Abstract
In contemporary total hip arthroplasty, instability has been a complication in approximately 2% to 5% of primary surgeries and 5% to 10% of revisions. Due to the reduction in the incidence of wear-induced osteolysis that has been achieved over the last decade, instability now stands as the single most common reason for revision surgery. Moreover, even without frank dislocation, impingement and subluxation are implicated in a set of new concerns arising with advanced bearings, associated with the relatively unforgiving nature of many of those designs. Against that backdrop, the biomechanical factors responsible for impingement, subluxation, and dislocation remain under-investigated relative to their burden of morbidity.
This manuscript outlines a 15-year program of laboratory and clinical research undertaken to improve the scientific basis for understanding total hip impingement and dislocation. The broad theme has been to systematically evaluate the role of surgical factors, implant design factors, and patient factors in predisposing total hip constructs to impinge, sublux, and/or dislocate. Because this class of adverse biomechanical events had not lent itself well to study with existing approaches, it was necessary to develop (and validate) a series of new research methodologies, relying heavily on advanced finite element formulations. Specific areas of focus have included identifying the biomechanical challenges posed by dislocation-prone patient activities, quantifying design parameter effects and component surgical positioning effects for conventional metal-on-polyethylene implant constructs, and the impingement/dislocation behavior of non-conventional constructs, quantifying the stabilizing role of the hip capsule (and of surgical repairs of capsule defects), and systematically studying impingement and edge loading of hard-on-hard bearings, fracture of ceramic liners, confounding effects of patient obesity, and subluxation-mediated worsening of third body particle challenge.
Introduction
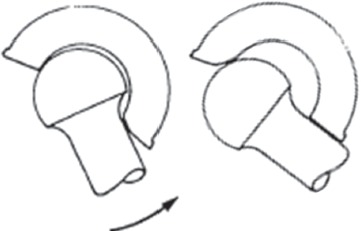
In total hip arthroplasty (THA), the inroads to particle-induced osteolysis that have accompanied low-wear advanced bearings have left instability as now the single most common cause for revision surgery1. Dislocation usually due to impingement and lever-out (Figure 1) has always been high on the list of reasons for clinical failures, despite THA as a whole being among the greatest successes of modern medicine. Increased research attention to instability is therefore justified even on grounds of its increased relative burden of morbidity. Additionally, due to the less-forgiving nature of various design features of contemporary advanced bearings, it has become increasingly evident that even without occurrence of frank dislocation, prelude impingement and subluxation events can cause serious problems in their own right. Some of these “new” concerns are particle and ion loads from edge loading of metal-on-metal bearings; stripe wear, squeaking, chipping, and liner fracture in ceramics; and impingement rim damage of thin liners in large diameter metal-on-polyethylene bearings.
Figure 1. Classic conceptual schematic (1975) of impingement/ dislocation, from work by Amstutz and colleagues 63.
Although dislocation is an unmistakable event at the clinical level, its unpredictability and its abruptness of occurrence have posed major hurdles to drawing direct causative conclusions from clinical experience. Rather, it has been possible only to infer general associations with plausible predisposing factors. Those associations have often been of only low or modest statistical power, even when working with patient cohorts that are unusually large by orthopaedic standards2-5. Moreover, using large patient cohorts for associative studies has usually required trading-off against heterogeneity of potentially confounding factors (surgeon variables, implant variables, patient variables.) One exception has been our group's opportunity for long-term follow-up of single-surgeon dislocation experience under conditions where relatively few changes of implant or surgical technique took place6, 7. This has been advantageous in terms of statistical power for documenting the effects of those few factors which varied (e.g., head size, usage of skirts), although there has been the obvious trade-off that many factors of potential interest unfortunately could not be studied since they did not vary. Despite the substantial challenges of clinical research in this area, reducing the incidence of problems in patients is of course the gold standard by which any presumed improvements for THA stability must be judged. However, clinical experience does not lend itself well to efficiently identifying direct cause-and-effect relationships in this area. Rather, impingement/dislocation research needs to take place in controlled settings that are reasonably representative of clinical circumstances, where individual influence factors can be systematically studied.
