Abstract
Objective
We sought to determine if serum citrulline (CIT), an amino acid produced by small bowel enterocytes, was associated with clinical and biochemical markers of gastrointestinal function in children undergoing hematopoietic cell transplantation (HCT).
Methods
We conducted a multi-center, prospective cohort study of 26 children to define time-related changes in serum CIT over the course of HCT. Markers of gastrointestinal function including oral energy intake, emesis, stool volume, presence of graft-versus-host disease (GVHD), oral mucositis severity, and cytokine and neurohormone levels were measured. Weekly serum CIT concentrations were obtained from 10 days prior until 30 days after HCT.
Results
Mean baseline CIT concentration was 22.7 µmol/L (95% CI 17.7 – 27.6) on day −10, which decreased to a nadir of 7.5 µmol/L (95% CI 3.1 – 18.0, p = 0.017) on day +8 following HCT before returning to baseline by day 30. After adjustment for IL-6 level (1.0% lower CIT per 10% increase in IL-6, p=0.004), presence of acute graft-versus-host disease (GVHD), (27% lower CIT, p=0.025) and oral energy intake (2.1% lower CIT per 10% decrease in energy intake, p=0.018), the nadir shifted to day +10, when mean CIT concentration was lower in patients with severe oral mucositis (6.7 µmol/L, 95% CI 3.4–13.1) than in those without severe mucositis (11.9 µmol/L, 95% CI 5.8–24.4, p=0.003). Change in CIT was not correlated with stool volume, C-reactive protein, TNF-alpha, leptin, or ghrelin.
Conclusion
In children undergoing HCT, serum CIT correlates with measures of gastrointestinal function (oral mucositis severity, dietary intake, acute GVHD) and may reflect mucosal injury to the gastrointestinal tract.
Keywords: Citrulline, biomarker, gastrointestinal function, hematopoietic cell transplantation
Introduction
During hematopoietic cell transplantation (HCT), children frequently suffer gastrointestinal toxicities such as mucositis, nausea, vomiting and diarrhea that limit enteral intake and prompt the use of parenteral nutrition. Many of these symptoms arise from injury to the gastrointestinal system caused by myeloablative conditioning therapy given in preparation for HCT. Myeloablative therapies produce gastrointestinal inflammation and mucosal barrier injury through complex processes that involve several overlapping mechanisms. Chemotherapeutic agents selectively target cells with high turnover, and therefore the mucosal barrier is particularly vulnerable to injury from such therapies (1). Additionally, chemotherapeutic agents produce reactive oxygen species and stimulate pro-inflammatory factors such as IL-6, nuclear factor -κB and TNF-alpha (2). These mediators become amplified and contribute to a positive feedback loop which causes damage to and apoptosis of the gastrointestinal cells of the basal epithelium, submucosa, and epithelial surface (3). Furthermore, in the setting of allogeneic HCT, alloreactivity can contribute to gastrointestinal toxicity through graft-versus-host disease (GVHD). Mucosal injury, however induced, contributes to bacterial translocation and bacteremia.
Citrulline (CIT) is a non-essential, non-protein amino acid synthesized by small bowel enterocytes that serves as a precursor for renal arginine production. Circulating CIT exists as a free amino acid (4) and can be measured in blood plasma or serum. CIT has been proposed as a biomarker of small intestinal enterocyte mass (5, 6) and function (7). Higher CIT concentrations have been correlated with longer bowel length in children with short bowel syndrome and may predict the necessity for long-term parenteral nutrition in these patients (8). Evidence also suggests that CIT is predictive of enterocyte loss and villous atrophy in HIV enteropathy (9). In pediatric patients with intestinal failure, lower CIT is associated with catheter-associated bloodstream infections, suggesting a potential role as a marker of impaired intestinal barrier integrity (10). CIT has been shown to decline with repeated cycles of chemotherapy in pediatric patients with acute myeloid leukemia, although pre-chemotherapy CIT concentrations were not reported (11). While CIT concentrations have been shown to decrease after myeloablative conditioning in adults, reaching a nadir of < 10 µmol/mL 10 days after the start of chemotherapy (12), the change in CIT over the course of HCT in children is unknown, as is the relationship between clinical markers of gastrointestinal function and CIT concentrations in this population. We hypothesized that serum CIT concentrations decrease significantly over the course of HCT in children, and that CIT values would correlate with clinical and biochemical markers of gastrointestinal function.
