Abstract
Breast cancer is the most common type of malignancy in females. Advances in systemic therapies and radiotherapy (RT) provided long survival rates in breast cancer patients. RT has a major role in the management of breast cancer. During the past 15 years several developments took place in the field of imaging and irradiation techniques, intensity modulated RT, hypofractionation and partial-breast irradiation. Currently, improvements in the RT technology allow us a subsequent decrease in the treatment-related complications such as fibrosis and long-term cardiac toxicity while improving the loco-regional control rates and cosmetic results. Thus, it is crucial that modern radiotherapy techniques should be carried out with maximum care and efficiency. Several randomized trials provided evidence for the feasibility of modern radiotherapy techniques in the management of breast cancer. However, the role of modern radiotherapy techniques in the management of breast cancer will continue to be defined by the mature results of randomized trials. Current review will provide an up-to-date evidence based data on the role of modern radiotherapy techniques in the management of breast cancer.
Keywords: Breast cancer, Radiotherapy, Intensity modulated radiotherapy, Partial breast irradiation, Hypofractionation
Core tip: Several randomized trials provided evidence for the feasibility of modern radiotherapy techniques in the management of breast cancer. Current review will provide an up-to-date evidence based data on the role of modern radiotherapy techniques in the management of breast cancer.
INTRODUCTION
Radiotherapy (RT) has a major role in the management of breast cancer for many years. It significantly reduces the risk of loco-regional recurrences after surgery by at least 70%[1]. RT has been shown to improve overall survival both for early stage breast cancer after breast-conserving surgery (BCS) and locally advanced disease after mastectomy[1]. However, its use is usually limited by late toxicity. In patients with a long life expectancy, only modern RT techniques could obtain survival benefit that is mostly dependent on the radiation dose to the cardiac structures[1-4].
During the past 15 years, several developments took place such as imaging and irradiation techniques, hypofractionation and partial-breast irradiation (PBI). Improvements in the RT technology now frequently allow us a subsequent decrease in treatment-related complications such as fibrosis and long-term cardiac toxicity while improving the loco-regional control rates and cosmetic results[5-7]. Computed tomography (CT) simulators, modern-day linear accelerators, three-dimensional (3D) planning techniques and treatment verification modalities provides improved targeting and smaller irradiated volumes of normal tissue.
Depending on the type of surgery and pathology reports, traditionally, conventional two-dimensional (2D) beams were used for whole-breast or chest wall irradiation[8]. The first important challenging step in the RT technique came with the introduction of the CT-based treatment planning and 3D conformal RT (3DCRT) that provides us precise target volume definition, dose distribution calculation, and virtual simulation. Optimal shielding of organs at risk (OARs), including the heart, lungs, brachial plexus, esophagus, trachea, thyroid, and spinal cord decreased normal tissue exposure. Additionally, more homogeneous dose distribution in the clinical target volume could be obtained.
INTENSITY MODULATED RADIOTHERAPY
Intensity modulated RT (IMRT) is an advanced form of 3DCRT that became increasingly available for breast cancer. Several important studies has been carried out on the use of IMRT for breast cancer patients requiring complex breast treatments[9]. Patients with larger breasts are more likely to have dose inhomogeneities and most likely to benefit from IMRT. It can also be the best alternative for left-sided breast cancers to decrease cardiac dose, re-irradiation, contralateral breast irradiation, PBI, and deeply seated tumor bed irradiation.
The modulation of beam intensities could be determined by allowing sculpting the dose to fit a patient’s anatomy. The major goal for IMRT technique is providing more homogenous dose distribution throughout the breast and concave structures such as the chest wall[10,11]. This technology also allows better conformality of dose to the target and better sparing of OARs compared to non-IMRT plans[7,10,12-17]. However it has some drawbacks, including decrease in surface build up dose which could adversely affect local control and increase risk for secondary malignancies[18-20].
For each individual case, adequate coverage of the primary tumor site and most of the breast can be achieved by changing the gantry angle, the collimator angle, or shaping [with small cardiac blocks or multileaf collimator (MLC) leaves] the borders of the medial and/or lateral tangential fields while the heart and lung can be excluded from the high dose region at the same time. The normal tissue anatomy, the location of the primary tumor bed, and the contour of the breast should be taken into consideration when applying treatment field modifications for each individual patient. Forward planning by using the “field-in-field” technique provides excellent dose homogeneity in the irradiated areas. Thus, it is the most widely used technique in breast IMRT[10,21] (Figure 1). There are also other methods, including forward-planned step-and-shoot breast IMRT, and an inversely planned breast IMRT technique, which can all improve dose homogeneity (Figure 2).
Figure 1.
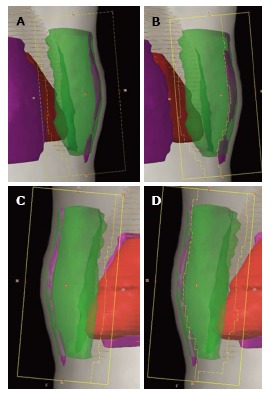
BEV shaped forward-planned intensity modulated radiotherapy (IMRT) (field-in field technique). Open tangential fields (A and C). DRR showing the multileaf collimator segments closing the volumes receiving ≥ 110% of the prescribed dose (B and D).
Figure 2.
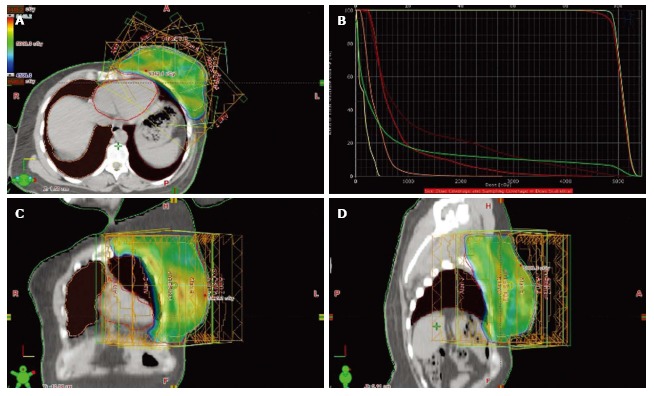
Treatment fields and dose distribution of inverse-planned intensity modulated radiotherapy: Axial section (A), dose-volume histogram (B), coronal section (C) and sagittal section (D).
IMRT planning should be performed based on 3D visualization of contours delineated on planning CT images. Proper patient positioning, immobilization, target localization, and management of breathing-related motion are essential for IMRT due to sharp dose gradient changes[22]. Also, sophisticated technical resources and longer period of time are required both for planning and quality assurance tests. In addition, radiation delivery turns out to be more complex. It requires specialized software to automate the process to reduce treatment time and the risk of delivery error. As a result of longer beam-on time, whole body and contralateral breast doses may increase.
Image-guided RT (IGRT) is required to precise localization of both target and normal tissues during planning and treatment procedure. The main advantage of IGRT is that it allows more accurate targeting in breast cancer by providing correct target volume delineation, obtaining simulation images, and set-up correction using images with the patient in the treatment position immediately prior to or during the treatment. A variety of imaging methods are used: (1) gantry-mounted systems [MV-electronic portal imaging device (EPID), kV/MV cone beam CT, MV systems-tomotherapy]; (2) room-mounted systems; and (3) non-ionizing systems (ultrasound, video-based systems)[23]. Besides, breath-holding techniques (using “active breathing control” devices or unassisted) and respiratory gating can effectively limit motion and decrease the dose to the heart and lungs, especially in cases in which the tumor bed is very close to the heart[24-26].
There are several IMRT techniques; dynamic or static MLC based IMRT, arc therapy, tomotherapy, and topotherapy[27,28]. Radiation is delivered to the patient as gantry rotates continuously around the patient in intensity modulated arc therapy. In tomotherapy, there is a helical radiation delivery that continues during treatment couch movement and binary MLC motion creates fluency. Topotherapy is performed with static gantry while as the patient translates through the treatment field instead of rotational delivery[28].
TREATMENT OUTCOMES AND TOXICITY AFTER IMRT
IMRT has changed our RT practice and it is used for palliative and curative indications throughout several tumor types. The highest level of evidence by using IMRT exists particularly for breast and nasopharyngeal carcinomas[29]. Although the clinical outcome of IMRT in breast cancers came mainly from retrospective studies, it has been assessed in three prospective randomized studies[30-33] (Table 1). The primary aim of these studies was to investigate treatment-induced toxicity and patients’ quality of life (QOL). They showed that IMRT increased the dose homogeneity and decreased the frequency and severity of toxicity in early-stage breast cancer after BCS. However, larger sample size and longer follow-up is required to see long-term clinical outcomes and evaluate for late deleterious effects.
Table 1.
