Abstract
Background
The medial patellofemoral ligament (MPFL) is essential for the maintenance of correct biomechanical function of the knee. Reconstruction of the MPFL is commonly used in the restoration of patellofemoral stability after traumatic lateral subluxation of the patella. Although a method to accurately determine the MPFL's insertion point has been described, it remains unclear if anatomic placement of MPFL graft tissue is essential for preservation of knee function after MPFL reconstruction. Thus, the purpose of this study was to determine the importance of anatomic placement of MPFL graft tissue for the preservation of knee function following MPFL reconstruction operations.
Methods
Twenty-seven subjects who underwent MPFL reconstruction operations were retrospectively analyzed. Postoperative radiographs were reviewed. Measurements were taken, and the placement of each patient's MPFL graft tissue was determined to be anatomic or non-anatomic based on radiographic methods previously described in the literature. Each subject's electronic medical record was then reviewed, and clinical data was recorded. Finally, the clinical outcomes of each patient were compared to placement location of the MPFL graft tissue in their procedure.
Results
Thirteen patients were found to have anatomic MPFL graft tissue placement, and 14 non-anatomic. A significant post-operative difference was found between groups in the following parameters: WOMAC pain (anatomic mean = 85.71 ± 11.34, non-anatomic mean = 75.00 ± 26.35 p = 0.018), function (anatomic mean = 85.85 ± 9.96, non-anatomic mean = 79.09 ± 24.45, p = 0.017) and in KOOS symptom (anatomic mean = 75.63 ± 11.79, non-anatomic mean = 67.83 ± 22.40, p = 0.024), pain (anatomic mean = 77.54 ± 8.61, non-anatomic mean = 71.39 ± 25.18, p = 0.01), ADL (anatomic mean = 85.85 ± 9.97, non-anatomic mean = 79.09 ± 24.45, p = 0.017) and overall (anatomic mean = 74.61 ± 10.33, non-anatomic mean = 69.41 ± 24.25, p = 0.01) scores. No significant difference was observed for post-op instability (p = 0.290) or apprehension (p = 0.496), improvement in WOMAC or KOOS, 2-week, 6-week, or final 1-year range of motion, WOMAC stiffness, or KOOS sport/recreation or QOL.
Conclusion
Within the range of graft placement values considered by this study, while no reduction in range of motion was seen, non-anatomic placement of MPFL graft tissue in MPFL reconstruction operations caused increased pain and decreased function, evidenced by post-operative KOOS and WOMAC scores.
Clinical Relevance
It seems that the pivotal step in MPFL reconstruction operations is ensuring correct patellofemoral tracking via intraoperative electrical femoral nerve stimulation. If this step of the procedure is performed correctly, non-anatomic placement will not limit range of motion, lead to continued apprehension, or affect the overall biomechanical functioning of the knee.
Keywords: medial patellofemoral ligament (MPFL), patellar instability, lateral patellar subluxation, MFPL graft tissue placement, anatomy, radiographic landmarks, outcome scores, WOMAC, KOOS
Introduction:
The medial patellofemoral ligament (MPFL) guides the patella into the trochlear groove during the first 30 degrees of knee flexion11, 22. With bony anatomy, other ligamentous restraints, and the dynamic action of the quadriceps, it keeps the patella in correct alignment in the early stages of knee flexion when the bone has yet to engage the trochlear groove11, 22. It provides a connection between the patella and the femur, stabilizing and tethering the patella as it travels in the groove11, 22. It has been established that the MPFL inhibits lateral subluxation of the patella. It is not an isometric structure, and is tighter in extension than in flexion11, 22. Because of this discrepancy, the ligament allows the knee to enter full flexion without the structure being damaged11, 22.
The tethering function that is present at the initiation of flexion ensures that the patella enters the trochlear groove, avoiding pain, apprehension, and loss of function associated with subluxation10'22. Population-wide lateral patellar subluxation is common12. Certain anatomic variants, such as patella alta and vertical positioning of the patella, make lateral subluxation more likely14. With lateral subluxation, the MPFL is often disrupted. Lateral patellar subluxation is most often traumatic, and commonly results from injuries sustained while engaging in sporting activities or other forms of vigorous exercise16. Although their exact mechanism varies, these injuries involve lateral translocation of the patella beyond the lateral border of the trochlear groove, resulting in rupture of the MPFL and the medial capsule16. They do, however, always involve valgus motion and external rotation of the extended knee, which cause the patella to miss entry to the trochlear groove leading to lateral translocation and patellar subluxation in flexion16.
