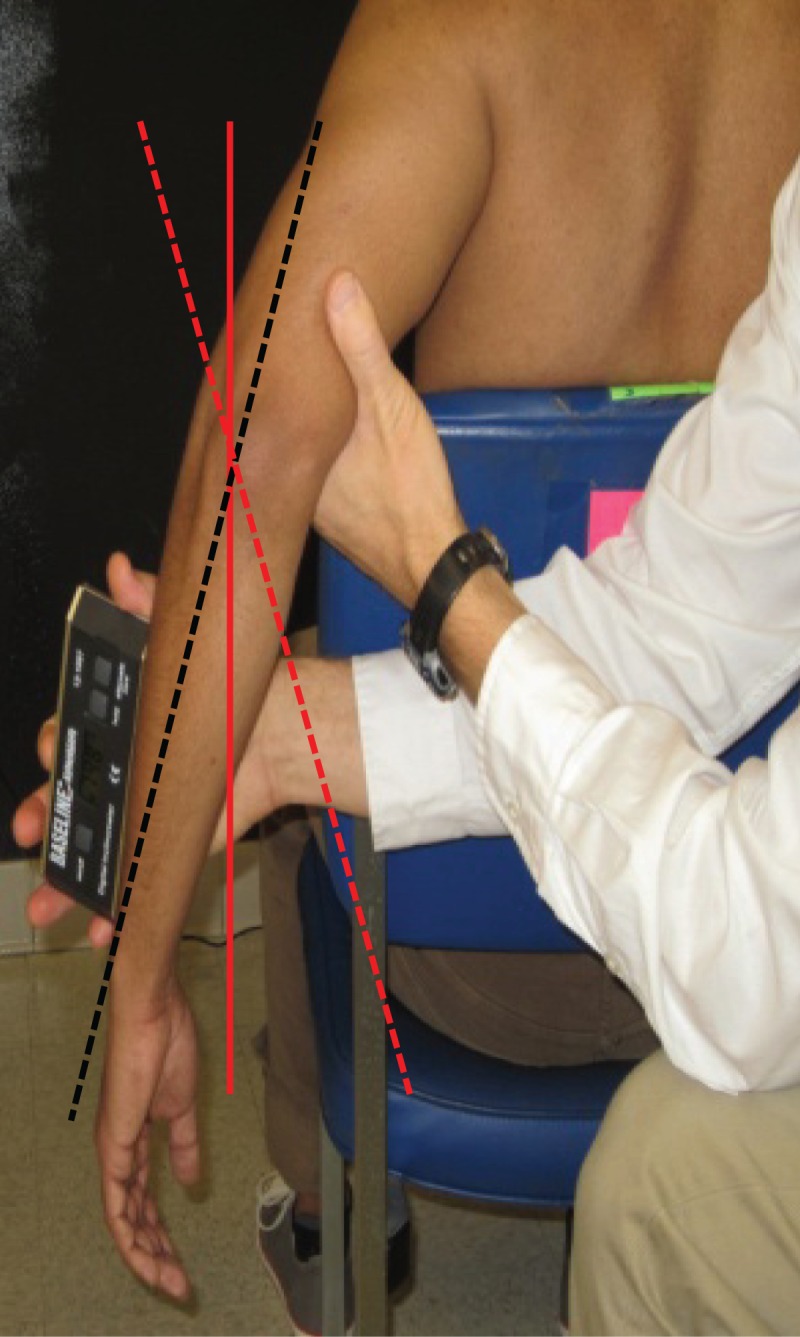
Forearm rest angle measurement. The forearm rest angle is defined as the angle that the forearm makes relative to the vertical. Forearm position is considered externally rotated (dashed black line) if oriented lateral to vertical (solid red line) and internally rotated if oriented medial to vertical (dashed red line).