Materials and Methods
We recently performed a randomized, blinded, multi-center trial (clinicaltrials.gov identifier 00115258) of varied amounts of energy provision in twenty-six children undergoing allogeneic HCT (13). The study design has been described previously (14). In brief, patients undergoing HCT were randomized to receive standard energy provision at 130–150% of estimated basal energy expenditure (BEE) as calculated by standard equations (15) versus 100% of resting energy expenditure (REE) as measured by indirect calorimetry. Patients were screened for enrollment 7–14 days prior to HCT. Parenteral nutrition was initiated when oral intake declined to less than baseline REE for more than 3 days, and no earlier than day 0 of HCT. After engraftment (defined as 3 days with absolute neutrophil count > 500/µL), parenteral nutrition was discontinued when enteral calorie intake reached 50% of REE, or at hospital discharge. Data collection continued through day +100 after HCT. Pediatric patients admitted for allogeneic HCT at Boston Children’s Hospital in Boston, MA or Mattel Children’s Hospital in Los Angeles, CA were deemed eligible for the study if they met the following inclusion criteria: 1) age greater than 6 years, 2) undergoing first myeloablative allogeneic HCT with conditioning therapy including either total body irradiation with 1400 cGy or busulfan, in addition to other chemotherapeutic drugs, 3) receiving GVHD prophylaxis with methotrexate and a calcineurin inhibitor, with or without systemic corticosteroids, 4) unrelated or related HCT donor with 6/6 human leukocyte antigen-match.
Exclusion criteria included the following conditions: 1) baseline underweight status (defined as BMI z-score less than −2 for age and sex), 2) baseline overweight (defined as BMI z-score greater than 2 for age and sex), 3) age less than 6 years, 4) previous recipient of an HCT, 5) unmatched HCT donor, 6) history of a soy or egg allergy, 7) receiving treatment for hyper- or hypothyroidism, 8) previously diagnosed insulin-dependent diabetes, 9) currently receiving parenteral nutrition, 10) unable to comply with study protocols, and 11) any other contraindication to parenteral nutrition.
Data collected included daily oral energy intake, stool output, emesis, number of bloodstream infections, daily peak mucositis scores, diagnosis and grade of GVHD, and the number of days that parenteral nutrition was administered. Additionally, we obtained biochemical measurements including serum electrolytes, C-reactive protein (CRP), IL-6, TNF-alpha, leptin, ghrelin, and protein status including albumin and prealbumin, as well as anthropometric measurements including BMI, skinfold measurements, and percent body fat (using whole body dual-energy X-ray absorptiometry). Serum was frozen and stored at −80C, allowing batched analysis of blood samples (16).
Demographic and clinical assessments of study participants were performed by concomitant review of the medical record. Data collected included medical record number, date of birth, sex, pubertal status, underlying diagnosis, type of donor, date of transplant, and the conditioning and prophylactic medication regimens administered. Results of routine laboratory testing, including white blood cell count and serum chemistries were recorded twice weekly. Blood urea nitrogen, serum creatinine, total fluid intake and total urine output were recorded twice weekly as markers of renal function and fluid status. Biochemical indices of liver function, including ALT, alkaline phosphatase, and total and direct bilirubin were recorded once weekly. Presumed catheter-related infections (including phlebitis, tunnel infections, pocket infections, and site infections) and all positive cultures from blood, urine, and cerebrospinal fluid were recorded throughout hospitalization. Stool output and the presence and volume of diarrhea were recorded daily during hospitalization. GVHD scoring was performed by the HCT attending according to the 1994 consensus (17) criteria.
Pediatric dental residents received standardized training in the assessment and scoring of oral mucositis, and performed an oral assessment with a standard dental mouth mirror and headlamp at baseline and biweekly throughout hospitalization. Oral mucositis was graded using the WHO and NCI CTC criteria and scoring, with scores being recorded as grade 0 (no mucositis) through grade 4 (life threatening mucositis) (18). Peak mucositis score on either scale was used to classify patients into the categories of no/minimal mucositis (score 0–1) or severe mucositis (score 2–4). The correlation coefficient among raters was 0.94.
Underivatized CIT concentrations in serum specimens were evaluated in the Clinical and Epidemiological Research Laboratory in the Department of Laboratory Medicine, Boston Children's Hospital. Samples were ultra-centrifuged to remove high molecular weight proteins. The separation selectivity of CIT was obtained using a C18 BEH column and the ion pairing agent pentadecafluorooctanoic acid on a Waters Acquity UPLC System (Waters Corporation, Milford, MA). Positive electrospray ionization and a Waters Quattro premier triple-quad mass spectrometer were used to detect CIT by its characteristic 176–70 and 176–113 m/z transitions.
Data management and analysis
Spline models were employed to construct a curve describing the change in serum CIT over time. Because CIT concentrations had a skewed distribution, (maximum:median concentration 4.8; skewness 1.5), CIT was log-transformed for analysis and the results retransformed and reported as percentage difference. Potentially influential covariates, including age, sex, infection, body weight, energy intake, presence of GVHD, and cytokine levels were added to the model as invariant or time-varying predictors and tested for significance in multivariable models. For all analyses, a two-tailed p-value <0.05 was considered statistically significant. SAS version 9.3 software (SAS Institute Inc., Cary, NC) was utilized for statistical analyses.