Randomized phase III trials of intensity modulated radiation therapy for breast cancer
| Ref. |
N |
FU (mo) | End-points | IMRT | Non IMRT | P value | Outcomes reported | |
| IMRT | 2D RT | |||||||
| Donovan et al[32], 2007 | 150 | 156 | N/A | Distribution of any change in breast appearance between the presence or absence of doses > 105% | OR, 2.6; 95%CI: 1.1-6 | 0.03 | DC, LAE, QOL | |
| Photographic assessment of any change in breast appearance at 1, 2 and 5 yr | OR, 1.7; 95%CI; 1.2-2.5 | 0.008 | ||||||
| Physician assessment of breast induration at 5-yr, % | ||||||||
| Centre of the breast | 21 | 32 | 0.02 | |||||
| Pectoral fold | 22 | 29 | 0.006 | |||||
| İnframammary fold | 17 | 24 | 0.009 | |||||
| Boost site | 37 | 61 | < 0.001 | |||||
| Pignol et al[30], 2008 | 170 | 161 | N/A | Acute skin toxicity (Gr 3-4), % | 27.1 | 36.7 | 0.06 | DC, AAE, QOL |
| Moist desquamation (all breast), % | 31.2 | 47.8 | 0.002 | |||||
| Moist desquamation (inframammary crease), % | 26.5 | 43.5 | 0.001 | |||||
| Pain (Gr 2-4), % | ||||||||
| Barnett et al[31], 2012 | 411 | 404 | 241 | Photographic assessment of breast shrinkage at 2 yr | OR, 1.51; 95%CI: 0.83-1.58 | 0.41 | AAE, LAE, QOL | |
| Acute toxicity (Gr ≥ 2) | OR, 1.00; 95%CI: 0.76-1.34 | 0.97 | ||||||
| Telangiectasia | OR, 1.68; 95%CI: 1.13-2.50 | 0.009 | ||||||
| Moderate or poor overall cosmesis (good baseline surgical cosmesis) | OR, 0.63; 95%CI: 0.39-1.03 | 0.061 | ||||||
| Patient reported | ||||||||
| Breast pain, % | 46.7 | 37.3 | 0.98 | |||||
| Oversensitivity, % | 47.1 | 35 | 0.43 | |||||
| Mukesh et al[33], 2013 | 228 | 237 | 601 | Photographic assessment of breast shrinkage at 5 yr | OR, 0.79; 95%CI: 0.55-1.14 | 0.21 | LAE, TRO | |
| Teleangiectasia | OR, 0.58; 95%CI: 0.36-0.92 | 0.021 | ||||||
| Overall cosmesis | OR, 0.68; 95%CI: 0.48-0.96 | 0.027 | ||||||
| Breast edema | OR, 0.74; 95%CI: 0.48-1.15 | 0.18 | ||||||
| Tumor bed induration | OR, 0.76; 95%CI: 0.54-1.06 | 0.11 | ||||||
| Pigmentation | OR, 0.80; 95%CI: 0.46-1.38 | 0.42 | ||||||
| 5-yr overall survival, % | 91.7 | 92.5 | 0.88 | |||||
| 5-yr locoregional recurrence, % | 1.35 | 2.56 | 0.36 | |||||
Minimum average follow-up. IMRT: Intensity modulated radiotherapy; 2D RT: Two dimensional radiotherapy; N: Number of patients; FU: Follow-up; DC: Dosimetry characteristics; AAE: Acute adverse effects; LAE: Late adverse effects; TRO: Treatment related outcomes; QOL: Quality of life; Gr: Grade; N/A: Not available; NS: Not significant.
There is only one randomized study of treatment efficacy comparing IMRT and non-IMRT[33]. Mukesh et al[33] reported 5-year results of 815 patients randomized to either standard wedged-based tangential fields or forward-planned IMRT. In this study, there was no statistically significant difference in 5-year loco-regional recurrence (2.56% and 1.35%) or overall survival (92.5% and 91.7%) rates. Additionally, there has been two reported trial designed to investigate breast cancer-related outcomes, the retrospective cohort study (n = 240) by McDonald et al[34] and the prospective cohort study (n = 332) by Morganti et al[35]. Findings did not show a statistically significant differences between IMRT and non-IMRT techniques for breast cancer related outcomes like survival, disease-specific survival and freedom from contralateral breast cancer recurrence.
Conventional RT causes acute skin toxicity, specifically moist desquamation in 30%-50% of patients[36,37]. An inhomogeneous dose distribution and consequential hot spots of whole breast irradiation increases the rate of acute and late skin toxicity including erythema, edema, desquamation, pain, telengiectasia and fibrosis, and effects negatively cosmetic results and patient’s QOL[32,38,39]. Large breast size and dose inhomogeneities > 10% associated with a poorer clinical outcomes[40-42]. IMRT significantly improved dose homogeneity with a median of 0.1% of the treatment volume receiving ≥ 110% of the prescribed dose vs 10% with conventional wedge-based breast RT[10,11]. Therefore, IMRT has been established as an effective treatment for adjuvant RT after BCS with a decrease in moderate or severe skin reaction of up to 44% and better cosmetic results as compared with the standard wedged tangential field techniques[30-32,34,42,43].
There are eight studies reported on acute radiation toxicity of IMRT[30,34,35,42-44]. Vicini et al[10] reported a prospective trial of breast IMRT and demonstrated a reduction in acute skin reactions. Similarly, Freedman et al[43] reported the results of a matched-pair analysis of 131 patients treated using either breast IMRT (n = 73) or standard wedge-based RT (N = 58). This study found a significant reduction in the rate of acute desquamation using IMRT compared with the wedge-based treatment. Harsolia et al[42] reported toxicity results of 172 patients at a median follow-up of 4.7 years, and demonstrated that the use of IMRT resulted in significantly less acute ≥ Grade 2 toxicity for dermatitis (41% vs 85%, P < 0.001), breast edema (1% vs 28%, P < 0.001), and hyperpigmentation (5% vs 50%, P < 0.001), compared with patients treated with conventional wedge-based plans (2D RT)[42]. In patients with larger breasts (≥ 1600 cm3, n = 64), use of IMRT was associated with a statistically significant decrease in ≥ Grade 2 acute breast edema (0% vs 36%, P < 0.001), and hyperpigmentation (3% vs 41%, P = 0.001), and chronic long-term edema (3% vs 30%, P = 0.007) compared with conventional wedge-based plans. McDonald et al[34] reported cohort analysis on long-term outcomes of IMRT (n = 121) with conventional RT (3D RT) (n = 124)[34]. Median dose to the whole breast was 50 Gy, and median total dose to the tumor cavity was 60 Gy for both IMRT and conventional RT patient groups. IMRT resulted in reduced Grade 2 or 3 dermatitis compared with conventional RT (39% vs 52%, P = 0.047) at median 6.3 years of follow-up. Pignol et al[30] reported the first multicenter randomized trial demonstrating a successful reduction an acute radiation skin toxicity using IMRT[30]. Three hundred and fifty-eight patients were randomized to forward-planned IMRT or standard conventional wedge technique after complete excision of an early-stage breast cancer. IMRT significantly reduced the occurrence of moist desquamation anywhere in the breast and in the inframammary fold, with an absolute reduction of 16.6% (P = 0.002) and 17% (P = 0.001), respectively. Smaller breast size (P < 0.001) and use of IMRT (P = 0.003) strongly associated with a decreased risk of moist desquamation. Despite no statistically significant difference in the QOL or pain between the two treatment arms, there was a highly significant correlation between the development of moist desquamation and grade 2 to 3 pain score (P < 0.0001), a decrease in the global health status scale (P = 0.0019), and an increase in the breast symptoms scale (P = 0.0028). The retrospective cohort study reported by Morganti et al[35] found that all skin-related acute toxicity reduced when standard 3D wedges were compared to simplified step and shoot IMRT technique (P < 0.05), despite with a lower total dose in the IMRT group[35]. Only the retrospective cohort study by Freedman et al[44] reported on the proportion of treatment time with acute dermatitis, finding a significant benefit for IMRT compared with conventional wedged-based plans (18% vs 71%, P < 0.0001)[44]. In that trial, 405 patients treated with conventional RT and 399 patients treated with IMRT. A subgroup analysis demonstrated that the time spent with radiation induced Grade 2-3 dermatitis was decreased in IMRT for all patients regardless of breast size (all P < 0.05). Barnett et al[31] reported the randomized trial demonstrating no significant differences were found in the incidence of any acute toxicity and development of any photographically assessed breast shrinkage between the IMRT or standard RT groups [odds ratio (OR), 1.51; 95% confidence interval (CI): 0.83-1.58; P = 0.41][31].
The decrease in acute toxicity achieved with IMRT translates into a decrease in late toxicity. There are two randomized trials showing a beneficial effect of forward-planned IMRT on late toxicity[31-33]. The first prospective randomized clinical trial testing the role of forward-planned IMRT in terms of 5 year outcome for adverse effects was reported by Donovan et al[32]. They randomized 306 patients after BCS to standard 2D wedge-based RT or to IMRT (either IMRT with “step-and-shoot” fields or a physical 3D compensator). All were treated with a dose of 50 Gy in 25 fractions followed by a 10-Gy boost with electrons. Forward-planned IMRT significantly decreased dose inhomogeneity (≥ 105% of the prescribed dose) comparing standard 2D wedge-based plans (19% vs 92%). Results of treatment were evaluated using photographic assessment performed before RT and at 1, 2, and 5 years follow-up. The standard arm patients were 1.7 times more likely to have a change in breast appearance than the IMRT arm patients after adjustment for the year of photographic assessment (95%CI: 1.2-2.5, P = 0.008). However, there were no significant differences in outcome between randomized groups in any of the self-assessed parameters, including breast pain, discomfort, hardness, body image, or QOL as measured by EORTC QLQ C30 and BR23 modules. The highest levels of dose inhomogeneity in the 2D RT were seen in the upper and lower third of the breast. This suggested that dose inhomogeneity in the breast increases late adverse events. The second one designed to investigate the effect of forward-planned IMRT on the incidence of late radiation toxicity reported by Barnett et al[31] from Cambridge. In their study, 815 patients with early stage breast cancer were randomized to either standard wedged-based tangential fields or forward-planned IMRT. Patients were randomized if ≥ 2 cm3 receiving > 107% of prescribed dose. In this study, breast dosimetry was significantly improved with the forward-planned IMRT. All patients were treated to a dose of 40 Gy in 15 fractions. The patients in the standard RT group were more likely to develop telangiectasia than those in the IMRT group at early follow-up of only 2 years after RT completion (OR, 1.68; 95%CI: 1.13-2.40; P = 0.009). In patients who had good baseline surgical cosmesis, those randomized to IMRT were less likely to deteriorate to a moderate or poor overall cosmesis than those in the standard RT group (OR, 0.63; 95% CI: 0.39-1.03, P = 0.061). Recently, Mukesh et al[33] reported 5-year results of this study[33]. On univariate analysis, patients receiving IMRT had superior overall cosmesis (OR, 0.68; 95%CI: 0.48 to 0.96; P = 0.027) and reduced skin telangiectasia (OR, 0.58; 95%CI: 0.36 to 0.92; P = 0.021) as compared with patients receiving standard RT arm. However, no significant difference was observed in the development of photographically assessed breast shrinkage or clinically assessed breast edema, tumor bed induration, or pigmentation. On multivariate analysis, use of IMRT was significantly associated with improved overall cosmesis (OR, 0.65; 95%CI: 0.44 to 0.98; P = 0.038) and decreased risk of skin telangiectasia (OR, 0.57; 95%CI; 0.34 to 0.95; P = 0.031). Large breast volume, poorer baseline surgical cosmesis, and tumor bed boost were also associated with suboptimal overall cosmesis on multivariate analysis. Patients with moderate to poor baseline surgical cosmesis more frequently developed suboptimal final cosmesis, tumor bed induration, and photographically assessed breast shrinkage at 5 years in the study.