STATISTICAL METHODS
Primary Study
Range of Motion: The primary dependent variable in this study was GHJ ROM change across the 4 PST measurements. The intra‐rater reliability estimates (Intraclass correlation coefficients (3,3) and standard error of measurement) for each ROM measurement at each time point were calculated. The GHJ ROM change for each measurement was calculated by subtracting the post fatigue, 24 hour, and 48 hour ROM from pre fatigue ROM, referred to as condition I (Pre‐fatigue ROM – Post‐ fatigue ROM), condition II (Pre‐fatigue ROM – 24 Hour ROM), and condition III (Pre‐ fatigue ROM‐ 48 Hour ROM) respectively. The change score was used because the authors were interested in identifying the measurement that shows maximum change in the ROM, and because it normalizes the GHJ ROM across subjects.
To examine the effect of measurement (4 levels) and condition (3 levels) on ROM change, a 2 factor repeated measures ANOVA was run. To assess assumptions of sphericity, Mauchly's test result was run and evaluated. If assumptions were violated, Greenhouse–Geisser (G–G) and Huynh–Feldt (H–F) corrections were planned to determine statistical significance, which was set at p < 0.05. Because the aim was to identify a position that showed greater ROM change, a separate 1 factor (measurement position) repeated measure ANOVA was also planned for each condition.
Muscle Stiffness: The within day reliability of the muscle stiffness measurements was examined by calculating the intraclass correlation coefficient (ICC3,3) among the 2 baseline measures in the subset of 15 participants. The infraspinatus and teres minor muscle AUC over time (4) was analyzed using separate 1 factor repeated measure ANOVA. Tukey‐Kramer post hoc comparisons were planned for significant main effects of time.
Secondary Study
Forearm Rest Angle: A separate 1 factor repeated measure ANOVA was run to examine the effects of time on forearm rest angle. Tukey‐ Kramer post hoc analyses were planned for significant main effects. All statistical analyses were performed using NCSS 2001 (Kaysville, Utah, USA) and the α level was set at 0.05.
RESULTS
Primary Study
Range of Motion: The within day intra‐rater ICC's and standard error of measurement (SEM) for ROM measured in each position at each time point are presented in Table 3. In general the ICCs ranged from 0.92 to 0.98 and SEM were lowest for HAD (1.2°‐1.7°) and highest for EIR (2.5°‐4.4°). There was a mean reduction of 59% in isometric external rotation force immediately after the fatigue protocol.
Table 3.
Intra‐rater reliability estimates for range of motion measured across the four measurements at each time point. ICC's were calculated from the repeated measures of ROM that were done consecutively on each subject by the same examiner.
| Measurement | Time | ICC | SEM |
|---|---|---|---|
| Extension with IR | Pre‐Fatigue | 0.98 | 2.5° |
| Post Fatigue | 0.93 | 4.4° | |
| 24 Hours | 0.98 | 2.5° | |
| 48 Hours | 0.98 | 2.5° | |
| SAB | Pre‐Fatigue | 0.95 | 2.4° |
| Post Fatigue | 0.96 | 2.2° | |
| 24 Hours | 0.92 | 2.6° | |
| 48 Hours | 0.95 | 2.3° | |
| HAD | Pre‐Fatigue | 0.97 | 1.7° |
| Post Fatigue | 0.95 | 1.6° | |
| 24 Hours | 0.94 | 1.6° | |
| 48 Hours | 0.96 | 1.2° | |
| LF | Pre‐Fatigue | 0.95 | 2.1° |
| Post Fatigue | 0.93 | 2.3° | |
| 24 Hours | 0.96 | 2.2° | |
| 48 Hours | 0.95 | 2.3° |
ICC = Intraclass correlation coefficient; SEM = Standard error of measurement;
SAB = Scapular plane abduction to 60 °, with internal rotation;
HAD = Horizontal adduction; LF = Low flexion, with internal rotation.
