Abstract
The first example of one-pot sequential Cope/Rauhut-Currier reactions are reported and used to make functionalized decalin structures with all-carbon quaternary stereocenters. The substrates for the new sequential reaction are generated through a six-step sequence including an enantioselective Birch reduction-allylation reaction which makes the overall process asymmetric.
Keywords: sequential, Cope, Rauhut-Currier, enantioselective
In an age when most any molecular target can be synthesized, a primary focus of current synthetic organic chemistry is to increase the efficiency of synthetic transformations. Tandem or sequential reactions have been an important tool in this regard1–12. Herein we report a one-pot intramolecular Cope/Rauhut-Currier sequence which illustrates the potential for the efficient enantioselective construction of functionalized decalin structures (3, Scheme 1). Building on the Birch-Cope sequence13–15 and Rauhut-Currier application16 that we communicated recently, we now report the ability to conduct the Cope rearrangement and an intramolecular Rauhut-Currier reaction in one pot. There are many tandem reactions involving a Cope rearrangement1,11,12 and there are also a collection of domino reactions involving the Rauhut-Currier reaction17–22, but to the best of our knowledge the two have yet to be combined into a one-pot sequential process.
Scheme 1.
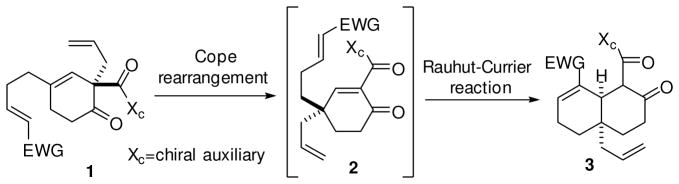
One-pot sequential Cope-Rauhut-Currier to build decalin structures.
The new Cope-Rauhut-Currier sequence may be used to enantioselectively generate valuable functionalized decalin cores with an all-carbon quaternary center. Decalin cores with quaternary stereocenters can be found at the heart of many bioactive natural product classes, including most notably terpenoid compounds. Some recent literature examples of bioactive natural produts with a decalin core include crotonolides23, kauranes24, crotogoudin25, walsucochin B26, cafestol27, and tubingensin A28.
Not surprisingly, given the prevalence of these structures with potentially useful therapeutic activity, there are many approaches to the enantioselective synthesis of decalin rings. Some classic common strategies employed in recent work include the Diels Alder [4+2] cycloaddition29–33, Weiland-Miescher ketone (WMK) synthesis34–39, and cascading polyene cyclizations40–42. Although these strategies have all proven successful and quite efficient, there is always a need for new tools to address the many idiosyncrasies of molecule construction. This is especially true for the particularly complex case of enantioselective synthesis of all-carbon quaternary stereocenters and the frequently used decalin structure, a common and valuable intermediate in bioactive natural product synthesis.
Development of the one-pot Cope/Rauhut-Currier sequence commenced with the synthesis of the starting materials for the Cope rearrangement in six steps (Scheme 2). Beginning with 5-iodosalicylate derivate 415, a Sonogashira cross-coupling reaction appended the necessary three carbon chain. Other procedures, such as Heck cross-coupling or Wittig reactions with a 5-formyl-salicylate derivative, were attempted, but the Sonogashira procedure was the most efficient. The tetrahydropyranyl (THP) ether protected propargylic coupling partner was also the most effective in this procedure as it eliminated a polymerization side reaction. The THP group also offered a stable protecting group for the alcohol functionality in the subsequent Birch reduction-alkylation step. Catalytic hydrogenation reduced the alkyne and enantioselective Birch reduction-allylation43,44 afforded the cyclohexadiene 7 in good yield and excellent enantioselectivity. Concomitant hydrolysis of the enol ether and the THP protecting group was followed by Dess-Martin periodinane oxidation of the primary alcohol to afford aldehyde 8. Finally, Horner-Wadsworth-Emmons conditions were used to add the requisite polarized alkene in 1. The six step process was accomplished in 32–49% overall yield.