When this occurs, a partial or total tear of the MPFL can result. In many cases after this trauma, surgical correction of the MPFL is not necessary and gentle medial force can be applied to the patella as the knee is extended to reduce the structure back into the correct anatomic position16, 22. In some cases, however, surgical MPFL reconstruction is indicated16, 18. One of the most common scenarios necessitating surgical MPFL reconstruction is correction of chronic lateral patellar subluxation16, 18. Chronic lateral patellar subluxation can greatly hinder the performance of an athlete, and lead to loss of functionality of the knee joint and great suffering in the individual16, 18. If this ligamentous laxity is not corrected surgically, the function of the knee joint may be chronically compromised16, 18.
Palmer first recognized the importance of correct graft positioning for ligamentous reconstruction operations in 19383, 6. In his research on anterior cruciate ligament (ACL) reconstruction, he found that placement of the graft tunnel in the correct anatomic position lead to improved clinical results1, 2, 6. As a result of his work, the clinical outcome of anatomic vs. non-anatomic placement of ACL graft tissue is now well-known4,5,6. It is hypothesized that the same parameter holds true with the placement of the MPFL graft tissue during surgical reconstruction of the MPFL16.
MPFL reconstruction procedures generally yield excellent results, even in the presence of degenerative conditions such as trochlear dysplasia15,16,18. In patients with recurrent lateral subluxation, however, a significantly higher failure rate has been demonstrated18. Patellofemoral joint hypermobility has been linked to below-average functional improvements after the procedure19. Case series and other previous work have suggested that incorrect graft placement may cause continued patellar apprehension, subluxation, and dislocation, as well as pain, limited motion, and arthritis7,10. Incorrect graft placement has also been shown to lead to lengthening of the graft post-operatively, and cause application of increased force to the medial patellofemoral cartilage23,24. Anatomic graft placement is technically difficult to achieve20. Nonetheless, redislocation after surgery is uncommon and patient satisfaction is high21.
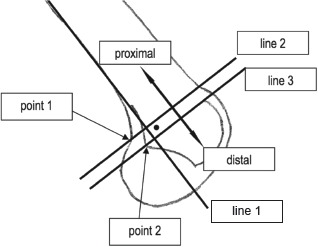
In determination of the importance of correct anatomic placement of MPFL graft tissue during MPFL reconstruction operations, location of this anatomic connection of the MPFL to the medial aspect of the femur is paramount16. Other authors have described a method to determine graft placement based on post-operative radiographs6. Schottle et al. outlines a method to reliably and systematically locate this point of MPFL insertion (Figure 1)6 and defines the anatomic point as 1.3 mm anterior to the extension of the posterior cortex, 2.5 mm distal to a perpendicular line through the origin of the posterior femoral condyle, and 3 mm proximal to a perpendicular line through the posterior aspect of the Blumensaat line6.
Figure 1. Schottle Anatomic MPFL Positioning Method6.
Bollier et al. published a case series that demonstrated the frequency by which the anatomic ideal point of femoral insertion is hit during MPFL reconstruction operations7. The purpose of our study is to further explore this issue, investigating the effect of non-anatomic graft placement on range of motion, pain in the knee, and functional outcome scores. We hypothesized that patients with MPFL placement closest to anatomic have the lowest incidence of patellar instability and apprehension, greatest improvement in [Western Ontario and McMaster Universities Arthritis Index (WOMAC)] and [Knee injury and Osteoarthritis Outcome Score (KOOS)] scores, and best achievement of early range of motion.
During MPFL reconstruction operations, including the Fulkerson Osteotomy and MPFL reconstruction, using the senior author's technique, femoral nerve stimulation is used to both determine correct tracking of the patella and MPFL isometry9,13,16. This step ensures that, during entry into knee flexion, the patella is centered on the lateral trochlear edge and is congruent.9,13,16. After correct patellar tracking is verified, reconstruction of the MPFL is performed to provide a check-reign and eliminate the apprehension sign9,13,16. Thus, the function of the MPFL is not to force the patella to track correctly, but rather to tether the patella (much like a dog on a leash) while it tracks in the trochlear groove. This function cannot be sufficiently performed if underlying patellofemoral biomechanics are disrupted.9,13,16,17.
