Abstract
Objective
Links between obesity, with its attendant estrogen abnormalities, and the endometrial carcinoma (EC) DNA Mismatch Repair Protein (MMR) system have recently been proposed. We investigated relationships between Body Mass Index (BMI) and clinicopathological correlates including MMR expression in a large single institution EC cohort.
Methods
Clinical and pathological databases from 2007 to 2012 were used to identify consecutive hysterectomy specimens with EC. Univariate and multivariate analyses were used to explore relationships between BMI, age, stage, tumor type and immunohistochemical results for MLH1, PMS2, MSH2 and MSH6.
Results
1049 EC were identified. Overall, BMI was higher amongst women with normal MMR (p=0.002). However, when stratified by age and specific MMR, statistically significant differences localized exclusively to women <50 years old with loss of MSH2 and/or MSH6 (p=0.003 and p=0.005 respectively). Higher BMI correlated with endometrioid FIGO 1 and 2 tumors (p<0.001) and with stage 1a (p<0.001). Conversely, MMR abnormalities did not show significant associations with stage (p=0.302) or histologic grade (p=0.097).
Conclusions
BMI showed statistically significant associations with MMR expression, tumor grade and stage amongst 1049 consecutive EC. Obesity correlates with lower grade and stage EC. A link between BMI and maintenance of the MMR system is not supported by our data because the only statistically significant association occurred in women <50 years old with MSH2 and/or MSH6 abnormalities where Lynch syndrome related cases are expected to cluster.
Keywords: DNA Mismatch Repair Protein, MMR, Endometrial Cancer, Body Mass Index, BMI
Introduction
Excess body weight is a well-established risk factor for endometrial carcinoma (EC) [1–3]. Obesity, defined as body mass index (BMI) >30 kg/m2, afflicts over 35% of women in the United States, resulting in a 41% population attributable risk for EC [4]. The relationship between obesity and EC is complex and involves multiple mechanisms including increased free estrogens [1] and a low grade inflammatory milieu [5]. Recently, other EC investigators have also suggested a link between BMI with its attendant estrogen levels and the highly conserved DNA mismatch repair (MMR) system [6]. Moreover, Miyamoto et al. have contributed in-vitro evidence that estradiol increases cell proliferation, MLH1/MSH2 expression, and MMR activity in cultured glandular endometrial cells as well as an endometrial cancer cell line [7]. Because the MMR system corrects DNA mismatches generated during replication [8] its impairment leads to accumulation of mutations and microsatellite instability (MSI), a phenotype observed in 17–31% of EC [9–10]. MSH2 and MSH6 (E. coli MutS homologs); and MLH1 and PMS2 (E. coli MutL homologs) work as heterodimers that recognize and initiate mismatch repair [11]. The pathologic loss of expression of one or more of these MMR proteins can be detected with immunohistochemistry (IHC) [12]. Since they are “obligatory partners” of their respective heterodimer, loss of expression of MLH1 or MSH2 results in concurrent loss of expression of PMS2 and MSH6 respectively [12]. Conversely, mutations of the PMS2 or MSH6 genes result in isolated protein losses. While most MSI in sporadic EC can be attributed to hypermethylation of the MLH1 gene promoter [13–14] instead of MMR gene mutations [15], EC is the second most common cancer in women with Lynch syndrome (LS), a condition caused by a hereditable autosomal dominant germ line mutation of one of the MMR genes [16–17].
The main objective of the present study is to correlate BMI with MMR IHC, including MLH1, PMS2, MSH2 and MSH6, in 1049 consecutive EC. Associations with other clinicopathological variables including tumor type, grade and stage are also presented.
Methods
The institutional pathology database at the Ohio State University Wexner Medical Center (OSUWMC) was searched for the terms endometrial carcinoma and endometrial carcinosarcoma in hysterectomy specimens received between July 1, 2007 and April 15, 2012. Tumor stage, histological type and MMR IHC results were extracted from original pathology reports. Our institution routinely performs MLH1 (NovoCastra, clone: ES05), PMS2 (BD, clone: A16-4), MSH2 (Calbiochem, clone: FE11), and MSH6 (Epitomics, clone: EP 49) on all endometrial carcinoma specimens using clinically-validated Bond (MLH1, MSH6) and Dako (MSH2, PMS2) immunostainers, each at a dilution of 1/200 and with colon cancer as control tissue. IHC for any of the four MMR proteins is considered positive if definite nuclear staining is detected in neoplastic cells. The patients were grouped by presence or absence of the MMR proteins (both individually and as a group), and further clinical data including height, weight, and age was extracted from the institution’s electronic medical record; BMI data was calculated. Endometrial carcinoma was classified as type 1 or low grade if the tumor was diagnosed as endometrioid carcinoma FIGO grade 1 or 2. FIGO grade 3 endometrioid tumors were classified as type 2 or high grade, along with serous carcinomas, carcinosarcomas, clear cell carcinomas, mixed carcinomas, and undifferentiated carcinomas.