Scheme 2.
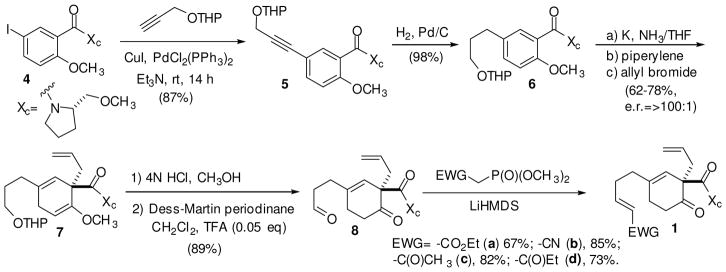
Synthesis of Cope/Rauhut-Currier reaction starting materials.
Initially, Cope rearrangement and the Rauhut-Currier process were explored independently. In the case of 1a and 1b, the Cope rearrangement to 2a and 2b occurred in an unoptimized 68 and 35% yield, respectively. Disappointingly, subjecting 2a or 2b to the Rauhut-Currier conditions that we previously reported16 on similar substrates were unsuccessful. Fortunately, a simple adjustment to a higher boiling alcohol solvent, 2-methyl-1-butanol, and heating the reaction to reflux temperature provided the necessary solution. In the event, 2a was successfully converted to 3a in the requisite Rauhut-Currier reaction with trimethylphosphine (0.3–0.6 eq., 1 M in toluene) and 2-methyl-1-butanol in 56% yield.
The need for even higher temperatures in the Rauhut-Currier reaction immediately suggested that the two processes might be coupled. Indeed, subjecting 1a to the Cope conditions and then, upon completion as monitored by NMR, adding PMe3 and 2-methyl-1-butanol and returning to 130°C, produced the identical product, 3a, in 69% yield; a 30% improvement in yield over the two-pot, two-step process (Table 1). Subjecting the nitrile (1b), methyl ketone (1c) and ethyl ketone (1d) to the same conditions produced good yields of the respective products, 3b-d. The t-butyl ester (not shown) decomposed under the Cope reaction conditions; presumably due to the instability of the t-butyl group at higher temperatures. Attempts to extend this procedure to the synthesis of a homologated seven-member carbocyclic ring with an ethyl ester derivative also failed. The ring junction stereochemistry of 3a-d is tentatively assigned as cis. The 2-carboxamide group is a mixture of stereoisomers and can isomerize.
Table 1.
One-pot sequential Cope/Rauhut-Currier reaction.
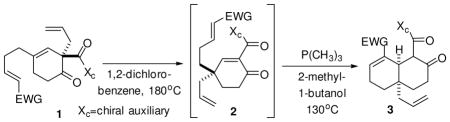
| ||
|---|---|---|
| entry | EWG | One-pot Sequential Cope/Rauhut-Currier yield of 3 |
| 1 | -CO2Et (a) | 69% |
| 2 | -CN (b) | 65 |
| 3 | -C(=O)CH3 (c) | 81 |
| 4 | -C(=O)CH2CH3 (d) | 76 |
In conclusion, the first example of a one-pot sequential Cope/Rauhut-Currier reaction has been accomplished. The current procedure for the synthesis of decalin cores compliments our previous work16 which generated 6–5 bicarbocyclic structures with Rauhut-Currier reactions. The highly functionalized decalin structure that results from the one-pot sequential Cope/Rauhut-Currier reaction has the potential to be a valuable intermediate in the synthesis of complex bioactive natural products.
Supplementary Material
Acknowledgments
The project described was supported by Award Number R15GM087291 from the National Institute Of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of General Medical Sciences or the National Institutes of Health. The authors would like to thank Bryn Mawr College for additional financial support. One author (TMR) is indebted to Johnson & Johnson Pharmaceutical Research Institute and Hoffmann-La Roche for financial support as well.
