Abstract
Nipple-sparing mastectomy (NSM) is a safe technique in patients who are candidates for conservation breast surgery. However, there is worry concerning its oncological safety and surgical outcome in terms of postoperative complications. The authors reviewed the literature to evaluate the oncological safety, patient selection, surgical techniques, and also to identify the factors influencing postoperative outcome and complication rates. Patient selection and safety related to NSM are based on oncological and anatomical parameters. Among the main criteria, the oncological aspects include the clinical stage of breast cancer, tumor characteristics and location including small, peripherally located tumors, without multicentricity, or for prophylactic mastectomy. Surgical success depends on coordinated planning with the oncological surgeon and careful preoperative and intraoperative management. In general, the NSM reconstruction is related to autologous and alloplastic techniques and sometimes include contra-lateral breast surgery. Choice of reconstructive technique following NSM requires accurate consideration of various patient related factors, including: breast volume, degree of ptosis, areola size, clinical factors, and surgeon’s experience. In addition, tumor related factors include dimension, location and proximity to the nipple-areola complex. Regardless of the fact that there is no unanimity concerning the appropriate technique, the criteria are determined by the surgeon’s experience and the anatomical aspects of the breast. The positive aspects of the technique utilized should include low interference with the oncological treatment, reproducibility, and long-term results. Selected patients can have safe outcomes and therefore this may be a feasible option for early breast cancer management. However, available data demonstrates that NSM can be safely performed for breast cancer treatment in selected cases. Additional studies and longer follow-up are necessary to define consistent selection criteria for NSM.
Keywords: Breast reconstruction, Skin-sparing mastectomy, Nipple-sparing mastectomy, Outcome, Complications, Silicone breast implants, Tissue expanders, Oncoplastic surgery
Core tip: In selected patients, nipple-sparing mastectomy (NSM) has allowed an adequate oncologic control with satisfactory aesthetic outcome. In addition, utilizing the native breast skin optimizes the aesthetic outcome of the reconstructed breast and minimizes post-mastectomy deformity. The satisfactory results are due to a close collaboration with the oncological surgical team in terms of incision selection and mastectomy flap dissection. In general, choice of reconstructive procedure requires careful consideration of various patient related factors, including: breast volume, degree of ptosis, areolar size, patient preference and expectation, and surgeon experience. With careful patient selection and well-planned surgical technique, NSM can provide satisfactory outcomes with acceptable complication rates. However, available data demonstrate that NSM can be safely performed for breast cancer treatment in selected cases. Although NSM reduces the psychological trauma associated with nipple-areola complex resection, the oncologic safety as well as functional and aesthetic outcomes needs additional investigation. Thus, additional clinical studies and longer follow-up are necessary to define consistent selection criteria for NSM.
INTRODUCTION
Early breast cancer treatment has advanced greatly in recent years. The introduction of skin-sparing mastectomy (SSM) technique has improved the aesthetic outcome of oncological breast surgery and immediate reconstruction[1]. In fact, breast reconstruction following mastectomy can result in a prominent scars and a paddle of skin that is of a different color. Thus, the SSM involves en-bloc resection of the glandular tissue, nipple-areola complex (NAC), and the skin overlying superficial tumours[2-5]. Simultaneously, the native breast skin envelope and infra-mammary fold are preserved therefore facilitating the reconstruction procedure. Utilizing the breast skin envelope optimizes the contour of the breast, resulting in a satisfactory aesthetic outcome and minimizing scarring and post-mastectomy deformity[6-9].
Recently, an argumentation has advanced about the opportunity of extending conservation of the skin to include the NAC[10-29]. In fact, although breast reconstruction following SSM may offer aesthetic advantages over mastectomy, removal of the NAC significantly impacts on the aesthetic outcome. Some surgical techniques have been developed to repair the NAC, including local skin flaps, skin grafts, and nipple-sharing procedures[30,31]. However, different surgical stages are usually necessary to achieve an acceptable aesthetic result and sometimes with an unpredictable outcome[30-32]. In one clinical series, Jabor et al[32] evaluated the satisfaction following NAC reconstruction and observed that almost 36% of patients mentioned dissatisfaction.
First described by Freeman in the 1960s as a subcutaneous mastectomy with NAC sparing, the author indicated the technique for benign diseases, however he did not report the procedure for oncological objectives or as a risk-reduction alternative[10,11]. Recently, there has been an increase in clinical experience studies of NSM for breast cancer prophylaxis or early cancer treatment, evidencing revived interest in this surgical procedure[12-30,33-44]. In fact, there is evidence that NSM provides aesthetic advantages, with reduced need for further surgery and NAC reconstruction[15,17,20-22,29,33-39,44-48]. However, it is important to emphasize that most of these clinical series do not have sufficient follow-up, thus definite conclusions based on the present data is precipitated. In addition, to date there have been no controlled clinical trials evaluating the oncological effectiveness of nipple-sparing mastectomy (NSM) vs traditional SSM surgery. In spite of the controversies involving risk of local relapse, some current clinical studies have shown that the NSM is a safe procedure for selected cases[11,14-16,18,23-27,29,30,33-39,44,47,48].
LITERATURE SEARCH/DATA EXTRACTION
Two independent reviewers have evaluated titles and abstracts without language restrictions to assess eligibility in terms of outcome measures and study design. A literature search was carried out up to October 2013 to identify studies of breast cancer patients submitted to NSM and determine if any technique of immediate reconstruction was recorded. In an attempt to minimize the omission of potentially relevant clinical studies, we also reviewed the reference lists of included studies and relevant reviews for additional eligible articles. Potential studies were identified by searches of MEDLINE and PubMed databases using the terms “Nipple-Areola Sparing Mastectomy”, “Total Skin-Sparing Mastectomy”, “Subcutaneous Mastectomy” and “Immediate Reconstruction”. Studies identified were screened for those that focused on techniques, surgical and oncological outcomes after NSM reconstruction and references of each study were further investigated to include all relevant published data. All types of reconstruction techniques were included (tissue expander, implant, autologous tissue, and combination of methods) and compared.
A total of 440 potential articles were identified during the primary evaluation. After appraisal of the inclusion criteria, 265 articles were identified for potential inclusion and reviewed in detail. A total of 150 articles were excluded, leaving 109 articles to form the basis of this review.
ONCOLOGICAL ASPECTS
Oncological safety/patient selection
The main criteria include the clinical stage of breast cancer and tumor aspects[11,15,27,37-39]. From the oncological point of view, the NAC is resected because of the traditional concept that the adjacent ducts may contain tumor cells and the possibility of local recurrence[40,41]. In addition, some clinical series observed that nipple involvement in mastectomy specimens ranges from 0% to 58%[12,38,42-54] (Table 1). One might surmise that this wide range is chiefly due to divergences in techniques used for pathology tests of the breast specimens, differences in technique and subgroup of patient populations. In fact, early anatomical studies proposed by Sappey described a centripetal lymphatic drainage toward the areolar plexus, thus justifing the rationale of NAC resection[15,44]. Contrarily, recent anatomical studies demonstrated a lymphatic drainage to the deep pectoral plexus[44,55,56].
Table 1.
Occult neoplastic involvement of the nipple areola complex
| Ref. | Year | n | Nipple areola complex involvement (%) |
| Santini et al[45] | 1989 | 1291 | 12 |
| Menon et al[46] | 1989 | 33 | 58 |
| Verma et al[47] | 1997 | 26 | 0 |
| Vyas et al[42] | 1998 | 140 | 16 |
| Laronga et al[38] | 1999 | 246 | 5.6 |
| Simmons et al[43] | 2002 | 217 | 10.6 |
| Loewen et al[48] | 2008 | 302 | 10 |
| Rusby et al[53] | 2008 | 130 | 24.6 |
| Banerjee et al[49] | 2008 | 219 | 20 |
| Voltura et al[50] | 2008 | 34 | 5.9 |
| Pirozzi et al[51] | 2010 | 50 | 28 |
| Reynolds et al[52] | 2011 | 29 | 7 |
| Wang et al[54] | 2012 | 787 | 7 |
Concerning the clinical aspects, recent studies have noted that the risk of tumor involvement of the NAC has been magnified[38,41-43]. Thus, some clinical series have demonstrated that the NSM is a safe technique for some group of patients[11,14-16,18,19,23-27,29,30,33-39,44,57]. In fact, some studies have considered NSM safe in patients with peripherally located tumors, small, without multicentricity, or for risk reduction[24]. Although there is no unanimity regarding the selection criteria, the major part of studies include tumor size up to 3 cm, lack of clinical involvement of the NAC and tumor to nipple distance greater than to 2 cm. In addition, patients with clinical axillary node involvement; whose tumors are centrally located; who have inflammatory breast cancer, or Paget disease are not candidates for NSM.
In a clinical experience of 286 SSM specimens, Laronga et al[38] observed that 5.6% were found to contain tumor in the NAC and did not define significant differences between groups regarding tumor size and histological subtype. However, sub-areolar tumor location and multi-centricity were important risk factors for NAC involvement. Based on these findings, the authors observed that in patients with negative axilla and tumors situated on the periphery, the probability of an occult tumor is less than 2%. Similarly, Vyas et al[42] in a clinical series of 140 mastectomies analyzed whether NAC correlated with areola-tumor distance, tumor size, nodal status and lymphatic embolization. In this sample, the authors also observed tumour size and nodal positivity as a potential risk factor for NAC involvement. Correspondingly, Simmons et al[43] analyzed 217 mastectomy specimens and evaluated tumor involvement of the NAC. Concerning the NAC involvement, the overall frequency was 10.6% and comparisons of patients with tumors < 2 cm with tumors ≥ 2 cm did not present a significant difference. The authors observed that only 6.7% of small tumors with up to two positive lymph nodes only had NAC involvement. For tumors located in central quadrants, the NAC was involved in 27.3% of cases. Contrarily, for those located in any of the four quadrants, the NAC was compromised in only 6.4% of cases. Gerber et al[57] in a series of 112 NSMs, evaluated patients whose tumors were more than 2 cm from the NAC. The frozen sections of the subareolar tissue were negative for tumor in 54.5% of cases, thus enabling NAC preservation. During the follow-up, 5.4% local recurrences (LR) occurred in patients who underwent SSM compared with 8.2% of 134 patients who had undergone conventional mastectomy during the same follow-up. Regolo et al[19] in a clinical study of 219 mastectomies observed that 20% of NACs were compromised by tumor, consisting of 9.4% of stage 1-2 tumors and 30% of stage III tumors. Concerning the tumor location, the NAC was compromised in 2.5% of peripheral tumors and in 68% of central quadrants. The authors failed to observe any cases of local relapse in patients undergoing NSM after an average of 16 mo follow-up (Table 2).
Table 2.
