Abstract
A 64-year-old woman presented with an acute onset of myelitis and optic neuritis after 47 months of etanercept use for rheumatoid arthritis. Etanercept was discontinued and pulse methylprednisolone therapy (1000 mg/day for 3 days) was started, followed by a quick and complete resolution. Demyelination associated with antitumor necrosis factor agents, reported to develop mostly from 1 week to 12 months after the initiation of the agents, could develop after a few years and thus warrants vigilant monitoring.
Background
Tumor necrosis factor-α (TNF-α) is a proinflammatory cytokine that plays an important role in the pathogenesis of rheumatoid arthritis (RA), ankylosing spondylitis, psoriatic arthritis, inflammatory bowel disease and various other autoimmune diseases. Currently, five anti-TNF agents are available and have been widely used.1–4 While these agents are generally very effective against these diseases, they have been associated with rare but serious adverse events, such as infectious diseases, neoplasm, autoimmune diseases, demyelination and heart failure.5–9
Demyelination associated with anti-TNF agents came to be widely known by the report of Mohan et al,8 which described 19 patients with demyelination development during anti-TNF therapy (17 patients with etanercept and 2 patients with infliximab). Furthermore, an aggravation of disease activity of multiple sclerosis during lenercept, a p55 TNF-receptor fusion protein conjugated to the Fc region of human IgG, also suggested the association between anti-TNF agents and demyelination.10 According to Mohan's report, demyelination associated with anti-TNF agents developed, on average, 5 months after their initiation (with the range from 1 week to 15 months).8 We experienced a case that developed demyelination 47 months after etanercept was started.
Case presentation
A 64-year-old woman was referred to our hospital for a recent onset of symmetrical wrist and digital joint pain with morning stiffness. Her comorbidity included autoimmune hepatitis and Sjögren's syndrome. Asymptomatic antiphospholipid antibody seropositivity had been also known. On examination, she was noted to have swelling and tenderness in the wrist, knees and multiple digital and toe joints. Rheumatoid factor and anticyclic citrullinated peptide antibody (anti-CCP antibody) were positive. She was therefore diagnosed with RA and was started on bucillamine and prednisolone 7.5 mg/day. As arthritic activity persisted, methotrexate 6 mg/week was started instead of bucillamine. Then etanercept 50 mg/week was subsequently added, leading to clinical remission. Prednisolone was tapered to 3 mg/day.
Forty-seven months after the addition of etanercept, she experienced an acute onset of muscle weakness of the left leg and of hypoesthesia and dysesthaesia in the left leg and left buttock area. These symptoms progressed and made her visit our hospital 3 days after the onset. Physical examination revealed decreased muscle strength in the left leg and hyper-reflexia in the left Achilles and patellar tendons. Tactile hypoesthesia and dysesthaesia in the left side below the Th9 level were observed.