Late toxicity results have been reported in only two retrospective cohort studies and one prospective study[34,42,45]. The study by Harsolia et al[42] (n = 172) showed a significant difference between IMRT and conventional wedged-based plans in favor of IMRT for ≥ Grade 2 breast edema (1% vs 25%, P < 0.001), with no differences in hyperpigmentation, fat necrosis, induration/fibrosis or overall cosmetic score[42]. The study by McDonald et al[34] (n = 240) found a trend towards a reduction in lymphedema rates (0% vs 4%, P = 0.06), with no differences in the reported occurrence of radiation pneumonitis, fat necrosis or second malignancies[34]. Freedman et al[45] reported the 5-year results of a phase II study of IMRT. Seventy-five patients were treated with simultaneous integrated boost (SIB)-IMRT; the whole breast received 2.25 Gy per fraction for a total of 45 Gy and the tumor bed received 2.8 Gy per fraction for a total of 56 Gy in 20 treatments over four weeks. After a median follow-up of 69 mo, the 5-year rate of local recurrence was 2.7%. There were no significant differences over time in patient-reported cosmesis, pain and arm function and physician-reported cosmesis through the 5-year period of the study.
ACCELERATED PARTIAL BREAST IRRADIATION
Multiple prospective randomized trials have demonstrated that BCS followed by whole breast RT (WBRT) as a standard treatment approach in early stage breast cancer[46-51]. A large meta-analysis showed that a reduction in ipsilateral breast tumor recurrence (IBTR) translated into a survival benefit of 5.4% after 15 years in early stage breast cancer patients treated with BCS[1]. This treatment allows preservation of the breast with equivalent survival to mastectomy. In WBRT, entire breast is treated with a standard fractionation, which consists of 45-50 Gy, daily Monday to Friday over a 5- to 6-wk period. Despite being well tolerated and good cosmetic results, some patients does not receive WBRT due to long treatment duration, limited geographical access, and cost of the treatment[52]. To address some of these issues accelerated partial breast irradiation (APBI) has gained popularity in selected patients. However, more data are needed about the use of different methods of APBI defining the optimal patient selection criteria, technique, dose and fractionation, side effects, and long-term outcomes.
APBI is a reasonable alternative to WBRT in patients with BCS who have more favorable tumor characteristics. Published studies indicated that 70%-90% of IBTRs after breast conservation therapy occurred at or in close proximity to the lumpectomy cavity[49,50,53]. Multiple phase II and several phase III trials confirmed that APBI may offer equivalent local control to WBRT with shortening conventional treatment duration from 5 to 6 wk to a single fraction or few days (1-3 wk). In APBI, only the lumpectomy cavity treated with a limited margin for potential microscopic spread. Potential advantages of APBI include shorter treatment interval, improved cosmesis due to the decreased volume of breast tissue treated, decreased heart and lung volume, and reduced cost compared with standard fractionation[54]. Additionally, decreased volume allows acceleration and hypofractionation which might be has some theoretical radiobiological advantages.
A consensus group by American Society for Radiation Oncology (ASTRO) and the European GEC-ESTRO Cancer Working Group developed guidelines for patient selection to APBI on the basis of a variety of clinical and pathologic factors (Table 2)[55,56]. These guidelines categorized patients into three groups: Low-risk or suitable, intermediate-risk or cautionary group, and high risk or unsuitable group. ASTRO defined suitable group which was include patients age ≥ 60 years, without BRCA mutation, T1 (≤ 2), > 2 mm surgical margins, no lymphovascular space invasion, ER positive, unicentric, invasive ductal or other favorable histology, no extensive intraductal component, and lymph node negative optimally as part of a clinical trial. Application of APBI to intermediate or cautionary group is considered acceptable only in the context of prospective clinical trials[57]. It is compatible with GEC-ESTRO recommendations except that tumor size (T1-2; ≤ 3 cm) and age (≥ 50 years).
Table 2.
American Society for Radiation Oncology and GEC-ESTRO recommendations on patient selection criteria for Accelerated Partial Breast Irradiation
| ASTRO | GEC-ESTRO | ASTRO | GEC-ESTRO | ASTRO | GEC-ESTRO | |
| Factor | Suitable | Low-risk | Cautionary | Intermediate-risk | Unsuitable | High-risk |
| Patient factors | ||||||
| Age (yr) | ≥ 60 | > 50 | 50-59 | 40-50 | < 50 | < 40 |
| BRCA1/2 mutation | Not present | Not defined | Not present | Not defined | Present | Not defined |
| Pathologic factors | ||||||
| Tumor size (cm) | ≤ 2 | ≤ 3 | 2.1-3.0 | ≤ 3 | > 3 | > 3 |
| T stage | T1 | T1-2 | T0 or T2 | T1-2 | T3-4 | T2 (> 3 cm), T3-4 |
| Histology | IDC or other favorable subtypes | IDC, mucinous, tubular, medullary and colloid carcinoma | ILC allowed | ILC allowed | Any | Any |
| Grade | Any | Any | Any | Any | Any | Any |
| Pure DCIS | Not allowed | Not allowed | ≤ 3 cm | Allowed | > 3 cm | Any |
| EIC | Not allowed | Not allowed | ≤ 3 cm | Not allowed | > 3 cm | Allowed |
| Associated LCIS | Allowed | Allowed | Allowed | Allowed | Allowed | Allowed |
| Multicentricity | Unicentric | Unicentric | Unicentric | Unicentric | Multicentric | Multicentric |
| Multifocality | Clinically unifocal ≤ 2 cm | Unifocal | Clinically unifocal 2.1-3 cm | Multifocal (limited within 2 cm of the index lesion) | Clinically multifocal, > 3 cm | Multifocal (> 2 cm from the index lesion) |
| LVSI | No | Not allowed | Limited/focal | Not allowed | Extensive | Allowed |
| ER status | Positive | Any | Negative | Any | Any | Any |
| Surgical margins | ≥ 2 mm | ≥ 2 mm | < 2 mm | < 2 mm | Positive | Positive |
| Nodal factors | ||||||
| N stage | pN0 (i-, i+) | pN0 | pN0 (i-, i+) | pN1mi, pN1a | ≥ pN1 | pNx, ≥ pN2a |
| Nodal surgery | SN biopsy or ALND | None performed | ||||
| Neoadjuvant therapy | Not allowed | Not allowed | Not allowed | Not allowed | If used | If used |
DCIS: Ductal carcinoma in situ; EIC: Extensive intraductal component; LCIS: Lobular carcinoma in situ; ASTRO: American Society for Radiation Oncology.
Modalities for APBI include brachytherapy; interstitial brachytherapy (multi-catheter interstitial implant), intracavitary brachytherapy [one balloon catheter (MammoSite®), multiple balloon catheter (Contura®), hybrid BRT (SAVI®)], intraoperative RT (IORT); intraoperative electrons (Liac®, Mobetron®, or Novac-7®;3-10 MeV) or low energy X-rays (Intrabeam®; 50 kV), external beam RT; 3DCRT and IMRT.
Multicatheter interstitial brachytherapy
The most mature follow-up and experience of all APBI technique is multicatheter interstitial brachytherapy (MIB) which is commonly used as a boost treatment[55-58]. It is an invasive approach that multiple interstitial catheters (up to 20) are placed surrounding the tumor bed at the time of surgery or postoperatively under ultrasound guidance. The number of the catheters may vary according to the size and the shape of the target volume. After catheter placement, tumor bed plus a 1-2 cm margin is treated with LDR, HDR or PDR devices. Radioactive sources are inserted temporarily into the catheter during treatment and then removed. Most commonly used regimen is 34 Gy in 10 fractions (twice daily) over 5 d. This modality only performed at a few institutions because of the specialized training required, costly equipment, and more complex technical support needed for the procedure. It is well tolerated but dose heterogeneity within the target volume can potentially lead to fat necrosis and subcutaneous toxicity[59]. Acute complications include pain and infection. Often oral antibiotic treatment is required and rarely requires removal of the catheters.
Intracavitary brachytherapy: Intracavitary brachytherapy is the most common form of brachytherapy for APBI because of the less invasive, simple, and requires less experience. It can be applied with a single-lumen (MammoSite®) or multilumen (Contura®) balloon catheter, and elliptically shaped cluster of catheters such as SAVI®[60]. It can be inserted into the lumpectomy cavity either at the time of surgery or postoperatively using ultrasound guidance[61]. The balloon is then filled with saline, and an HDR radioactive source (commonly 192Ir) is inserted. After the balloon is inflated, it should be symmetric and conform to the cavity. Dose is usually prescribed to 1 cm from the balloon surface. The minimum distance between the balloon and the skin/chest wall should be ≥ 5 mm, with a shorter distance leading to a poorer cosmesis. Therefore, this technique may not be suitable for small breast size. The most commonly used regimen is 3.4 Gy per fraction, given twice daily, total of 34 Gy over 5 consecutive days[58]. Potential advantage of this technique is that final pathology is known. Multi-lumen balloon catheters are more suitable for irregularly shaped lumpectomy cavities[62]. The morbidity rates were significantly higher in patients treated with intracavitary brachytherapy with reported infection rates of 9.5% and seroma formation of 26.8% than IORT which were 1.3% and 12.9%, respectively[63,64]. Fat necrosis was observed less compared to interstitial brachytherapy[65,66].