Ethics
Written informed consent (or assent where indicated) was obtained from all study participants and/or their guardians, and the Institutional Review Boards at both institutions approved the study protocol.
Results
Thirty subjects were enrolled between February 2004 and February 2009. Prior to the start of the study, 3 patients became ineligible and 1 withdrew. Therefore prospective data was obtained on 26 patients throughout the course of HCT. Table 1 displays baseline demographic characteristics for the study participants. The average age at enrollment was 14.9 years. Fifty-four of the study participants were female, and 42% were non-white race. The majority of study participants had an underlying diagnosis of hematologic malignancy prior to HCT. Fifty-four percent of patients underwent unrelated donor HCT. For conditioning chemotherapy, 21/26 (81%) received cyclophosphamide and total body irradiation (TBI), whereas the remaining 5/26 (19%) received cyclophosphamide and busulfan.
Table 1.
Baseline characteristics of 26 children undergoing HCT
| Mean +/− SD or N (%) |
|
|---|---|
| Age (y) | 14.9 ± 4.2 |
| Female sex | 14 (54) |
| Nonwhite race | 11 (42) |
| Diagnosis | |
| Acute lymphoblastic leukemia | 7 (27) |
| Acute myelogenous leukemia | 7 (27) |
| Myelodysplastic syndrome | 3 (12) |
| Aplastic anemia | 1 (4) |
| Chronic myelogenous leukemia | 3 (12) |
| Lymphoma | 2 (8) |
| Other | 3 (12) |
| Conditioning regiment | |
| Cyclophosphamide + TBI | 21 (81) |
| Cyclophosphamide + busulfan | 5 (19) |
| Anthropometric measures | |
| Height (cm) | 154.7 ± 19.3 |
| Weight (kg) | 52.6 ± 17.9 |
| BMI (kg/m2) | 21.1 ± 3.63 |
| BMI (z score) | 0.31 ± 0.83 |
| Diet and energy measures | |
| Oral intake at baseline (kcal/day) | 2006 ± 778 |
| REE at baseline (kcal/day) | 1430 ± 282 |
| Laboratory values | |
| Baseline serum CIT (µmol/ | 22.7 ± 12.2 |
| IL-6 | 5.2 ± 5.1 |
| Baseline mucositis score | |
| NCI | |
| NCI = 0 | 23 (88) |
| NCI = 1 | 2 (8) |
| NCI = 2 | 1 (4) |
| WHO | |
| WHO = 0 | 23(88) |