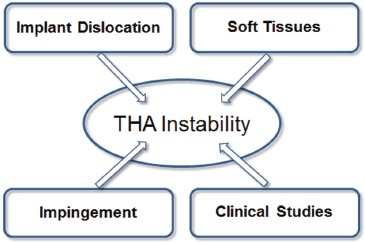
Historically, dislocation and impingement/subluxation events have been an under-developed area of laboratory orthopaedic investigation, owing in large part to the complexity of the phenomena involved and the logistical difficulty of implementing appropriate models. Up until the mid-1990s, only a handful of investigations had even been attempted in this area, largely restricted to geometric range-of-motion samplings either with bench surrogates or in simplified cadaver preparations8. Recognizing the un-met clinical need and the scientific opportunity in this area, in 1996 our group began developing platform technology to enable systematic study of surgical factors, implant design factors, and patient activity factors bearing upon THA dislocation propensity. A key consideration in that work from the very beginning continuing up into the present has been that dislocations and impingement/subluxation events need to be addressed fundamentally as kinetic phenomena, i.e., that forces and moments, and local stresses in the tissues and materials involved, are what matter clinically. While inter-related with traditional kinematic (geometric) factors such as range-of-motion, it is these kinetic factors that govern whether a given impinging implant will or will not dislocate, and whether tissues and/or implant components will or will not be harmed during a given impingement event. Necessarily, however, including kinetic considerations greatly increases the complexity and difficulty of quantifying impingement/dislocation events. The present paper summarizes our group's now 15-year research effort in investigating of THA instability kinetics, results from which have been reported in 31 original full length articles and 4 graduate theses. The problem of instability has been addressed from four inter-related perspectives (Figure 2): implant dislocation studies, soft tissue involvement, impingement models, and clinical studies. The principal laboratory research methodology adopted has been finite element analysis (FEA). Contemporary capabilities in the field of computational modeling have reached a level of sophistication such that the role of physical experimentation has withered in many areas within the broad field of mechanics. An FEA approach to THA impingement/dislocation held the attraction that once appropriate investments were made in model development and validation, individual variables or combinations of variables could be investigated systematically and efficiently, in virtually unlimited depth and detail. Of course, to enable applying FEA in this area, it has been necessary to design and conduct a number of novel experimental studies to collect input data that previously had been unavailable. And, even more importantly, physical experimentation has been indispensible for model validation. Specific questions appropriate for laboratory study have been informed by clinical experience, both of the THA community as a whole, and within our own group.
Figure 2. THA instability was investigated from the primary perspectives of Implant Dislocation 9-20; Soft Tissues 21-25; Impingement 8, 26-32 and Clinical Studies 6, 7, 33-40.
Dislocation FE Model Development and Validation:
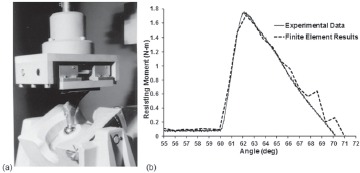
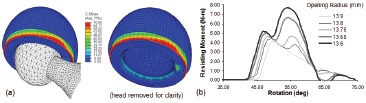
A necessary first step was to develop capability to simulate dislocations computationally, in three dimensions. While 3D stress analyses of THA components with well-prescribed external loadings had become relatively routine even as of the late 1980s, dealing with stresses due to internal interface contact was problematic, especially in situations where the respective contacting surfaces were undergoing large relative sliding motions due to bearing surface articulation. Building on some then-recent success with performing sliding contact FEA simulations of walking in the context of THA wear 41, numerical trials were undertaken in which the femoral head was driven to rotate further within the acetabular component, until the neck made contact with the liner rim. Head rotation through the normal range of motion involves only a small amount of resistance, from bearing surface frictional torque. Making head rotation continue after the onset of impingement, however, required overcoming the much larger resisting moment developing due to (head-center-eccentric) contact force buildup at the impingement site18. This resisting moment proved crucially important, for two reasons. First, its maximum achievable value was useful as an overall dislocation resistance metric for given set of THA construct parametric conditions (i.e., specific implant design, component surgical positioning, and patient activity challenge.) Second, resisting moment was a discrete entity also lending itself to direct physical measurement at any instant of an impingement/subluxation event, thus providing a basis for validation of the computational results (Figure 3).
Figure 3. (a) Laboratory fixture for imposing THA dislocation (b) Comparison of experimental versus computational results.