Two‐factor repeated measure ANOVA for ROM change indicated a statistically significant interaction effect between measurement and condition (F = 2.47, p = 0.02). Separate one factor repeated measure ANOVA revealed that for condition I, extension with IR measurement showed statistically significant greater ROM change (19.9°) compared to LF (7.5°), SAB (6.8°) and HAD (0.5°). In addition, both LF and SAB ROM change were also statistically significant compared to HAD ROM change. In conditions II and III, extension with IR showed greater ROM change (12.9° and 12.3°) than LF (3.3° and 3.8°), SAB (1.9° and 2.5°) and HAD (−0.9° and 0.0°), respectively and was statistically significant (Figure 2).
Figure 2.
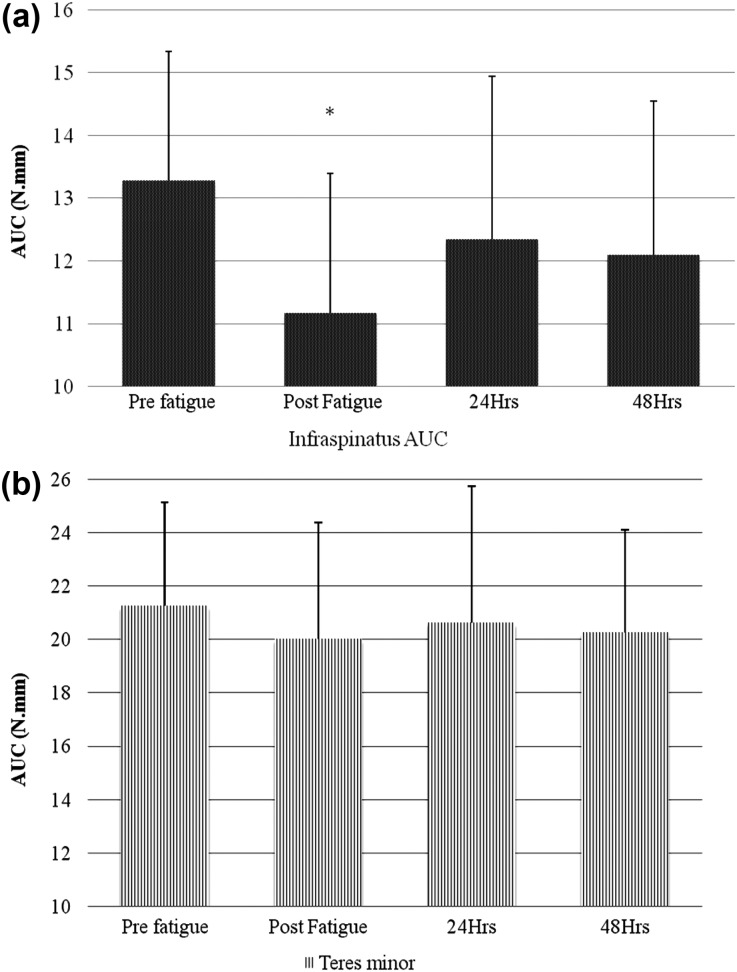
Area under the curve (AUC) of a) Infraspinatus; and b) Teres minor. Y‐axis‐ area under the curve (in N.mm), X‐Axis‐ time points at which the stiffness was measured. * indicates statistically significant infraspinatus AUC reduction immediately after the external rotator fatigue. Error bars represent standard deviations.
Muscle Stiffness: Muscle stiffness was measured as the area under the curve (AUC) of the force displacement graph gen II‐B erated by the Myotonometer. The within day ICC's (3,3) for the pre fatigue muscle stiffness were 0.95 and 0.97 for infraspinatus and teres minor respectively. One factor repeated measure ANOVA indicated a significant main effect of time for infraspinatus AUC (F = 10.5, p<0.001). Teres minor AUC did not reach statistical significance (F = 0.74, p = 0.53) (Figure 4).