Methods
Patients who underwent Fulkerson Osteotomy procedures involving MPFL reconstruction performed by the senior author between the years of 2006 and 2012 were considered for the study. Before being included in the study population, patients had to meet a series of criteria. These criteria included the following:
Patient had adequate post-operative radiographs that clearly displayed MPFL tunnel and surgical placement of MPFL graft tissue in the femur.
Patient's electronic medical record contained both pre-operative and post-operative functional scores (WOMAC and KOOS) as well as range of motion at two weeks, six weeks and final 1 year follow-up.
Twenty-seven subjects were ultimately considered as the study population. These 27 subjects who underwent MPFL reconstruction were retrospectively analyzed for MFPL graft tissue placement relative to the anatomic ideal. The total distance from anatomic ideal was determined trigonometrically by first measuring the two distances (anterior or posterior, and proximal or distal to ideal), then determining the actual geographic distance from anatomic ideal using the Pythagorean theorem (Figure 2). The Pythagorean theorem states that for any right triangle, the length of the side opposite the right angle is equal to the square root of the square of one side plus the square of the other side, or 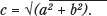 . Using this method, ‘c,’ or the actual geographic distance between point of MPFL graft placement and anatomic ideal, was calculated.
. Using this method, ‘c,’ or the actual geographic distance between point of MPFL graft placement and anatomic ideal, was calculated.
Figure 2. Sample radiograph, showing the distance anterior and proximal of the femoral tunnel to the anatomic ideal described by Schottle et al. 6.
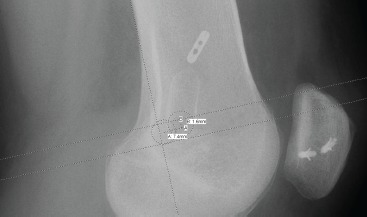
Figure 3. Anatomic vs. Non-anatomic Determination Method 7.
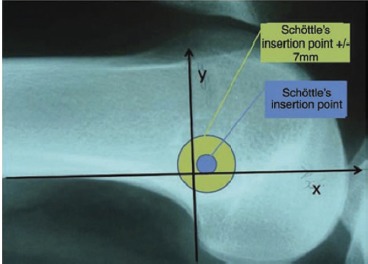
A guide pin is placed intraoperatively to mark the desired location of the MPFL tunnel, then a cannulated drill bit (7 mm in diameter) is placed over the pin. Since the drill bit is 7 mm in diameter (used for radiographic location of the intended tunnel), the distance from the center of the drill bit to the edge (its radius, 3.5 mm) plus a one-drill-bit-diameter (7 mm) margin of error was found to equal 10.5 mm. Clinical exam by intraoperative femoral nerve stimulation of the quadriceps muscle is used in each case to determine isometry of the graft and maximum patellofemoral congruency.
Anatomic placement was determined using the method described by Schottle et al6. Placement of the MPFL tunnel center less than 10.5 mm from the anatomic ideal was designated to be anatomic, and placement greater than 10.5 mm was designated to be non-anatomic. This determination was calculated using a 7 mm margin of error from the edge of Schottle's ideal femoral tunnel point, and based on intra-operative practices during MPFL reconstruction operations.
Functional scores including WOMAC (pain, stiffness, and function) and KOOS (symptom, pain, function in daily living (ADL), sport/recreation, knee related quality of life (QOL), and overall scale) were then recorded and analyzed at two weeks, six weeks and final 1 year follow-up. Range of motion at two weeks, six weeks and final 1 year follow-up was recorded, and patient-reported problems with knee flexion were recorded. Inter- and intra-rater reliability were pursued by performance of all measurements twice each by two investigators.
In the final step of the data analysis, the clinical data that was gathered was compared with the placement of the MPFL graft tissue tunnel on the lateral radiographs. SPSS Statistical software (IBM Corp) was used to perform statistical comparison and analysis of the data gathered. Chi square and independent samples t-tests were performed.
Results
The study population was comprised of 10 males and 17 females, with a mean age of 23.48 ± 8.31, an average height of 171.5 cm ± 11.15, and an average weight of 79.83 kg ± 19.5 (Table 1). Thirteen patients had their surgery on the right knee, and 14 on the left. All patients had both Fulkerson Osteotomy procedures involving MPFL reconstruction with intraoperative femoral nerve stimulation.
Table 1.