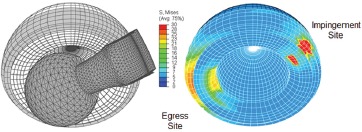
After developing numerical techniques to allow computation of the sudden high stress concentrations at the site of de-novo impingement contact, the numerical model was then extended to address lift-out head subluxation associated with pivoting about the impingement fulcrum. Onset of subluxation involved progressive diminution of the initially hemispherical (bearing) contact surface, with progressive build-up of contact stress at the site of head egress (Figure 4), diametrically opposite from the neck impingement site. Often, peak stress magnitudes computed for small contact cusp at the head egress site even exceeded stress magnitudes at the neck impingement site. (This phenomenon of subluxation-associated cusp stress concentration at the acetabular rim, initially encountered in 1996, has nowadays come to be familiarly known as edge-loading.) A final step in first-generation model development was to generalize from single-axis to multi-axial head rotations, toward being able to simulate dislocation-prone physiologic patient maneuvers. This proved challenging computationally because the impingement site became then no longer a statically-located fulcrum, but rather a patch of extreme stress concentration, traveling along a substantial swath on the liner rim. In some instances of relatively glancing neck-liner contact, there could be up to between 10 to 20 degrees of additional head rotation between initial impingement and frank dislocation, reinforcing that conventional geometric range of motion is only a very loose surrogate for the true angular range of THA kinetic stability.
Figure 4. Finite element model of total hip dislocation.
Kinetics and Kinematics of Dislocation-Prone Maneuvers:
While successful computational execution of the dislocation FEA formulation was a necessary step, deriving clinically meaningful information from that model depended also on physiologically realistic input data. At the time, unfortunately, there had been very little precedent for dislocation as a laboratory research topic, so data were lacking as to kinetics and kinematics of patient motions typically associated with dislocation. For that reason, studies were undertaken of THA age-matched individuals executing a battery of dislocation-prone maneuvers (leg-crossing, rising from a low seat, bending to tie a shoe, and four others), using an Optotrak® motion analysis system to record segmental kinematics of the pelvis and lower extremities, along with inverse Newtonian equilibrium analysis and optimization to determine muscle forces and articular contact force at the hip13. Several dislocation-prone maneuvers involved hip joint contact forces that were dramatically (sometimes even > 2x) higher than those conventionally reported for locomotion activities, owing to the upper body's center of gravity being shifted well anterior of the hip centers, thus requiring high force outputs from the hip extensor muscles to maintain sagittal plane equilibrium, in turn therefore elevating hip joint contact force.
Parametric Effects in Conventional Constructs:
Having a physically validated three-dimensional FE model driven by physiologically grounded inputs opened the way for parametric studies of implant design factors, component surgical positioning factors, and patient motion challenge factors bearing upon THA dislocation 12-15, 17, 20. Variables parametrically considered included head size, liner lip chamfer angle, liner lip breadth, cup inset depth, cup backing diameter, cup liner offset, femoral stem offset, femoral component head/neck diameter ratio, presence/absence of a skirt, liner material characterization (UHMWPE elastic modulus and several variants of elasto-plastic behavior), component surgical orientation (cup abduction, cup anteversion, femoral component anteversion), and patient dislocation challenge (five posterior and two anterior risk maneuvers). The FE model was extended to also include peri-implant osseous structures, for purposes of quantifying dislocation propensity due to component-on-bone and bone-on-bone impingement (a consideration especially for larger head sizes.) Concurrently, work also was undertaken to extend the breadth of model validation by linking in with ongoing cadaveric testing at Baylor University19.
From among the body of results for the many individual parameters considered, two broader-level sets of relationships became evident. First, regarding implant design factors, while both the peak moment developing to resist dislocation and the range of kinetic stability (i.e., the range of motion prior to frank dislocation) were sensitive to many individual attributes of implant design, there was nearly always a very direct trade-off between those two considerations. That is, individual design parameter changes that achieved improvements in peak resisting moment involved reduction in kinetic range of stability, whereas improved range of stability came at the expense of lower peak resisting moment. The second broader-level set of relationships involved surgical positioning. While it was always possible to identify a zone of component orientations that protected very well against dislocation for any given patient challenge maneuver, those orientations' level of protection against certain other challenge maneuvers was much less. For example, cups that were ideally well positioned to avoid posterior dislocation from activities such as shoe tying or rising from a low seat were highly vulnerable to anterior dislocation from activities such as exorotation pivot or roll-over in bed, and vice-versa.