Secondary Study
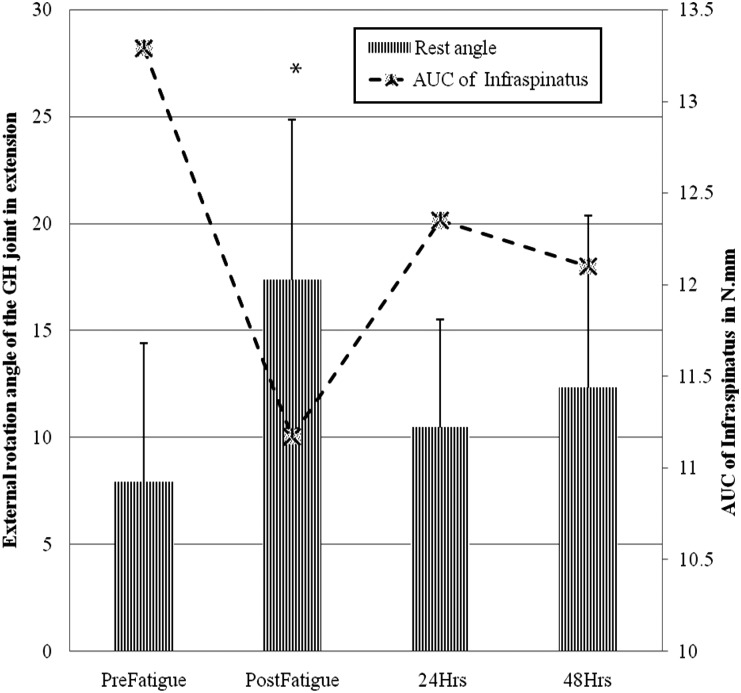
Forearm Rest Angle: Separate 1 factor repeated measure ANOVA for forearm rest angle indicated a statistically significant main effect of time (F = 13.54, p < 0.001). Tukey‐Kramer post hoc analysis indicated that the forearm rest angle immediately after fatigue was significantly greater than at 24 and 48 hours (Figure 3).
Figure 3.
Forearm rest angle variation across the time points. * indicates statistically significant increase in forearm rest angle post fatigue and decrease in Infraspinatus area under the curve (AUC). The primary Y‐axis denotes the external rotation angle of the forearm; secondary Y‐axis denotes the Infraspinatus AUC in N.mm. Note that the secondary Y‐axis starts at 10 N.mm.
DISCUSSION
The results of this study demonstrated that the magnitude of ROM change quantified after an external rotator fatigue protocol differs among tested PST measurements. The hypothesis of this study was that maximum ROM changes after an external rotator fatigue protocol would be observed in a measurement combining humeral extension with internal rotation or in horizontal adduction. This hypothesis was generated based on the evidence from cadaver studies where maximum strains on the posterior muscles were observed in these positions.22,23 The results show that after external rotator fatigue maximum ROM reductions were observed in extension with IR but not in horizontal adduction. This finding is supported by a previous cadaveric study that quantified the strain on the infraspinatus muscle and reported that infraspinatus is maximally lengthened in extension with IR.23 The results of the present study taken together with past findings suggests that a position of extension with internal rotation could be useful in assessing the IR ROM loss that might be due to infraspinatus tightness.
Horizontal adduction is often used to assess PST however; in this study HAD ROM after fatigue was not significantly changed. This finding is also supported by previous studies where no significant infraspinatus strain increases were reported in horizontal adduction.22 A possible reason for this may be that the motion of the humeral head occurring in horizontal adduction may be limited by posterior deltoid, latissimus dorsi, teres minor, and infraspinatus.16 In this study the fatigue protocol was aimed at the external rotator muscles however, the muscle stiffness of teres minor was not significantly increased following the fatigue protocol. Posterior deltoid muscle stiffness was not measured in this study. It is possible that the ROM measured in HAD is influenced by teres minor and posterior deltoid and to a lesser degree by infraspinatus. Another explanation for no significant changes in horizontal adduction may be that ROM in this position is influenced by posterior capsule.37 Harryman et al37 in a cadaveric study reported increased anterior and superior humeral translations in horizontal adduction after experimental posterior capsular tightening. The increase in antero‐superior humeral translation may result in limitation of humeral motion by bony approximation before the infraspinatus is fully lengthened.