| WHO = 1 | 2 (8) |
| WHO = 2 | 1 (4) |
HCT = hematopoietic cell transplantation; REE = resting energy expenditure; BMI = body mass index; NCI = National Cancer Institute; WHO = World Health Organization, TBI = total body irradiation
Mean (SD) BMI of the study participants was 21.1 (3.63) and mean (SD) BMI z-score was 0.31 (0.83). Over the course of HCT, study participants received 21.6 ± 10.1 (mean ±SD) days of parenteral nutrition, indicating significant limitations in their oral intake.
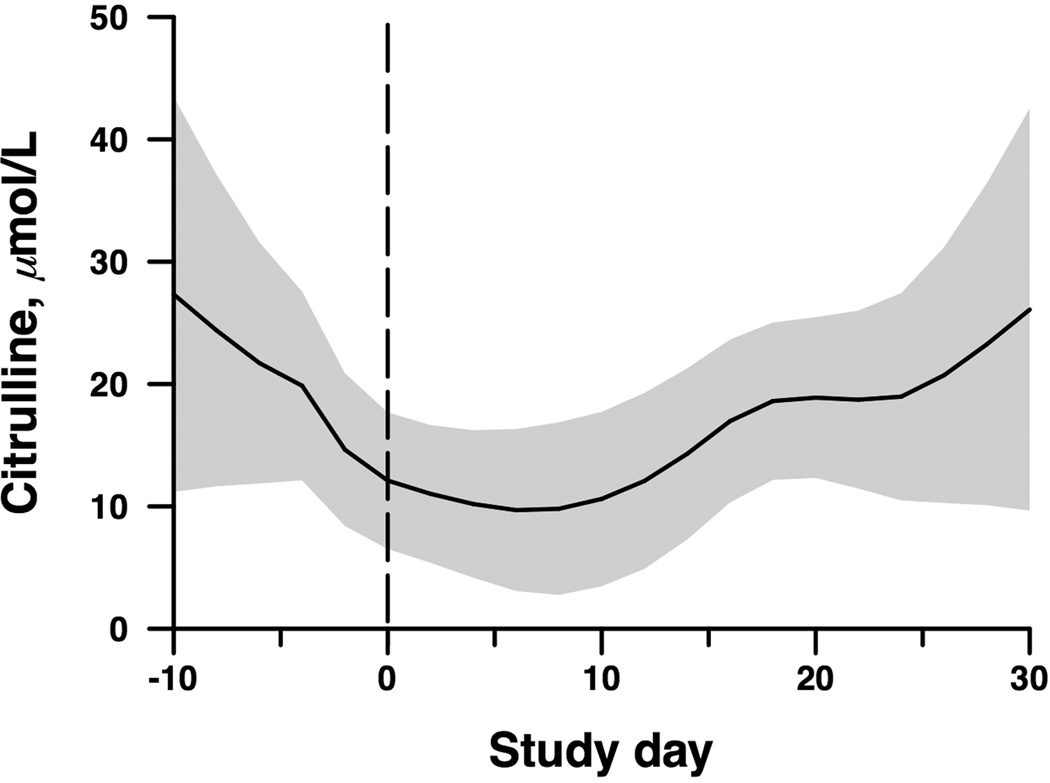
Serum CIT concentration decreased following administration of myeloablative conditioning, after which a recovery in CIT concentration was observed (Figure 1). Mean baseline serum CIT concentration at day −10 was 22.7 µmol/L (95% CI 17.7 – 27.6), which decreased to a nadir on day +8 following HCT of 7.5 µmol/L (95% CI 3.1 – 18.0, p = 0.017). CIT concentrations then recovered, approaching pre-transplant levels by day +30 following HCT. Serum CIT concentrations were not significantly different at any point between arms receiving different energy intakes.
Figure 1.
Change in serum citrulline over time (p=0.017) in 26 children undergoing HCT. Band indicates ±1 standard error for fitted spline curve. Study day −10 indicates 10 days before HCT, and study day 0 is the day of HCT.
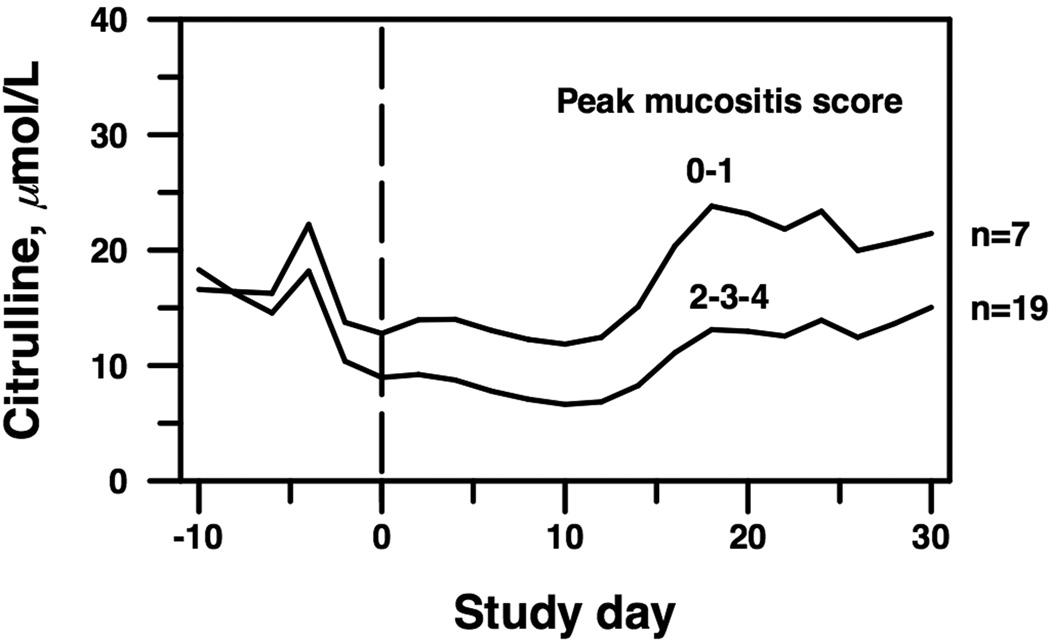
Table 2 and Figure 2 display serum CIT concentrations by peak mucositis score. Severe oral mucositis was defined as a peak mucositis score of 2, 3, or 4 on either the WHO or NCI oral mucositis scales. Seven subjects had a peak mucositis score of 0–1, while 19 subjects had a peak mucositis score of 2, 3, or 4. Concordance between the WHO and NCI scales was 92.3%. After adjustment for significant covariates in a multivariable spline model (detailed below), the nadir of serum CIT concentration shifted to day +10 and was lower in patients with severe oral mucositis (6.7 µmol/L, 95% CI 3.4–13.1) than in those without severe oral mucositis (11.9 µmol/L, 95% CI 5.8–24.4, p = 0.003).