Studies of Non-Conventional Constructs:
Besides quantifying dislocation propensity and tradeoffs for conventional unconstrained THA designs, the FE model lent itself also to exploring unconventional design concepts. One of these (Figure 5) was bi-curvilinear impingement surfaces16: convex meridional curvature of the acetabular lip, and concave meridional curvature of the femoral neck, such that any neck-on-lip impingement would have the tendency to cause the neck contact site to “roll” radially outward on the lip (and radially downward on the neck), thus progressively building up more resisting moment than would occur for a conventional (non-rolling) impingement fulcrum. This indeed turned out to be the case: depending on the specific dislocation challenge considered, there was up to a 29% improvement in peak resisting moment, and (i.e., without trade-off) up to a 14° improvement in kinetic stability range. Also, because the mating radial curvatures between neck and liner led to line-like contact rather than point-like contact at the impingement site, there was up to 50% reduction in peak polyethylene contact stress. Another study of alternative design concepts involved constrained liners9. This class of specialty devices had been conceived for last-resort attempts to maintain stability in patients with recurrent dislocations, and had been the subject of a number of clinical studies at our institution8,35-40. Experiences had been highly variable both at our center and elsewhere, with some patients tending to re-dislocate34 even despite positive head capture (i.e., cup rim extending past 180° of arc), and with some implants undergoing dissociation due to hoop-stress-induced fracture of the equatorial metal restraining ring. A competing consideration was that intra-operative assembly or in select circumstances, even closed reduction after an initial constrained liner dislocation37 requires that the surgeon forcefully push the head into the cup, in order to achieve the interference fit necessitated by the “undersize” cup opening. The FE model allowed systematically addressing these issues (Figure 6), to identify amount of cup opening undersizing that afforded a balance between difficulty of interference fit assembly versus resistance to head lever-out, and corresponding retaining ring dimensions. Physical validations were provided both by lever-out testing of resisting moments, and by “push-in” measurements of interference fit resistance in a series of implants with parametrically varied cup openings, custom-fabricated for this study by one of the collaborating manufacturers.
Figure 5. Meridional curvature of potential impingement surfaces. (a) Design concept. (b) FEA model of performance during an impingement/ subluxation event.
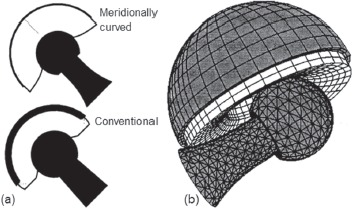
Figure 6. (a) FE stress contours illustrating high hoop stress in the retaining ring, and (b) resisting moment parametric line plot, showing effects of change in interference fit.
An alternative approach to recurrent dislocation lies in increasing the implant's kinetic range of motion, rather than increasing its peak resisting moment. In concert with a clinical series at our institution38, laboratory studies of ranges of motion of tri-polar implant were undertaken, which showed than these devices indeed functioned very closely as intended40. (Those laboratory assessments needed to be done experimentally rather than computationally, because the FEA model's capabilities at that time (1997-1998) were limited to dealing with just a single surface of articulation, whereas tri-polar implants involve two concentric “in series” surfaces of articulation.) This favorable short-term experience with tri-polar constrained implants for patients with recurrent dislocations has continued into the intermediate term36,39. We also have found that in cases where the (dislocated) primary implant's acetabular shell remains well fixed and undamaged, very good performance can be achieved by cementing in a tri-polar liner35, similarly to what is often done for worn conventional liners42.
Capsule Contributions to Construct Stability:
Another set of considerations bearing upon THA impingement/subluxation and dislocation involves the hip capsule. It has long been recognized that patients with capsular biomechanical deficit are at elevated risk of dislocation4, prior hip surgery being one major cause for capsule compromise. This is widely felt to be a principal reason why dislocation rates are consistently higher for revision THAs than for primaries2, especially for surgeons using extensive or even full7 capsule resection. Despite the intuitive attraction of implant designs and surgical component positioning that provide intrinsically maximum dislocation resistance, these intrinsic factors need to be viewed within the context of the overall THA construct, of which the capsule is an important part.