External beam radiotherapy: The newest of the three major techniques with the most amounts of ongoing randomized studies is APBI with 3DCRT or IMRT. Potential advantages include noninvasiveness, knowledge of final pathology, a more homogenous dose distribution, widespread availability, less user experience, less seroma formation and infection. Additionally irregular cavities can be treated without concern for distance from the skin[55,67]. The most common regimen is 38.5 Gy in 10 fractions given twice daily over 5 d. Shortcomings are the delivering more radiation to uninvolved quadrants of the breast and critical organs compared to other forms of APBI, short follow-up, uncertainty regarding the optimal dose and fractionation, and patient set-up requirement before each fraction.
Intra-operative radiotherapy: The least prevalent technique is IORT using electrons (Liac®, Mobetron®, or Novac-7®; 3-10 MeV) or low energy X-rays (Intrabeam®; 50 kV). This technique is most commonly used in Europe and firstly used as a boost treatment. Most commonly applied after quadrantectomy and sentinel lymph node biopsy. A single fraction treatment can be delivered with either electrons (21 Gy in one fraction) or low energy photons (20 Gy in one fraction) immediately after surgery in the operating room[68]. Direct visualization of the operative bed before treatment delivery reduces the likelihood of missing the target. This modality allows shielding of the skin. The potential disadvantages include increased operating times, the lack of final pathological result before delivering the RT, technical expertise and limited availability of this technique. Long-term radiobiological and cosmetic effects of such a single high fraction dose to the breast are largely unknown; however, an acceptable toxicity is achieved based on a randomized trial and a large, nonrandomized cohort studies[63,69]. The risk of toxicity was low: 1.3% infections, 12.9% seroma formation, and 4.2% fat necrosis[63].
Accelerated partial breast irradiation trials: Multiple modern phase II studies regarding APBI have reported promising local control and excellent cosmetic results[57,70-77]. These studies showed 3%-6% of patients with 5-year local recurrence rates and 56%-99% of patients with good or excellent cosmesis. However, many Phase III studies have not been completed and primary outcome data of the largest randomized trial of WBRT vs APBI with the longest follow-up (NSABP B-39/RTOG 0413) is not yet reported. Regarding the efficacy of APBI in a higher risk population of patients, this trial including patients with > 18 years of age, DCIS, 1-3 positive lymph nodes, and ER negative tumors will provide valuable data on literature. This trial also allows comparison of the efficacies and toxicities of three most common techniques of APBI: MammoSite®, MIB and 3DCRT.
So far, only 5 randomized controlled trials have presented the final results of a completed trial[69,78-81] (Table 3). The first two published studies from the United Kingdom have important limitations including patient selection criteria and variety of techniques[78,79]. At 8-year follow-up, local recurrence was increased using APBI. However, surgical margins and axillary nodal status were not evaluated and larger tumors (< 4 cm) were included in the Christie Hospital study[78]. Similarly, in the Yorkshire Hospital trial, surgical margin status was not assessed[79]. After the publication of these trials, there has been growing interest and published studies on APBI using more strict patient selection criteria and modern radiation technique. The first of these studies was Hungarian trial compared WBRT with APBI using either HDR MIB or electrons in 258 patients with early stage breast cancer[80]. At a median follow-up of 66 mo, the 5-year local recurrence rate was 4.7% for APBI and 3.4% for WBRT arm (P = 0.50). Excellent to good cosmesis was noted in 77.6% and 62.9%, respectively (P = 0.009). There were no significant differences in disease-free or overall survival. Since another study opened with the same patients group covering GEC-ESTRO trial, this study was stopped early. The second completed randomized trial, the TARGIT-A trial (n = 2232), compared WBRT with single dose IORT with or without boost after BCS[69]. With a median follow-up of 2 years, the estimated 4-year local recurrence rate was 1.2% for IORT group and 0.95% for WBRT group (P = 0.41). The incidence of major toxicities were 3.9% and 3.3%, respectively (P = 0.44). Fourteen percent of patients received WBRT in addition to IORT according to the final pathology report. Five-year results of this trial recently published[81]. Supplemental WBRT after IORT was applied in 15.2% of patients who received IORT in the prepathology stratum. The 5-year risk for local recurrence in the conserved breast was 3.3% for IORT vs 1.3% for WBRT (P = 0.042). Overall, breast cancer mortality was similar between two groups (2.6% vs 1.9%, P = 0.56) but there were significantly fewer non-breast cancer deaths with IORT (1.4% vs 3.5%, P = 0.0086). Grade 3-4 skin complications were significantly reduced with IORT (P = 0.029). The last randomized trial was reported by Veronesi et al[82] in 2013. They randomized 1305 patients after BCS to WBRT or IORT with electrons (21 Gy). After a median follow-up of 5.8 years, 35 patients in the IORT group and four patients in the WBRT group had had an IBTR (P < 0.0001). Five-year overall survival did not differ between the groups (96.8% in the IORT vs 96.9% in the WBRT, P = 0.59). Skin complications were significantly reduced with IORT (P = 0.0002). However, longer follow-up is required before routinely adopting these modern radiation techniques into clinical practice.
Table 3.
Prospective randomized phase III trials of Accelerated Partial Breast Irradiation
| Institution/trial | Number of patients | Inclusion criteria | Control arm | Experimental arm |
| National Institute of Oncology, Budapest, Hungary[80] | 258 | Wide local excision, > 40 yr, Tm ≤ 20 mm, Invasive ductal carcinoma (non-lobular), Node negative, Margin negative | WBRT (50 Gy in 25 fx) | (1) MIB (36.4 Gy in 7 fx) (2)Electrons (50 Gy in 25 fx) |
| European Institute of Oncology ELIOT[63,82] | 1305 | Quadrantectomy, ≥ 48 yr, Tm ≤ 2.5 cm, Invasive carcinoma, Node negative | WBRT (50 Gy in 25 fx) ± 10 Gy boost | IORT (21 Gy in 1 fx, electrons up to 9 MeV) |
| TARGIT-A[69,81] | 3451 | Lumpectomy, ≥ 45 yr, Invasive ductal carcinoma (non-lobular), Node negative | WBRT 40–56 Gy ± 10–16 Gy boost | IORT (20 Gy in 1 fx, low-energy X-rays) |
Tm: Tumor; WBRT: Whole breast radiotherapy; fx: Fraction; MIB: Multicatheter interstitial brachytherapy; IORT: Intraoperative radiotherapy; DCIS: Ductal carcinoma in situ; 3DCRT: Three dimensional conformal radiotherapy; Gy: Gray.
With regard to cosmesis, the analysis of all major studies shows conflicting data about the outcomes with APBI. Vicini et al[83] reported the long-term experience of APBI with Mammosite A total of 1440 patients were treated. With a median follow-up of 4.5 years, 5-year local recurrence rate was 3.8% and 91% of patients had a good or excellent cosmetic result. The prospective study by Jagsi et al[84] reported an early closure of an APBI study with IMRT. They showed unacceptable cosmesis in 7 of 34 patients with a median follow-up of 2.5 years. Toxicity of 3DCRT APBI was reported by Hepel et al[85] in accordance with the technique and dose-volume constraints of the NSABP/RTOG 0413 protocol. At 15 mo, grade 2-4 late toxicity was observed in 10% of patients. The preliminary results of the RAPID study was presented at American Society for Radiation Oncology (ASTRO) 2012 and showed that the toxicity with 3DCRT APBI (32%) was more severe than WBRT (19%) at 3 years[86]. In another prospective study (n = 50), 54% of incidence of moderate to severe fibrosis and 35% of fat necrosis was seen with long-term follow-up after the use of interstitial brachytherapy[65]. The total dose was significantly correlated with the poor cosmetic results. Livi et al[87] detected significant improvements in acute grade 1-2 skin toxicity, favoring APBI with IMRT over conventionally fractionated WBRT. The preliminary results of the NSABP B-39/RTOG 0413 trial also have been reported equivalence of cosmesis to WBRT[88].
Veronesi et al[63] reported on 1822 patients treated with IORT (electron, 21 Gy) after quadrantectomy. With a mean follow-up of 36.1 mo, the local recurrence rate and the recurrence rate outside the treatment area were 2.3% and 1.4%, respectively. Recently, ELIOT study was evaluated regarding GEC-ESTRO recommendations and local recurrence rates were reported[89]. They found that 5-year local recurrence was 1.9% for good candidates, 7.4% for possible candidates, and 7.7% for contraindication groups. It is clearly shows for this technique that accurate patient selection is so important. In order to reach a conclusion that APBI is an acceptable alternative to WBRT, further studies with longer follow-up are needed. Until this date; when treating patients with APBI, consensus guidelines should be considered.
HYPOFRACTIONATED WHOLE BREAST RADIOTHERAPY
Hypofractionated RT involves fewer treatments, delivers a higher dose per treatment, and shorter overall treatment time (approximately 5 wk) compared to conventional RT. The role of hypofractionated WBRT after BCS has been clearly defined by four prospective randomized trials (Table 4)[90-95]. The Royal Marsden Hospital and Sutton and Gloucestershire Oncology Centre trial randomizing patients in the same 5-wk length of treatment between conventional RT (50 Gy in 25 fractions) and hypofractionated WBRT (39 Gy or 42.9 Gy in 13 fractions)[90,91]. There was a significant reduction in the rate of local recurrence using hypofractionated WBRT (12.1% for 50 Gy, 14.8% for 39 Gy, and 9.6% for 42.9 Gy; P = 0.027). However, there was a statistically significant change in breast appearance with the largest daily fraction size to 42.9 Gy compared with 39 Gy and 50 Gy. START trials (A and B) reported the experience of WBRT and hypofractionation[92,93]. Trial A compared 50 Gy in 25 fractions, 41.6 Gy in 13 fractions, or 39 Gy in 13 fractions within same 5 wk length of treatment[92]. Trial B compared 50 Gy in 25 fractions over 5 wk vs 40 Gy in 15 fractions over 3 wk[93]. The 5-year local control, disease free survival, and overall survival with the hypofractionation arms similar to conventional RT arm. Ten-year results of these studies have been published recently and similar breast cancer related outcomes have been reported[94]. In trial A, moderate or marked breast induration, telangiectasia, and breast edema were significantly less common in the 39 Gy group than in the 50 Gy group. Normal tissue effects did not differ significantly between 41.6 Gy and 50 Gy groups. In trial B, breast shrinkage, telangiectasia, and breast edema were significantly less common in the 40 Gy group than in the 50 Gy group. Whelan et al[95] randomized 1234 patients to either 42.5 Gy in 16 fractions over 22 d vs 50 Gy in 25 fractions over 35 d. At 10 years, a non-significant trend was seen for a lower local recurrence in the hypofractionated arm than in the conventional RT arm (6.2% and 6.7%, respectively). There were no differences in the survival, breast cancer mortality, and cosmetic outcomes. Additionally, ongoing UK FAST trial comparing 50 Gy in 25 fractions vs 28.5 or 30 Gy in 5 once-weekly fractions of 5.7 or 6 Gy, respectively[96]. Preliminary results of this study showed inferior outcome for the ultra short fractionation regimen.