Clinical outcome and local recurrences following nipple-sparing mastectomy
| Ref. | Year | n | Stage | Follow-up (mo) | Nipple areola complex recurrence | Local recurrence |
| Gerber et al[25] | 2009 | 61 | 0-I | 59 | 1.6 | 5.4 |
| Garcia-Etienne et al[15] | 2006 | 42 | 0-I | 10.5 | 0 | 0 |
| Bistoni et al[106] | 2006 | 10 | 0-I | 36 | 0 | 0 |
| Voltura et al[50] | 2008 | 51 | 0-III | 18 | 0 | 5.9 |
| Crowe et al[14] | 2004 | 54 | 0-II | 41 | 0 | 0 |
| Petit et al[104] | 2005 | 579 | 0-I | 19 | 0 | 0.9 |
| Sacchini et al[18] | 2006 | 192 | 0-III | 24.6 | 0 | 3 |
| Paepke et al[103] | 2009 | 109 | 0-III | 34 | 0 | 1.83 |
| Babiera et al[107] | 2010 | 54 | 0-III | 15 | 0 | 0 |
| Benediktsson et al[105] | 2008 | 216 | 0-III | 156 | 0 | 8.5 |
| Munhoz et al[33] | 2013 | 158 | 0-II | 65.6 | 0 | 3.7 |
Caruso et al[16] indicated NSM in patients with tumors that were peripherally situated. Their study included 50 patients with a 12% overall recurrence rate. Similarly, Sacchini et al[18] evaluated patients who had NSM with reconstruction for either risk reduction, treatment of cancer, or both. With a median follow-up of 24 mo, two breast cancer patients and two patients who had NSM for prophylaxis presented a local recurrence outside of the NAC. Based on this clinical experience, the authors concluded that the risk of local relapse is low and the procedure is feasible in the risk-reducing and breast cancer-treatment.
Munhoz et al[33] evaluated 158 consecutive patients submitted to NSM. In almost 35% of patients the procedure was indicated for cancer prophylaxis including high-risk lesions, prophylactic, familial history and carriers of the BRCA1 or BRCA2 mutation. In the remaining breast cancer patients, almost 75% of tumors measured 2 cm or less (T1) and the majority were stage 0 and I. Similarly as observed by other authors, the present study also included a few stage III breast carcinomas; however in the preoperative period these patients were staged as earlier-stage carcinoma[9,58]. Additionally, the authors excluded patients with NAC infiltration, NAC bleeding or with the tumor at less than 5 cm from the NAC. Considering these parameters, the authors believe that NSM is feasible with low local recurrence. With a mean follow-up of 65.6 mo, local recurrence rate was 3.7% and the incidence of distant metastases was 1.8%.
In a comprehensive review, Tokin et al[24] observed that the local recurrence following NSM was between 0%-20%, with studies varying widely in inclusion criteria and follow-up period. Boneti et al[26] reported in a series of 281 NSM with 25.3 mo mean follow-up, a 4.6% local recurrence rate. Jensen et al[27] published results from 149 patients without local recurrences at a mean 5-years follow-up.
In a recent review, Mallon et al[59] quantified the incidence of occult NAC cancer and identified the factors influencing occult nipple malignancy, local recurrence rates, and complication rates. According to the authors, the overall nipple (0.8%) and flap (3.4%) recurrence rates were similar to those reported after mastectomy and conservative breast surgery. However, care must be taken to distinguish that follow-up periods for NSM clinical studies are briefer than those for mastectomy and partial mastectomy. For definitive conclusions, a longer and similar follow-up is necessary, as the greater part of recurrences occur within 5 years.
Therefore, it would appear oncologically safe to perform NSM, provided the tumor is not close to the NAC, small, peripherally located, without multicentricity and a frozen section protocol is performed. Although various clinical series including SSM and NSM aided in the selection of patients for NSM using tumor to NAC distance values, the ideal tumor to NAC distance has yet to be clarified, since the total number of patients analyzed in these clinical series is insufficient and requires validation[41,59]. Additionally, patients must be informed that NAC resection may still be necessary if residual tumor is identified on frozen sections of the subareolar tissue or definitive histology.
Timing: One stage x two stage approach
NSM may be planned in one setting with immediate reconstruction (one-stage approach)[39,57,60], or in two settings with partial glandular resection or NAC autonomization followed by additional breast tissue resection and total reconstruction weeks to months afterwards (two-stages approach)[30,34,39,61-65].
Preoperative planning should include the breast ptosis and volume and mostly addressing singular reconstructive requirements, enabling each patient to receive an individual “custom-made” planning. In addition, an in-depth discussion concerning alternatives for NSM reconstruction should be undertaken with the patients and her family, including the risks and positive aspects of one vs two-stages approaches.
One-stage approach: With one-stage approach both procedures (breast cancer treatment/risk reduction and reconstruction procedures) are associated in one operative setting. Additionally, the emotional benefit of having begun reconstruction at the time of NSM procedure may decrease the impact of the loss of the breast. In fact, Sahin et al[60] in a series of 21 bilateral prophylactic NSM due to higher risk for cancer indicated the one stage approach and simultaneous breast reconstruction using submuscular silicone implants. According to the authors, better projection and shape may be achieved with serial expansion of the submuscular pocket, but this has to be weighed against the morbidity associated with two surgical procedures. In their clinical experience, a one-stage procedure using high-profile implants resulted in very good projection while avoiding the morbidity of a second surgery.
Other centers indicated both approaches according to the quality and the width of the remaining breast skin flap. Chen et al[30] in a series of 115 NSM evaluated the risks and benefits of the procedure associated with immediate breast reconstruction. In all patients, reconstruction with tissue expander or silicone implant was performed immediately following the NSM. Of the 66 patients, 58 underwent tissue expansion followed by implant placement in a two-stage reconstruction (87.9%) and eight patients underwent one-stage reconstruction (12.1%). According to the authors, nineteen patients had wound-healing problems. Full and partial necrosis of the NAC was not associated to initial expander volume but was more prevalent in thin flaps and larger breasts.
Although NSM and immediate implant reconstruction can be accomplished in a single stage, this is not the first option in some cancer centers[30]. In fact, Chen et al[30] emphasized that with two the stage approach it is possible to have a better control over the NSM skin flap. First, some aspects relating to implant asymmetry can be treated at the time of the second stage. Second, by limiting the volume of the expander such that the skin flap is not redundant but also not under tension, the risk of necrosis is reduced. Finally, patients usually desire a volume change, and starting the reconstruction with a two stage approach allows the surgeon to customize the outcome to patient preference.
In spite of these aspects, for some group of patients the one-stage approach can be advantageous. In fact, patients with small breasts, without ptosis and cardiovascular clinical diseases are the best candidates for one-stage NSM. Caruso et al[16] considered NSM in patients with small to moderate-sized breasts with moderate to minimal ptosis and a healthy breast skin. Similarly, in a systematic review Endara et al[39] examined current trends with NSM, including selection criteria, incision choice, and reconstructive techniques. In the major part of the cases, NSM requires no skin resection, however with increasing breast volume (> 500 g) or breast ptosis, higher rates of NAC or breast skin flap necrosis are expected. In addition, low BMI and minimal ptosis were consistently used to screen patients for NSM in these studies.
Conversely, with the one-stage approach the surgical time can be lengthened and potential complications of the NSM (e.g., skin/NAC necrosis, dehiscence, infection) can adversely influence the postoperative outcome. In addition, the procedure can be compromised by positive margins, especially in the sub-areolar region. In fact, Mallon et al[59] in a recent comprehensive review demonstrated that the greater part of the NSM studies performed biopsy of the retroareolar tissue separately from the mastectomy specimen. Concerning the technique, some studies used frozen section analysis, however, this technique has a false-negative rate as high as 8.7% according to the present review. Therefore many cancer centers await definitive pathologic evaluation of sub-areolar specimens before deciding on NAC resection. Thus, it is advocated that all patients submitted to one stage therapeutic NSM have a retroareolar sampling. In addition, these patients must be informed that the NAC may need to be ultimately resected if result of the retroareolar biopsy is compromised.
Two-stage approach: With two-stage approach, the surgical process is less extensive than NSM and immediate reconstruction in one operative setting. Some patients are so distressed by their cancer diagnosis, that they are not able to cooperate in reconstructive decisions. Additionally, some potential complications of the NSM and reconstruction techniques (e.g., skin necrosis, dehiscence, infection) can unfavorably defer the adjuvant therapy. However, while the rationale for this approach is reasonable, the addition of a different surgical stage may introduce possibilities for complications[65].
First proposed by Palmieri et al[66], the two-stage concept of delayed NSM had the objective of complete removal of all breast tissue, including the lactiferous ducts. According to the authors, the first stage involves NAC autonomization by performing a periareolar incision to detach the ductus from the nipple. The second stage is then performed 2-3 wk later. The authors observed one case of NAC necrosis that occurred during the NAC autonomization, delaying the NSM for 6 wk to allow complete revascularization with a satisfactory outcome. Similarly, Jensen et al[67] indicated the two-stage approach with NAC surgical delay in 20 patients who were at high risk for NAC necrosis following NSM. The authors performed the delay technique 7-21 d prior to NSM mastectomy. Sub-areolar biopsy was performed at the time of the delay procedure and if the biopsy revealed malignancy, the NAC was removed at the time of NSM. All of the NAC survived and in 2 patients the subareolar biopsy was positive and 3 NAC were removed.
Another important point is related to the possibility of another stage to improve the aesthetic outcome[30,34]. In fact, Blechman et al[34] in a series of 55 NSM performed in 29 consecutive patients evaluated the technical aspects and outcome. After tissue expansion the implant volume can be selected during the second stage without causing flap tension. Also, this strategy provides an opportunity to refine the breast contour such as by fat grafting.
In the greater part of the clinical series, NSM are related to patients with relatively small, minimally ptotic breasts or for risk reduction[14,39,61,62]. However, the NSM reconstruction of large and/or ptotic breasts poses a more troublesome challenge than the NSM of small sized breasts because of an excessively large skin flap[33]. In addition, the Wise-pattern skin excision best addresses this excess skin but is associated with a high incidence of flap necrosis with subsequent reconstruction failure[22,33]. Munhoz et al[33] in a series of 158 patients submitted to NSM observed a significantly higher incidence of complications in the obese and larger specimen group. This aspect can be partially explained by a decreased perfusion of the relatively large skin flaps that result from SSM in much larger breasts. According to the authors, after adjusting for other risk factors (BMI, weight of breast specimen), the probability of complications tends to be higher for the Wise pattern with superior pedicle incision approaches.
Although large breasts and severe ptosis may represent a contraindication for NSM, surgical strategies based on the two-stage concept were planned to correct the ptosis followed by NSM in a second stage. Introduced by Spear et al[61] the NSM staged procedure includes patients with large or ptotic breasts and candidates to NAC preservation. In fact, the authors observed that although there are breasts that are too large to be considered for a NSM, it is possible to extend the indications by using the two stage approach and reducing the breast volume and ptosis previously. Thus, the main objective in these sub-group of patients is to preserve the oncological objective of the NSM (therapeutic or risk reduction) while expanding the aesthetic outcome and minimizing complications. For this objective, some authors divided the one-stage Wise-pattern skin excision into a two-stage procedure[61,63,64]. In the first stage, the mastopexy or reduction mammoplasty is performed, keeping periareolar dermis preserved to maintain the adequate NAC blood supply at the time of the future definitive NSM. At the time of the second stage, care must be taken to guarantee consistent flap thickness in order to avoid damage to the skin flap blood supply.