Investigations
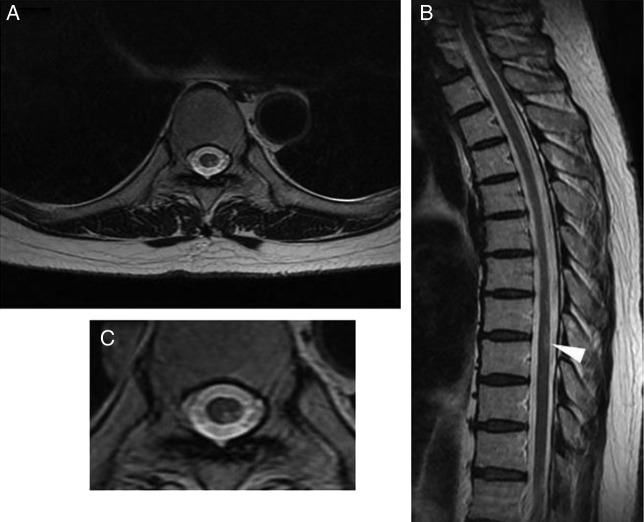
Laboratory tests revealed normal blood cell counts and normal liver and kidney functions. Cerebrospinal fluid analysis revealed normal cell count (1 cell/mm3), normal protein (30 mg/dL) and glucose levels (57 mg/dL), but an elevated IgG index (0.94, normal range <0.6). Oligoclonal band was noted. Myelin basic protein or antiaquaporin-4 antibody (examined by ELISA) was not detected. T2-weighted MRI revealed a high intensity lesion in the left posterior area of the spinal cord at the Th8–9 level (figure 1A–C). Abnormal signal was not detected in the cerebrum. We tested for lupus serology because anti-TNF agents are associated with a new-onset systemic lupus erythematosus (SLE),7 only to find a slight elevation in IgG antidouble-stranded and antisingle-stranded DNA antibody titres (13 IU/mL (normal range <12) and 28 U/mL (normal range <25), examined by ELISA, respectively) and normal complement levels. Although she did not notice any visual change, we performed a visual evoked potential in search of subclinical optic nerve lesions, which showed an extension of P100 latency in both eyes suggesting optic nerve damage.
Figure 1.
T2-weighted MRI showed a high intensity lesion in the left posterior area (A and C) of the spinal cord at the Th8–9 (B, arrowhead).
Treatment
Since etanercept was known to cause myelitis and optic neuritis, it was discontinued on admission. As her clinical course was acutely progressive, we started pulse methylprednisolone therapy (1000 mg/day for 3 days). Pulse therapy was followed by oral prednisolone 60 mg/day (1 mg/kg/day) with a quick tapering over 4 weeks down to 15 mg/day and then gradually to her maintenance dose of 3 mg/day.
Outcome and follow-up
Her muscle weakness started to show significant improvement on the second day of pulse therapy and quickly normalised within a few days and her sensory abnormality resolved gradually over 2 weeks. Abnormal signal in the spinal cord was not observed in the MRI obtained in 4 weeks. When the dose of prednisolone reached 3 mg/day her arthritis flared. Tocilizumab, a humanised anti-interleukin-6 receptor antibody, was started and she quickly achieved clinical remission again and has since remained neurologically symptom-free for 3 years.
Discussion
When patients present with a new-onset encephalomyelitis, potential causes include clinically isolated syndrome, neuromyelitis optica (NMO), autoimmune diseases (SLE, mixed connective tissue disease, Sjögren’s syndrome, Behçet disease and antiphospholipid antibody syndrome, thrombosis, infectious diseases (virus, bacteria and mycobacteria), vaccination, drugs (anti-TNF agents) and paraneoplastic syndrome.11 12 Furthermore, in many such cases, it is not possible to definitively identify a single cause.
In our case, clinical presentation, serological and radiographic findings, and quick responses to discontinuation of potentially offending drugs, administration of high-dose glucocorticoids, or both all suggested myelitis and optic neuritis associated with an anti-TNF agent, etanercept. Sjögren's syndrome remained possible, but we considered it less likely to be a sole cause in our case as myelitis and optic neuritis associated with Sjögren's syndrome have been reported to be generally refractory to glucocorticoids.13 14 Although it is possible that this episode denotes the onset of multiple sclerosis, the patient has not experienced additional neurological events so far. In addition, she did not fulfil the criteria of NMO,15 as she neither showed contiguous spinal cord lesion extending three nor more segments nor had detectable antiaquaporin-4 antibodies in serum. Furthermore, oligoclonalband, which is typically absent in patients with NMO, was detected in our case.16
Mohan et al8 experienced a case that developed demyelination while receiving an anti-TNF agent and subsequent investigation using the database of Adverse Events Reporting System of the US Food and Drug Administration (FDA) revealed that the other 18 patients also developed demyelination during anti-TNF therapy. The most common symptom was sensory disturbance (13/19), followed by visual disturbance (8/19), gait disorder, psychotic manifestation and paralysis of the facial nerve. In most cases, anti-TNF agents were discontinued and glucocorticoids were used temporarily, followed by a prompt resolution of presenting symptoms.8 Others reported a reappearance of symptoms after re-exposure.17 Our case developed myelitis and optic neuritis 47 months after etanercept was added. A PubMed search revealed that most cases developed demyelination during the first year of anti-TNF agent administration.17 18 However, some cases started suffering from demyelination more than 30 months after anti-TNF agents were started. Among patients with RA, the longest period from the initiation of anti-TNF agent to the development of demyelination was 42 months (infliximab) and that was 49 months among all recipients (infliximab).17 19 20 These reports indicate that demyelination could develop long after anti-TNF agents are started.