Capsule abnormality can take various forms, including thickness anomaly, stiffening/scarring, substance tears, detachments from bony insertions, and surgical incisions. Dealing with these different forms of capsule mechanical deficit involves different technical considerations intraoperatively. Ideally definitive repairs almost always involve trade-offs, especially the need for additional surgical exposure. Intraoperative decision-making would benefit from better information linking site and severity of capsule defect(s) to the corresponding dislocation propensity. Unfortunately for FEA purposes, however, this was another area for which there was almost no precedent knowledge base, so work was undertaken to biomechanically characterize the hip capsule, to enable its representation within the THA impingement/dislocation model. This involved systematic dissection of fresh-frozen normal cadaver hips, with mappings of anatomic attachments around the acetabula and femora, of capsule thickness, and of visually apparent fiber directions. Tensile testing of capsule sub-sections allowed compiling a database of mechanical properties as a function of anatomic location23.
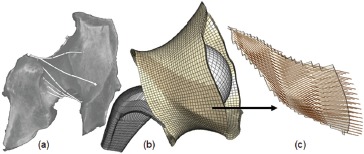
The recorded loci of capsule attachment locations were registered to the bony surfaces of the existing FE model, so that the (three-dimensional) space encompassed by capsule tissue could be zoned into elements, and the corresponding distribution of mechanical properties assigned. Since the inner (i.e., synovial) surface of the capsule could sometimes wrap around portions of the enclosed implant and/or bony surfaces during various hip angulation maneuvers, and since the capsule could sometimes locally infold upon itself and/or be externally pinched, it was necessary to make provision computationally for an extensive set of surface-to-surface contact contingencies. Mainly because of these new complexities of contact, the computational run times for the FE dislocation models lengthened considerably to days, or sometimes even weeks when the capsule was included. However, despite this (initial) unwieldiness computationally, it was very clear from even the earliest capsule-inclusive FEA models that the capsule's mechanical status was the single most important determinant of THA construct stability24. This provided motivation for investing additional developmental effort to further refine the sophistication of capsule representation in the model, and to streamline computational execution. The current capsule embodiment (Figure 7) involves 27 distinct material regions, each with fiber directionbased anisotropic local mechanical properties derived from additional cadaver dissections, along with CT and MR imaging. Building on the earlier work with tensile testing of strips of excised capsule tissue, the present capsule mechanical property values are grounded also in optimization-based matches with intact-joint load/ deformation data. The computational runs now typically execute in 10 to 20 hours of clock time.
Figure 7. Fiber-direction-based FE representation of the hip capsule. (a) CT delineation of fiber direction, (b) overall capsule meshing structure, and (c) fiber direction distribution in a sub-section of the capsule mesh.
Given this identified importance of capsule integrity to THA stability, the need for capsule-focused physical validation of the overall construct FE model became paramount. This posed a unique challenge experimentally, in that baseline physical data ideally needed to be available that would reflect the full degree of THA stabilization provided by an intact capsule, for purposes of comparison with the corresponding baseline situation computationally. Conventional THA implantation in cadaver hip specimens was an unattractive option, since this would necessarily have required a capsule incision, which in a cadaver preparation could only be passively re-approximated with sutures, a very different situation than the full active healing that normally would occur clinically.
To address this challenge, specialty implant hardware was designed and built which replicated the intra-capsular geometry of conventional THA implants, but which could be implanted entirely without need for capsule incision25. Briefly, there was a large metal collar which could be screw-anchored to the inner pelvic wall (Figure 8a), and whose underside had a recess which had been machined for purposes of subsequently seating a conventional THA acetabular component in a surgically appropriate position. After screw attachment to the inner pelvic wall, the collar served as a cutting guide for a circular hole to be sawed retrograde through the acetabulum. This exposed the native femoral head, which then was piecemeal-ablated and removed, working through the sawn acetabular portal. Next, from an entry point on the greater trochanter, a hole was bored approximately along the femoral neck axis, through which a metal rod was inserted. This rod, whose distal end was then screw-anchored in the proximal femur, had a proximal end that replicated the intra-capsular geometry of the THA femoral component neck and trunnion, and onto which a femoral component head could be seated through the acetabular access portal. The pelvic wall collar was then unscrewed, the THA acetabular component seated and screw-anchored into the collar's underside recess, and the collar was then re-affixed to the pelvic wall. This resulted in replication of the intra-capsular aspects of conventional THA, while preserving full capsule integrity (Figure 8b). Thus-implanted hemipelvis specimens were in turn mounted within a purpose-built four degree-of-freedom servo-hydraulic hip simulator (Figure 8c), which had been programmed to replicate the (previously measured13) kinematics and kinetics of various dislocation-prone maneuvers. A six degree-of-freedom load cell installed in the hip simulator allowed measurement of resisting moment during impingement/ dislocation, directly corresponding to the resisting moment determined computationally. The agreement achieved between the computational and FE simulations was gratifying (Figure 8d.)