Table 2.
Change in citrulline concentration by mucositis severity
| Citrulline concentration, mean (95% CI), µ mol/L | |||
|---|---|---|---|
| Study day | Peak mucositis score 0–1 |
Peak mucositis score 2–4 |
p* |
| −10 | 16.6 (2.9–94.5) | 18.3 (3.5–95.1) | 0.81 |
| 0 (transplant) | 12.8 (8.7–18.8) | 9.0 (6.3–12.8) | 0.006 |
| +10 (nadir) | 11.9 (5.8–24.4) | 6.7 (3.4–13.1) | 0.003 |
| +20 | 23.1 (12.3–43.4) | 13.0 (7.2–23.4) | 0.0007 |
| +30 | 21.5 (3.8–122.6) | 15.1 (3.0–76.4) | 0.42 |
Comparing fitted spline curves at indicated time, adjusted for IL-6, GVHD and oral energy intake.
Figure 2.
Change in serum CIT over time by peak mucositis score, adjusted for IL-6, GVHD, and oral energy intake. The groups differed overall (p=0.008) and in particular at the nadir (day +10, p=0.003). Study day −10 indicates 10 days before HCT, and study day 0 is the day of HCT.
Five of the twenty-six subjects (19%) developed acute GVHD, with a median day of diagnosis of +19 (range 9–29). The overall median consensus acute GVHD grade was 3 (range 1–4). Three subjects had a clinical diagnosis of gastrointestinal GVHD (stages 2, 3 and 3). Two of these subjects had among the lowest median CIT values in the cohort (3.3 and 7.6 µmol/L). Serum CIT was 27% lower in subjects who developed acute GVHD compared to those without GVHD (p=0.025, adjusted for other covariates in the multivariable model). Additionally, serum CIT was inversely associated with IL-6 level (1.0% decrease in serum CIT concentration per 10% increase in IL-6, p=0.004). Change in CIT concentration was significantly correlated with daily oral intake, measured as daily oral kilocalories (kcal) as a percentage of predicted Schofield REE (2.1% decrease in CIT per 10% decrease in energy intake as %REE, p=0.018). However, oral energy intake by macronutrient category (carbohydrate, protein, or fat) and REE showed no significant association with change in CIT concentrations.
Twenty-two subjects lost weight over the course of HCT, while the remaining 4 subjects gained weight. The mean change in weight between day −7 and +30 was −2.7 (range −9.1 to 3.9) kg. Change in body weight showed a simple correlation with change in CIT concentration (r = 0.48, p = 0.013), but no influence as a time-varying covariate in the spline model. Change in serum CIT concentration was not associated with age, sex, infection, days on parenteral nutrition, conditioning regimen, stool output, stool consistency, or CRP, TNF-α, ghrelin, or leptin levels.
Discussion
In this prospective study of children undergoing HCT, we found that CIT concentrations decreased after initiation of myeloablative conditioning therapy and then recovered, and that these changes in CIT were significantly and independently associated with peak mucositis score, IL-6 concentration, daily energy intake, and the development of acute GVHD. CIT concentration was not associated with other clinical measures of gastrointestinal function such as stool consistency, volume of stool output, or duration of parenteral nutrition. Additionally, CIT concentrations were not associated with REE.
These results build upon what has been demonstrated in adults undergoing myeloablative conditioning therapy for HCT (12, 19), namely a significant decrease in CIT concentrations after treatment with myeloablative therapies followed by recovery. While studies in adults have correlated CIT concentration with intestinal damage following HCT (20–22), to our knowledge, this is the first study to demonstrate temporal changes in CIT in children undergoing HCT, as well as a significant relationship between CIT and oral mucositis, pro-inflammatory cytokines, oral energy intake, body weight change, and GVHD.
The potential for CIT concentration to serve as an objective biomarker for oral mucositis severity is appealing for several reasons. The WHO and NCI oral mucositis assessment scores rely on subjective assessment and may vary with the skill and experience of the observer. While a thorough oral exam may not always be possible in children due to lack of patient cooperation or developmental state, serum CIT concentration is easy to measure and would be an attractive alternative tool for reliable assessment of oral mucositis severity.