Patient Demographic Data
| Parameter | Mean | Standard Deviation |
|---|---|---|
| Age (yrs) | 23.48 | 8.318 |
| Height (cm) | 171.5 | 11.15 |
| Weight (kg) | 79.83 | 19.5 |
Inter- and intra-rater reliability values were found to be very strong. Intraclass correlation values for intra-rater reliability of investigator 1 were 0.998 (95% CI 0.996-0.999, p < 0.05) for single measures and 0.999 (95% CI 0.998-1.000, p < 0.05) for average measures. Intraclass correlation values for intra-rater reliability of investigator 2 were 0.995 (95% CI 0.988-0.998, p < 0.05) for single measures and 0.997 (95% CI 0.994-0.999, p < 0.05) for average measures. Intraclass correlation values for inter-rater reliability were 0.993 (95% CI 0.987-0.996, p < 0.05) for single measures and 0.998 (95% CI 0.997-0.999, p < 0.05) for average measures (Tables 2-4).
Table 2.
Intra-rater reliability, Investigator 1
| Interclass Correlation | Value | 95% CI | P Value |
|---|---|---|---|
| Single Measures | .998 | .996-.999 | <.001 |
| Average Measures | .999 | .998-1.000 | <.001 |
Table 4.
Inter-rater reliability
| Interclass Correlation | Value | 95% CI | P Value |
|---|---|---|---|
| Single Measures | .993 | .987-.996 | <.001 |
| Average Measures | .998 | .997-.999 | <.001 |
Table 3.
Intra-rater reliability, Investigator 2
| Interclass Correlation | Value | 95% CI | P Value |
|---|---|---|---|
| Single Measures | .995 | .988-.998 | <.001 |
| Average Measures | .997 | .994-.999 | <.001 |
A significant post-operative difference was found between groups in the following parameters: WOMAC pain (anatomic mean = 85.71 ± 11.34, non-anatomic mean = 75.00 ± 26.35 p = 0.018), function (anatomic mean = 85.85 ± 9.96, non-anatomic mean = 79.09 ± 24.45, p = 0.017) and in KOOS symptom (anatomic mean = 75.63 ± 11.79, non-anatomic mean = 67.83 ± 22.40, p = 0.024), pain (anatomic mean = 77.54 ± 8.61, non-anatomic mean = 71.39 ± 25.18, p = 0.01), ADL (anatomic mean = 85.85 ± 9.97, non-anatomic mean = 79.09 ± 24.45, p = 0.017) and overall (anatomic mean = 74.61 ± 10.33, non-anatomic mean = 69.41 ± 24.25, p = 0.01) scores. No significant difference was observed for post-op instability (p = 0.290) or apprehension (p = 0.496), improvement in WOMAC or KOOS, 2-week, 6-week, or final 1-year range of motion, WOMAC stiffness, or KOOS sport/recreation or QOL (Table 5).
Table 5.
WOMAC, KOOS, and ROM Results
| Parameter (all values post-op) | Anatomic Mean | Non-Anatomic Mean | P Value |
|---|---|---|---|
| WOMAC pain | 85.71 ± 11.34 | 75.00 ± 26.35 | .018 |
| WOMAC stiffness | 67.86 ± 18.98 | 71.25 ± 24.33 | .436 |
| WOMAC function | 85.85 ± 9.96 | 79.09 ± 24.45 | .017 |
| KOOS symptom | 75.63 ± 11.79 | 67.83 ± 22.40 | .024 |
| KOOS pain | 77.54 ± 8.61 | 71.39 ± 25.18 | .01 |
| KOOS ADL | 85.85 ± 9.97 | 79.09 ± 24.45 | .017 |
| KOOS sport/rec | 36.90 ± 18.79 | 38.50 ± 32.92 | .098 |
| KOOS QOL | 66.66 ± 23.08 | 43.32 ± 32.81 | .277 |
| KOOS overall | 74.61 ± 10.33 | 69.41 ± 24.25 | .01 |
| 2-week ROM | 55.00 ± 19.49 | 65.36 ± 20.89 | .922 |
| 6-week ROMH 1 | 95.50 ± 12.34 | 100.45 ± 20.06 | .248 |
| 1-year ROM | 129.70 ± 7.056 | 126.50 ± 11.80 | .320 |
Non-anatomic graft placement did not predispose patients to reported flexion problems (p = 0.163), postop chondromalacia (p = 0.148), or continued post-op patellofemoral articulation pain (p = 0.586), as there was no statistically significant difference noted between the anatomic and non-anatomic groups in these parameters.