In the present study ROM loss was also significant in LF and SAB measurements however the magnitude of the measured differences was much smaller. Because a greater observable change in a measurement may be easier to detect in a clinical setting, the use of extension with IR may be preferable to LF and SAB. The ROM change in supine internal rotation with humerus abducted to 90° was not measured. This decision was made because it has been reported that the IR ROM measured in this position is greatly affected by humeral torsion.38 The aim of the current study was only to compare the ROM changes that occurred due to muscle fatigue and future studies should explore the influence of humeral torsion on ROM measured in extension with internal rotation position.
An external rotator fatigue protocol was used to induce immediate ROM changes.36 These changes have been attributed to microstructural damage resulting in edema, increased muscle passive tension and, muscle stiffness.29,39,40 Although the protocol consisted of both concentric and eccentric components, the researchers emphasized the eccentric component by not giving any assistance during the eccentric phase of the protocol. Microstructural damage to the muscle following the protocol was not directly measured however, reduction in the isometric force is considered an indicator of muscle damage.41 A mean reduction of 59% in the isometric external rotation force following the protocol suggests that the fatigue protocol used successfully affected the muscle condition.
Muscle stiffness of infraspinatus and teres minor were analyzed using two separate 1 factor repeated measure ANOVAs. They were analyzed separately because descriptive analysis showed different pre fatigue infraspinatus (AUC 13.2 ± 2 N.mm) and teres minor (AUC 21.2 ± 3.8 N.mm) measures of muscle stiffness (Figure 2). Because the magnitude of muscle stiffness difference between infraspinatus and teres minor was greater than the magnitude of muscle stiffness change over time, including muscle as a factor in ANOVA model would have reduced the power of the test to detect an interaction between muscle stiffness and time. Before the initiation of the fatigue protocol muscle stiffness was measured twice to assess the within day reliability of the hardness measurement. High ICC's (0.95 & 0.97) among the repeated baseline hardness measurements and a significant change in muscle stiffness after the fatigue protocol suggests that in the present study Myotonometer was able to detect muscle stiffness changes.
There was no objective measure of the force applied by the examiner during extension with IR measurement, which may have introduced examiner bias. To address the lack of objective measure of the examiner force, a secondary study was conducted. In the secondary study the forearm rest angle rest in the starting position of extension with IR (Figure 1) was measured. Because this measurement excludes examiner force while keeping other conditions the same, the potential for examiner bias is minimized. There was a statistically significant change in the forearm rest angle immediately after the fatigue protocol, corroborating the results of the primary study where maximum ROM change was observed in the EIR position. Although a statistical relationship among the infraspinatus muscle stiffness measurements and forearm rest angle was not explored, it is worth noting that forearm rest angle change coincided with infraspinatus muscle stiffness over time (Figure 3) similar to changes noted in previous studies.42,43 The variation of the forearm rest angle with infraspinatus muscle stiffness change suggests that the results of the primary study that ROM measured in EIR were not influenced by the external force applied by the examiner.