Liu et al[63] in a series of 12 patients achieved successful outcome using the two staged Wise-pattern excision. In the first stage, the NSM and reconstruction were performed using a vertical excision. In the second stage, the redundant skin at the inframammary fold was excised, tightening the breast skin envelope vertically. According to the authors, the addition of the two staged incisions recreates the Wise pattern, breaking up the T point into two straightforward primary closures. Similarly, Spear et al[61] reported a successful two-stage NSM in 15 patients (24 breasts). All patients underwent NSM after mastopexy or reduction (71% prophylactic and 29% therapeutic) with an average follow-up of 13 mo. Four of the 24 operated breasts (17%) presented a complication. Besides the satisfactory outcome, it is important to emphasize that although the two-stage NSM is acceptable in the prophylactic group, patient selection is somewhat more complex in the group with breast cancer. Thus, the two-staged procedure must be correctly planned so that it does not significantly delay the oncological treatment in this patient population. Yacoumettis[64] in a retrospective study of 52 patients evaluated the results of bilateral subcutaneous mastectomy for breast cancer prophylaxis. All reconstructions were completed in two-stages with tissue expanders followed by textured gel filled silicone implants. According to the authors and during the average follow-up of 7.2 years, no cases of invasive cancer were observed, and the aesthetic outcome was considered satisfactory.
Thus, the two-stage concept can be, in theory, advantageous when compared to the one stage NSM. However, as we observed in any procedure this approach can present some limitations. The main negative aspects are related to some technical difficulties, i.e., scar tissue and fibrosis. Additionally, the procedure can be time consuming and demanding additional costs, which can represent some limitations to the insurance coverage and resource implications for community hospitals.
Incision selection
Numerous incisions have been described by a variety of designs incorporating a periareolar approach, or other variations in the shape around the NAC[11-16,18-22,30,33-37,39,59,61,63,66-68]. Although the incision types vary with configuration, the impasse of the access incision with no complications has drawn attention in the great part of the studies[20,25-28,30,33] (Figure 1).
Figure 1.
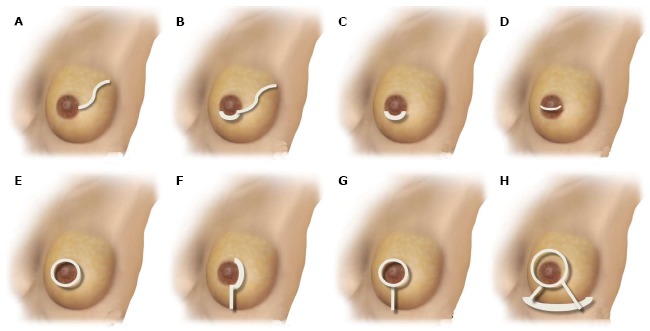
Schematic representation of nipple-sparing mastectomy incisions. A: Radial lateral incision; B: Periareolar with lateral extension; C: Hemi-periareolar (superior and inferior); D: Transareolar; E: Circumareolar (periareolar total); F: Periareolar with vertical extension; G: Circumareolar (periareolar total) with vertical extension; H: Wise-pattern mastectomy.
A critical survey shows that the procedure is normally performed by numerous approaches, but the greater part more than one type of incision is performed[11-16,18-22,30,33-37,39,59,61,63,66-69]. In fact, Endara et al[39] analyzed 48 NSM studies, of which 41 described details related to the type of NSM incision. A total of 15 diverse approaches were described and the greater part of the studies (70%) more than one type of incision was indicated. According to the authors, the most common incision described were radial, followed by periareolar, inframammary, mastopexy, and transareolar (Figures 2-4).
Figure 2.
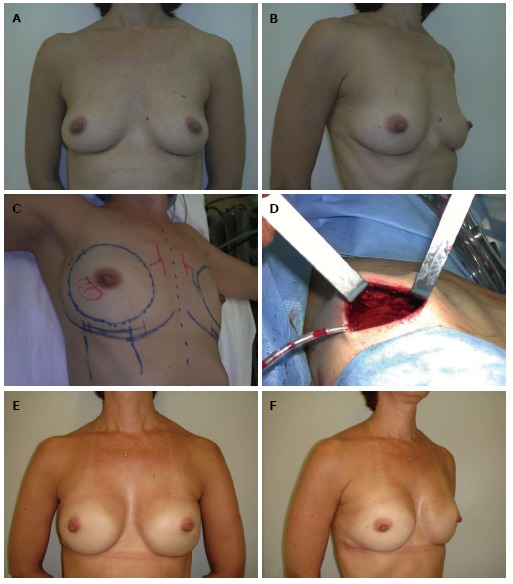
Nipple-sparing mastectomy/inframammary incision. A and B: A 44-year-old patient with an invasive ductal carcinoma in the right breast (1.4 cm) and a familial history of breast cancer; C and D: Nipple-sparing mastectomy preoperative planning was based on a bilateral through a inframammary approach and immediate reconstruction with biodimensional implant-expander (Allergan 150 SH, 285 cm3). Intraoperative frozen sections demonstrated nipple-areola complex free of tumor; E and F: Five years postoperative appearance with a very good outcome.
Figure 4.
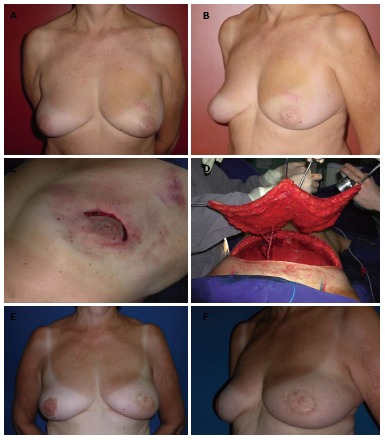
Nipple-sparing mastectomy/superior periareolar incision. A and B: A 56-year-old patient with invasive ductal carcinoma of the left breast (2.3 cm); C and D: The patient underwent a left nipple-sparing mastectomy mastectomy with a superior periareolar incision and sentinel lymph node biopsy. The oncological procedure was immediately followed by a free deep inferior epigastric perforator flap reconstruction; E and F: Five years postoperative appearance with a very good outcome. The superior periareolar incision was converted to a total circumareolar incision in order to achieve a better symmetry during the second stage of reconstruction.
Figure 3.
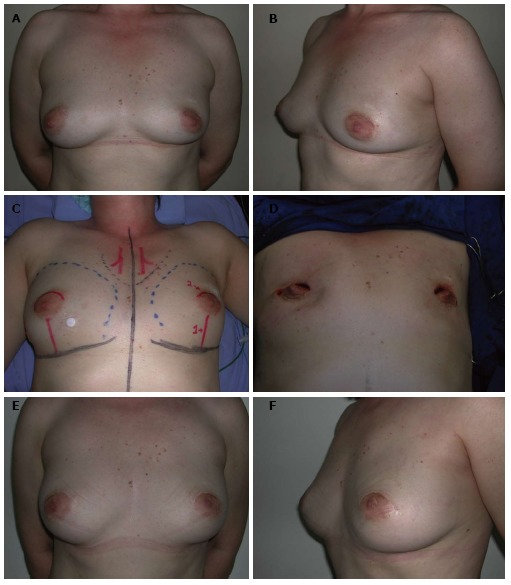
Nipple-sparing mastectomy/superior periareolar incision. A and B: A 52-year-old patient with in situ multifocal carcinoma in the right breast (4.8 cm) and atypical hyperplasia in the left breast; C and D: The patient underwent a bilateral nipple-sparing mastectomy mastectomy through a superior periareolar incision and sentinel lymph node biopsy; E and F: The oncological procedure was immediately followed by a bilateral pedicled transverse rectus abdominis myocutaneous flap reconstruction. Four years postoperative appearance with a very good outcome.
The radial incision is one of the most performed techniques for NSM. Endara et al[39] reported that this incision represented almost 46% of all incisions performed. Stolier et al[20] in a series of 82 NSM for risk reduction and cancer treatment described that the most common incisions utilized were related to the radial incision and a lateral incision beginning from outside the NAC. According to the authors, this incision allowed an adequate exposure to all regions including the axillary tail and the internal thoracic vessels for free flap anastomosis. Colwell et al[70] performed an inferolateral approach with the incision located in the lateral quadrant. Similarly, Chung and Sacchini evaluated NSM incisions, which the greater part associated the periareolar to the radial incisions[65]. The same group reported NSM through different incisions and the periareolar incision with lateral extension was used in 42% of cases[11]. The authors mentioned a satisfactory exposure as advantages of the use of the radial extensions. Compared to other incisions, complications were observed in 67% of cases with an inferior lateral incision (inframammary fold extended laterally). Wijayanayagam et al[71] in a series of 64 NSMs performed in 43 patients evaluated the technical aspects and surgical outcome. Using different types of incisions, the authors observed that the radial incision provided the best approach and had the greatest likelihood of maintaining viable NAC without necrosis, which was observed in almost 97% of the sample. Despite the benefits, some authors do not advocate this approach due to aesthetic disadvantages. In fact, this technique creates a scar that is especially visible in the oblique and profile views[60].
The periareolar incisions are the second most performed techniques for NSM. In fact, Endara et al[39] reported that the periareolar approach represent almost 27% of all incisions performed for NSM. The main benefits are related to scar camouflage with a more satisfactory outcome. Despite its advantages, the periareolar incision is not adequate for all patients candidate to NSM. In fact, the more suitable indication is in patients with small breasts with an adequate areola diameter. A limited exposure and difficulty in breast flaps dissection are commonly observed in small areola patients and inexperienced breast surgeon. For patients with large areola diameter without breast ptosis, a hemicircumareolar incision is usually indicated. Another important indication is the presence of a marked color transition between the NAC and the breast skin and small/medium volume breasts (cup size A-B). Sahin et al[60], in a series of prophylactic NSM usually indicated the periareolar incision for small-breasted patients. According to the authors, the NSM and the reconstruction are performed through this incision, extending circumareolar or semicircular in the lower half of the NAC. Rivolin et al[35] in a series of 22 patients submitted to NSM evaluated the benefits of the periareolar approach associated with mastopexy for patients with ptotic breasts. All patients in the periareolar group were submitted to a one-stage reconstruction, while a two-stage approach was selected in 20% of patients. The complication rate was higher in the periareolar group, although the difference did not reach significance. Despite the satisfactory outcome, the mastopexy technique was inadequate if repositioning the NAC was more than 3 cm or in sufficiently large reductions to reduce excess skin. In women with larger and more ptotic breasts, Chen et al[30] advocated the omega-type elliptical incision. Similar to the periareolar incision with lateral extension, the omega-type approach gave the surgeon wide access to the breast regions and axilla.
