Abstract
Cutaneous drug reactions make up the largest proportion of adverse events in the medical field. Causality, in particular, is difficult to determine, and therefore, preventing recurrent reactions can be challenging. Bendamustine was initially thought to be a well-tolerated chemotherapy agent with few side effects aside from bone marrow suppression. However, the incidence of cutaneous reactions reported is rising. We describe three such reactions in relation to bendamustine administration in hopes of adding to the awareness of such side effects.
Key words: Bendamustine, Adverse events, Rash, Dermatologic reaction, Lymphoma, Chemotherapy
Introduction
Drug eruptions are common, affecting 2–3% of all hospitalized patients, and complications associated with medications make up the largest proportion of adverse events seen in hospitals [1]. The incidence of cutaneous drug reactions is likely to be higher among outpatients. Drug eruptions can be difficult to diagnose, particularly causality, as even biopsies are nonspecific and patients are often on more than one potentially offending medication [1]. We present 3 cases that highlight these difficulties and the broad spectrum of drug eruptions in the setting of lymphoma patients receiving multidrug regimens including bendamustine.
Case Presentations
Case 1: Drug Reaction with Eosinophilia and Systemic Symptoms Syndrome
A 60-year-old Caucasian female presented with a 6-month history of constitutional symptoms, dyspnea on exertion, lymphadenopathy and pleural effusions and was diagnosed with stage IV marginal zone lymphoma with bone marrow involvement. Four to six weeks prior to admission, she had developed a progressive generalized erythematous rash and edema involving her trunk and extremities. A biopsy of the rash showed mild superficial perivascular and focal interface lymphocytic dermatitis. These findings were characterized as consistent with drug eruption, although no new drugs were identified in her history.
The decision was made to treat with bendamustine and rituxan as an inpatient due to nonresolving malignant pleural effusion. Her initial rash on presentation was thought to be due to possible lymphomatous involvement and lymphedema as it improved over the 2–3 days following chemotherapy.
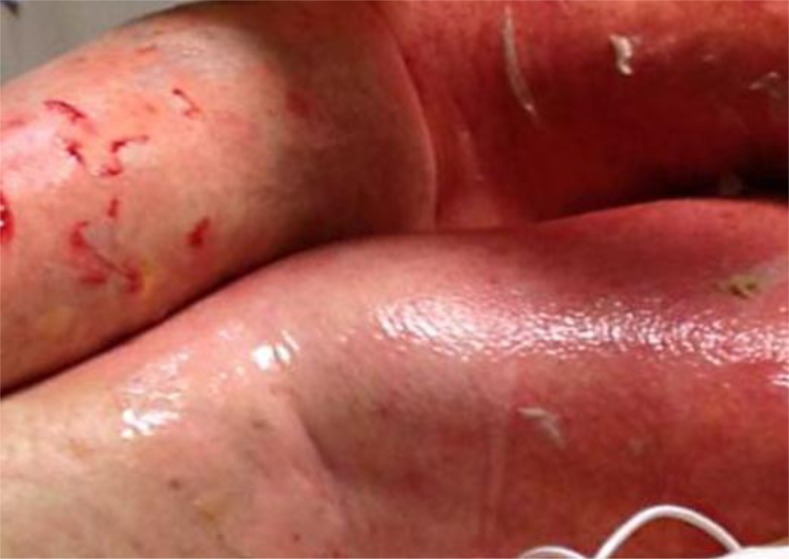
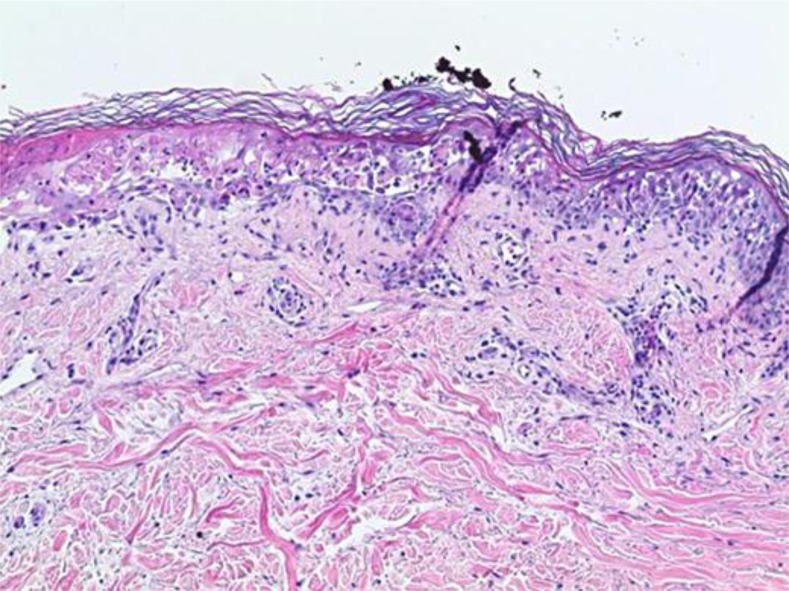