The posterior capsule of the GHJ may also affect the ROM in the positions tested in this study however, it is safe to assume that the GHJ posterior capsule did not influence the ROM changes observed. First, if the exercise protocol used in this study did alter the capsule, one would expect increased, not decreased ROM because repeated internal and external rotations are used to precondition the capsule to gain maximum GHJ ROM.25 Second, the participants were not overhead athletes, a population known to have GHJ posterior capsular adaptations in response to long duration of repeated overloading.1,8 Third, any permanent structural or mechanical changes in the capsule would take longer than 48 hour to develop.44
The results of the present study should be interpreted in the light of several limitations. First, the electromyographic (EMG) activity of the muscles during the ROM measurement was not recorded. This was done to simulate the ROM measurements, as they will be done in clinics where monitoring EMG activity during the examination is not the norm. Although subject relaxation during the measurements was not quantified, the examiner used his 20 years of musculoskeletal clinical experience to subjectively determine when relaxation was achieved, which parallels standard clinical measurement procedures. Muscle stiffness measured using the Myotonometer may be influenced by subcutaneous tissue thickness. However, use of a within‐subject design means that any effect of subcutaneous tissue on muscle stiffness measurements is consistent for each subject. Third, because participants were young adults without shoulder pain, and had no history of participation in overhead throwing activities, the results of the study should not be generalized to elderly or adolescent individuals, to those with shoulder pain, or to overhead athletes who could have increased humeral torsion or altered scapular position that may influence the PST measurements.13,45
CONCLUSION
The ROM changes that were measured across the four tested PST measurements varied after an external rotation fatigue protocol. Maximum ROM change was measured in a measurement combining GHJ extension and internal rotation. The results of this study might help clinicians to evaluate ROM loss due to infraspinatus muscle tightness. However, before the clinical use of extension with internal rotation can be recommended, further evaluation of this measurement in different populations with known humeral torsion and scapular position changes is necessary.
REFERENCES
- 1.Pappas AM Zawacki RM McCarthy CF Rehabilitation of the pitching shoulder. Am J Sports Med. 1985; 13: 223‐235 [DOI] [PubMed] [Google Scholar]
- 2.Warner JJP Micheli LJ Arslanian LE et al. Patterns of flexibility, laxity, and strength in normal shoulders and shoulders with instability and impingement. Am J Sports Med. 1990; 18: 366. [DOI] [PubMed] [Google Scholar]
- 3.Bigliani LU Codd TP Connor PM et al. Shoulder motion and laxity in the professional baseball player. Am J Sports Med. 1997; 25: 609‐613 [DOI] [PubMed] [Google Scholar]
- 4.Crockett HC Gross LB Wilk KE et al. Osseous adaptation and range of motion at the glenohumeral joint in professional baseball pitchers. Am J Sports Med. 2002; 30: 20‐6 [DOI] [PubMed] [Google Scholar]
- 5.Ellenbecker TS Roetert EP Bailie DS et al. Glenohumeral joint total rotation range of motion in elite tennis players and baseball pitchers. Med Sci Sports Exerc. 2002; 34: 2052‐2056 [DOI] [PubMed] [Google Scholar]
- 6.Osbahr DC Cannon DL Speer KP Retroversion of the humerus in the throwing shoulder of college baseball pitchers. Am J Sports Med. 2002; 30: 347‐53 [DOI] [PubMed] [Google Scholar]
- 7.Reagan KM Meister K Horodyski MB et al. Humeral retroversion and its relationship to glenohumeral rotation in the shoulder of college baseball players. Am J Sports Med. 2002; 30: 354‐60 [DOI] [PubMed] [Google Scholar]