Besides the limited exposure, the periareolar incision can result in an impairment to blood supply, which can induce NAC necrosis. In fact, Regolo et al[19], in a series of 32 NSM utilizing the periareolar incision observed a high rate of complications of the NAC (60%). Consequently, Munhoz et al[21] developed an approach to improve the surgical exposure based on total circumareolar incision. This technique was based on the double concentric periareolar incision to resect the glandular tissue, while maintaining the vascularization of the NAC through the subdermal vascular plexus. In addition, the authors advocated de-epithelializing the whole periareolar incision to allow for triple-layer closure of the wound. Therefore, no part of the suture lines present only one layer, thus lessening the risk of breast implant exposure.
The inframammary incision is the third most performed approach for NSM. According to Endara et al[39] the inframammary technique represents almost 20% of all incisions performed for NSM. Blechman et al[34] in a clinical series of 55 NSM through a lateral infra-mammary incision performed in 29 consecutive patients evaluated the technical aspects and outcome. The authors indicated the lateral IMF approach for a variety of breast volumes, and were able to place different volumes of implants. According to the authors, the benefits are related to hiding the scar and the incision is the furthest from the NAC and thus it is the least likely to threaten its vascular stability. In addition, rotating the IMF incision laterally facilitates easier access to the sentinel lymph node biopsy. Contrarily, Chen et al[30] in their review of a series of 115 NSMs evaluated the risks and benefits of the procedure associated with immediate breast reconstruction. The IMF approach was indicated for patients with smaller breasts. Stolier et al[20] observed that the inframammary fold incision was uncertain. According to the authors, surgical access to the recipient vessels may be problematic, making this incision more adequate to implant reconstruction. In addition, they reported an inaccurate dissection around the NAC and in the upper quadrants. Similarly, Wijayanayagam et al[71] in a series of 64 NSMs performed in 43 women observed that the inframammary incision provided a large exposure. However, they were concerned about the ability to access the upper quadrants in patients with large breasts and limited this incision to patients with very small breasts. Thus, the authors recommended using an incision of at least 10 cm because the larger incision enables easier eversion of the skin for improved visualization of sub-areolar region. Saliban et al[36] analyzed 118 NSMs in 80 consecutive patients and observed that patients with different breast sizes underwent inframammary approach, except those patients who had very large breasts or those who requested a breast lift.
Contrarily, some authors avoid the inframammary fold incision due to the technical limitation to dissect the upper pole breast tissue and inadequate resection[20,30,33,36,60,70]. In fact, Chen et al[30] observed that although the inframammary incision allows a better final position of the scar, the resection of glandular tissue superiorly could be more challenging. Additionally, in some cases the authors believe that it is difficult to place the incision on the right position once the final implant volume is decided at the end of the surgery[33]. Besides these limitations, some authors believe that the inframammary incision could impair the inframammary blood supply[36,72]. Proano and Perbeck compared skin blood supply in patients having either an inframammary approach or a lateral lazy S incision using laser Doppler and fluorescein flometry[72]. In a series of 69 patients, they observed a significant reduction in flow to an area of skin 2 cm below the NAC in the group submitted to inframammary approach.
The mammoplasty incision has been previously described for planning SSM/NSM in ptotic breasts[1]. Classified by Carlson et al[5,6] as a Type IV, it involves breasts that require a reduction of the skin flap and offers a wide exposure[22,33,39,73-76]. According to Endara et al[39] the mammoplasty approach represents almost 4% of all incisions performed for NSM. The main benefits are related to a better surgical access in patients with large breast and moderate/severe ptosis. Another potential advantage is related to reduction of the skin envelope and the dead space between the skin and the implant. Rusby observed that a limited volume of fluid collecting between skin flaps and reconstruction allows the preserved skin to redrape over the breast mound to a variable and uncontrolled extent[75]. In fact, by reducing the skin flap, such that it is closer to the breast mound size, movement is reduced.
Munhoz et al[33] reported that almost 35% of the patients were submitted to the mammoplasty incision. The superior pedicle and inferior pedicle techniques were indicated for moderate ptosis and severe ptosis cases respectively. In spite of the main benefits, this technique has some limitations since the lateral and medial skin flaps that close down to the inframammary fold may become ischemic, and implant exposure can be observed[33,74,75]. Another negative aspect is related to the relative lack of space in the inferior and medial aspects of the submuscular pocket. It is possible to release the inferior aspect of the pectoralis muscle, however a subcutaneous pocket could become an implant exposed, in the situation of an ischemic NSM flap[22]. According to Toth and Lappert[1], this aspect is critical and not rare if the general surgeon during dissection needs to leave very thin poorly vascularized NSM flaps. Thus, the technique requires close collaboration between the oncologic and reconstructive surgeons. In higher risk patients or severe breast ptosis, Munhoz et al[33] preferred the inferior pedicle technique since the well-vascularized pedicle provides a stable soft-tissue cover for the implant, which protects against exposure. Similarly, Nava et al[22] in a series of 28 patients with ptotic breasts proposed a combined flap technique to reconstruct by use of anatomical silicone implants. After preoperative planning, a large area in the lower half of the breast was deepithelialized according to the conventional Wise pattern.
Skin flap and NAC complications
In spite of the NSM advantages, the outcome is not always predictable. Surgical concerns are related to increased complications such as wound healing problems or ischemic necrosis[19,24-29,33]. In fact, one of the most problematic complications of NSM is skin flap and NAC necrosis, which can lead to unsatisfactory aesthetic result (Table 3) (Figure 5).
Table 3.
Clinical outcome and vascular related complications following nipple-sparing mastectomy
| Ref. | Year | n |
Nipple necrosis |
|
| Total (n) | Partial (n) | |||
| Petit et al[109] | 2009 | 1001 | 35 | 55 |
| Nahabedian et al[17] | 2006 | 11 | 0 | 1 |
| Spear et al[108] | 2011 | 49 | 3 | 3 |
| Voltura et al[50] | 2008 | 51 | 0 | 0 |
| De Alcantara Filho et al[23] | 2011 | 341 | 0 | 1 |
| Wijayanayagam et al[71] | 2008 | 64 | 3 | 10 |
| Jensen et al[27] | 2011 | 127 | 0 | 8 |
| Paepke et al[103] | 2009 | 109 | 1 | 23 |
| Babiera et al[107] | 2010 | 54 | 0 | 4 |
| Munhoz et al[33] | 2013 | 158 | 1 | 8 |
Figure 5.
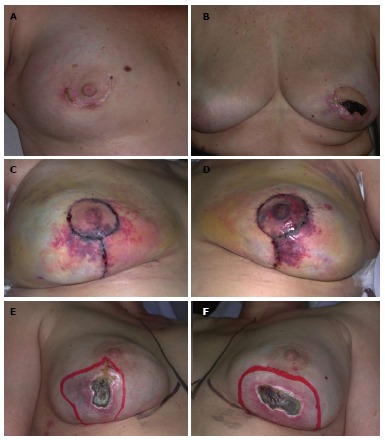
Local complications following nipple-sparing mastectomy. A: Inferior periareolar incision with partial wound dehiscence; B: Superior periareolar incision with partial nipple areola complex necrosis; C and D: Wise pattern incision with partial mastectomy and nipple areola complex necrosis; E and F: Inframammary incision with partial mastectomy necrosis.
Early reports on the NSM technique described high rates of complications[18,28,30,57]. Gerber et al[57] in one of the first clinical series of NSM evaluated the NAC outcome in 61 patients. The authors observed that 9.8% of patients presented partial nipple necrosis with no cases of total necrosis. Komorowski et al[28] observed a 7.9%incidence of total nipple necrosis and a 5.3% of partial nipple loss. In 2006, Sacchini et al[18] observed necrosis of the nipple in 11% of the sample and it was judged minimal in 59% of patients. Munhoz et al[33] identified patient and breast related factors that increased complication rates. Concerning the NAC outcome, the majority of NAC demonstrated some degree of immediate ischemia manifested by coolness. However, the NAC skin survived in almost 95% of cases and partially survived in 4.4%. In these cases, the NAC developed epidermolysis/partial-thickness necrosis and most of these healed conservatively.
Previous studies have reported some risk of skin flap/NAC necrosis[20,24-30,33,36,39]. Although comparing NAC necrosis rates between different populations, techniques and experiences can be challenging, most studies report rates from 0 to 19.5%[23,25]. As techniques have improved, the rates of local complications have been reduced to satisfactory levels[19-22,24-27,33]. Some authors advocate the use of lateral incisions, avoidance of periareolar incisions which require more skin traction, limiting dissection beyond the lateral aspect of the anterior axillary line and over the sternum to preserve blood supply to the skin flap, and the use of scissors to avoid thermal lesion[11,44]. In addition, the option of the adequate surgical approach is critical and depends on previous scars, tumor location, breast volume, degree of ptosis and NAC anatomy. Although large studies are necessary to evaluate the best incision type, reduced NAC necrosis have been described with radial areolar incisions[20,36,39,71,76].
In a recent review, Endara et al[39] evaluated the incision type and outcome following NSM. Based on 48 clinical studies in a pooled analysis, the authors reported similar rates of NAC necrosis between radial and inframammary incisions (8.83% and 9.09%, respectively) but an increased rate of necrosis following periareolar approaches (17.81%). In this review, the transareolar incision presented the highest incidence of nipple necrosis (81.82%). Based on the results of this review, the preferred incision is either the inframammary fold or the radial with a lateral extension.
Contrarily, Munhoz et al[33] observed that the type of incision was not significantly predictive of complications in univariate analysis. However, after adjusting for other risk factors (BMI and weight of specimen), the probability of complications tends to be higher for hemi-periareolar and Wise-pattern superior pedicle incision. In addition, they observed a lower incidence of NAC necrosis with the double circle incision technique. This aspect is probably due to the full access along the inferior border of NAC, which seems to allow adequate blood supply to the NAC. The authors believed that besides the limited access, the hemiperiareolar technique can potentially result in vascular impairment to blood supply due to traction, which can induce partial necrosis. In fact, Regolo et al[19] in a series utilizing the periareolar incision observed a high rate of necrotic complication of the NAC, which they abandoned in favor of a lateral incision. Similarly, Stolier et al[20] reported no cases of nipple necrosis when using a lateral or radial incision and Chung et al[65] found adequate postoperative NAC viability by using a periareolar and lateral skin incision or an inframammary approach.
Some authors suggest that clinical co-morbidities are relevant risk factors for complications[5,6,28,33,77-82]. Komorowski et al[28] analyzed such factors and concluded that age below 45 years is associated with a reduced risk of necrosis. Contrarily, Munhoz et al[33] did not observe age as a significant factor for NAC necrosis. However, in an univariate analysis the authors showed a significantly higher incidence of complications in the obese, hypertensive and larger specimen group. In fact, the deleterious effect of obesity on breast reconstruction was previously studied[77,78,80]. One might suppose that increased BMI may predispose the flap necrosis due to the compromised sub-dermal plexus brought about by the increased surface of the flap[83]. In addition, obese patients are likely to have additional complications due to associated vascular disease. Similarly as observed by Wooderman et al[79], the authors observed that specimen weight more than the mean weight seems to be associated with statistically significant odds ratios to develop complications[33]. This aspect can be partially explained by a decreased perfusion of the relatively large skin flaps that result from SSM in much larger breasts.