Discussion
The medial patellofemoral ligament (MPFL) is essential for the maintenance of correct biomechanical function of the knee. Reconstruction of the MPFL is commonly used in the restoration of patellofemoral stability after traumatic lateral subluxation of the patella. Although a method to accurately determine the MPFL's insertion point has been described, it remains unclear if anatomic placement of MPFL graft tissue is essential for preservation of knee junction after MPFL reconstruction. Thus, the purpose of this study was to determine the importance of anatomic placement of medial patellofemoral ligament (MPFL) graft tissue for the preservation of knee function following MPFL reconstruction operations.
Intra- and inter-rater reliability were likely strong due to measurement simplicity and investigator agreement regarding key parameters prior to their performance. The measurements were relatively easy to perform, and the investigators agreed on placement of the line perpendicular to the posterior femoral cortex, the line tangential to the posterior condyle, and the line tangential to the posterior aspect of the Blumensaat line. Within the range of graft placement values considered by this study, nonanatomic placement of the femoral MPFL tunnel appears to cause increased pain and decreased function as evidenced by post-operative KOOS and WOMAC scores. However, no significant difference was noted in apprehension, range of motion, quality of life, sport and recreation, patellofemoral pain, or incidence of chondromalacia. These parameters commonly serve as clinical benchmarks, and are generally considered to be the most important indicators of early success of the MPFL reconstruction operation.
This study had a number of limitations. It was a small retrospective review of cases that were performed at a single institution (University of Iowa Hospitals and Clinics). It was a case series. Follow-up length only extended to one year, as dictated by the information available in the electronic medical record. Also, one surgeon performed all operations in the cases considered by the study.
It is recommended that particular attention be paid during surgery to the tightness of the graft during active extension and passive flexion to 90 degrees16. Clinical exam is performed intra-operatively using femoral nerve stimulation to determine the isometry of the graft. If it were felt that there was tightening of the ligament in flexion, then loosening of the graft would be allowed without compromising its check-reign function in the extended position. If this pivotal portion of the MPFL reconstruction procedure is performed correctly, it seems that the patella tracks correctly into the trochlear groove post-operatively regardless of graft tissue placement site. Also, the graft tissue will not be damaged by the normal flexion and extension of the knee joint, and no limits to range of motion or apprehension should occur if correct isometry is achieved intra-operatively16.
References
- 1.Aglietti P, Buzzi R, Giron F, Simeone AJ, Zaccherotti G. Arthroscopic- assisted anterior cruciate ligament reconstruction with the central third patellar tendon: a 5-8-year follow-up. Knee Surg Sports Traumatol Arthrosc. 1997;5:138–144. doi: 10.1007/s001670050041. [DOI] [PubMed] [Google Scholar]
- 2.Allen CR, Giffin JR, Harner CD. Revision anterior cruciate ligament reconstruction. Orthop Clin North Am. 2003;34:79–98. doi: 10.1016/s0030-5898(02)00066-4. [DOI] [PubMed] [Google Scholar]
- 3.Palmer I. On injuries to the ligaments of the ankle joint: a clinical study. Acta Chir Scand. 1938;53(suppl):1–282. [Google Scholar]
- 4.Bernard M, Hertel P, Hornung H, Cierpinski T. Femoral insertion of the ACL: radiographic quadrant method. Am J Knee Surg. 1997;10:14–21. discussion 21-22. [PubMed] [Google Scholar]
- 5.Sommer C, Friederich NF, Muller W. Improperly placed anterior cruciate ligament grafts: correlation between radiological parameters and clinical results. Knee Surg Sports Traumatol Arthrosc. 2000;8:207–213. doi: 10.1007/s001670000125. [DOI] [PubMed] [Google Scholar]
- 6.Schottle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35:801–804. doi: 10.1177/0363546506296415. [DOI] [PubMed] [Google Scholar]