- 8.Burkhart SS Morgan CD Kibler WB The disabled throwing shoulder: spectrum of pathology Part I: pathoanatomy and biomechanics. Arthroscopy. 2003; 19: 404‐420 [DOI] [PubMed] [Google Scholar]
- 9.Borsa PA Dover GC Wilk KE et al. Glenohumeral range of motion and stiffness in professional baseball pitchers. Med Sci Sports Exerc. 2006; 38: 21‐26 [DOI] [PubMed] [Google Scholar]
- 10.Borsa PA Wilk KE Jacobson JA et al. Correlation of range of motion and glenohumeral translation in professional baseball pitchers. Am J Sports Med. 2005; 33: 1392‐1399 [DOI] [PubMed] [Google Scholar]
- 11.Myers JB Laudner KG Pasquale MR et al. Glenohumeral range of motion deficits and posterior shoulder tightness in throwers with pathologic internal impingement. Am J Sports Med. 2006; 34: 385‐91 [DOI] [PubMed] [Google Scholar]
- 12.Ruotolo C Price E Panchal A Loss of total arc of motion in collegiate baseball players. J Shoud Elbow Surg. 2006; 15: 67‐71 [DOI] [PubMed] [Google Scholar]
- 13.Chant CB Litchfield R Griffin S et al. Humeral head retroversion in competitive baseball players and its relationship to glenohumeral rotation range of motion. J Orthop Sports Phys Ther. 2007; 37: 514‐520 [DOI] [PubMed] [Google Scholar]
- 14.Tyler TF Nicholas SJ Roy T et al. Quantification of posterior capsule tightness and motion loss in patients with shoulder impingement. Am J Sports Med. 2000; 28: 668‐73 [DOI] [PubMed] [Google Scholar]
- 15.Tyler TF Roy T Nicholas SJ et al. Reliability and validity of a new method of measuring posterior shoulder tightness. J Orthop Sports Phys Ther. 1999; 29: 262‐269 [DOI] [PubMed] [Google Scholar]
- 16.Laudner KG Myers JB Pasquale MR et al. Scapular dysfunction in throwers with pathologic internal impingement. J Orthop Sports Phys Ther. 2006; 36: 485‐494 [DOI] [PubMed] [Google Scholar]
- 17.Myers JB Oyama S Wassinger CA et al. Reliability, precision, accuracy, and validity of posterior shoulder tightness assessment in overhead athletes. Am J Sports Med. 2007; 35: 1922‐1930 [DOI] [PubMed] [Google Scholar]
- 18.McClure P Balaicuis J Heiland D et al. A randomized controlled comparison of stretching procedures for posterior shoulder tightness. J Orthop Sports Phys Ther. 2007; 37: 108‐114 [DOI] [PubMed] [Google Scholar]
- 19.Laudner KG Stanek JM Meister K Assessing posterior shoulder contracture: the reliability and validity of measuring glenohumeral joint horizontal adduction. J Athl Train. 2006; 41: 375‐380 [PMC free article] [PubMed] [Google Scholar]
- 20.Poser A Casonato O Posterior glenohumeral stiffness: Capsular or muscular problemϿ. A case report. Man Ther. 2008; 13: 165‐170 [DOI] [PubMed] [Google Scholar]
- 21.Bach HG Goldberg BA Posterior capsular contracture of the shoulder. J Amer Acad Orthop Surg. 2006; 14: 265‐77 [DOI] [PubMed] [Google Scholar]
- 22.Borstad JD Dashottar A Quantifying strain on posterior shoulder tissues during 5 simulated clinical tests: A cadaver study. J Orthop Sports Phys Ther. 2011; 41: 90‐99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muraki T Aoki M Uchiyama E et al. The effect of arm position on stretching of the supraspinatus, infraspinatus, and posterior portion of deltoid muscles: a cadaveric study. Clin Biomech. 2006; 21: 474‐480 [DOI] [PubMed] [Google Scholar]
- 24.Fitzpatrick MJ Tibone JE Grossman M et al. Development of cadaveric models of a thrower's shoulder. J Should Elbow Surg. 2005; 14: 49S‐57S [DOI] [PubMed] [Google Scholar]
- 25.Grossman MG Tibone JE McGarry MH et al. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior‐to‐posterior lesions. JBJS. 2005; 87: 824‐831 [DOI] [PubMed] [Google Scholar]
- 26.Medvecky MJ Ong BC Rokito AS et al. Thermal capsular shrinkage: Basic science and clinical applications. Arthroscopy. 2001; 17: 624‐635 [DOI] [PubMed] [Google Scholar]