Table 4.
Prospective randomized phase III trials of whole breast radiotherapy vs conventional fractionation radiotherapy
| Institution/trial | N | Median F/U | Eligibility criteria | Treatment arms | Primary endpoint | Secondary endpoint |
| Royal Marsden Hospital/Sutton and Gloucestershire Oncology Centre[90,91] | 1410 | 5 yr1 | Invasive breast cancer, T1-3N0-1M0, < 75 yr, BCS (complete macroscopic resection),Level II/III AD | 50 Gy in 25 fx 39 Gy in 13 fx 42.9 Gy in 13 fx | Late changes in breast appearance | Palpable breast induration Ipsilateral tumor recurrence |
| UK START A[92,94] | 2236 | 9.3 yr | Invasive breast cancer, T1-3aN0-1M0, > 18 yr, Clear tm margins (≥ 1 mm), No immediate surgical reconstruction, Available for follow-up | 50 Gy in 25 fx 41.6 Gy in 13 fx 39 Gy in 13 fx | Loco-regional tumor recurrence | Late normal tissue effects QOL |
| UK START B[93,94] | 2215 | 9.9 yr | Invasive breast cancer, T1-3aN0-1M0,> 18 yr, Clear tm margins (≥ 1 mm), No immediate surgical reconstruction, Available for follow-up | 50 Gy in 25 fx 40 Gy in 15 fx | Loco-regional tumor recurrence | Late normal tissue effects QOL |
| Ontario Clinical Oncology Group[95] | 1234 | 12 yr | Invasive breast cancer, BCS + Level I/II AD,Tm ≤ 5 cm, Negative axillary nodes, Maximum width of breast tissue ≤ 25 cm, No multicentric disease | 50 Gy in 25 fx 42.5 Gy in 16 fx | Local recurrence | Regional and distant recurrence Second cancers Breast cosmesis Late toxic effects of radiation |
Minimum follow-up. N: Number of patients; F/U: Follow-up; WBRT: Whole breast radiotherapy; RT: Radiotherapy; BCS: Breast conserving surgery; AD: Axillary dissection; fx: Fraction; Gy: Gray; UK START; United Kingdom Standardization of Breast Radiotherapy; QOL: Quality of life.
In a meta-analysis of the START A and B trials and pilot study from the Ontario Clinical Oncology Group found no significant difference between the hypofractionated WBRT and conventional RT for grade 3 tumors[97]. Cochrane review comparing the major trials of hypofractionated WBRT with conventional RT have shown that there is no difference in local recurrence rate (RR, 0.97; P = 0.78), breast appearance (RR, 1.17; P = 0.09), or 5-year survival (RR, 0.89; P = 0.16)[98]. However, acute skin toxicity was significantly lower with conventional RT (RR, 0.21; P = 0.007).
Despite the successful outcomes of hypofractionated WBRT, there are many unanswered questions regarding this issue. Firstly, all of these randomized studies did not have routine boost irradiation which was standard after WBRT for invasive breast cancer. Therefore, the optimal boost method after hypofractionated WBRT is still unknown. However, three phase I-II studies have reported favorable early local control and cosmesis of hypofractionated WBRT with a concurrent boost for early-stage breast cancer[99-101]. Grade 3 or higher skin toxicity was not reported in these trials. Secondly, patients who underwent hypofractionated WBRT are often low risk patients, and have small breast size and small chest wall separation. Especially, application of this technique to high risk patients whom required chemotherapy remains investigational until mature data from IMPORT and RTOG 1005 can provide efficacy and safety of hypofractionated WBRT in this group. Finally, Coles et al[102] suggested that hypofractionated WBRT should be applied to only right-sided breast cancer to reduce the RT doses per fraction received by the heart and coronary arteries.
Nowadays, the ASTRO has published guidelines for the implementation of hypofractionated WBRT in early-stage breast cancer[103]. Hypofractionated WBRT can be an acceptable treatment option outside of a clinical trial including patients with pT1-2 tumors, N0 nodal disease, age > 50 years old, patients who do not receive chemotherapy, and patients who do not require tumor bed boost.
CONCLUSION
Currently, phase III randomized trials demonstrated superiority of IMRT over conventional techniques in terms of both acute and late complications after breast conserving surgeries. Dosimetric trials showed that IMRT also improves breast and regional lymphatic coverage while decreasing radiation doses to heart, lungs, and contralateral breast tissues compared to old-fashioned radiotherapy techniques.
Hypofractionated regimens such as APBI may improve therapeutic index after breast conserving surgery. Furthermore, the duration of therapy will be shorter, and the workload in radiotherapy department will be minimized by those hypofractionated regimens. However, current standard of care after breast conserving surgery is still whole breast irradiation, not APBI. The role of hypofractionated regimens will be defined by mature results of both completed and ongoing randomized trials in the next decade.
Finally, it is noteworthy that quality assurance is crucial for the application of those challenging radiotherapy techniques. Even minor errors may result in catastrophic outcomes. Therefore, planning and implementing of modern radiotherapy techniques in breast cancer should be carried out with maximal care.
Footnotes
P- Reviewer: Ceresoli GL, Onishi H, Vinh-Hung V S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
References
- 1.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, Godwin J, Gray R, Hicks C, James S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 2.Paszat LF, Vallis KA, Benk VM, Groome PA, Mackillop WJ, Wielgosz A. A population-based case-cohort study of the risk of myocardial infarction following radiation therapy for breast cancer. Radiother Oncol. 2007;82:294–300. doi: 10.1016/j.radonc.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Ragaz J, Jackson SM, Le N, Plenderleith IH, Spinelli JJ, Basco VE, Wilson KS, Knowling MA, Coppin CM, Paradis M, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956–962. doi: 10.1056/NEJM199710023371402. [DOI] [PubMed] [Google Scholar]
- 4.Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, Kamby C, Kjaer M, Gadeberg CC, Rasmussen BB, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353:1641–1648. doi: 10.1016/S0140-6736(98)09201-0. [DOI] [PubMed] [Google Scholar]
- 5.Muren LP, Maurstad G, Hafslund R, Anker G, Dahl O. Cardiac and pulmonary doses and complication probabilities in standard and conformal tangential irradiation in conservative management of breast cancer. Radiother Oncol. 2002;62:173–183. doi: 10.1016/s0167-8140(01)00468-6. [DOI] [PubMed] [Google Scholar]
- 6.Hurkmans CW, Borger JH, Bos LJ, van der Horst A, Pieters BR, Lebesque JV, Mijnheer BJ. Cardiac and lung complication probabilities after breast cancer irradiation. Radiother Oncol. 2000;55:145–151. doi: 10.1016/s0167-8140(00)00152-3. [DOI] [PubMed] [Google Scholar]
- 7.Hurkmans CW, Cho BC, Damen E, Zijp L, Mijnheer BJ. Reduction of cardiac and lung complication probabilities after breast irradiation using conformal radiotherapy with or without intensity modulation. Radiother Oncol. 2002;62:163–171. doi: 10.1016/s0167-8140(01)00473-x. [DOI] [PubMed] [Google Scholar]
- 8.Katz A, Strom EA, Buchholz TA, Thames HD, Smith CD, Jhingran A, Hortobagyi G, Buzdar AU, Theriault R, Singletary SE, et al. Locoregional recurrence patterns after mastectomy and doxorubicin-based chemotherapy: implications for postoperative irradiation. J Clin Oncol. 2000;18:2817–2827. doi: 10.1200/JCO.2000.18.15.2817. [DOI] [PubMed] [Google Scholar]
- 9.McCormick B, Hunt M. Intensity-modulated radiation therapy for breast: is it for everyone? Semin Radiat Oncol. 2011;21:51–54. doi: 10.1016/j.semradonc.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Vicini FA, Sharpe M, Kestin L, Martinez A, Mitchell CK, Wallace MF, Matter R, Wong J. Optimizing breast cancer treatment efficacy with intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2002;54:1336–1344. doi: 10.1016/s0360-3016(02)03746-x. [DOI] [PubMed] [Google Scholar]
- 11.Kestin LL, Sharpe MB, Frazier RC, Vicini FA, Yan D, Matter RC, Martinez AA, Wong JW. Intensity modulation to improve dose uniformity with tangential breast radiotherapy: initial clinical experience. Int J Radiat Oncol Biol Phys. 2000;48:1559–1568. doi: 10.1016/s0360-3016(00)01396-1. [DOI] [PubMed] [Google Scholar]
- 12.Hong L, Hunt M, Chui C, Spirou S, Forster K, Lee H, Yahalom J, Kutcher GJ, McCormick B. Intensity-modulated tangential beam irradiation of the intact breast. Int J Radiat Oncol Biol Phys. 1999;44:1155–1164. doi: 10.1016/s0360-3016(99)00132-7. [DOI] [PubMed] [Google Scholar]
- 13.Lo YC, Yasuda G, Fitzgerald TJ, Urie MM. Intensity modulation for breast treatment using static multi-leaf collimators. Int J Radiat Oncol Biol Phys. 2000;46:187–194. doi: 10.1016/s0360-3016(99)00382-x. [DOI] [PubMed] [Google Scholar]
- 14.Krueger EA, Fraass BA, Pierce LJ. Clinical aspects of intensity-modulated radiotherapy in the treatment of breast cancer. Semin Radiat Oncol. 2002;12:250–259. doi: 10.1053/srao.2002.32468. [DOI] [PubMed] [Google Scholar]