In contrast to oral mucositis, the intestinal mucositis that follows myeloablative conditioning is more difficult to assess. Endoscopic evaluation and intestinal biopsy is an invasive procedure that subjects a patient to sedation or anesthesia. Additionally, intestinal biopsy carries the risk of bleeding, which is more pronounced in patients undergoing HCT due to associated thrombocytopenia. Other methods of measuring small intestine function, such as dual sugar absorption tests, involve cumbersome, timed specimen collection, and may be less sensitive and specific than CIT concentration (22). Additional biomarkers of intestinal function have also been proposed, including intestinal fatty-acid binding protein (23) and flagellin (24), however neither have been extensively studied in children. In our cohort, CIT concentration was not significantly associated with standard gastrointestinal function assessments such as stool output or consistency. It is important to note that colonic function also regulates stool consistency and is not reflected by CIT concentration. Moreover, clinical measures such as stool frequency and appearance are subjective and may be insufficiently sensitive to subtle changes in small intestinal function. Furthermore, our sample size likely resulted in limited power to detect these associations.
We found that CIT concentration was inversely associated with IL-6, generally held to be a marker of inflammation. There is conflicting data in the literature regarding the relationship between CIT concentration and systemic inflammation. In a study of adult patients with Crohn disease, Papadia et al. (25) found no association between plasma CIT concentrations and laboratory markers of inflammation including erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), white blood cell count, or platelet count, and concluded that CIT concentrations were independent of intestinal inflammation. Alternatively, Lee et al. (26) reported lower plasma citrulline concentrations in pediatric Crohn disease patients with systemic inflammation as measured by erythrocyte sedimentation rate and CRP. Van der velden et al.(21) showed that the decrease in CIT concentration occurs before the onset of inflammation in HCT as measured by CRP. Furthermore, a study by Burpee et al. (27) demonstrated that IL-6 concentrations were significantly different between non-anemic pediatric patients with Crohn disease compared with healthy controls, hypothesizing that IL-6 may be a more sensitive serum marker for intestinal inflammation than ESR and CRP.
We found a correlation between the decrease in serum CIT concentration and IL-6, and found that this correlation persists throughout HCT, suggesting a similar time course for inflammation and intestinal damage. However, the changes in CIT concentration did not correlate with other systemic markers of inflammation such as CRP or TNF-α concentrations. Further study in both children and adults is needed to better elucidate the relationship between intestinal damage, systemic inflammation, CIT concentration and various inflammatory markers and cytokines. Assessing the role of intestinal-specific markers of inflammation such as fecal lactoferrin or calprotectin, independently and in relation to CIT, may also be of value in this population.
Previous work has demonstrated that oral intake declines significantly over the course of HCT in children, followed by recovery (28). In our study, daily oral intake, as measured by percentage of Schofield REE, was significantly correlated with change in CIT over time. Lower serum CIT concentrations could be a marker of more advanced enteritis, induced by either chemotherapy or radiation, leading to anorexia and diminished oral intake. Alternatively, decreased oral intake could result in mucosal atrophy and decreased CIT concentration. In either case, CIT might be an indirect marker of dietary energy intake. Further dietary analysis by macronutrient category, including amount of daily protein, carbohydrate, or fat intake, did not show significant association with change in CIT.
Change in body weight showed a simple correlation with change in CIT. A similar association was also recently demonstrated in a study of 282 children from Burkina Faso undergoing zinc supplementation in whom weight gain was significantly associated with increase in CIT concentrations (29). This association between CIT and weight gain may mean that changes in enterocyte mass parallel changes in body weight. Alternatively, higher CIT concentrations may be indicative of better absorptive function and thus a greater ability to maintain or gain weight over the course of HCT. In either case, these associations likely demonstrate the ability of CIT to indicate more extensive small bowel injury than is clinically apparent.
Nineteen percent of subjects in our study developed acute GVHD, and CIT was significantly lower in this subgroup. In 2 subjects with acute GI GVHD, median CIT levels were very low (less than 8 µmol/L). Lower CIT concentrations in these subjects could be the result of enterocyte damage secondary to acute GVHD. Alternatively, acute GVHD could lead to anorexia and poor oral intake resulting in decreased enterocyte mass and CIT concentrations. Clearly, there is an association between acute GVHD, systemic inflammation, and poor oral intake, which likely contributes to reduction in CIT concentrations in this subgroup. Since the nadir in CIT concentration (median day +10, adjusting for covariates) preceded the diagnosis of acute GVHD (median day +19), it is possible that CIT may be a pre-clinical marker of GVHD. This finding deserves further study.
Strengths of our study included (1) the prospective nature of the data collection, (2) the multi-center enrollment, (3) the enrollment of children, in whom there are limited data regarding serum CIT concentrations, and (4) the large number of clinical, anthropometric and biochemical variables that were collected. However, there were several limitations to our study. Our sample size was relatively small, which likely limited our power to detect the association of serum CIT concentrations with various clinical outcomes. Additionally, some clinical variables such as days on parenteral nutrition were managed per protocol as part of the initial trial design, which may have limited our ability to detect an association between CIT and duration of parenteral nutrition. Also, the oral mucositis assessments were performed by different practitioners throughout the course of data collection (although the inter-examiner correlation was high). Furthermore, all our subjects received myeloablative conditioning therapy, and therefore we are unable to comment on the role of CIT in patients undergoing HCT with non-myeloablative conditioning regimens. Finally, for ethical reasons, we did not routinely obtain intestinal biopsies and therefore are unable to compare CIT concentrations with small bowel histopathology.