Relationships between MMR defects, BMI, age, tumor type, and tumor stage were investigated. Comparison groups for MLH1, PMS2, MSH2, and MSH6 deficiencies were defined as the subjects with each absent individual protein. The normal or control group for the comparisons of each of the individual protein deficiencies consisted of the 814 patients in the cohort who had all four MMR proteins present by IHC. Age and BMI as continuous variables were compared between tumor types and protein defects using Wilcoxon rank-sum tests. Categorical variables were compared using Chi-square tests; however, Fisher’s exact test was utilized when the number of patients in any comparison group was small. Since numerous tests were performed, and to control the type I error rate, a p-value of 0.01 or less was considered significant for these tests. An analysis of variance model with Tukey-Kramer adjustment for multiple comparisons was used to compare the continuous BMI between tumor stages. All statistical analyses were performed using SAS Version 9.2 (SAS Institute Inc., Cary, NC).
The study was performed under appropriate IRB approvals (OSU 2007C0081 and 2001C0203).
Results
Patient Characteristics
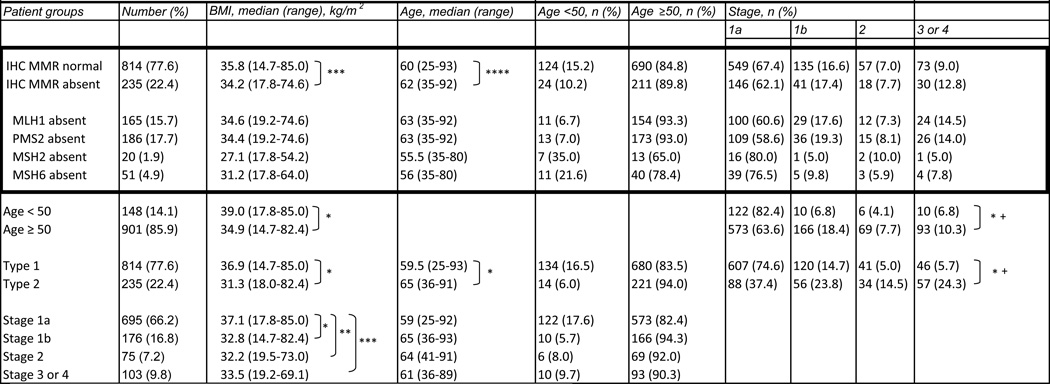
A total of 1054 eligible patients were identified within the study dates. Five patients were removed from the study; four of these had insufficient tumor for MMR IHC and one had complete coagulative necrosis from previous endometrial ablation precluding accurate assessment. Overall patient age ranged from 25 to 93 years (median 61). 814 patients had type 1 tumors (age range 25–93, median 59.5) and 235 patients had type 2 tumors (age range 36–91, median 65) including 65 FIGO 3 endometrioid adenocarcinomas, 52 serous carcinomas, 11 clear cell carcinomas, 36 carcinosarcomas, 2 poorly or undifferentiated carcinomas, and 69 mixed pattern carcinomas. The majority of patients presented with stage 1a disease (695, 66.3%) while 176 (16.8%), 75 (7.2%), and 103 (9.8%) patients presented with stage 1b, stage 2, and stage 3 or 4 disease, respectively. BMI ranged from 14.7 kg/m2 to 85.0 kg/m2 (median 35.3 kg/m2). Overall, BMI was significantly higher for women younger than 50 years (n=148, median BMI=39.0 kg/m2, range 17.8–85.0 kg/m2) versus women ≥50 years (n=901, median BMI=34.9 kg/m2, range 14.7–82.4 kg/m2), p<0.001. (Table 1)
Table 1.
Relationships between selected clinicopathologic variables and MMR expression
 |
Indicates significance in distribution of stages among 1a, 1b, 2, and 3 or 4
p<0.001;
p=0.008;
p=0.002,
p=0.010
MMR associations (Fig. 1, 2)
Figure 1.