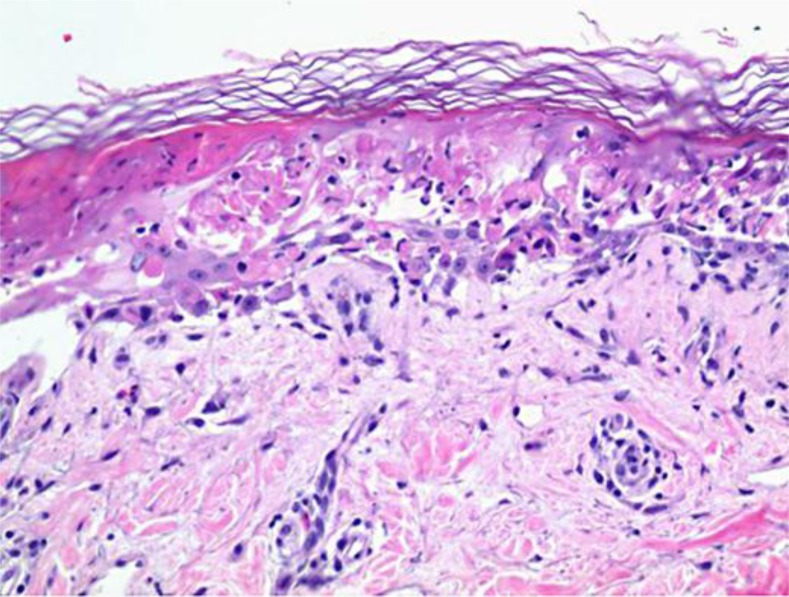
On day 7 following chemotherapy, the characteristics of her eruption changed. She developed a bullous rash involving 60–70% of her skin as well as mucosa (fig. 1). Bullae were fragile and superficial. A new biopsy revealed vacuolar interface dermatitis with necrosis of keratinocytes in the epidermis, with the underlying dermis displaying a superficial perivascular lymphocytic inflammatory infiltrate with eosinophils (fig. 2; fig. 3). She began to deteriorate rapidly with a clinical course further complicated by the development of severe hepatic and renal failure. A diagnosis of DRESS syndrome was made.
Fig. 1.
DRESS exanthema.
Fig. 2.
Low power of the DRESS lesion.
Fig. 3.
High power of the DRESS lesion.
Despite a high dose of steroids, the rash spread, eventually involving 100% of her body surface area, including her eyelids, conjunctiva, oral and vaginal mucus membranes. Despite the use of filgrastim and broad spectrum antibiotics, she developed neutropenic fever and Gram-negative sepsis. She expired on day 28.
Case 2: Stevens-Johnson Syndrome
The second case was a 66-year-old male diagnosed in 2009 with chronic lymphocytic leukemia associated with the deletion of chromosome 13. In 2012, his lymphocyte count reached 253,000, and he developed autoimmune hemolytic anemia. He initially received one cycle of intravenous immunoglobulin, cyclophosphamide, vincristine and prednisone (without rituxan).
His outpatient therapy was then changed to bendamustine the following month. The initial cycle was again given without rituxan due to the high burden of the disease. The following 14 days he developed nonneutropenic fevers and pneumonia. He received antibiotic therapy with cefepime, fluconazole, azithromycin and levofloxacin during two separate hospitalizations. Two days following the second admission (day 7 following bendamustine), he developed a generalized erythematous rash with desquamation that did not resolve with the discontinuation of cefepime or the administration of rituxan. His rash slowly resolved. A second episode of nonneutropenic fever, hypotension and acute renal failure occurred with the second cycle of bendamustine and rituxan 2 months later. This resolved as well. Given the timing of the reaction, it was questioned that the offending drug was bendamustine in both events.