In conclusion, serum CIT concentrations decreased after administration of myeloablative conditioning therapy in children undergoing HCT. In our cohort, adjusted mean CIT concentrations reached a nadir at day +10 following HCT and then recovered to baseline by day +30. CIT was a valid biomarker for oral mucositis severity in children undergoing HCT, and change in CIT over HCT was independently and significantly associated with IL-6 levels, daily oral intake and the occurrence of GVHD. Further study is needed to better define the relationship between gastrointestinal mucosal injury, gastrointestinal and systemic inflammation, and CIT concentration. CIT concentration may indicate more extensive mucosal injury to the gastrointestinal system than is able to be observed clinically, and CIT may be a valuable biomarker in the nutritional and gastrointestinal assessment of pediatric HCT patients.
Acknowledgements
Funding
The authors wish to thank Alice Kim, DMD, Zamerra Fida, DMD, Quyen Vu, DMD and Lisa DeLucia, DMD. CD, LJB, HAF, and ECG designed the research; all authors conducted the research; KBG and HAF analyzed the data; KBG wrote the paper; CD had primary responsibility for final content; all authors contributed to and approved the final manuscript. This study was supported by the Massachusetts Vitamin Litigation Grant, the Harvard Clinical and Translational Science Center UL1 RR-025758, and the NIH grants K24 HD 058795 and T32 DK 7477-30. CD was supported in part by Bill and Melinda Gates Foundation Award OPP1066203.
Footnotes
Conflict of Interest:
The authors report no conflict of interest
References
- 1.Logan RM, Stringer AM, Bowen JM, et al. The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: pathobiology, animal models and cytotoxic drugs. Cancer Treat Rev. 2007;33(5):448–460. doi: 10.1016/j.ctrv.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Sonis ST. The pathobiology of mucositis. Nat Rev Cancer. 2004;4(4):277–284. doi: 10.1038/nrc1318. [DOI] [PubMed] [Google Scholar]
- 3.Sonis ST, Elting LS, Keefe D, et al. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer. 2004;100(9 Suppl):1995–2025. doi: 10.1002/cncr.20162. [DOI] [PubMed] [Google Scholar]
- 4.Rabier D, Kamoun P. Metabolism of citrulline in man. Amino Acids. 1995;9:299–316. doi: 10.1007/BF00807268. [DOI] [PubMed] [Google Scholar]
- 5.Crenn P, Vahedi K, Lavergne-Slove A, et al. Plasma citrulline: A marker of enterocyte mass in villous atrophy-associated small bowel disease. Gastroenterology. 2003;124(5):1210–1219. doi: 10.1016/s0016-5085(03)00170-7. [DOI] [PubMed] [Google Scholar]
- 6.Crenn P, Coudray-Lucas C, Thuillier F, et al. Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology. 2000;119(6):1496–1505. doi: 10.1053/gast.2000.20227. [DOI] [PubMed] [Google Scholar]
- 7.Crenn P, Messing B, Cynober L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr. 2008;27(3):328–339. doi: 10.1016/j.clnu.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgibbons S, Ching YA, Valim C, et al. Relationship between serum citrulline levels and progression to parenteral nutrition independence in children with short bowel syndrome. J Pediatr Surg. 2009;44(5):928–932. doi: 10.1016/j.jpedsurg.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crenn P, De Truchis P, Neveux N, et al. Plasma citrulline is a biomarker of enterocyte mass and an indicator of parenteral nutrition in HIV-infected patients. Am J Clin Nutr. 2009;90(3):587–594. doi: 10.3945/ajcn.2009.27448. [DOI] [PubMed] [Google Scholar]
- 10.Hull MA, Jones BA, Zurakowski D, et al. Low serum citrulline concentration correlates with catheter-related bloodstream infections in children with intestinal failure. JPEN J Parenter Enteral Nutr. 2011;35(2):181–187. doi: 10.1177/0148607110381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Vliet MJ, Tissing WJ, Rings EH, et al. Citrulline as a marker for chemotherapy induced mucosal barrier injury in pediatric patients. Pediatr Blood Cancer. 2009;53(7):1188–1194. doi: 10.1002/pbc.22210. [DOI] [PubMed] [Google Scholar]