- 15.Evans PM, Donovan EM, Partridge M, Childs PJ, Convery DJ, Eagle S, Hansen VN, Suter BL, Yarnold JR. The delivery of intensity modulated radiotherapy to the breast using multiple static fields. Radiother Oncol. 2000;57:79–89. doi: 10.1016/s0167-8140(00)00263-2. [DOI] [PubMed] [Google Scholar]
- 16.Andratschke N, Maurer J, Molls M, Trott KR. Late radiation-induced heart disease after radiotherapy. Clinical importance, radiobiological mechanisms and strategies of prevention. Radiother Oncol. 2011;100:160–166. doi: 10.1016/j.radonc.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Offersen B, Højris I, Overgaard M. Radiation-induced heart morbidity after adjuvant radiotherapy of early breast cancer - Is it still an issue? Radiother Oncol. 2011;100:157–159. doi: 10.1016/j.radonc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Yokoyama S, Roberson PL, Litzenberg DW, Moran JM, Fraass BA. Surface buildup dose dependence on photon field delivery technique for IMRT. J Appl Clin Med Phys. 2004;5:71–81. doi: 10.1120/jacmp.v5i2.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glatstein E. Intensity-modulated radiation therapy: the inverse, the converse, and the perverse. Semin Radiat Oncol. 2002;12:272–281. doi: 10.1053/srao.2002.32433. [DOI] [PubMed] [Google Scholar]
- 20.Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys. 2006;65:1–7. doi: 10.1016/j.ijrobp.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 21.Fraass BA, Kessler ML, McShan DL, Marsh LH, Watson BA, Dusseau WJ, Eisbruch A, Sandler HM, Lichter AS. Optimization and clinical use of multisegment intensity-modulated radiation therapy for high-dose conformal therapy. Semin Radiat Oncol. 1999;9:60–77. doi: 10.1016/s1053-4296(99)80055-1. [DOI] [PubMed] [Google Scholar]
- 22.Hartford AC, Palisca MG, Eichler TJ, Beyer DC, Devineni VR, Ibbott GS, Kavanagh B, Kent JS, Rosenthal SA, Schultz CJ, et al. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) Practice Guidelines for Intensity-Modulated Radiation Therapy (IMRT) Int J Radiat Oncol Biol Phys. 2009;73:9–14. doi: 10.1016/j.ijrobp.2008.04.049. [DOI] [PubMed] [Google Scholar]
- 23.Topolnjak R, Sonke JJ, Nijkamp J, Rasch C, Minkema D, Remeijer P, van Vliet-Vroegindeweij C. Breast patient setup error assessment: comparison of electronic portal image devices and cone-beam computed tomography matching results. Int J Radiat Oncol Biol Phys. 2010;78:1235–1243. doi: 10.1016/j.ijrobp.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 24.Korreman SS, Pedersen AN, Aarup LR, Nøttrup TJ, Specht L, Nyström H. Reduction of cardiac and pulmonary complication probabilities after breathing adapted radiotherapy for breast cancer. Int J Radiat Oncol Biol Phys. 2006;65:1375–1380. doi: 10.1016/j.ijrobp.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 25.Sixel KE, Aznar MC, Ung YC. Deep inspiration breath hold to reduce irradiated heart volume in breast cancer patients. Int J Radiat Oncol Biol Phys. 2001;49:199–204. doi: 10.1016/s0360-3016(00)01455-3. [DOI] [PubMed] [Google Scholar]
- 26.Remouchamps VM, Vicini FA, Sharpe MB, Kestin LL, Martinez AA, Wong JW. Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation. Int J Radiat Oncol Biol Phys. 2003;55:392–406. doi: 10.1016/s0360-3016(02)04143-3. [DOI] [PubMed] [Google Scholar]
- 27.Caudrelier JM, Morgan SC, Montgomery L, Lacelle M, Nyiri B, Macpherson M. Helical tomotherapy for locoregional irradiation including the internal mammary chain in left-sided breast cancer: dosimetric evaluation. Radiother Oncol. 2009;90:99–105. doi: 10.1016/j.radonc.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez VJ, Buchholz DJ, Langen KM, Olivera GH, Chauhan B, Meeks SL, Ruchala KJ, Haimerl J, Lu W, Kupelian PA. Evaluation of two tomotherapy-based techniques for the delivery of whole-breast intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2006;65:284–290. doi: 10.1016/j.ijrobp.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 29.Veldeman L, Madani I, Hulstaert F, De Meerleer G, Mareel M, De Neve W. Evidence behind use of intensity-modulated radiotherapy: a systematic review of comparative clinical studies. Lancet Oncol. 2008;9:367–375. doi: 10.1016/S1470-2045(08)70098-6. [DOI] [PubMed] [Google Scholar]
- 30.Pignol JP, Olivotto I, Rakovitch E, Gardner S, Sixel K, Beckham W, Vu TT, Truong P, Ackerman I, Paszat L. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26:2085–2092. doi: 10.1200/JCO.2007.15.2488. [DOI] [PubMed] [Google Scholar]
- 31.Barnett GC, Wilkinson JS, Moody AM, Wilson CB, Twyman N, Wishart GC, Burnet NG, Coles CE. Randomized controlled trial of forward-planned intensity modulated radiotherapy for early breast cancer: interim results at 2 years. Int J Radiat Oncol Biol Phys. 2012;82:715–723. doi: 10.1016/j.ijrobp.2010.10.068. [DOI] [PubMed] [Google Scholar]
- 32.Donovan E, Bleakley N, Denholm E, Evans P, Gothard L, Hanson J, Peckitt C, Reise S, Ross G, Sharp G, et al. Randomised trial of standard 2D radiotherapy (RT) versus intensity modulated radiotherapy (IMRT) in patients prescribed breast radiotherapy. Radiother Oncol. 2007;82:254–264. doi: 10.1016/j.radonc.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Mukesh MB, Barnett GC, Wilkinson JS, Moody AM, Wilson C, Dorling L, Chan Wah Hak C, Qian W, Twyman N, Burnet NG, et al. Randomized controlled trial of intensity-modulated radiotherapy for early breast cancer: 5-year results confirm superior overall cosmesis. J Clin Oncol. 2013;31:4488–4495. doi: 10.1200/JCO.2013.49.7842. [DOI] [PubMed] [Google Scholar]
- 34.McDonald MW, Godette KD, Butker EK, Davis LW, Johnstone PA. Long-term outcomes of IMRT for breast cancer: a single-institution cohort analysis. Int J Radiat Oncol Biol Phys. 2008;72:1031–1040. doi: 10.1016/j.ijrobp.2008.02.053. [DOI] [PubMed] [Google Scholar]
- 35.Morganti AG, Cilla S, Valentini V, Digesu’ C, Macchia G, Deodato F, Ferrandina G, Cece MG, Cirocco M, Garganese G, et al. Phase I-II studies on accelerated IMRT in breast carcinoma: technical comparison and acute toxicity in 332 patients. Radiother Oncol. 2009;90:86–92. doi: 10.1016/j.radonc.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 36.Back M, Guerrieri M, Wratten C, Steigler A. Impact of radiation therapy on acute toxicity in breast conservation therapy for early breast cancer. Clin Oncol (R Coll Radiol) 2004;16:12–16. doi: 10.1016/j.clon.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Fisher J, Scott C, Stevens R, Marconi B, Champion L, Freedman GM, Asrari F, Pilepich MV, Gagnon JD, Wong G. Randomized phase III study comparing Best Supportive Care to Biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: Radiation Therapy Oncology Group (RTOG) 97-13. Int J Radiat Oncol Biol Phys. 2000;48:1307–1310. doi: 10.1016/s0360-3016(00)00782-3. [DOI] [PubMed] [Google Scholar]
- 38.Collette S, Collette L, Budiharto T, Horiot JC, Poortmans PM, Struikmans H, Van den Bogaert W, Fourquet A, Jager JJ, Hoogenraad W, et al. Predictors of the risk of fibrosis at 10 years after breast conserving therapy for early breast cancer: a study based on the EORTC Trial 22881-10882 ‘boost versus no boost’. Eur J Cancer. 2008;44:2587–2599. doi: 10.1016/j.ejca.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 39.Vrieling C, Collette L, Fourquet A, Hoogenraad WJ, Horiot JH, Jager JJ, Pierart M, Poortmans PM, Struikmans H, Maat B, et al. The influence of patient, tumor and treatment factors on the cosmetic results after breast-conserving therapy in the EORTC ‘boost vs. no boost’ trial. EORTC Radiotherapy and Breast Cancer Cooperative Groups. Radiother Oncol. 2000;55:219–232. doi: 10.1016/s0167-8140(00)00210-3. [DOI] [PubMed] [Google Scholar]
- 40.Neal AJ, Mayles WP, Yarnold JR. Invited review: tangential breast irradiation--rationale and methods for improving dosimetry. Br J Radiol. 1994;67:1149–1154. doi: 10.1259/0007-1285-67-804-1149. [DOI] [PubMed] [Google Scholar]
- 41.Moody AM, Mayles WP, Bliss JM, A’Hern RP, Owen JR, Regan J, Broad B, Yarnold JR. The influence of breast size on late radiation effects and association with radiotherapy dose inhomogeneity. Radiother Oncol. 1994;33:106–112. doi: 10.1016/0167-8140(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 42.Harsolia A, Kestin L, Grills I, Wallace M, Jolly S, Jones C, Lala M, Martinez A, Schell S, Vicini FA. Intensity-modulated radiotherapy results in significant decrease in clinical toxicities compared with conventional wedge-based breast radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:1375–1380. doi: 10.1016/j.ijrobp.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 43.Freedman GM, Anderson PR, Li J, Eisenberg DF, Hanlon AL, Wang L, Nicolaou N. Intensity modulated radiation therapy (IMRT) decreases acute skin toxicity for women receiving radiation for breast cancer. Am J Clin Oncol. 2006;29:66–70. doi: 10.1097/01.coc.0000197661.09628.03. [DOI] [PubMed] [Google Scholar]