RECONSTRUCTIVE ASPECTS
In general, the NSM reconstruction are related to autologous and alloplastic techniques and sometimes include contra-lateral breast surgery. Various reconstructive techniques have been described, and aesthetic outcomes of NSM reconstruction continue to be met with variable satisfaction rates. Choice of reconstructive technique requires consideration of numerous patient related factors, including: breast volume, degree of ptosis, areola size, patient preference and expectation, clinical factors, smoking and surgeon experience. In addition, tumor related factors include size, location and proximity to the skin and NAC. Regardless of the fact that there is no unanimity regarding the best procedure, the criteria are determined by the surgeon’s experience and the anatomical aspects of the breast. The main advantages of the technique utilized should include low interference with the oncological treatment, reproducibility and long-term outcome.
During a NSM, the NAC is preserved and incisions are located in more aesthetically regions. The breast volume, consisting of the breast tissue and fat, is entirely removed and reconstruction of the breast skin is not necessary. Thus, the objectives are to repair contour, volume and position.
In a recent systematic review, Endara et al[39] examined current trends with NSM, including the reconstructive options. Based on 48 clinical studies that met the inclusion criteria, yielding 6615 NSM, the authors observed that 2373 (45.5%) were two-stage expander to implant, 2126 (40.7%) were one-stage direct to implant, and 719 (13.8%) were autologous tissue.
Autologous reconstructions involve pedicle flaps such as the latissimus dorsi myocutaneous (LDMF) or transverse rectus abdominis myocutaneous (TRAM) flaps. Although these techniques presents positive aspects some limitations have arisen regarding the muscle resection[83-88]. Thus, alternatively free tissue transfer including the deep inferior epigastric perforator (DIEP), pedicled thoracodorsal perforator flap (TAP), free TRAM or the gluteal artery perforator (GAP) flaps can be indicated with a lower donor site morbidity. In fact, the DIEP flap diverges from abdominal myocutaneous flaps with maintenance of well-vascularized tissue and total abdominal muscular and aponeurotic layer preservation[89-94]. Mosahebi et al[83] in a series of 61 NSM reconstructions compared alloplastic and autologous tissue in terms of aesthetic outcome and satisfaction survey. According to the authors, all three reconstruction methods (implant, LDMF and DIEP) achieved good evaluation scores. However, in patients who had adjuvant radiotherapy, tonometry demonstrated that the breast remained softer in DIEP flap reconstruction.
In spite of the positive aspects, the outcome following SSM and NSM reconstruction with autologous tissue is not frequently predictable[39,95-97]. Utilizing autologous tissue and particularly free flaps require special considerations in terms of recipient vessels and a monitoring skin flap. Preoperatively the plastic surgeon should evaluate the incision approach, the recipient vessels and the width of the remaining skin flaps for adequate skin preservation. Munhoz et al[8] in a series of SSM DIEP reconstructions utilized five different incision approaches. According to the authors, the criteria decision was based on the breast anatomy (volume, ptosis and areola), the biopsy incision and the tumor location. The periareolar incision was the second most common incision selected and the restricted surgical exposure and difficulty in DIEP anastomosis were the main negative technical aspects. Thus, a correct selection of a suitable recipient pedicle is decisive for a successful outcome[90,92-94]. In NSM, the use of internal thoracic recipient vessels can be troublesome since the surgical exposure is restricted[8,95]. Thus, longer instruments and the use of endoscopic lighting are necessary[95]. In this situation, some authors advocated the periareolar approach with a lateral extension to obtain a better exposure[94,95]. In addition, some authors advocate that use of the internal thoracic vessels may result in a higher rate of NAC necrosis compared with using the thoracodorsal vessels[39,95]. Yang et al[95] in a series of 92 NSM free flap reconstructions utilized the internal mammary vessels if the mastectomy flap did not restrict the access. The authors observed that the thoracodorsal vessels were indicated in 59 cases, and internal mammary vessels in 33 cases including 4 cases with perforators of the internal thoracic vessels. In a selected group of patients, the internal thoracic branches can be used as an alternative to the internal mammary pedicle. The main advantages are sparing the internal mammary vessels and decreasing the operative time by limited dissection. However, Munhoz et al[92] reported that the internal thoracic branches are potentially available in only 55% of patients, therefore, this should not be the first option as recipient site.
Although autologous tissue presents advantages, it is not adequate in all cases especially in those without donor areas. In these cases, alloplastic techniques are usually indicated, and involve two-stage approach with tissue expanders followed by silicone gel implant replacement or one-stage reconstruction with conventional silicone implants. Although NSM reconstruction can be performed in a single stage, this is not the standard practice in several cancer centers[39]. Enthusiasts of single-stage reconstruction promote lower costs, however supporters of the two-stage approach advocate a second operation to improve symmetry and the unpredictability of the NSM flaps. Chen et al[30] evaluated reconstruction with tissue expander or silicone implant performed immediately following the 115 NSM. Of the 66 patients, 58 patients underwent tissue expansion followed by subsequent implant placement in a two-stage reconstruction (87.9%) and eight patients underwent one-stage reconstruction (12.1%). The authors advocate that with two-stage reconstruction, it is possible to achieve the maximum control over the skin flap and by limiting the volume of the tissue expander such that the skin envelope is not redundant, the risk of ischemia is reduced. In addition, future aspects relating to NAC position, asymmetry and implant asymmetry can be managed at the time of the replacement of the tissue expander with a silicone implant. Starting the reconstruction with a tissue expander allows the reconstructive surgeon to customize the results to patient preference. Endara et al[39] has demonstrated that the incidence of NAC necrosis is little increased with one-stage approach (4.50% x 3.90%), however the overall complication rates were higher in the two-stage group (52.4% x 18.6%). The authors emphasized that there is no ideal algorithm for reconstruction and the decision to proceed with reconstruction and the technique should be made by the surgeon based on assessment of skin flap viability.
The introduction of biodimensional implant-expander system (BIES) has proved increasingly popular over the last years[9,21,33,97-102]. Designed with the objective of combining the advantages of the silicone gel implant and tissue expander into one system, it may present a superior breast form compared with what might be achieved using unshaped implants or expanders. The system design permits postoperative adjustments in implant volume and contra-lateral symmetry[9,97-102].
In spite of the positive aspects, complications can be expected and are best avoided by placing the BIES under a submuscular pocket. Regardless of the good results observed with total muscular coverage, in some patients this technique is not free of unpredictable outcome[9,21,74,101,102]. Total muscular coverage can limit lower pole expansion and can result in a high-riding device[74,102]. Mahdi et al[99] in a series of BEIS reconstructions, observed that some patients failed to develop adequate lower pole projection and 35% required inferior muscular release to obtain a satisfactory result. Munhoz et al[33] advocated performing only minimal immediate expansion of the skin flaps in order to avoid tissue tension[29,33]. In fact, in a series of patients submitted to NSM reconstruction, Peled et al[29] observed that NAC necrosis greatly decreased after the technical refinements of incision selection and performing implant reconstruction in a two-stage fashion.
Another option for implant coverage in NSM reconstruction is the use of acellular dermal matrices (ADM). Boneti et al[26] in a series of 281 NSM reconstructions utilized the alloplastic tissue situated in a partial muscular pocket, with ADM bridging the lateral and inferior edge of the muscle and the chest wall. The authors observed an overall complication rate of 7.1% (20 of 281) and the most frequent complication was breast skin flap ischemia. Spear et al[61] described a successful two staged NSM in 15 patients (24 breasts) utilizing the ADM. According to the authors, four of the 24 operated breasts (17%) experienced a complication, in that 2 patients (8%) developed flap necrosis and two patients developed partial NAC necrosis. Endara et al[39] in a systematic review could not asses the impact of acellular dermal matrix on reconstructive outcomes following NSM. According to the authors, the studies either did not report acellular dermal matrix use, or did indeed place acellular dermal matrix for all cases in only three studies, totaling NSM reconstructions. Given the insufficient number of patients comparison of complication rates between the two groups was not possible.
CONCLUSION
NSM is not a new concept but is becoming increasingly accepted by breast surgeons. In selected patients, this approach has allowed an adequate oncologic control with satisfactory aesthetic outcome. Although NSM requires more intraoperative care, the concept can optimize the aesthetic result in early-stage breast cancer patients.
The satisfactory outcome are due to a close collaboration with the oncological surgical team in terms of incision selection flexibility and skin flap dissection. Alternately, care must be taken during the oncological procedure with meticulous surgical technique and gentle handling of tissues to avoid complications. In general, choice of reconstructive procedure requires careful consideration of various patient related factors, including: breast volume, degree of ptosis, areolar size, patient preference and expectation, clinical factors, and surgeon experience. Regardless of the fact that there is no consensus concerning the best technique, the criteria are determined by the surgeon’s experience and the anatomical aspects of the breast. Probably, all these objectives are not achieved by any single procedure and each technique has advantages and limitations. With careful patient selection and well-planned surgical technique, NSM can provide satisfactory outcomes with acceptable complication rates. However, available data demonstrate that NSM can be safely performed for breast cancer treatment in selected cases. Additional studies and longer follow-up are necessary to define consistent selection criteria for NSM.