De novo development of demyelination in recipients of anti-TNF agents and aggravation of disease activity in multiple sclerosis patients treated with lenercept and infliximab10 21 suggest either direct or indirect contribution of anti-TNF agents on demyelination. One proposed hypothesis is that systemically administered anti-TNF agents may inhibit the apoptosis of self-reactive T cells but fail to penetrate and reach the central nervous system (CNS), thereby inducing and exaggerating an autoimmune process of demyelination in the CNS. The other hypothesis is that an activation of latently infecting microorganisms may result in demyelination.22 Further research should be conducted to elucidate the mechanism of this complication of anti-TNF agents.
Recent systematic review again suggested a possible causal relationship between demyelinating disease and anti-TNF agents.20 However, no risk factor has so far been identified. Our case was immunologically active, having multiple autoantibodies including antinuclear antibodies, rheumatoid factor, anti-CCP antibody, anti-ss-DNA antibody, anti-SS-A antibody, and antiphospholipid antibody, which might have contributed to immunological dysregulation by the anti-TNF agent. In addition, it is interesting to note that the anti-IL-6 agent, tocilizumab, has not evoked autoimmune diseases such as SLE, vasculitis syndrome or demyelinating disease, all of which have been induced by anti-TNF agents.23
Demyelination caused by anti-TNF agents is a rare but serious adverse event. Our experience and others indicate that demyelination could develop long after anti-TNF agents are started and thus warrants vigilant monitoring in their recipients.
Learning points.
Demyelination could develop long after anti-TNF agents are started.
Acknowledgments
Kayoko Kaneko; Akio Yamamoto; Hidehiro Mizusawa; Mutsufusa Watanabe; Takayuki Kubodera.
Footnotes
Contributors: WY was involved in the acquisition of the data and drafting of the manuscript. KT contributed to the critical revision of the manuscript and the interpretation of the data. NM and HK were involved in the critical revision of the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Feldmann M. Development of anti-TNF therapy for rheumatoid arthritis. Nat Rev Immunol 2002;2:364–71 [DOI] [PubMed] [Google Scholar]
- 2.McLeod C, Bagust A, Boland A, et al. Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation. Health Technol Assess 2007;11:1–158, iii–iv [DOI] [PubMed] [Google Scholar]
- 3.Heiberg MS, Kaufmann C, Rødevand E, et al. The comparative effectiveness of anti-TNF therapy and methotrexate in patients with psoriatic arthritis: 6 month results from a longitudinal, observational, multicentre study. Ann Rheum Dis 2007;66:1038–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dretzke J, Edlin R, Round J, et al. A systematic review and economic evaluation of the use of tumour necrosis factor-alpha (TNF-α) inhibitors, adalimumab and infliximab, for Crohn's disease. Health Technol Assess 2011;15:1–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakai R, Komano Y, Tanaka M, et al. Time-dependent increased risk for serious infection from continuous use of tumor necrosis factor antagonists over three years in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:1125–34 [DOI] [PubMed] [Google Scholar]