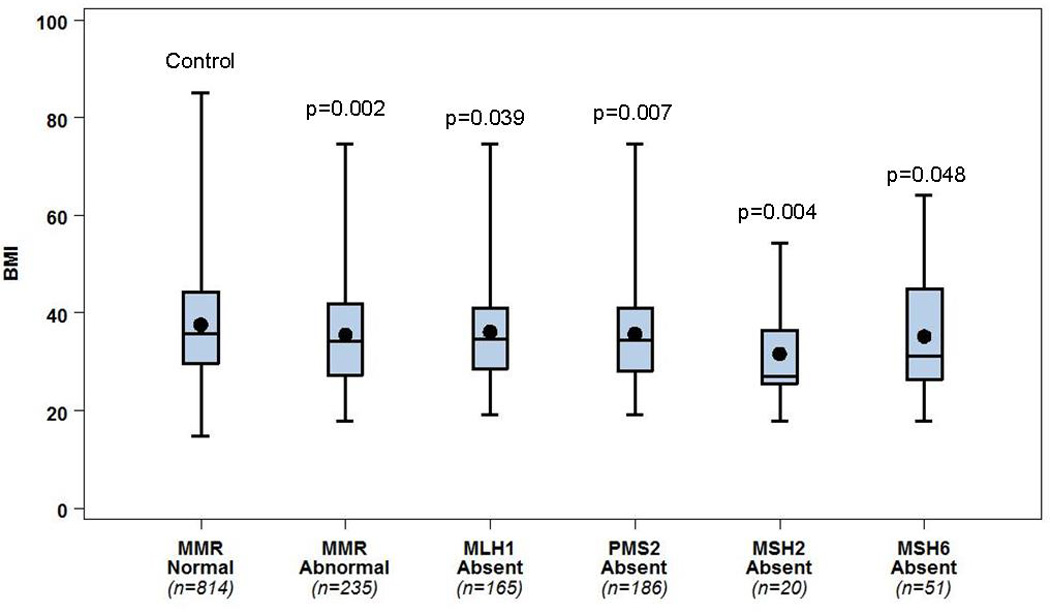
BMI data stratified by MMR protein absence (any protein absent, and individual MLH1, PMS2, MSH2, or MSH6 absent), compared to a control group of women with all MMR proteins present. Comparison demonstrates that women with EC MMR intact have a higher BMI than those with any abnormal MMR protein (p=0.002). This largely holds true when stratified by the individual abnormal protein lost (remaining comparisons).
Boxes: 25–75th percentile; whiskers: range; central line: mean; central dot: median.
Figure 2.
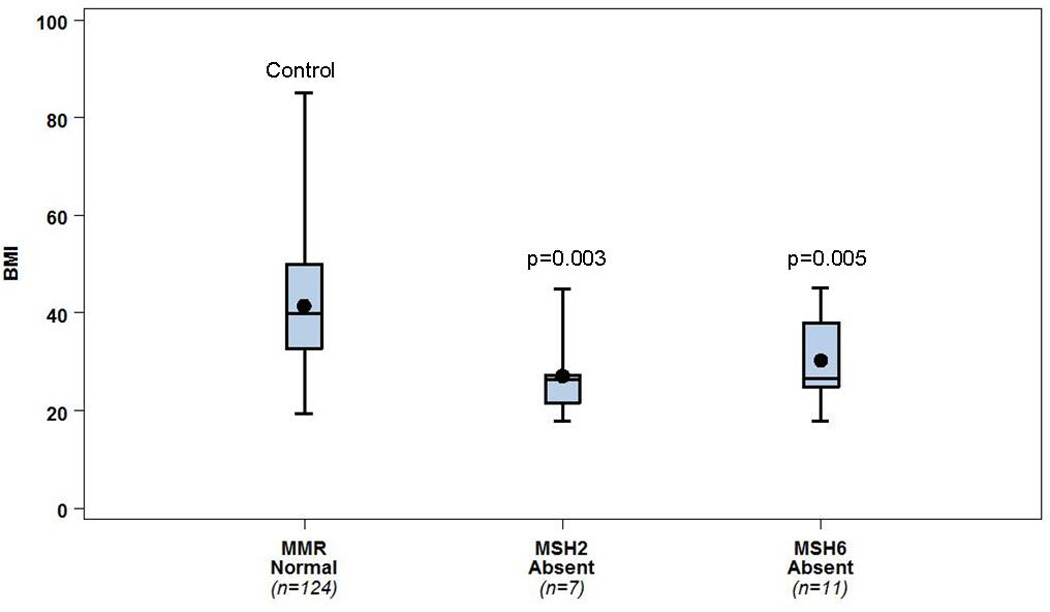
Women <50 years of age who lack MSH2 and/or MSH6 in their EC specimens have markedly lower BMIs than their <50 year counterparts with all proteins intact (p=0.003 and p=0.005, respectively).
Boxes: 25–75th percentile; whiskers: range; central line: mean; central dot: median.
One or more MMR proteins were absent in 235 (22.4%) of the 1049 tumors investigated. PMS2 loss was the most common abnormality, followed by absence of MLH1 (Table 1). Combined loss of MLH1 and PMS2 occurred in 165 cases (15.7%), while isolated PMS2 loss occurred in 21 cases (2.0%). Combined loss of MSH2 and MSH6 occurred in 20 (1.9%) cases while isolated loss of MSH6 occurred in 31 (3%) cases.