Footnotes
P- Reviewer: Heo CY, Jun Y S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
References
- 1.Toth BA, Lappert P. Modified skin incisions for mastectomy: the need for plastic surgical input in preoperative planning. Plast Reconstr Surg. 1991;87:1048–1053. [PubMed] [Google Scholar]
- 2.Kroll SS, Ames F, Singletary SE, Schusterman MA. The oncologic risks of skin preservation at mastectomy when combined with immediate reconstruction of the breast. Surg Gynecol Obstet. 1991;172:17–20. [PubMed] [Google Scholar]
- 3.Singletary SE. Skin-sparing mastectomy with immediate breast reconstruction: the M. D. Anderson Cancer Center experience. Ann Surg Oncol. 1996;3:411–416. doi: 10.1007/BF02305673. [DOI] [PubMed] [Google Scholar]
- 4.Simmons RM, Adamovich TL. Skin-sparing mastectomy. Surg Clin North Am. 2003;83:885–899. doi: 10.1016/S0039-6109(03)00035-5. [DOI] [PubMed] [Google Scholar]
- 5.Carlson GW, Bostwick J, Styblo TM, Moore B, Bried JT, Murray DR, Wood WC. Skin-sparing mastectomy. Oncologic and reconstructive considerations. Ann Surg. 1997;225:570–55; discussion 570-55;. doi: 10.1097/00000658-199705000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlson GW. Skin sparing mastectomy: anatomic and technical considerations. Am Surg. 1996;62:151–155. [PubMed] [Google Scholar]
- 7.Slavin SA, Schnitt SJ, Duda RB, Houlihan MJ, Koufman CN, Morris DJ, Troyan SL, Goldwyn RM. Skin-sparing mastectomy and immediate reconstruction: oncologic risks and aesthetic results in patients with early-stage breast cancer. Plast Reconstr Surg. 1998;102:49–62. doi: 10.1097/00006534-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Munhoz AM, Arruda E, Montag E, Aldrighi C, Aldrighi JM, Gemperli R, Ferreira MC. Immediate skin-sparing mastectomy reconstruction with deep inferior epigastric perforator (DIEP) flap. Technical aspects and outcome. Breast J. 2007;13:470–478. doi: 10.1111/j.1524-4741.2007.00467.x. [DOI] [PubMed] [Google Scholar]
- 9.Munhoz AM, Aldrighi C, Montag E, Arruda EG, Aldrighi JM, Filassi JR, Ferreira MC. Periareolar skin-sparing mastectomy and latissimus dorsi flap with biodimensional expander implant reconstruction: surgical planning, outcome, and complications. Plast Reconstr Surg. 2007;119:1637–149; discussion 1637-149;. doi: 10.1097/01.prs.0000246406.68739.e4. [DOI] [PubMed] [Google Scholar]
- 10.FREEMAN BS. Subcutaneous mastectomy for benign breast lesions with immediate or delayed prosthetic replacement. Plast Reconstr Surg Transplant Bull. 1962;30:676–682. doi: 10.1097/00006534-196212000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Etienne CA, Cody Iii HS, Disa JJ, Cordeiro P, Sacchini V. Nipple-sparing mastectomy: initial experience at the Memorial Sloan-Kettering Cancer Center and a comprehensive review of literature. Breast J. 2009;15:440–449. doi: 10.1111/j.1524-4741.2009.00758.x. [DOI] [PubMed] [Google Scholar]
- 12.Cense HA, Rutgers EJ, Lopes Cardozo M, Van Lanschot JJ. Nipple-sparing mastectomy in breast cancer: a viable option? Eur J Surg Oncol. 2001;27:521–526. doi: 10.1053/ejso.2001.1130. [DOI] [PubMed] [Google Scholar]
- 13.Stanec Z, Zic R, Stanec S, Budi S, Hudson D, Skoll P. Skin-sparing mastectomy with nipple-areola conservation. Plast Reconstr Surg. 2003;111:496–48; author reply 498. doi: 10.1097/00006534-200301000-00099. [DOI] [PubMed] [Google Scholar]
- 14.Crowe JP, Kim JA, Yetman R, Banbury J, Patrick RJ, Baynes D. Nipple-sparing mastectomy: technique and results of 54 procedures. Arch Surg. 2004;139:148–150. doi: 10.1001/archsurg.139.2.148. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Etienne CA, Borgen PI. Update on the indications for nipple-sparing mastectomy. J Support Oncol. 2006;4:225–230. [PubMed] [Google Scholar]
- 16.Caruso F, Ferrara M, Castiglione G, Trombetta G, De Meo L, Catanuto G, Carillio G. Nipple sparing subcutaneous mastectomy: sixty-six months follow-up. Eur J Surg Oncol. 2006;32:937–940. doi: 10.1016/j.ejso.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Nahabedian MY, Tsangaris TN. Breast reconstruction following subcutaneous mastectomy for cancer: a critical appraisal of the nipple-areola complex. Plast Reconstr Surg. 2006;117:1083–1090. doi: 10.1097/01.prs.0000202103.78284.97. [DOI] [PubMed] [Google Scholar]
- 18.Sacchini V, Pinotti JA, Barros AC, Luini A, Pluchinotta A, Pinotti M, Boratto MG, Ricci MD, Ruiz CA, Nisida AC, et al. Nipple-sparing mastectomy for breast cancer and risk reduction: oncologic or technical problem? J Am Coll Surg. 2006;203:704–714. doi: 10.1016/j.jamcollsurg.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Regolo L, Ballardini B, Gallarotti E, Scoccia E, Zanini V. Nipple sparing mastectomy: an innovative skin incision for an alternative approach. Breast. 2008;17:8–11. doi: 10.1016/j.breast.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 20.Stolier AJ, Sullivan SK, Dellacroce FJ. Technical considerations in nipple-sparing mastectomy: 82 consecutive cases without necrosis. Ann Surg Oncol. 2008;15:1341–1347. doi: 10.1245/s10434-007-9753-5. [DOI] [PubMed] [Google Scholar]
- 21.Munhoz AM, Aldrighi C, Montag E, Arruda E, Aldrighi JM, Filassi JR, Ricci M, Brasil JA, Rezende V, Ferreira MC. Optimizing the nipple-areola sparing mastectomy with double concentric periareolar incision and biodimensional expander-implant reconstruction: aesthetic and technical refinements. Breast. 2009;18:356–367. doi: 10.1016/j.breast.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Nava MB, Cortinovis U, Ottolenghi J, Riggio E, Pennati A, Catanuto G, Greco M, Rovere GQ. Skin-reducing mastectomy. Plast Reconstr Surg. 2006;118:603–10; discussion 611-3. doi: 10.1097/01.prs.0000233024.08392.14. [DOI] [PubMed] [Google Scholar]
- 23.de Alcantara Filho P, Capko D, Barry JM, Morrow M, Pusic A, Sacchini VS. Nipple-sparing mastectomy for breast cancer and risk-reducing surgery: the Memorial Sloan-Kettering Cancer Center experience. Ann Surg Oncol. 2011;18:3117–3122. doi: 10.1245/s10434-011-1974-y. [DOI] [PubMed] [Google Scholar]
- 24.Tokin C, Weiss A, Wang-Rodriguez J, Blair SL. Oncologic safety of skin-sparing and nipple-sparing mastectomy: a discussion and review of the literature. Int J Surg Oncol. 2012;2012:921821. doi: 10.1155/2012/921821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerber B, Krause A, Dieterich M, Kundt G, Reimer T. The oncological safety of skin sparing mastectomy with conservation of the nipple-areola complex and autologous reconstruction: an extended follow-up study. Ann Surg. 2009;249:461–468. doi: 10.1097/SLA.0b013e31819a044f. [DOI] [PubMed] [Google Scholar]
- 26.Boneti C, Yuen J, Santiago C, Diaz Z, Robertson Y, Korourian S, Westbrook KC, Henry-Tillman RS, Klimberg VS. Oncologic safety of nipple skin-sparing or total skin-sparing mastectomies with immediate reconstruction. J Am Coll Surg. 2011;212:686–693; discussion 693-695. doi: 10.1016/j.jamcollsurg.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 27.Jensen JA, Orringer JS, Giuliano AE. Nipple-sparing mastectomy in 99 patients with a mean follow-up of 5 years. Ann Surg Oncol. 2011;18:1665–1670. doi: 10.1245/s10434-010-1475-4. [DOI] [PubMed] [Google Scholar]
- 28.Komorowski AL, Zanini V, Regolo L, Carolei A, Wysocki WM, Costa A. Necrotic complications after nipple- and areola-sparing mastectomy. World J Surg. 2006;30:1410–1413. doi: 10.1007/s00268-005-0650-4. [DOI] [PubMed] [Google Scholar]
- 29.Warren Peled A, Foster RD, Stover AC, Itakura K, Ewing CA, Alvarado M, Hwang ES, Esserman LJ. Outcomes after total skin-sparing mastectomy and immediate reconstruction in 657 breasts. Ann Surg Oncol. 2012;19:3402–3409. doi: 10.1245/s10434-012-2362-y. [DOI] [PubMed] [Google Scholar]
- 30.Chen CM, Disa JJ, Sacchini V, Pusic AL, Mehrara BJ, Garcia-Etienne CA, Cordeiro PG. Nipple-sparing mastectomy and immediate tissue expander/implant breast reconstruction. Plast Reconstr Surg. 2009;124:1772–1780. doi: 10.1097/PRS.0b013e3181bd05fd. [DOI] [PubMed] [Google Scholar]
- 31.Teimourian B, Duda G. The propeller flap: a one-stage procedure for nipple-areola reconstruction. Aesthetic Plast Surg. 1994;18:81–84. doi: 10.1007/BF00444253. [DOI] [PubMed] [Google Scholar]
- 32.Jabor MA, Shayani P, Collins DR, Karas T, Cohen BE. Nipple-areola reconstruction: satisfaction and clinical determinants. Plast Reconstr Surg. 2002;110:457–463; discussion 464-465. doi: 10.1097/00006534-200208000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Munhoz AM, Aldrighi CM, Montag E, Arruda EG, Aldrighi JM, Gemperli R, Filassi JR, Ferreira MC. Clinical outcomes following nipple-areola-sparing mastectomy with immediate implant-based breast reconstruction: a 12-year experience with an analysis of patient and breast-related factors for complications. Breast Cancer Res Treat. 2013;140:545–555. doi: 10.1007/s10549-013-2634-7. [DOI] [PubMed] [Google Scholar]
- 34.Blechman KM, Karp NS, Levovitz C, Guth AA, Axelrod DM, Shapiro RL, Choi M. The lateral inframammary fold incision for nipple-sparing mastectomy: outcomes from over 50 immediate implant-based breast reconstructions. Breast J. 2013;19:31–40. doi: 10.1111/tbj.12043. [DOI] [PubMed] [Google Scholar]
- 35.Rivolin A, Kubatzki F, Marocco F, Martincich L, Renditore S, Maggiorotto F, Magistris A, Ponzone R. Nipple-areola complex sparing mastectomy with periareolar pexy for breast cancer patients with moderately ptotic breasts. J Plast Reconstr Aesthet Surg. 2012;65:296–303. doi: 10.1016/j.bjps.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 36.Salibian AH, Harness JK, Mowlds DS. Inframammary approach to nipple-areola-sparing mastectomy. Plast Reconstr Surg. 2013;132:700e–708e. doi: 10.1097/PRS.0b013e3182a4d64f. [DOI] [PubMed] [Google Scholar]