- 6.Askling J, van Vollenhoven RF, Granath F, et al. Cancer risk in patients with rheumatoid arthritis treated with anti-tumor necrosis factor alpha therapies: does the risk change with the time since start of treatment? Arthritis Rheum 2009;60:3180–9 [DOI] [PubMed] [Google Scholar]
- 7.De Bandt M, Sibilia J, Le Loët X, et al. Systemic lupus erythematosus induced by anti-tumour necrosis factor alpha therapy: a French national survey. Arthritis Res Ther 2005;7:R545–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohan N, Edwards ET, Cupps TR, et al. Demyelination occurring during anti-tumor necrosis factor alpha therapy for inflammatory arthritides. Arthritis Rheum 2001;44:2862–9 [DOI] [PubMed] [Google Scholar]
- 9.Kwon HJ, Coté TR, Cuffe MS, et al. Case reports of heart failure after therapy with a tumor necrosis factor antagonist. Ann Intern Med 2003;138:807–11 [DOI] [PubMed] [Google Scholar]
- 10.[No authors listed] TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. Neurology 1999;53:457–65 [PubMed] [Google Scholar]
- 11.Bhat A, Naguwa S, Cheema G, et al. The epidemiology of transverse myelitis. Autoimmun Rev 2010;9:A395–9 [DOI] [PubMed] [Google Scholar]
- 12.Tristano AG. [Autoimmune diseases associated with transverse myelitis. Review]. Invest Clin 2009;50:251–70 [PubMed] [Google Scholar]
- 13.Alexander E. Central nervous system disease in Sjögren's syndrome. New insights into immunopathogenesis. Rheum Dis Clin North Am 1992;18:637–72 [PubMed] [Google Scholar]
- 14.Alexander GE, Provost TT, Stevens MB, et al. Sjögren syndrome: central nervous system manifestations. Neurology 1981;31:1391–6 [DOI] [PubMed] [Google Scholar]
- 15.Wingerchuk DM, Lennon VA, Pittock SJ, et al. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006;66:1485–9 [DOI] [PubMed] [Google Scholar]
- 16.Jarius S, Paul F, Franciotta D, et al. Cerebrospinal fluid findings in aquaporin-4 antibody positive neuromyelitis optica: results from 211 lumbar punctures. J Neurol Sci 2011;306:82–90 [DOI] [PubMed] [Google Scholar]
- 17.Seror R, Richez C, Sordet C, et al. Pattern of demyelination occurring during anti-TNF-α therapy: a French national survey. Rheumatology (Oxford) 2013;52:868–74 [DOI] [PubMed] [Google Scholar]
- 18.Tanno M, Nakamura I, Kobayashi S, et al. New-onset demyelination induced by infliximab therapy in two rheumatoid arthritis patients. Clin Rheumatol 2006;25:929–33 [DOI] [PubMed] [Google Scholar]
- 19.Mercieca C, Vella N, Borg AA. Demyelination during anti-TNFα therapy for ankylosing spondylitis. Mod Rheumatol 2012;22:303–7 [DOI] [PubMed] [Google Scholar]
- 20.Cruz Fernández-Espartero M, Pérez-Zafrilla B, Naranjo A, et al. Demyelinating disease in patients treated with TNF antagonists in rheumatology: data from BIOBADASER, a pharmacovigilance database, and a systematic review. Semin Arthritis Rheum 2011;41:524–33 [DOI] [PubMed] [Google Scholar]
- 21.van Oosten BW, Barkhof F, Truyen L, et al. Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology 1996;47:1531–4 [DOI] [PubMed] [Google Scholar]
- 22.Robinson WH, Genovese MC, Moreland LW. Demyelinating and neurologic events reported in association with tumor necrosis factor alpha antagonism: by what mechanisms could tumor necrosis factor alpha antagonists improve rheumatoid arthritis but exacerbate multiple sclerosis? Arthritis Rheum 2001;44:1977–83 [DOI] [PubMed] [Google Scholar]
- 23.Ramos-Casals M, Brito-Zerón P, Muñoz S, et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore) 2007;86:242–51 [DOI] [PubMed] [Google Scholar]