MMR abnormalities were associated with patient BMI. Women whose tumors had abnormal MMR IHC were thinner than those women with normal MMR expression (median BMI 34.2 kg/m2, range 17.8–74.6 kg/m2 vs. 35.8 kg/m2, range 14.7–85.0 kg/m2, p=0.002, Wilcoxon Rank Sum test) (Fig. 1). However, women whose tumors lacked both MLH1 and PMS2 (the most common defect, n=165 cases) had a similar BMI compared with those who had normal MMR expression (n=814) (median BMI 34.6 kg/m2, range 19.2–85 kg/m2 vs. 35.8 kg/m2, range 14.7–85.0 kg/m2; respectively, p=0.040, Wilcoxon Rank Sum test). On the other hand, the BMIs of those women whose tumors lacked MSH2 and/or MSH6 (n=51) were markedly lower than women with normal MMR expression (MSH2 deficient women with median BMI 27.1 kg/m2, range 17.8–54.2; MSH6 deficient women with median BMI 31.2 kg/m2, range 17.8–64.0) (see Fig. 1 and Table 1 for breakdown by individual protein loss).
Loss of MMR was also associated with age at diagnosis. Women with absent MLH1 (median age 63, range 35–92) and/or PMS2 (median age 63, range 35–92) were older than women who had all proteins present (median age 60, range 25–93), p<0.001 for both comparisons. In contrast,, women with absent MSH2 and/or MSH6 on IHC (median age 56, range 35–80 for each) were significantly younger than those with all proteins detected (median age 60, range 25–93), p=0.006 and p=0.003, respectively.
More interestingly, when stratified by age, significant BMI differences were seen only in women <50 years old with loss of MSH2 and/or MSH6. BMI was significantly higher in women when all proteins were present (n=124, median BMI=39.3 kg/m2) than in the young women with MSH2 (n=7) and/or MSH6 (n=11) loss (median BMI=26.4 kg/m2 for each), p=0.003 and p=0.005, respectively (Fig. 2).
There was no BMI difference in women ≥50 years old who retained all proteins or lost expression of MSH2 and/or MSH6 (35.0 kg/m2 compared to 31.0 kg/m2 and 33.8 kg/m2, respectively), p=0.174 and p=0.460. Likewise, no significant differences in BMI were seen between those who retained all proteins or lost MLH1 and/or PMS2, regardless of age.
There was no difference in stage distribution between those who had MMR protein loss by IHC and those who did not, p=0.302. Additionally, there were no differences in MMR protein loss between type 1 and type 2 tumors (21.3% type 1 v. 26.4% type 2), p=0.097.
BMI, Tumor Type, Age, and Stage
BMI, tumor type, age, and stage were compared. Women with type 1 tumors (n=814) were significantly younger (median age 59.5 years) than women with type 2 tumors (n=235, median=65), p<0.001. Stage at presentation was significantly associated with tumor type. Type 1 tumors were more likely to present at a lower stage than type 2 tumors, p<0.001. Specifically, type 1 tumors were mostly stage 1a (74.6%) or stage 1b (14.7%) compared to type 2 tumors (37.5% stage 1a and 23.9% stage 1b). BMI was higher for women with type 1 tumors (median BMI= 36.9 kg/m2, range 14.7 – 85.0 kg/m2) than for women with type 2 tumors (median BMI=31.3 kg/m2, range 18.0–82.4 kg/m2), p<0.001. Of the 750 women classified as obese, 82.1% were diagnosed with type 1 tumors in comparison to 66.3% of overweight women and 66.7% of thin or normal weight women, p<0.001. Additionally, the BMI of stage 1a patients was significantly higher than stage 1b, stage 2, and stage 3 or 4 patients (Tukey-Kramer adjusted p-values of p<0.001, p=0.008, and p=0.002, respectively).
Discussion
We have analyzed a cohort of 1049 consecutive hysterectomy specimens with EC. Standard clinicopathological features including histologic tumor grade and pathological stage were fairly typical and consistent with other large published series [3,18–20]. Accordingly, a majority of tumors were type 1 (77.5%) and stage 1a (66.3%) and higher BMI correlated with lower EC stage and younger age [3,18,20]. Higher BMI was also associated with lower histologic grade, “type 1 EC”, as reported in most [18–19,21–24] but not all series [3,6].