- 37.Jensen JA. When can the nipple-areola complex safely be spared during mastectomy? Plast Reconstr Surg. 2002;109:805–807. doi: 10.1097/00006534-200202000-00060. [DOI] [PubMed] [Google Scholar]
- 38.Laronga C, Kemp B, Johnston D, Robb GL, Singletary SE. The incidence of occult nipple-areola complex involvement in breast cancer patients receiving a skin-sparing mastectomy. Ann Surg Oncol. 1999;6:609–613. doi: 10.1007/s10434-999-0609-z. [DOI] [PubMed] [Google Scholar]
- 39.Endara M, Chen D, Verma K, Nahabedian MY, Spear SL. Breast reconstruction following nipple-sparing mastectomy: a systematic review of the literature with pooled analysis. Plast Reconstr Surg. 2013;132:1043–1054. doi: 10.1097/PRS.0b013e3182a48b8a. [DOI] [PubMed] [Google Scholar]
- 40.Singletary SE, Robb GL. Oncologic safety of skin-sparing mastectomy. Ann Surg Oncol. 2003;10:95–97. doi: 10.1245/aso.2003.01.910. [DOI] [PubMed] [Google Scholar]
- 41.Patani N, Mokbel K. Oncological and aesthetic considerations of skin-sparing mastectomy. Breast Cancer Res Treat. 2008;111:391–403. doi: 10.1007/s10549-007-9801-7. [DOI] [PubMed] [Google Scholar]
- 42.Vyas JJ, Chinoy RF, Vaidya JS. Prediction of nipple and areola involvement in breast cancer. Eur J Surg Oncol. 1998;24:15–16. doi: 10.1016/s0748-7983(98)80117-0. [DOI] [PubMed] [Google Scholar]
- 43.Simmons RM, Brennan M, Christos P, King V, Osborne M. Analysis of nipple/areolar involvement with mastectomy: can the areola be preserved? Ann Surg Oncol. 2002;9:165–168. doi: 10.1007/BF02557369. [DOI] [PubMed] [Google Scholar]
- 44.Murthy V, Chamberlain RS. Nipple-sparing mastectomy in modern breast practice. Clin Anat. 2013;26:56–65. doi: 10.1002/ca.22185. [DOI] [PubMed] [Google Scholar]
- 45.Santini D, Taffurelli M, Gelli MC, Grassigli A, Giosa F, Marrano D, Martinelli G. Neoplastic involvement of nipple-areolar complex in invasive breast cancer. Am J Surg. 1989;158:399–403. doi: 10.1016/0002-9610(89)90272-9. [DOI] [PubMed] [Google Scholar]
- 46.Menon RS, van Geel AN. Cancer of the breast with nipple involvement. Br J Cancer. 1989;59:81–84. doi: 10.1038/bjc.1989.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verma GR, Kumar A, Joshi K. Nipple involvement in peripheral breast carcinoma: a prospective study. Indian J Cancer. 1997;34:1–5. [PubMed] [Google Scholar]
- 48.Loewen MJ, Jennings JA, Sherman SR, Slaikeu J, Ebrom PA, Davis AT, Fitzgerald TL. Mammographic distance as a predictor of nipple-areola complex involvement in breast cancer. Am J Surg. 2008;195:391–394; discussion 391-394. doi: 10.1016/j.amjsurg.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 49.Banerjee A, Gupta S, Bhattacharya N. Preservation of nipple-areola complex in breast cancer--a clinicopathological assessment. J Plast Reconstr Aesthet Surg. 2008;61:1195–1198. doi: 10.1016/j.bjps.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 50.Voltura AM, Tsangaris TN, Rosson GD, Jacobs LK, Flores JI, Singh NK, Argani P, Balch CM. Nipple-sparing mastectomy: critical assessment of 51 procedures and implications for selection criteria. Ann Surg Oncol. 2008;15:3396–3401. doi: 10.1245/s10434-008-0102-0. [DOI] [PubMed] [Google Scholar]
- 51.Pirozzi PR, Rossetti C, Carelli I, Ruiz CA, Pompei LM, Piato S. Clinical and morphological factors predictive of occult involvement of the nipple-areola complex in mastectomy specimens. Eur J Obstet Gynecol Reprod Biol. 2010;148:177–181. doi: 10.1016/j.ejogrb.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 52.Reynolds C, Davidson JA, Lindor NM, Glazebrook KN, Jakub JW, Degnim AC, Sandhu NP, Walsh MF, Hartmann LC, Boughey JC. Prophylactic and therapeutic mastectomy in BRCA mutation carriers: can the nipple be preserved? Ann Surg Oncol. 2011;18:3102–3109. doi: 10.1245/s10434-011-1908-8. [DOI] [PubMed] [Google Scholar]
- 53.Rusby JE, Brachtel EF, Othus M, Michaelson JS, Koerner FC, Smith BL. Development and validation of a model predictive of occult nipple involvement in women undergoing mastectomy. Br J Surg. 2008;95:1356–1361. doi: 10.1002/bjs.6349. [DOI] [PubMed] [Google Scholar]
- 54.Wang J, Xiao X, Wang J, Iqbal N, Baxter L, Skinner KA, Hicks DG, Hajdu SI, Tang P. Predictors of nipple-areolar complex involvement by breast carcinoma: histopathologic analysis of 787 consecutive therapeutic mastectomy specimens. Ann Surg Oncol. 2012;19:1174–1180. doi: 10.1245/s10434-011-2107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turner-Warwick RT. The lymphatics of the breast. Br J Surg. 1959;46:574–582. doi: 10.1002/bjs.18004620004. [DOI] [PubMed] [Google Scholar]
- 56.Handley RS. The early spread of breast carcinoma and its bearing on operative treatment. Br J Surg. 1964;51:206–208. doi: 10.1002/bjs.1800510310. [DOI] [PubMed] [Google Scholar]
- 57.Gerber B, Krause A, Reimer T, Müller H, Küchenmeister I, Makovitzky J, Kundt G, Friese K. Skin-sparing mastectomy with conservation of the nipple-areola complex and autologous reconstruction is an oncologically safe procedure. Ann Surg. 2003;238:120–127. doi: 10.1097/01.SLA.0000077922.38307.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Foster RD, Esserman LJ, Anthony JP, Hwang ES, Do H. Skin-sparing mastectomy and immediate breast reconstruction: a prospective cohort study for the treatment of advanced stages of breast carcinoma. Ann Surg Oncol. 2002;9:462–466. doi: 10.1007/BF02557269. [DOI] [PubMed] [Google Scholar]
- 59.Mallon P, Feron JG, Couturaud B, Fitoussi A, Lemasurier P, Guihard T, Cothier-Savay I, Reyal F. The role of nipple-sparing mastectomy in breast cancer: a comprehensive review of the literature. Plast Reconstr Surg. 2013;131:969–984. doi: 10.1097/PRS.0b013e3182865a3c. [DOI] [PubMed] [Google Scholar]
- 60.Sahin I, Isik S, Alhan D, Yıldız R, Aykan A, Ozturk E. One-staged silicone implant breast reconstruction following bilateral nipple-sparing prophylactic mastectomy in patients at high-risk for breast cancer. Aesthetic Plast Surg. 2013;37:303–311. doi: 10.1007/s00266-012-0044-6. [DOI] [PubMed] [Google Scholar]
- 61.Spear SL, Rottman SJ, Seiboth LA, Hannan CM. Breast reconstruction using a staged nipple-sparing mastectomy following mastopexy or reduction. Plast Reconstr Surg. 2012;129:572–581. doi: 10.1097/PRS.0b013e318241285c. [DOI] [PubMed] [Google Scholar]
- 62.Djohan R, Gage E, Gatherwright J, Pavri S, Firouz J, Bernard S, Yetman R. Patient satisfaction following nipple-sparing mastectomy and immediate breast reconstruction: an 8-year outcome study. Plast Reconstr Surg. 2010;125:818–829. doi: 10.1097/PRS.0b013e3181ccdaa4. [DOI] [PubMed] [Google Scholar]
- 63.Liu TS, Crisera CA, Festekjian JH, Da Lio AL. Staged wise-pattern skin excision for reconstruction of the large and ptotic breast. Plast Reconstr Surg. 2010;126:1831–1839. doi: 10.1097/PRS.0b013e3181f5278f. [DOI] [PubMed] [Google Scholar]
- 64.Yiacoumettis AM. Two staged breast reconstruction following prophylactic bilateral subcutaneous mastectomy. Br J Plast Surg. 2005;58:299–305. doi: 10.1016/j.bjps.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 65.Chung AP, Sacchini V. Nipple-sparing mastectomy: where are we now? Surg Oncol. 2008;17:261–266. doi: 10.1016/j.suronc.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 66.Palmieri B, Baitchev G, Grappolini S, Costa A, Benuzzi G. Delayed nipple-sparing modified subcutaneous mastectomy: rationale and technique. Breast J. 2005;11:173–178. doi: 10.1111/j.1075-122X.2005.21520.x. [DOI] [PubMed] [Google Scholar]
- 67.Jensen JA, Lin JH, Kapoor N, Giuliano AE. Surgical delay of the nipple-areolar complex: a powerful technique to maximize nipple viability following nipple-sparing mastectomy. Ann Surg Oncol. 2012;19:3171–3176. doi: 10.1245/s10434-012-2528-7. [DOI] [PubMed] [Google Scholar]
- 68.Salgarello M, Visconti G, Barone-Adesi L. Nipple-sparing mastectomy with immediate implant reconstruction: cosmetic outcomes and technical refinements. Plast Reconstr Surg. 2010;126:1460–1471. doi: 10.1097/PRS.0b013e3181ef8bce. [DOI] [PubMed] [Google Scholar]
- 69.Moyer HR, Ghazi B, Daniel JR, Gasgarth R, Carlson GW. Nipple-sparing mastectomy: technical aspects and aesthetic outcomes. Ann Plast Surg. 2012;68:446–450. doi: 10.1097/SAP.0b013e3182394bba. [DOI] [PubMed] [Google Scholar]
- 70.Colwell AS, Gadd M, Smith BL, Austen WG. An inferolateral approach to nipple-sparing mastectomy: optimizing mastectomy and reconstruction. Ann Plast Surg. 2010;65:140–143. doi: 10.1097/SAP.0b013e3181c1fe77. [DOI] [PubMed] [Google Scholar]
- 71.Wijayanayagam A, Kumar AS, Foster RD, Esserman LJ. Optimizing the total skin-sparing mastectomy. Arch Surg. 2008;143:38–45; discussion 45. doi: 10.1001/archsurg.143.1.38. [DOI] [PubMed] [Google Scholar]
- 72.Proano E, Perbeck LG. Influence of the site of skin incision on the circulation in the nipple-areola complex after subcutaneous mastectomy in breast cancer. Scand J Plast Reconstr Surg Hand Surg. 1996;30:195–200. doi: 10.3109/02844319609062814. [DOI] [PubMed] [Google Scholar]
- 73.Toth BA, Daane SP. Purse-string mastectomy with immediate prosthetic reconstruction: an improved skin-sparing technique for small breasts. Plast Reconstr Surg. 2003;111:2333–2337. doi: 10.1097/01.PRS.0000060799.03866.57. [DOI] [PubMed] [Google Scholar]