- 12.van der Velden WJ, Herbers AH, Feuth T, et al. Intestinal damage determines the inflammatory response and early complications in patients receiving conditioning for a stem cell transplantation. PLoS One. 2010;5(12):e15156. doi: 10.1371/journal.pone.0015156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma TS, Bechard LJ, Feldman HA, et al. Effect of titrated parenteral nutrition on body composition after allogeneic hematopoietic stem cell transplantation in children: a double-blind, randomized, multicenter trial. Am J Clin Nutr. 2012;95(2):342–351. doi: 10.3945/ajcn.111.026005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bechard LJ, Feldman HA, Gordon C, et al. A multi-center, randomized, controlled trial of parenteral nutrition titrated to resting energy expenditure in children undergoing hematopoietic stem cell transplantation ("PNTREE"): rationale and design. Contemp Clin Trials. 2010;31(2):157–164. doi: 10.1016/j.cct.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39(Suppl 1):5–41. [PubMed] [Google Scholar]
- 16.Harder U, Koletzko B, Peissner W. Quantification of 22 plasma amino acids combining derivatization and ion-pair LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(7–8):495–504. doi: 10.1016/j.jchromb.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 18.Kostler WJ, Hejna M, Wenzel C, et al. Oral mucositis complicating chemotherapy and/or radiotherapy: options for prevention and treatment. CA Cancer J Clin. 2001;51(5):290–315. doi: 10.3322/canjclin.51.5.290. [DOI] [PubMed] [Google Scholar]
- 19.Herbers AH, Blijlevens NM, Donnelly JP, et al. Bacteraemia coincides with low citrulline concentrations after high-dose melphalan in autologous HSCT recipients. Bone Marrow Transplant. 2008;42(5):345–349. doi: 10.1038/bmt.2008.170. [DOI] [PubMed] [Google Scholar]
- 20.Herbers AH, Feuth T, Donnelly JP, et al. Citrulline-based assessment score: first choice for measuring and monitoring intestinal failure after high-dose chemotherapy. Ann Oncol. 2010;21(8):1706–1711. doi: 10.1093/annonc/mdp596. [DOI] [PubMed] [Google Scholar]
- 21.van der Velden WJ, Herbers AH, Bruggemann RJ, et al. Citrulline and albumin as biomarkers for gastrointestinal mucositis in recipients of hematopoietic SCT. Bone Marrow Transplant. 2013 doi: 10.1038/bmt.2012.278. [DOI] [PubMed] [Google Scholar]
- 22.Lutgens LC, Blijlevens NM, Deutz NE, et al. Monitoring myeloablative therapy-induced small bowel toxicity by serum citrulline concentration: a comparison with sugar permeability tests. Cancer. 2005;103(1):191–199. doi: 10.1002/cncr.20733. [DOI] [PubMed] [Google Scholar]
- 23.Derikx JP, Evennett NJ, Degraeuwe PL, et al. Urine based detection of intestinal mucosal cell damage in neonates with suspected necrotising enterocolitis. Gut. 2007;56(10):1473–1475. doi: 10.1136/gut.2007.128934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziegler TR, Luo M, Estivariz CF, et al. Detectable serum flagellin and lipopolysaccharide and upregulated anti-flagellin and lipopolysaccharide immunoglobulins in human short bowel syndrome. Am J Physiol Regul Integr Comp Physiol. 2008;294(2):R402–R410. doi: 10.1152/ajpregu.00650.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papadia C, Sherwood RA, Kalantzis C, et al. Plasma citrulline concentration: a reliable marker of small bowel absorptive capacity independent of intestinal inflammation. Am J Gastroenterol. 2007;102(7):1474–1482. doi: 10.1111/j.1572-0241.2007.01239.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee EH, Ko JS, Seo JK. Correlations of plasma citrulline levels with clinical and endoscopic score and blood markers according to small bowel involvement in pediatric crohn disease. J Pediatr Gastroenterol Nutr. 2013;57(5):570–575. doi: 10.1097/MPG.0b013e31829e264e. [DOI] [PubMed] [Google Scholar]
- 27.Burpee T, Mitchell P, Fishman D, et al. Intestinal ferroportin expression in pediatric Crohn's disease. Inflamm Bowel Dis. 2011;17(2):524–531. doi: 10.1002/ibd.21367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bechard LJ, Guinan EC, Feldman HA, et al. Prognostic factors in the resumption of oral dietary intake after allogeneic hematopoietic stem cell transplantation (HSCT) in children. JPEN J Parenter Enteral Nutr. 2007;31(4):295–301. doi: 10.1177/0148607107031004295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wessells KR, Hess SY, Rouamba N, et al. Associations Between Intestinal Mucosal Function and Changes in Plasma Zinc Concentration Following Zinc Supplementation. J Pediatr Gastroenterol Nutr. 2013 doi: 10.1097/MPG.0b013e31829b4e9e. [DOI] [PMC free article] [PubMed] [Google Scholar]