- 44.Freedman GM, Li T, Nicolaou N, Chen Y, Ma CC, Anderson PR. Breast intensity-modulated radiation therapy reduces time spent with acute dermatitis for women of all breast sizes during radiation. Int J Radiat Oncol Biol Phys. 2009;74:689–694. doi: 10.1016/j.ijrobp.2008.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freedman GM, Anderson PR, Bleicher RJ, Litwin S, Li T, Swaby RF, Ma CM, Li J, Sigurdson ER, Watkins-Bruner D, et al. Five-year local control in a phase II study of hypofractionated intensity modulated radiation therapy with an incorporated boost for early stage breast cancer. Int J Radiat Oncol Biol Phys. 2012;84:888–893. doi: 10.1016/j.ijrobp.2012.01.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blichert-Toft M, Rose C, Andersen JA, Overgaard M, Axelsson CK, Andersen KW, Mouridsen HT. Danish randomized trial comparing breast conservation therapy with mastectomy: six years of life-table analysis. Danish Breast Cancer Cooperative Group. J Natl Cancer Inst Monogr. 1992;(11):19–25. [PubMed] [Google Scholar]
- 47.van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, van der Schueren E, Helle PA, van Zijl K, Bartelink H. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92:1143–1150. doi: 10.1093/jnci/92.14.1143. [DOI] [PubMed] [Google Scholar]
- 48.Poggi MM, Danforth DN, Sciuto LC, Smith SL, Steinberg SM, Liewehr DJ, Menard C, Lippman ME, Lichter AS, Altemus RM. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute Randomized Trial. Cancer. 2003;98:697–702. doi: 10.1002/cncr.11580. [DOI] [PubMed] [Google Scholar]
- 49.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 50.Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, Aguilar M, Marubini E. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 51.Arriagada R, Lê MG, Rochard F, Contesso G. Conservative treatment versus mastectomy in early breast cancer: patterns of failure with 15 years of follow-up data. Institut Gustave-Roussy Breast Cancer Group. J Clin Oncol. 1996;14:1558–1564. doi: 10.1200/JCO.1996.14.5.1558. [DOI] [PubMed] [Google Scholar]
- 52.Kuerer HM, Julian TB, Strom EA, Lyerly HK, Giuliano AE, Mamounas EP, Vicini FA. Accelerated partial breast irradiation after conservative surgery for breast cancer. Ann Surg. 2004;239:338–351. doi: 10.1097/01.sla.0000114219.71899.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fisher B, Anderson S. Conservative surgery for the management of invasive and noninvasive carcinoma of the breast: NSABP trials. National Surgical Adjuvant Breast and Bowel Project. World J Surg. 1994;18:63–69. doi: 10.1007/BF00348193. [DOI] [PubMed] [Google Scholar]
- 54.Greenup RA, Camp MS, Taghian AG, Buckley J, Coopey SB, Gadd M, Hughes K, Specht M, Smith BL. Cost comparison of radiation treatment options after lumpectomy for breast cancer. Ann Surg Oncol. 2012;19:3275–3281. doi: 10.1245/s10434-012-2546-5. [DOI] [PubMed] [Google Scholar]
- 55.Smith BD, Arthur DW, Buchholz TA, Haffty BG, Hahn CA, Hardenbergh PH, Julian TB, Marks LB, Todor DA, Vicini FA, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO) Int J Radiat Oncol Biol Phys. 2009;74:987–1001. doi: 10.1016/j.ijrobp.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 56.Polgár C, Van Limbergen E, Pötter R, Kovács G, Polo A, Lyczek J, Hildebrandt G, Niehoff P, Guinot JL, Guedea F, et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009) Radiother Oncol. 2010;94:264–273. doi: 10.1016/j.radonc.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 57.Antonucci JV, Wallace M, Goldstein NS, Kestin L, Chen P, Benitez P, Dekhne N, Martinez A, Vicini F. Differences in patterns of failure in patients treated with accelerated partial breast irradiation versus whole-breast irradiation: a matched-pair analysis with 10-year follow-up. Int J Radiat Oncol Biol Phys. 2009;74:447–452. doi: 10.1016/j.ijrobp.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 58.Beitsch PD, Shaitelman SF, Vicini FA. Accelerated partial breast irradiation. J Surg Oncol. 2011;103:362–368. doi: 10.1002/jso.21785. [DOI] [PubMed] [Google Scholar]
- 59.Moser EC, Vrieling C. Accelerated partial breast irradiation: the need for well-defined patient selection criteria, improved volume definitions, close follow-up and discussion of salvage treatment. Breast. 2012;21:707–715. doi: 10.1016/j.breast.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 60.Edmundson GK, Vicini FA, Chen PY, Mitchell C, Martinez AA. Dosimetric characteristics of the MammoSite RTS, a new breast brachytherapy applicator. Int J Radiat Oncol Biol Phys. 2002;52:1132–1139. doi: 10.1016/s0360-3016(01)02773-0. [DOI] [PubMed] [Google Scholar]
- 61.Stewart AJ, Khan AJ, Devlin PM. Partial breast irradiation: a review of techniques and indications. Br J Radiol. 2010;83:369–378. doi: 10.1259/bjr/11505970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilder RB, Curcio LD, Khanijou RK, Eisner ME, Kakkis JL, Chittenden L, Agustin J, Lizarde J, Mesa AV, Ravera J, et al. A Contura catheter offers dosimetric advantages over a MammoSite catheter that increase the applicability of accelerated partial breast irradiation. Brachytherapy. 2009;8:373–378. doi: 10.1016/j.brachy.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 63.Veronesi U, Orecchia R, Luini A, Galimberti V, Zurrida S, Intra M, Veronesi P, Arnone P, Leonardi MC, Ciocca M, et al. Intraoperative radiotherapy during breast conserving surgery: a study on 1,822 cases treated with electrons. Breast Cancer Res Treat. 2010;124:141–151. doi: 10.1007/s10549-010-1115-5. [DOI] [PubMed] [Google Scholar]
- 64.Nelson JC, Beitsch PD, Vicini FA, Quiet CA, Garcia D, Snider HC, Gittleman MA, Zannis VJ, Whitworth PW, Fine RE, et al. Four-year clinical update from the American Society of Breast Surgeons MammoSite brachytherapy trial. Am J Surg. 2009;198:83–91. doi: 10.1016/j.amjsurg.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 65.Hattangadi JA, Powell SN, MacDonald SM, Mauceri T, Ancukiewicz M, Freer P, Lawenda B, Alm El-Din MA, Gadd MA, Smith BL, et al. Accelerated partial breast irradiation with low-dose-rate interstitial implant brachytherapy after wide local excision: 12-year outcomes from a prospective trial. Int J Radiat Oncol Biol Phys. 2012;83:791–800. doi: 10.1016/j.ijrobp.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khan AJ, Arthur D, Vicini F, Beitsch P, Kuerer H, Goyal S, Lyden M, Haffty BG. Six-year analysis of treatment-related toxicities in patients treated with accelerated partial breast irradiation on the American Society of Breast Surgeons MammoSite Breast Brachytherapy registry trial. Ann Surg Oncol. 2012;19:1477–1483. doi: 10.1245/s10434-011-2133-1. [DOI] [PubMed] [Google Scholar]
- 67.Vicini FA, Chen P, Wallace M, Mitchell C, Hasan Y, Grills I, Kestin L, Schell S, Goldstein NS, Kunzman J, et al. Interim cosmetic results and toxicity using 3D conformal external beam radiotherapy to deliver accelerated partial breast irradiation in patients with early-stage breast cancer treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2007;69:1124–1130. doi: 10.1016/j.ijrobp.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 68.Offersen BV, Overgaard M, Kroman N, Overgaard J. Accelerated partial breast irradiation as part of breast conserving therapy of early breast carcinoma: a systematic review. Radiother Oncol. 2009;90:1–13. doi: 10.1016/j.radonc.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 69.Vaidya JS, Joseph DJ, Tobias JS, Bulsara M, Wenz F, Saunders C, Alvarado M, Flyger HL, Massarut S, Eiermann W, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet. 2010;376:91–102. doi: 10.1016/S0140-6736(10)60837-9. [DOI] [PubMed] [Google Scholar]
- 70.Arthur DW, Winter K, Kuske RR, Bolton J, Rabinovitch R, White J, Hanson WF, Wilenzick RM, McCormick B. A Phase II trial of brachytherapy alone after lumpectomy for select breast cancer: tumor control and survival outcomes of RTOG 95-17. Int J Radiat Oncol Biol Phys. 2008;72:467–473. doi: 10.1016/j.ijrobp.2007.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vicini F, Winter K, Wong J, Pass H, Rabinovitch R, Chafe S, Arthur D, Petersen I, White J, McCormick B. Initial efficacy results of RTOG 0319: three-dimensional conformal radiation therapy (3D-CRT) confined to the region of the lumpectomy cavity for stage I/ II breast carcinoma. Int J Radiat Oncol Biol Phys. 2010;77:1120–1127. doi: 10.1016/j.ijrobp.2009.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khan AJ, Vicini FA, Beitsch P, Goyal S, Kuerer HM, Keisch M, Quiet C, Zannis V, Keleher A, Snyder H, et al. Local control, toxicity, and cosmesis in women & gt; 70 years enrolled in the American Society of Breast Surgeons accelerated partial breast irradiation registry trial. Int J Radiat Oncol Biol Phys. 2012;84:323–330. doi: 10.1016/j.ijrobp.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 73.Chen PY, Vicini FA, Benitez P, Kestin LL, Wallace M, Mitchell C, Pettinga J, Martinez AA. Long-term cosmetic results and toxicity after accelerated partial-breast irradiation: a method of radiation delivery by interstitial brachytherapy for the treatment of early-stage breast carcinoma. Cancer. 2006;106:991–999. doi: 10.1002/cncr.21681. [DOI] [PubMed] [Google Scholar]