Footnotes
General experimental details; copies of 1H and 13C NMR spectra, IR spectra, gas and liquid chromatographs and mass spectra for compounds 1a-d, 3a-d, and 5–9. This material is available free of charge via the Internet at doi:.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jones AC, May JA, Sarpong R, Stoltz BM. Angewandte Chemie International Edition. 2014;53:2556. doi: 10.1002/anie.201302572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pellissier H. Tetrahedron. 2013;69:7171. [Google Scholar]
- 3.Pellissier H. Tetrahedron. 2006;62:1619. [Google Scholar]
- 4.Pellissier H. Tetrahedron. 2006;62:2143. [Google Scholar]
- 5.Dömling A. Chemical Reviews. 2005;106:17. doi: 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]
- 6.Multicomponent Reactions. Wiley-VCH; Weinheim: 2005. [Google Scholar]
- 7.Guillena G, Ramón DJ, Yus M. Tetrahedron: Asymmetry. 2007;18:693. [Google Scholar]
- 8.Ramón DJ, Yus M. Angewandte Chemie International Edition. 2005;44:1602. doi: 10.1002/anie.200460548. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Wan JP. Organic & Biomolecular Chemistry. 2011;9:6873. doi: 10.1039/c1ob05769c. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Lee D. European Journal of Organic Chemistry. 2011;2011:4269. [Google Scholar]
- 11.Davies HML, Lian Y. Accounts of Chemical Research. 2012;45:923. doi: 10.1021/ar300013t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Touré BB, Hall DG. Chemical Reviews. 2009;109:4439. doi: 10.1021/cr800296p. [DOI] [PubMed] [Google Scholar]
- 13.Paul T, Malachowski WP, Lee J. J Org Chem. 2007;72:930. doi: 10.1021/jo0621423. [DOI] [PubMed] [Google Scholar]
- 14.Malachowski WP, Paul T, Phounsavath S. J Org Chem. 2007;72:6792. doi: 10.1021/jo070976v. [DOI] [PubMed] [Google Scholar]
- 15.Paul T, Malachowski WP, Lee J. Org Lett. 2006;8:4007. doi: 10.1021/ol0615228. [DOI] [PubMed] [Google Scholar]
- 16.Qiao Y, Kumar S, Malachowski WP. Tetrahedron Letters. 2010;51:2636. [Google Scholar]
- 17.Hu FL, Wei Y, Shi M. Advanced Synthesis & Catalysis. 2014;356:736. [Google Scholar]
- 18.Zhou R, Wang J, Yu J, He Z. The Journal of Organic Chemistry. 2013;78:10596. doi: 10.1021/jo401363u. [DOI] [PubMed] [Google Scholar]
- 19.Xie P, Huang Y. European Journal of Organic Chemistry. 2013;2013:6213. [Google Scholar]
- 20.Hu C, Geng Z, Ma J, Huang Y, Chen R. Chemistry – An Asian Journal. 2012;7:2032. doi: 10.1002/asia.201200308. [DOI] [PubMed] [Google Scholar]
- 21.Liu W, Zhou J, Zheng C, Chen X, Xiao H, Yang Y, Guo Y, Zhao G. Tetrahedron. 2011;67:1768. [Google Scholar]
- 22.Yao W, Wu Y, Wang G, Zhang Y, Ma C. Angewandte Chemie International Edition. 2009;48:9713. doi: 10.1002/anie.200905091. [DOI] [PubMed] [Google Scholar]
- 23.Liu CP, Xu JB, Zhao JX, Xu CH, Dong L, Ding J, Yue JM. Journal of Natural Products. 2014;77:1013. doi: 10.1021/np500042c. [DOI] [PubMed] [Google Scholar]
- 24.Wu HY, Zhan R, Wang WG, Jiang HY, Du X, Li XN, Li Y, Pu JX, Sun HD. Journal of Natural Products. 2014;77:931. doi: 10.1021/np4010135. [DOI] [PubMed] [Google Scholar]