- 7.Bollier M, Fulkerson J, Cosgarea A, Tanaka M. Case report: technical failure of medial patellofemoral ligament reconstruction. Arthroscopy. 2011;27:1153–1159. doi: 10.1016/j.arthro.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Fulkerson JP. Anteromedialization of the tibial tuberosity for patellofemoral malalignment. Clinical Orthopaedics and Related Research. 1983;177:176–81. [PubMed] [Google Scholar]
- 9.Ebinger TP, Boezaart A, Albright JP. Modifications of the Fulkerson Osteotomy: a pilot study assessment of a novel technique of dynamic intraoperative determination of the adequacy of tubercle transfer. The Iowa Orthopaedic Journal. 61:61–64. [PMC free article] [PubMed] [Google Scholar]
- 10.Elias JJ, Cosgarea AJ. Technical errors during medial patellofemoral ligament reconstruction could overload medial patellofemoral cartilage. The American Journal of Sports Medicine. 2006;34(9):1478–1485. doi: 10.1177/0363546506287486. [DOI] [PubMed] [Google Scholar]
- 11.Feller JA, Amis AA, Andrish JT, Arendt EA, Erasmus PJ, Powers CM. Surgical biomechanics of the patellofemoral joint. The Journal of Arthroscopic and Related Surgery. 2007;23(5):542–53. doi: 10.1016/j.arthro.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 12.O'Donnell P, Johnstone C, Watson M, McNally E, Ostlere S. Evaluation of patellar tracking in symptomatic and asymptomatic individuals by magnetic resonance imaging. Skeletal Radiology. 2005;34:130–5. doi: 10.1007/s00256-004-0867-6. [DOI] [PubMed] [Google Scholar]
- 13.Lavery M, Bell J, Rickelman T, Boezaart A, Albright JP. Patellofemoral realignment: dynamic intraoperative assessment. The Iowa Orthopaedic Journal. 25:160–3. [PMC free article] [PubMed] [Google Scholar]
- 14.Ward SR, Terk MR, Powers CM. Patella Alta: Association with Patellofemoral Alignment and changes in Contact Area During Weight-Bearing. Journal of Bone and Joint Surgery. 2007;89:1749–55. doi: 10.2106/JBJS.F.00508. [DOI] [PubMed] [Google Scholar]
- 15.Steiner TM, Teitge RA, Torge-Spak R. “Medial patellofemoral ligament reconstruction in patients with lateral patellar instability and trochlear dysplasia.”. American Journal of Sports Medicine. 2006;34.8:1254–261. doi: 10.1177/0363546505285584. [DOI] [PubMed] [Google Scholar]
- 16.Albright J. Personal interview. 2012. 11 Jun. < http://www.uihealthcare.org/physician.aspx?id=19111>.
- 17.Servien E, Verdonk PC, Neyret P. Tibial tuberosity transfer for episodic patellar dislocation. Sports Med Arthrosc. 2007;15(2):61–7. doi: 10.1097/JSA.0b013e3180479464. [DOI] [PubMed] [Google Scholar]
- 18.Arendt EA. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;1909.14 doi: 10.1007/s00167-011-1516-y. n. pag. Web. [DOI] [PubMed] [Google Scholar]
- 19.Howells NR, Eldridge JD. Medial patellofemoral ligament reconstruction for patellar instability in patients with hypermobility: a case control study. J Bone Joint Surg Br. 2012;94(12):1655–9. doi: 10.1302/0301-620X.94B12.29562. [DOI] [PubMed] [Google Scholar]
- 20.Servien E, Fritsch B, Lustig S, Demey G, Debarge R, Lapra C, Neyret P. In vivo positioning analysis of medial patellofemoral ligament reconstruction. Am J Sports Med. 2011;39:134–139. doi: 10.1177/0363546510381362. [DOI] [PubMed] [Google Scholar]
- 21.Panni AS, Alam M, Cersiello S, Vasso M, Maffulli N. “Medial patellofemoral ligament reconstruction with a divergent patellar transverse 2-tunnel technique.”. Am J Sports Med. 2011;39.12:2647–55. doi: 10.1177/0363546511420079. Print. [DOI] [PubMed] [Google Scholar]
- 22.Schepsis AA, Rogers AJ. Medial patellofemoral ligament reconstruction: Indications and technique. Sports Med Arthrosc Rev. 2012;20:162–170. doi: 10.1097/JSA.0b013e318264188b. [DOI] [PubMed] [Google Scholar]
- 23.Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA. The medial patellofemoral ligament - location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachements. Am J Sports Med. 2012;40:1871–1879. doi: 10.1177/0363546512449998. [DOI] [PubMed] [Google Scholar]
- 24.Elias JJ, Cosgarea AJ. Technical errors during medial patellofemoral ligament reconstruction could overload medial patellofemoral cartilage - a computational analysis. Am J Sports Med. 2006;34:1478–1485. doi: 10.1177/0363546506287486. [DOI] [PubMed] [Google Scholar]