Case 3: Bullous Pemphigoid
The third patient was a 59-year-old female diagnosed with stage IVb splenic marginal zone lymphoma after presenting with constitutional symptoms and urticaria. She was found to have a leukemic phase and massive splenomegaly. She was treated with splenectomy and systemic chemotherapy consisting of bendamustine and rituxan. Prior to the third cycle, the patient developed a mild pruritic eruption with raised plaques. The eruption worsened 2 weeks later as the lesions became intensely pruritic, erythematous and ulcerative. Despite the treatment with antibiotics and corticosteroids, she progressed over the course of 1 week to a tense bullous rash covering the extremities. Dermatological evaluation, including biopsies, confirmed a diagnosis of bullous pemphigoid. Immunofluorescence studies revealed IgG positivity, and morphology showed subepidermal eosinophil rich bullae. One month later, her rash was controlled with prednisone and mycophenolate mofetil.
Discussion
Severe drug eruptions associated with high morbidity and mortality include Stevens-Johnson syndrome and toxic epidermal necrolysis [1]. These syndromes are often confused and may represent a continuum of each other, with toxic epidermal necrolysis having >30% of epidermal detachment and Stevens-Johnson syndrome having only 10% or less. Both syndromes may include mucosal lesions. Morbidity and mortality from these conditions stem from their complications including massive fluid losses, electrolyte imbalances, infections and diffuse interstitial pneumonitis, leading to acute respiratory distress syndrome. Patients require weeks of intensive care before the epidermis replaces itself [1].
DRESS syndrome represents another severe drug eruption with a 10% mortality rate, typically in the setting of hepatic failure [2]. The syndrome was first described for a skin reaction associated with anticonvulsants in 1936 [3]. The term DRESS syndrome was later coined in 1996 as a syndrome specifically describing a drug eruption with internal organ involvement and hematologic abnormalities, particularly eosinophilia or atypical lymphocytosis [4]. It can also be associated with facial edema, conjunctivitis and pharyngeal mucosa erythema [2]. DRESS syndrome can be difficult to diagnose, as onset is typically 2–6 weeks after the drug was administered but is more probable when associated with hypereosinophilia, liver involvement, fever and lymphadenopathy [5]. Other possible internal organ involvement includes interstitial pneumonitis, interstitial nephropathy and myocardial involvement [4]. Etiology, as with other severe drug eruptions, is unclear but postulated to be associated with human herpes virus reactivation. Many drugs have been implicated in DRESS syndrome; however, the ones most often reported are allopurinol, penicillins, sulfonamides and antipsychotics. Severity and likelihood of the diagnosis of DRESS as well as other severe cutaneous drug reactions are scored using the RegiSCAR in Europe [5]. The mainstay of treatment remains the removal of the offending drug and supportive care with the use of steroids remaining controversial in their effectiveness [2, 5].
Bendamustine was first synthesized in 1963 as a chemotherapeutic agent with both antimetabolite and alkylating properties. These allow the induction of cell death with a variety of mechanisms [6]. Bendamustine was rediscovered and more extensively studied in the 1990s. Since that time, bendamustine has demonstrated significant efficacy in many lymphoma subtypes, particularly indolent lymphomas resistant to other alkylators. In 2008, it was approved by the FDA for the treatment of rituximab-refractory chronic lymphocytic leukemia and indolent B-cell non-Hodgkin lymphomas [7]. Its side effect profile has been shown to be well tolerated, with the most common side effects being cytopenias and fatigue [7].
The FDA prescribing guidelines state that skin reactions have been reported with bendamustine, most often in combination with another agent (rituximab or allopurinol), so that true causality cannot be determined. However, these reactions have been reported to be severe and progressive with several deaths mentioned [8].
Several reports of bendamustine skin reactions describe underlying dermatologic features at presentation. Most described rashes develop following the first administration of bendamustine and generally worsen in severity over the following days. Biopsies often reveal a diffuse or perivascular eosinophilic infiltration within the dermis [9].