- 25.Breitler S, Carreira EM. Angewandte Chemie International Edition. 2013;52:11168. doi: 10.1002/anie.201305822. [DOI] [PubMed] [Google Scholar]
- 26.Xu S, Gu J, Li H, Ma D, Xie X, She X. Organic Letters. 2014;16:1996. doi: 10.1021/ol500553x. [DOI] [PubMed] [Google Scholar]
- 27.Zhu L, Luo J, Hong R. Organic Letters. 2014;16:2162. doi: 10.1021/ol500623w. [DOI] [PubMed] [Google Scholar]
- 28.Goetz AE, Silberstein AL, Corsello MA, Garg NK. Journal of the American Chemical Society. 2014;136:3036. doi: 10.1021/ja501142e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henderson JR, Parvez M, Keay BA. Organic Letters. 2009;11:3178. doi: 10.1021/ol901372m. [DOI] [PubMed] [Google Scholar]
- 30.Yuan C, Du B, Yang L, Liu B. Journal of the American Chemical Society. 2013;135:9291. doi: 10.1021/ja4040335. [DOI] [PubMed] [Google Scholar]
- 31.Schubert M, Metz P. Angewandte Chemie International Edition. 2011;50:2954. doi: 10.1002/anie.201007551. [DOI] [PubMed] [Google Scholar]
- 32.Yoshimura F, Tanino K, Miyashita M. Accounts of Chemical Research. 2012;45:746. doi: 10.1021/ar200267a. [DOI] [PubMed] [Google Scholar]
- 33.Phoenix S, Reddy MS, Deslongchamps P. Journal of the American Chemical Society. 2008;130:13989. doi: 10.1021/ja805097s. [DOI] [PubMed] [Google Scholar]
- 34.Schmalzbauer B, Herrmann J, Müller R, Menche D. Organic Letters. 2013;15:964. doi: 10.1021/ol400156u. [DOI] [PubMed] [Google Scholar]
- 35.Jung ME, Guzaev M. The Journal of Organic Chemistry. 2013;78:7518. doi: 10.1021/jo400909t. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen TX, Dakanali M, Trzoss L, Theodorakis EA. Organic Letters. 2011;13:3308. doi: 10.1021/ol200966z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Enomoto M, Morita A, Kuwahara S. Angewandte Chemie International Edition. 2012;51:12833. doi: 10.1002/anie.201206299. [DOI] [PubMed] [Google Scholar]
- 38.Yokoe H, Mitsuhashi C, Matsuoka Y, Yoshimura T, Yoshida M, Shishido K. Journal of the American Chemical Society. 2011;133:8854. doi: 10.1021/ja202874d. [DOI] [PubMed] [Google Scholar]
- 39.Bradshaw B, Etxebarria-Jardí G, Bonjoch J. Journal of the American Chemical Society. 2010;132:5966. doi: 10.1021/ja101994q. [DOI] [PubMed] [Google Scholar]
- 40.Jeker OF, Kravina AG, Carreira EM. Angewandte Chemie International Edition. 2013;52:12166. doi: 10.1002/anie.201307187. [DOI] [PubMed] [Google Scholar]
- 41.Domingo V, Arteaga JF, López Pérez JL, Peláez R, Quílez del Moral JF, Barrero AF. The Journal of Organic Chemistry. 2011;77:341. doi: 10.1021/jo201968t. [DOI] [PubMed] [Google Scholar]
- 42.Zhao YJ, Loh TP. Organic Letters. 2008;10:2143. doi: 10.1021/ol800499p. [DOI] [PubMed] [Google Scholar]
- 43.Schultz AG. Chemical Communications. 1999:1263. [Google Scholar]
- 44.Schultz AG, Macielag M, Sundararaman P, Taveras AG, Welch M. J Am Chem Soc. 1988;110:7828. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