MMR protein IHC and genotyping for MSI have both been used to screen for LS, carrying similar specificity and sensitivity rates [6,16,25–27]. While testing selected cases based on clinical criteria and histopathologic findings has been suggested by some [27–28], the practice at our institution is to screen all EC hysterectomy specimens with IHC for MLH1, PMS2, MSH2 and MSH6. Consequently, we are uniquely powered to describe MMR associations with clinicopathological variables including BMI across very large numbers of unselected EC. Overall, we documented MMR abnormalities in 22.4% of EC, consistent with our institution’s previous reports [29–30]. Women with MMR loss were older (mean 62 vs. 60 years, p=0.008) with such loss being detected in 16.2% and 23.4% of women <50 years old and ≥50 years old, respectively. Garg et al [28] in New York and Matthews et al [31] in Alabama report abnormal MMR IHC in 30% and 34% amongst their <50 year-old patients with EC. However, we documented only 16.2% abnormal MMR IHC in women of this age, a proportion closer to the 19.6% documented by Grzankowski et al amongst 158 women with EC in Hawaii [32]. Although these differences could be entirely explained by the much larger number of cases included in the current study, bona fide biological factors such as ethnicity and the body weight distribution of the population itself could have played a role. Importantly, women with MLH1 and/or PMS2 abnormalities were noted to be older, while women with MSH2 and/or MSH6 abnormalities were younger, when compared to women with retained MMR expression. Moreover, patients with absent MLH1 and/or PMS2 had a median age of 63 while those with absent MSH2 and/or MSH6 had a median age of 56. This is most likely due to the high prevalence of age-related hypermethylation of the MLH1 promoter [33–34] in our MLH1 and/or PMS2 deficient cohort. If a predictably small number [12] of germ line mutations present in this group were extracted, the differences seen could theoretically be even more striking.
Body mass and MMR associations are of special interest. In women with MMR germ line mutations, estrogen and obesity may be less crucial drivers in the initiation of EC, as deficient MMR activity would account for the accumulation of mutations throughout the genome [11,35–36]. In fact, EC is diagnosed at an overall significantly earlier age in LS patients, including a large proportion of premenopausal women in whom progesterone provides an estrogen balance [35,37–38]. Smaller published EC series in premenopausal women support this view. Lu et al compared nine EC patients with deleterious MMR mutations and abnormal MMR IHC to 91 EC patients without deleterious MMR mutations [39]. Mean BMI was significantly higher in the latter (35 kg/m2 vs 29.2 kg/m2) [39]. Similar findings were subsequently reported by Matthews et al [31] and Grzankowski et al [32]. When analyzing all 1049 women included in the present study we determined that obese patients had a lower overall MMR protein loss. However, when age and the specific MMR protein absent were taken into account, statistically significant BMI differences remained only in women <50 years with MSH2 and/or MSH6 loss versus the <50 year control group. Specifically, women of this age with intact MSH2 and MSH6 had a median BMI of over 39 kg/m2, versus 26.4 kg/m2 when MSH2 and/or MSH6 were absent. Women with Lynch syndrome presenting with EC before the age of 50 years and demonstrating IHC loss of MSH2 and MSH6 likely account for this large BMI difference. In fact, two out of these eleven MSH2 or MSH6 deficient women <50 years old subsequently demonstrated deleterious mutations (one in MSH2, one in MSH6, data not shown). Other women in this group have not been tested. Our group has discussed relevant challenges and opportunities on genetic counseling of women with EC and possible LS elsewhere [40].
Conversely, BMI did not differ in either age category in women with and without IHC loss of MLH1 and/or PMS2, probably reflecting the lesser contribution of mutations of the corresponding genes to the LS EC pool [41]. As previously mentioned, hypermethylation of the MLH1 promoter likely accounts for the majority of these cases, explaining the insignificant BMI relationships in this patient group. The single value trending toward significance in the group, the difference in BMI in women 50 years of age or older who retained or lost PMS2 (p=0.026), cannot be considered statistically significant due to the large number of comparisons we analyzed.
In vitro studies by Miyamoto et al showed that the MMR system is sensitive to estrogen in non-neoplastic endometrial glandular cells and in the estrogen receptor-positive Ishikawa endometrial cancer cell line. MLH1 and MSH2 mRNA and protein levels increased in a dose dependent manner when exposed to estradiol including physiologic range concentrations [7]. MMR activity was also upregulated [7]. Furthermore, Hamid et al [42] and Irving et al [43] showed increased expression of MSH2 and MLH1 respectively during the proliferative phase of the menstrual cycle. Hence, MMR expression in our group of EC could correlate with BMI as it relates to estrogen levels. However, we documented no statistically significant BMI differences between women with intact MMR expression and women <50 years old with loss of MLH1 and/or PMS2 or women ≥ 50 years old with any MMR loss. Therefore our data does not lend support to an estrogen role in the maintenance of the MMR system in the majority of women with EC. However, important limitations are of note. First, BMI is only an indirect indicator of the estrogenic milieu and our study does not account for potentially significant confounders such as diet, supplements, drugs and comorbidities. Secondly, as was expected, our cohort’s BMI distribution was skewed by most patients falling within the obese category (BMI ≥ 30 kg/m2). Hence, women with lower BMI and presumably lower estrogen were clearly underrepresented. Additionally, small cell counts for some of the categories precluded reliably modeling each protein absence with the covariates of interest, thus posing statistical limitations to our study.