One patient with B-cell prolymphocytic leukemia developed rash with palpable purpura and hemorrhagic plaques while thrombocytopenic. This rash was preceded by a severe generalized maculopapular rash with most bendamustine skin reactions. A biopsy revealed a diffuse eosinophilic infiltrate [9]. In a case series of 16 patients with chronic lymphocytic leukemia or follicular lymphoma, over 50% of the patients developed an erythematous maculopapular rash [10]. Another case report described a severe desquamating rash involving >75% of the skin as well as oral mucosa. The rash developed 5 days after the first cycle of bendamustine. A biopsy revealed interface dermatitis with a lymphohistiocytic infiltrate [11].
In our case of DRESS syndrome, the characteristic rash appeared only 1 week after drug exposure. This led to the question of the offending agent; however, the patient did not have a reasonable alternate medication exposure. We also postulate that both the patient's lymphoma and bendamustine itself may have induced a quicker response based on already reduced immunoglobulin levels and a rapid reduction in B-cell lymphocytes. This initiating factor in the mechanism of DRESS was proposed by Criado et al. [12] and has been cited as the reason for the long latency in cases [13].
Case 2 represents the typical scenario of a drug exanthem where the causative agent is in question. Rash developed following bendamustine, but the patient was also exposed to antibiotics that are known to cause dermatologic complications. However, unlike most reported cases, rituximab was not a questionable cause, as it was not administered with the bendamustine initially. Case 3 also represents a more atypical presentation than initially reported, with a reaction after the second cycle rather than after initial exposure to the drug. However, no other causative agent was found.
References
- 1.Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med. 1994;331:1272–1285. doi: 10.1056/NEJM199411103311906. [DOI] [PubMed] [Google Scholar]
- 2.Cardoso CS, Vieira AM, Oliveira AP. Dress syndrome: a case report and literature review. BMJ Case Rep. 2011;2011 doi: 10.1136/bcr.02.2011.3898. pii: bcr0220113898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saltzstein SL, Ackerman LV. Lymphadenopathy induced by anticonvulsant drugs and mimicking clinically pathologically malignant lymphomas. Cancer. 1959;12:164–182. doi: 10.1002/1097-0142(195901/02)12:1<164::aid-cncr2820120122>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 4.Bocquet H, Bagot M, Roujeau JC, et al. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (Drug Rash with Eosinophilia and Systemic Symptoms: DRESS) Semin Cutan Med Surg. 1996;15:250–257. doi: 10.1016/s1085-5629(96)80038-1. [DOI] [PubMed] [Google Scholar]
- 5.Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124:588–597. doi: 10.1016/j.amjmed.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Cheson BD, Leoni L. Bendamustine: mechanism of action and clinical data. Clin Adv Hematol Oncol. 2011;9(suppl 19):1–11. [PubMed] [Google Scholar]
- 7.Tageja N. Bendamustine: safety and efficacy in the management of indolent non-Hodgkins lymphoma. Clin Med Insights Oncol. 2011;5:145–156. doi: 10.4137/CMO.S6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Treanda Prescribing Information. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022249s002lbl.pdf.
- 9.Gavini A, Telang GH, Olszewski AJ. Generalized purpuric drug exanthem with hemorrhagic plaques following bendamustine chemotherapy in a patient with B-prolymphocytic leukemia. Int J Hematol. 2012;95:311–314. doi: 10.1007/s12185-012-1012-2. [DOI] [PubMed] [Google Scholar]
- 10.Malipatil B, Ganesan P, Sundersingh S, Sagar TG. Preliminary experience with the use of bendamustine: a peculiar skin rash as the commonest side effect. Hematol Oncol Stem Cell Ther. 2011;4:157–160. doi: 10.5144/1658-3876.2011.157. [DOI] [PubMed] [Google Scholar]
- 11.Alamdari HS, Pinter-Brown L, Cassarino DS, Chiu MW. Severe cutaneous interface drug eruption associated with bendamustine. Dermatol Online J. 2010;16:1. [PubMed] [Google Scholar]
- 12.Criado PR, Criado RF, Avancini JM, Santi CG. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Drug-Induced Hypersensitivity Syndrome (DIHS): a review of current concepts. An Bras Dermatol. 2012;87:435–449. doi: 10.1590/s0365-05962012000300013. [DOI] [PubMed] [Google Scholar]
- 13.Kano Y, Shiohara T. The variable clinical picture of drug-induced hypersensitivity syndrome/drug rash with eosinophilia and systemic symptoms in relation to the eliciting drug. Immunol Allergy Clin North Am. 2009;29:481–501. doi: 10.1016/j.iac.2009.04.007. [DOI] [PubMed] [Google Scholar]