- 27.Reinold MM Wilk KE Macrina LC et al. Changes in shoulder and elbow passive range of motion after pitching in professional baseball players. Am J Sports Med. 2008; 36: 523‐7 [DOI] [PubMed] [Google Scholar]
- 28.Lauritzen F Paulsen G Raastad T et al. Gross ultrastructural changes and necrotic fiber segments in elbow flexor muscles after maximal voluntary eccentric action in humans. J Appl Phys. 2009; 107: 1923‐1934 [DOI] [PubMed] [Google Scholar]
- 29.Reisman S Allen TJ Proske U Changes in passive tension after stretch of unexercised and eccentrically exercised human plantarflexor muscles. Exp Brain Res. 2009; 193: 545‐554 [DOI] [PubMed] [Google Scholar]
- 30.Laudner KG Williams JG The relationship between latissimus dorsi stiffness and altered scapular kinematics among asymptomatic collegiate swimmers. Phys Ther in Sports. 2013; 14: 50‐53 [DOI] [PubMed] [Google Scholar]
- 31.Leonard CT Deshner WP Romo JW et al. Myotonometer intra‐and interrater reliabilities. Arch Phys Med Rehab. 2003; 84: 928‐932 [DOI] [PubMed] [Google Scholar]
- 32.Zinder SM Padua DA Reliability, validity, and precision of a handheld myometer for assessing in vivo muscle stiffness. J Sport Rehab. 2011; Technical Notes 1. Epub. [DOI] [PubMed] [Google Scholar]
- 33.Hung CJ Hsieh CL Yang PL et al. Relationships between posterior shoulder muscle stiffness and rotation in patients with stiff shoulder. J Rehabil Med. 2010; 42: 216‐220 [DOI] [PubMed] [Google Scholar]
- 34.Kerins CM Moore SD Butterfield TA et al. Reliability of the myotonometer for assessment of posterior shoulder tightness. Int J Sports Phys Ther. 2013; 8: 248‐255 [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai NT McClure PW Karduna AR Effects of muscle fatigue on 3‐dimensional scapular kinematics. Arch Phys Med Rehabil. 2003; 84: 1000‐1005 [DOI] [PubMed] [Google Scholar]
- 36.Ebaugh DD McClure PW Karduna AR Scapulothoracic and glenohumeral kinematics following an external rotation fatigue protocol. J Orthop Sports Phys Ther. 2006; 36: 557‐571 [DOI] [PubMed] [Google Scholar]
- 37.Harryman DT Translation of the humeral head on the glenoid with passive glenohumeral motion. JBJS. 1990; 72: 1334‐1343 [PubMed] [Google Scholar]
- 38.Myers JB Oyama S Goerger BM et al. Influence of Humeral Torsion on Interpretation of Posterior Shoulder Tightness Measures in Overhead Athletes. Clin J Sport Med. 2009; 19: 366‐71 [DOI] [PubMed] [Google Scholar]
- 39.Newham DJ McPhail G Mills KR et al. Ultrastructural changes after concentric and eccentric contractions of human muscle. J Neuro Sci. 1983; 61: 109‐122 [DOI] [PubMed] [Google Scholar]
- 40.Proske U Allen TJ Damage to skeletal muscle from eccentric exercise. Exerc Sports Sci Rev. 2005; 33: 98‐104 [DOI] [PubMed] [Google Scholar]
- 41.Byrne C Eston R Maximal‐intensity isometric and dynamic exercise performance after eccentric muscle actions. J Sports Sci. 2002; 20: 951‐959 [DOI] [PubMed] [Google Scholar]
- 42.Murayama M Nosaka K Yoneda T et al. Changes in hardness of the human elbow flexor muscles after eccentric exercise. Eur J Appl Phys. 2000; 82: 361‐367 [DOI] [PubMed] [Google Scholar]
- 43.Whitehead NP Weerakkody NS Gregory JE et al. Changes in passive tension of muscle in humans and animals after eccentric exercise. J Phys. 2001; 533: 593‐604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hildebrand KA Zhang M Germscheid NM et al. Cellular, matrix, and growth factor components of the joint capsule are modified early in the process of posttraumatic contracture formation in a rabbit model. Acta orthopaedica. 2008; 79: 116‐125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myers JB Laudner KG Pasquale MR et al. Scapular position and orientation in throwing athletes. Am J Sports Med. 2005; 33: 263‐271 [DOI] [PubMed] [Google Scholar]