In keeping with the previous report by Backes et al [30], who found no differences in tumor histology among patients who lost or retained MMR expression, we found no significant differences in MMR expression between tumor types: 21.3% of type 1 tumors and 26.4% of type 2 tumors showed MMR protein abnormalities (p=0.097). Matthews et al reported similar findings amongst sixty-one patients [31]. However, in their study of 473 EC, McCourt et al reported a higher frequency of MSI in type 1 than in type 2 tumors [6]. While raising awareness of the disproportionate number of uncommon dedifferentiated carcinomas Garg et al also reported most of their EC with MMR abnormalities in women <50 years old to be of endometrioid subtype FIGO grades 1 and 2 [28]. While endometrioid carcinomas are still reported as the most common histologic type of EC overall, other groups have reported a greater proportion of non-endometrioid tumors in LS when compared to sporadic EC [32,37,44].
In summary, we report MMR IHC clinicopathological associations in the largest single-institution series in the literature. The work is based on universal screening for LS of 1049 consecutive hysterectomy specimens using IHC for MSH2, MSH6, MLH1 and PMS2. Loss of MMR expression was not associated with tumor grade or pathologic stage. Likewise, there were no MMR IHC associations with BMI in women >50 years old or in women <50 years old with loss of MLH1 or PMS2. Women with MSH2 or MSH6 expression abnormalities, however, had a significantly lower BMI, potentially reflecting an increased prevalence of LS cases in this group.
Footnotes
Disclosure: The authors report no conflicts of interest or disclosures associated with this work.
References
- 1.Allen NE, Key TJ, Dossus L, Rinaldi S, Cust A, Lukanova A, et al. Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC) Endocr Relat Cancer. 2008;15(2):485–497. doi: 10.1677/ERC-07-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjorge T, Stocks T, Lukanova A, Tretli S, Selmer R, Manjer J, et al. Metabolic syndrome and endometrial carcinoma. Am J Epidemiol. 2010;171(8):892–902. doi: 10.1093/aje/kwq006. [DOI] [PubMed] [Google Scholar]
- 3.Reeves KW, Carter GC, Rodabough RJ, Lane D, McNeeley SG, Stefanick ML, et al. Obesity in relation to endometrial cancer risk and disease characteristics in the Women's Health Initiative. Gynecol Oncol. 2011;121:376–382. doi: 10.1016/j.ygyno.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polednak AP. Estimating the number of US incident cancers attributable to obesity and the impact on temporal trends in incidence rates for obesity-relatd cancers. Cancer Detect Prev. 2008;32(3):190–199. doi: 10.1016/j.cdp.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Dossus L, Rinaldi H, Becker S, Lukanova A, Tjonneland A, Olsen A. Obesity, inflammatory markers, and endometrial cancer risk: a prospective case-control study. Endocr Relat Cancer. 2010;17(4):1007–1019. doi: 10.1677/ERC-10-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCourt CK, Mutch DG, Gibb RK, Rader JS, Goodfellow PJ, Trinkaus K, et al. Body mass index: relationship to clinical, pathologic and features of microsatellite instability in endometrial cancer. Gynecol Oncol. 2007;104:535–539. doi: 10.1016/j.ygyno.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Miyamoto T, Shiozawa T, Kashima H, Feng YZ, Suzuki A, Kurai M, et al. Estrogen up-regulates mismatch repair activity in normal and malignant endometrial glandular cells. Endocrinol. 2006;147:4863–4870. doi: 10.1210/en.2006-0632. [DOI] [PubMed] [Google Scholar]
- 8.Li GM. Mechanisms and functions of DNA mismatch repair. Cell Res. 2008;18:85–98. doi: 10.1038/cr.2007.115. [DOI] [PubMed] [Google Scholar]
- 9.Risinger JI, Berchuck A, Kohler MF, Watson P, Lynch HT, Boyd J. Genetic instability of microsatellites in endometrial carcinoma. Cancer Res. 1993;53(21):5100–5103. [PubMed] [Google Scholar]
- 10.Peterson LM, Kipp BR, Halling KC, Kerr SE, Smith DI, Distad TJ, et al. Molecular characterization of endometrial cancer: a correlative study assessing microsatellite instability, MLH1 hypermethylation, DNA mismatch repair protein expression, and PTEN, PIK3CA, KRAS, and BRAF mutation analysis. Int J Gynecol Pathol. 2012;31(3):195–205. doi: 10.1097/PGP.0b013e318231fc51. [DOI] [PubMed] [Google Scholar]