- 74.Hammond DC, Capraro PA, Ozolins EB, Arnold JF. Use of a skin-sparing reduction pattern to create a combination skin-muscle flap pocket in immediate breast reconstruction. Plast Reconstr Surg. 2002;110:206–211. doi: 10.1097/00006534-200207000-00035. [DOI] [PubMed] [Google Scholar]
- 75.Rusby JE, Gui GP. Nipple-sparing mastectomy in women with large or ptotic breasts. J Plast Reconstr Aesthet Surg. 2010;63:e754–e755. doi: 10.1016/j.bjps.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 76.Niemeyer M, Paepke S, Schmid R, Plattner B, Müller D, Kiechle M. Extended indications for nipple-sparing mastectomy. Breast J. 2011;17:296–299. doi: 10.1111/j.1524-4741.2011.01079.x. [DOI] [PubMed] [Google Scholar]
- 77.Alderman AK, Wilkins EG, Kim HM, Lowery JC. Complications in postmastectomy breast reconstruction: two-year results of the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg. 2002;109:2265–2274. doi: 10.1097/00006534-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 78.Gamboa-Bobadilla GM, Killingsworth C. Large-volume reduction mammaplasty: the effect of body mass index on postoperative complications. Ann Plast Surg. 2007;58:246–249. doi: 10.1097/01.sap.0000248108.52837.6c. [DOI] [PubMed] [Google Scholar]
- 79.Woerdeman LA, Hage JJ, Hofland MM, Rutgers EJ. A prospective assessment of surgical risk factors in 400 cases of skin-sparing mastectomy and immediate breast reconstruction with implants to establish selection criteria. Plast Reconstr Surg. 2007;119:455–463. doi: 10.1097/01.prs.0000246379.99318.74. [DOI] [PubMed] [Google Scholar]
- 80.Bailey MH, Smith JW, Casas L, Johnson P, Serra E, de la Fuente R, Sullivan M, Scanlon EF. Immediate breast reconstruction: reducing the risks. Plast Reconstr Surg. 1989;83:845–851. doi: 10.1097/00006534-198905000-00011. [DOI] [PubMed] [Google Scholar]
- 81.Davies K, Allan L, Roblin P, Ross D, Farhadi J. Factors affecting post-operative complications following skin sparing mastectomy with immediate breast reconstruction. Breast. 2011;20:21–25. doi: 10.1016/j.breast.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 82.Spear SL, Hannan CM, Willey SC, Cocilovo C. Nipple-sparing mastectomy. Plast Reconstr Surg. 2009;123:1665–1673. doi: 10.1097/PRS.0b013e3181a64d94. [DOI] [PubMed] [Google Scholar]
- 83.Mosahebi A, Ramakrishnan V, Gittos M, Collier J. Aesthetic outcome of different techniques of reconstruction following nipple-areola-preserving envelope mastectomy with immediate reconstruction. Plast Reconstr Surg. 2007;119:796–803. doi: 10.1097/01.prs.0000251999.52374.09. [DOI] [PubMed] [Google Scholar]
- 84.Grotting JC, Urist MM, Maddox WA, Vasconez LO. Conventional TRAM flap versus free microsurgical TRAM flap for immediate breast reconstruction. Plast Reconstr Surg. 1989;83:828–41; discussion 842-4. doi: 10.1097/00006534-198905000-00009. [DOI] [PubMed] [Google Scholar]
- 85.Feller AM, Hörl HW, Biemer E. The transverse rectus abdominis musculocutaneous free flap: a reliable alternative for delayed autologous tissue breast reconstruction. Ann Plast Surg. 1990;25:425–434. doi: 10.1097/00000637-199012000-00001. [DOI] [PubMed] [Google Scholar]
- 86.Kroll SS, Marchi M. Comparison of strategies for preventing abdominal-wall weakness after TRAM flap breast reconstruction. Plast Reconstr Surg. 1992;89:1045–1051; discussion 1045-1051. [PubMed] [Google Scholar]
- 87.Schusterman MA, Kroll SS, Weldon ME. Immediate breast reconstruction: why the free TRAM over the conventional TRAM flap? Plast Reconstr Surg. 1992;90:255–61; discussion 262. [PubMed] [Google Scholar]
- 88.Elliott LF, Eskenazi L, Beegle PH, Podres PE, Drazan L. Immediate TRAM flap breast reconstruction: 128 consecutive cases. Plast Reconstr Surg. 1993;92:217–227. doi: 10.1097/00006534-199308000-00004. [DOI] [PubMed] [Google Scholar]
- 89.Koshima I, Soeda S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br J Plast Surg. 1989;42:645–648. doi: 10.1016/0007-1226(89)90075-1. [DOI] [PubMed] [Google Scholar]
- 90.Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg. 1994;32:32–38. doi: 10.1097/00000637-199401000-00007. [DOI] [PubMed] [Google Scholar]
- 91.Blondeel PN. One hundred free DIEP flap breast reconstructions: a personal experience. Br J Plast Surg. 1999;52:104–111. doi: 10.1054/bjps.1998.3033. [DOI] [PubMed] [Google Scholar]
- 92.Munhoz AM, Ishida LH, Sturtz GP, Cunha MS, Montag E, Saito FL, Gemperli R, Ferreira MC. Importance of lateral row perforator vessels in deep inferior epigastric perforator flap harvesting. Plast Reconstr Surg. 2004;113:517–524. doi: 10.1097/01.PRS.0000100812.37842.A8. [DOI] [PubMed] [Google Scholar]
- 93.Munhoz AM, Ishida LH, Montag E, Sturtz GP, Saito FL, Rodrigues L, Gemperli R, Ferreira MC. Perforator flap breast reconstruction using internal mammary perforator branches as a recipient site: an anatomical and clinical analysis. Plast Reconstr Surg. 2004;114:62–68. doi: 10.1097/01.prs.0000129074.88594.d7. [DOI] [PubMed] [Google Scholar]
- 94.Munhoz AM, Sturtz G, Montag E, Arruda EG, Aldrighi C, Gemperli R, Ferreira MC. Clinical outcome of abdominal wall after DIEP flap harvesting and immediate application of abdominoplasty techniques. Plast Reconstr Surg. 2005;116:1881–1893. doi: 10.1097/01.prs.0000191186.20698.0d. [DOI] [PubMed] [Google Scholar]
- 95.Yang SJ, Eom JS, Lee TJ, Ahn SH, Son BH. Recipient vessel selection in immediate breast reconstruction with free abdominal tissue transfer after nipple-sparing mastectomy. Arch Plast Surg. 2012;39:216–221. doi: 10.5999/aps.2012.39.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim HJ, Park EH, Lim WS, Seo JY, Koh BS, Lee TJ, Eom JS, Lee SW, Son BH, Lee JW, et al. Nipple areola skin-sparing mastectomy with immediate transverse rectus abdominis musculocutaneous flap reconstruction is an oncologically safe procedure: a single center study. Ann Surg. 2010;251:493–498. doi: 10.1097/SLA.0b013e3181c5dc4e. [DOI] [PubMed] [Google Scholar]
- 97.Gui GP, Tan SM, Faliakou EC, Choy C, A’Hern R, Ward A. Immediate breast reconstruction using biodimensional anatomical permanent expander implants: a prospective analysis of outcome and patient satisfaction. Plast Reconstr Surg. 2003;111:125–138; discussion 139-140. doi: 10.1097/01.PRS.0000037752.95854.41. [DOI] [PubMed] [Google Scholar]
- 98.Maxwell GP. Immediate breast reconstruction using biodimensional anatomical permanent expander implants: a prospective analysis of outcome and patient satisfaction (discussion) Plast Reconstr Surg. 2003;111:139–145. doi: 10.1097/01.PRS.0000037752.95854.41. [DOI] [PubMed] [Google Scholar]
- 99.Mahdi S, Jones T, Nicklin S, McGeorge DD. Expandable anatomical implants in breast reconstructions: a prospective study. Br J Plast Surg. 1998;51:425–430. doi: 10.1054/bjps.1997.0111. [DOI] [PubMed] [Google Scholar]
- 100.Mandrekas AD, Zambacos GJ, Katsantoni PN. Immediate and delayed breast reconstruction with permanent tissue expanders. Br J Plast Surg. 1995;48:572–578. doi: 10.1016/0007-1226(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 101.Salgarello M, Seccia A, Eugenio F. Immediate breast reconstruction with anatomical permanent expandable implants after skin-sparing mastectomy: aesthetic and technical refinements. Ann Plast Surg. 2004;52:358–64; discussion 365-6. doi: 10.1097/01.sap.0000105914.69611.c7. [DOI] [PubMed] [Google Scholar]
- 102.Hayes AJ, Jenkins MP, Sandhu SS, Baum M. Subpectoral breast reconstruction using the biodimensional system. Ann R Coll Surg Engl. 1997;79:355–360. [PMC free article] [PubMed] [Google Scholar]
- 103.Paepke S, Schmid R, Fleckner S, Paepke D, Niemeyer M, Schmalfeldt B, Jacobs VR, Kiechle M. Subcutaneous mastectomy with conservation of the nipple-areola skin: broadening the indications. Ann Surg. 2009;250:288–292. doi: 10.1097/SLA.0b013e3181b0c7d8. [DOI] [PubMed] [Google Scholar]
- 104.Petit JY, Veronesi U, Orecchia R, Luini A, Rey P, Intra M, Didier F, Martella S, Rietjens M, Garusi C, et al. Nipple-sparing mastectomy in association with intra operative radiotherapy (ELIOT): A new type of mastectomy for breast cancer treatment. Breast Cancer Res Treat. 2006;96:47–51. doi: 10.1007/s10549-005-9033-7. [DOI] [PubMed] [Google Scholar]
- 105.Benediktsson KP, Perbeck L. Survival in breast cancer after nipple-sparing subcutaneous mastectomy and immediate reconstruction with implants: a prospective trial with 13 years median follow-up in 216 patients. Eur J Surg Oncol. 2008;34:143–148. doi: 10.1016/j.ejso.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 106.Bistoni G, Rulli A, Izzo L, Noya G, Alfano C, Barberini F. Nipple-sparing mastectomy. Preliminary results. J Exp Clin Cancer Res. 2006;25:495–497. [PubMed] [Google Scholar]
- 107.Babiera G, Simmons R. Nipple-areolar complex-sparing mastectomy: feasibility, patient selection, and technique. Ann Surg Oncol. 2010;17 Suppl 3:245–248. doi: 10.1245/s10434-010-1256-0. [DOI] [PubMed] [Google Scholar]
- 108.Spear SL, Willey SC, Feldman ED, Cocilovo C, Sidawy M, Al-Attar A, Hannan C, Seiboth L, Nahabedian MY. Nipple-sparing mastectomy for prophylactic and therapeutic indications. Plast Reconstr Surg. 2011;128:1005–1014. doi: 10.1097/PRS.0b013e31822b6456. [DOI] [PubMed] [Google Scholar]
- 109.Petit JY, Veronesi U, Orecchia R, Rey P, Martella S, Didier F, Viale G, Veronesi P, Luini A, Galimberti V, et al. Nipple sparing mastectomy with nipple areola intraoperative radiotherapy: one thousand and one cases of a five years experience at the European institute of oncology of Milan (EIO) Breast Cancer Res Treat. 2009;117:333–338. doi: 10.1007/s10549-008-0304-y. [DOI] [PubMed] [Google Scholar]