- 74.Vicini F, Beitsch PD, Quiet CA, Keleher AJ, Garcia D, Snider HC, Gittleman MA, Zannis VJ, Kuerer HM, Lyden M. Three-year analysis of treatment efficacy, cosmesis, and toxicity by the American Society of Breast Surgeons MammoSite Breast Brachytherapy Registry Trial in patients treated with accelerated partial breast irradiation (APBI) Cancer. 2008;112:758–766. doi: 10.1002/cncr.23227. [DOI] [PubMed] [Google Scholar]
- 75.Chen PY, Wallace M, Mitchell C, Grills I, Kestin L, Fowler A, Martinez A, Vicini F. Four-year efficacy, cosmesis, and toxicity using three-dimensional conformal external beam radiation therapy to deliver accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2010;76:991–997. doi: 10.1016/j.ijrobp.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 76.Borruto F, Ferraro F. Adenocarcinoma of a neovagina constructed according to the Baldwin-Mori technique. Eur J Gynaecol Oncol. 1990;11:403–405. [PubMed] [Google Scholar]
- 77.Kuske RR, Winter K, Arthur DW, Bolton J, Rabinovitch R, White J, Hanson W, Wilenzick RM. Phase II trial of brachytherapy alone after lumpectomy for select breast cancer: toxicity analysis of RTOG 95-17. Int J Radiat Oncol Biol Phys. 2006;65:45–51. doi: 10.1016/j.ijrobp.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 78.Ribeiro GG, Magee B, Swindell R, Harris M, Banerjee SS. The Christie Hospital breast conservation trial: an update at 8 years from inception. Clin Oncol (R Coll Radiol) 1993;5:278–283. doi: 10.1016/s0936-6555(05)80900-8. [DOI] [PubMed] [Google Scholar]
- 79.Dodwell DJ, Dyker K, Brown J, Hawkins K, Cohen D, Stead M, Ash D. A randomised study of whole-breast vs tumour-bed irradiation after local excision and axillary dissection for early breast cancer. Clin Oncol (R Coll Radiol) 2005;17:618–622. doi: 10.1016/j.clon.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 80.Polgár C, Fodor J, Major T, Németh G, Lövey K, Orosz Z, Sulyok Z, Takácsi-Nagy Z, Kásler M. Breast-conserving treatment with partial or whole breast irradiation for low-risk invasive breast carcinoma--5-year results of a randomized trial. Int J Radiat Oncol Biol Phys. 2007;69:694–702. doi: 10.1016/j.ijrobp.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 81.Vaidya JS, Wenz F, Bulsara M, Tobias JS, Joseph DJ, Keshtgar M, Flyger HL, Massarut S, Alvarado M, Saunders C, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet. 2014;383:603–613. doi: 10.1016/S0140-6736(13)61950-9. [DOI] [PubMed] [Google Scholar]
- 82.Veronesi U, Orecchia R, Maisonneuve P, Viale G, Rotmensz N, Sangalli C, Luini A, Veronesi P, Galimberti V, Zurrida S, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol. 2013;14:1269–1277. doi: 10.1016/S1470-2045(13)70497-2. [DOI] [PubMed] [Google Scholar]
- 83.Vicini F, Beitsch P, Quiet C, Gittleman M, Zannis V, Fine R, Whitworth P, Kuerer H, Haffty B, Keisch M, et al. Five-year analysis of treatment efficacy and cosmesis by the American Society of Breast Surgeons MammoSite Breast Brachytherapy Registry Trial in patients treated with accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2011;79:808–817. doi: 10.1016/j.ijrobp.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 84.Jagsi R, Ben-David MA, Moran JM, Marsh RB, Griffith KA, Hayman JA, Pierce LJ. Unacceptable cosmesis in a protocol investigating intensity-modulated radiotherapy with active breathing control for accelerated partial-breast irradiation. Int J Radiat Oncol Biol Phys. 2010;76:71–78. doi: 10.1016/j.ijrobp.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hepel JT, Tokita M, MacAusland SG, Evans SB, Hiatt JR, Price LL, DiPetrillo T, Wazer DE. Toxicity of three-dimensional conformal radiotherapy for accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2009;75:1290–1296. doi: 10.1016/j.ijrobp.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 86.Whelan TJ, Olivotto I, Parpia S, Berrang T, Truong P, Cochrane B, Julian JA, RAPID Trial Investigators, Ontario Clinical Oncology Group . Interim toxicity results from RAPID: a randomized trial of accelerated partial breast irradiation (APBI) using 3D conformal external beam radiation therapy (3D CRT) ASTRO; 2012. [DOI] [PubMed] [Google Scholar]
- 87.Livi L, Buonamici FB, Simontacchi G, Scotti V, Fambrini M, Compagnucci A, Paiar F, Scoccianti S, Pallotta S, Detti B, et al. Accelerated partial breast irradiation with IMRT: new technical approach and interim analysis of acute toxicity in a phase III randomized clinical trial. Int J Radiat Oncol Biol Phys. 2010;77:509–515. doi: 10.1016/j.ijrobp.2009.04.070. [DOI] [PubMed] [Google Scholar]
- 88.Julian TB, Costantino JP, Vicini FA, White JR, Cecchini RS, Winter KA, Arthur DW, Kuske R, Rabinovitch R, Parda DS, et al. National Surgical Adjuvant Breast & Bowel Project (NSABP) A randomized phase III study of conventional whole breast irradiation (WBI) vs partial breast irradiation (PBI) for women with stage 0, 1, or 2 breast cancer: NSABP B-39/RTOG 0413. San Antonio Breast Cancer Symposium; 2011. pp. Abstract OT2–06-02. [Google Scholar]
- 89.Leonardi MC, Maisonneuve P, Mastropasqua MG, Morra A, Lazzari R, Dell’Acqua V, Ferrari A, Rotmensz N, Sangalli C, Luini A, et al. Accelerated partial breast irradiation with intraoperative electrons: using GEC-ESTRO recommendations as guidance for patient selection. Radiother Oncol. 2013;106:21–27. doi: 10.1016/j.radonc.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 90.Yarnold J, Ashton A, Bliss J, Homewood J, Harper C, Hanson J, Haviland J, Bentzen S, Owen R. Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: long-term results of a randomised trial. Radiother Oncol. 2005;75:9–17. doi: 10.1016/j.radonc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 91.Owen JR, Ashton A, Bliss JM, Homewood J, Harper C, Hanson J, Haviland J, Bentzen SM, Yarnold JR. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: long-term results of a randomised trial. Lancet Oncol. 2006;7:467–471. doi: 10.1016/S1470-2045(06)70699-4. [DOI] [PubMed] [Google Scholar]
- 92.Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bliss JM, Brown J, Dewar JA, Dobbs HJ, Haviland JS, et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol. 2008;9:331–341. doi: 10.1016/S1470-2045(08)70077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bentzen SM, Bliss JM, Brown J, Dewar JA, Dobbs HJ, et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet. 2008;371:1098–1107. doi: 10.1016/S0140-6736(08)60348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haviland JS, Owen JR, Dewar JA, Agrawal RK, Barrett J, Barrett-Lee PJ, Dobbs HJ, Hopwood P, Lawton PA, Magee BJ, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 95.Whelan TJ, Pignol JP, Levine MN, Julian JA, MacKenzie R, Parpia S, Shelley W, Grimard L, Bowen J, Lukka H, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 96.Agrawal RK, Alhasso A, Barrett-Lee PJ, Bliss JM, Bliss P, Bloomfield D, Bowen J, Brunt AM, Donovan E, Emson M, et al. First results of the randomised UK FAST Trial of radiotherapy hypofractionation for treatment of early breast cancer (CRUKE/04/015) Radiother Oncol. 2011;100:93–100. doi: 10.1016/j.radonc.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 97.Haviland JS, Yarnold JR, Bentzen SM. Hypofractionated radiotherapy for breast cancer. N Engl J Med. 2010;362:1843; author reply 1843–1844. doi: 10.1056/NEJMc1002798. [DOI] [PubMed] [Google Scholar]
- 98.James ML, Lehman M, Hider PN, Jeffery M, Hickey BE, Francis DP. Fraction size in radiation treatment for breast conservation in early breast cancer. Cochrane Database Syst Rev. 2010;(11):CD003860. doi: 10.1002/14651858.CD003860.pub3. [DOI] [PubMed] [Google Scholar]
- 99.Freedman GM, Anderson PR, Goldstein LJ, Ma CM, Li J, Swaby RF, Litwin S, Watkins-Bruner D, Sigurdson ER, Morrow M. Four-week course of radiation for breast cancer using hypofractionated intensity modulated radiation therapy with an incorporated boost. Int J Radiat Oncol Biol Phys. 2007;68:347–353. doi: 10.1016/j.ijrobp.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 100.Formenti SC, Gidea-Addeo D, Goldberg JD, Roses DF, Guth A, Rosenstein BS, DeWyngaert KJ. Phase I-II trial of prone accelerated intensity modulated radiation therapy to the breast to optimally spare normal tissue. J Clin Oncol. 2007;25:2236–2242. doi: 10.1200/JCO.2006.09.1041. [DOI] [PubMed] [Google Scholar]
- 101.Chadha M, Woode R, Sillanpaa J, Lucido D, Boolbol SK, Kirstein L, Osborne MP, Feldman S, Harrison LB. Early-stage breast cancer treated with 3-week accelerated whole-breast radiation therapy and concomitant boost. Int J Radiat Oncol Biol Phys. 2013;86:40–44. doi: 10.1016/j.ijrobp.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 102.Coles CE, Brunt AM, Wheatley D, Mukesh MB, Yarnold JR. Breast radiotherapy: less is more? Clin Oncol (R Coll Radiol) 2013;25:127–134. doi: 10.1016/j.clon.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 103.Smith BD, Bentzen SM, Correa CR, Hahn CA, Hardenbergh PH, Ibbott GS, McCormick B, McQueen JR, Pierce LJ, Powell SN, et al. Fractionation for whole breast irradiation: an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;81:59–68. doi: 10.1016/j.ijrobp.2010.04.042. [DOI] [PubMed] [Google Scholar]