- 11.Kunkel TA, Erie DA. DNA mismatch repair. Annu Rev Biochem. 2005;74:681–710. doi: 10.1146/annurev.biochem.74.082803.133243. [DOI] [PubMed] [Google Scholar]
- 12.Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. J Molec Diagn. 2008;10(4):293–300. doi: 10.2353/jmoldx.2008.080031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esteller M, Levine R, Baylin SB, Ellenson LH, Herman JG. MLH1 promoter hypermethylation is associated with the microsatellite instability phenotype in sporadic endometrial carcinomas. Oncogene. 1998;17(18):2413–2417. doi: 10.1038/sj.onc.1202178. [DOI] [PubMed] [Google Scholar]
- 14.Simpkins SB, Bocker T, Swisher EM, Mutch DG, Gersell DJ, Kovatich AJ, et al. MLH1 promoter methylation and gene silencing is the primary cause of microsatellite instability in sporadic endometrial cancers. Hum Mol Genet. 1999;8(4):661–666. doi: 10.1093/hmg/8.4.661. [DOI] [PubMed] [Google Scholar]
- 15.Katabuchi H, van Rees B, Lambers AR, Ronnet BM, Blazes MS, Leach FS, et al. Mutations in DNA mismatch repair genes are not responsible for microsatellite instability in most sporadic endometrial carcinomas. Cancer Res. 1995;55(23):5556–5560. [PubMed] [Google Scholar]
- 16.Hampel H, Frankel W, Panescu J, Lockman J, Sotamaa K, Fix D, et al. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2006;66:7810–7817. doi: 10.1158/0008-5472.CAN-06-1114. [DOI] [PubMed] [Google Scholar]
- 17.Bonadona V, Bonaiti B, Olschwang S, Grandjouan S, Huiart L, Longy M, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305(22):2304–2310. doi: 10.1001/jama.2011.743. [DOI] [PubMed] [Google Scholar]
- 18.Munstedt K, Wagner M, Kullmer U, Hackethal A, Franke FE. Influence of body mass index on prognosis in gynecological malignancies. Cancer Causes Control. 2008;19(9):909–916. doi: 10.1007/s10552-008-9152-7. [DOI] [PubMed] [Google Scholar]
- 19.Felix AS, Weissfeld JL, Stone RA, Bowser R, Chivukula M, Edwards RP, et al. Factors associated with Type I and Type II endometrial cancer. Cancer Causes Control. 2010;21:1851–1856. doi: 10.1007/s10552-010-9612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mauland KK, Trovik J, Wik E, Raeder MB, Njolstad TS, Stefansson IM, et al. High BMI is significantly associated with positive progesterone receptor status and clinico-pathological markers for non-aggressive disease in endometrial cancer. Br J Cancer. 2011;104:921–926. doi: 10.1038/bjc.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 22.Everett E, Tamimi H, Greer B, Swisher E, Paley P, Mandel L, et al. The effect of body mass index on clinical/pathologic features, surgical morbidity, and outcome in patients with endometrial cancer. Gynecol Oncol. 2003;90(1):150–157. doi: 10.1016/s0090-8258(03)00232-4. [DOI] [PubMed] [Google Scholar]
- 23.Pavelka JC, Ben-Shachar I, Fowler JM, Ramirez NC, Copeland LJ, et al. Morbid obesity and endometrial cancer: surgical, clinical, and pathologic outcomes in surgically managed patients. Gynecol Oncol. 2004;95(3):588–592. doi: 10.1016/j.ygyno.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 24.Anderson B, Connor JP, Andrews JI, Davis CS, Buller RE, Sorosky JI, et al. Obesity and prognosis in endometrial cancer. Am J Obstet Gynecol. 1996;174:1171–1179. doi: 10.1016/s0002-9378(96)70659-2. [DOI] [PubMed] [Google Scholar]
- 25.Modica I, Soslow RA, Black D, Tornos C, Kauff N, Shia J. Utility of immunohistochemistry in predicting microsatellite instability in endometrial carcinoma. Am J Surg Pathol. 2007;31:744–751. doi: 10.1097/01.pas.0000213428.61374.06. [DOI] [PubMed] [Google Scholar]
- 26.Pino MS, Chung DC. Application of molecular diagnostics for the detection of Lynch syndrome. Expert Rev Mol Diagn. 2010;10:651–665. doi: 10.1586/erm.10.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh CS, Blum A, Walts A, Alsabeh R, Tran H, Koeffler HP, et al. Lynch syndrome among gynecologic oncology patients meeting Bethesda guidelines for screening. Gynecol Oncol. 2010;116:516–521. doi: 10.1016/j.ygyno.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garg K, Leitao MM, Jr, Kauff ND, Hansen J, Kosarin K, Shia J, et al. Selection of endometrial carcinomas for DNA mismatch repair protein immunohistochemistry using patient age and tumor morphology enhances detection of mismatch repair abnormalities. Am J Surg Pathol. 2009;33:925–933. doi: 10.1097/PAS.0b013e318197a046. [DOI] [PubMed] [Google Scholar]
- 29.Cohn DE, Pavelka JC, Frankel WL, Morrison CD, Hampel H, Copeland LJ, et al. Correlation between patient weight and defects in DNA mismatch repair: is this the link between an increased risk of previous cancer in thinner women with endometrial cancer? Int J Gynecol Cancer. 2008;18:136–140. doi: 10.1111/j.1525-1438.2007.00964.x. [DOI] [PubMed] [Google Scholar]
- 30.Backes FJ, Leon ME, Ivanov I, Suarez A, Frankel WL, Hampel H, et al. Prospective evaluation of DNA mismatch repair protein expression in primary endometrial cancer. Gynecol Oncol. 2009;114:486–490. doi: 10.1016/j.ygyno.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 31.Matthews KS, Estes JM, Conner MG, Manne U, Whitworth JM, Huh WK, et al. Lynch syndrome in women less than 50 years of age with endometrial cancer. Obstet Gynecol. 2008;111:1161–1166. doi: 10.1097/AOG.0b013e31817051d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grzankowski KS, Shimizu DM, Kimata C, Black M, Terada KY. Clinical and pathologic features of young endometrial cancer patients with loss of mismatch repair expression. Gynecol Oncol. 2012;126(3):408–412. doi: 10.1016/j.ygyno.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Yiu R, Qui H, Lee SH, Garcia-Aguilar J. Mechanisms of microsatellite instability in colorectal cancer patients in different age groups. Dis Colon Rectum. 2005;48:2061–2069. doi: 10.1007/s10350-005-0171-0. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa H, Nuovo GJ, Zervos EE, Martin EW, Jr, Salovaara R, Aaltonen LA, de la Chapelle A. Age-related hypermethylation of the 5’ region of MLH1 in normal colonic mucosa is associated with microsatellite-unstable colorectal cancer. Cancer Res. 2001;61:6991–6995. [PubMed] [Google Scholar]
- 35.Meyer LA, Broaddus RR, Lu KH. Endometrial cancer and Lynch syndrome: clinical and pathologic considerations. Cancer Control. 2009;16(1):14–22. doi: 10.1177/107327480901600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Backes FJ, Cohn DE. Lynch syndrome. Clin Obst and Gynecol. 2011;54(2):199–214. doi: 10.1097/GRF.0b013e3182185a41. [DOI] [PubMed] [Google Scholar]
- 37.Broaddus RR, Lynch HT, Chen LM, Daniels MS, Conrad P, Munsell MF, et al. Pathologic features of endometrial carcinoma associated with HNPCC: a comparison with sporadic endometrial carcinoma. Cancer. 2006;106:87–94. doi: 10.1002/cncr.21560. [DOI] [PubMed] [Google Scholar]
- 38.Daniels MS. Genetic testing by cancer site, uterus. Cancer J. 2012;18(4):338–342. doi: 10.1097/PPO.0b013e3182610cc2. [DOI] [PubMed] [Google Scholar]
- 39.Lu KH, Schorge JO, Rodabaugh KJ, Daniels MS, Sun CC, Soliman PT, et al. Prospective determination of prevalence of Lynch Syndrome in young women with endometrial cancer. J Clin Oncol. 2007;25(33):5158–5164. doi: 10.1200/JCO.2007.10.8597. [DOI] [PubMed] [Google Scholar]
- 40.Backes FJ, Mitchell E, Hampel H, Cohn DE. Endometrial cancer patients and compliance with genetic counseling: room for improvement. Gynecol.Oncol. 2011;123:532–536. doi: 10.1016/j.ygyno.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Pollak M. Insulin and insulin-like growth factor signaling in neoplasia. Nat Rev Cancer. 2011;8(12):915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 42.Hamid AA, Mandai M, Konishi I, Nabu K, Tsuruta Y, Kusakari T, et al. Cyclical change of hMSH2 protein expression in normal endometrium during the menstrual cycle and its overexpression in endometrial hyperplasia and sporadic endometrial carcinoma. Cancer. 2002;94(4):997–1005. [PubMed] [Google Scholar]
- 43.Irving JA, Dupuis B, Wang L, Gilks CB. Cyclical expression of the DNA mismatch repair enzyme hMLH1 in normal endometrium. Fertil Steril. 2002;78(1):195–196. doi: 10.1016/s0015-0282(02)03159-x. [DOI] [PubMed] [Google Scholar]
- 44.Carcangiu ML, Radice P, Casalini P, Bertario L, Merola M, Sala P. Lynch syndrome--related endometrial carcinomas show a high frequency of nonendometrioid types and of high FIGO grade endometrioid types. Int J Surg Pathol. 2010;18:21–26. doi: 10.1177/1066896909332117. [DOI] [PubMed] [Google Scholar]