Figure 8. (a) Retrograde sawing of the acetabular access portal; (b) Radiographic appearance of the intra-capsular construct; (c) Hemi pelvis specimen mounted in the servohydraulic hip simulator; (d) Computed versus experimentally measured resisting moments, versus simulation time, during a simulated sit-to-stand maneuver.

Impingement of Hard-on-Hard Bearings:
Following cadaver validations, parametric computational series were undertaken to determine the degree to which resistance to dislocation depended on capsule thickness, on the locations and extent of capsule detachment from bony insertions, and on (surgical) longitudinal incisions at various sites21. Simulations were also run to assess stability improvements accompanying alternative suture repairs, and to assess the risk of failure of those repairs. Computationally, suture repairs could be conveniently simulated by numerically equivalencing (effectively, pinning together) pairs of finite element nodes on either side of the two tissue edges being attached. Nodal equivalencing also provided a direct basis for assessing suture failure risk, by means of querying the FEA algorithm's internally-maintained datafile of resultant forces on all individual nodes. In the case of a pair of equivalenced nodes, these resultants were the forces necessary to keep the nodal pair held together, i.e., the local pull-apart force being resisted by the suture.
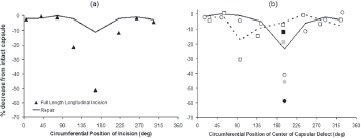
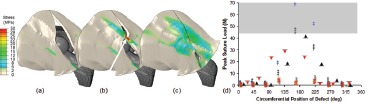
Posterior and poster o-lateral capsule detachments, either from the acetabulum or the femur, involved pronounced decreases of construct stability21. A useful single numerical metric for case-to-case stability comparisons is the mechanical energy required to cause dislocation, a parameter whose value could be readily calculated from the area under the curve of resisting moment versus imposed hip rotation angle. For flexion-dominated motion challenges, even relatively small (˜1/8 circumference) posterior or postero-lateral detachments typically involved dislocation energy being reduced by 50% or more below levels for the intact capsule (Figure 9a). Repairs of such defects typically returned peak resisting moment values to within 10-20% of baseline levels. Unrepaired full-length longitudinal capsule incisions likewise were found to substantially compromise construct stability, also in a very site-dependent manner (Figure 9b).
Figure 9. Effect of capsule defect and repair on dislocation energy for (a) longitudinal full-length capsule incisions and (b) bony attachment-site releases. Caption: Acetabular 1/8 release ( ), 1/4 release ( ), 3/8 release ( ), and repair ( ); Femoral 1/8 release ( ), 1/4 release ( ), 3/8 release ( ), and repair ( ). Posterior position corresponds to the 180° circumferential location.
Computed pull-out forces for individual sutures for various repair alternatives for various capsule defects are shown in Figure 10. It can be appreciated (shaded band in Figure 10d) that many repair arrangements that ideally would restore near-normal construct stability for severe defects unfortunately involved the suture sites being at substantial risk of failure43. These often dramatic decreases of hip stability occurring for adversely located capsule deficits serve to underscore that capsule compromise may be the predominant predisposing factor for THA instability. Also, the substantial stress concentrations developed adjacent to local detachment sites, and the high tensile stresses at some of the suture repair sites, are consistent with the high incidence of early failures often seen in posterior structure repairs44,45. Since most THA dislocations occur for flexion-dominated motion challenges, the model's results help explain the lower dislocation rates documented in clinical series where the posterior capsular structures either have not been violated or have been robustly repaired.
Figure 10. (a) Posterior capsule incision as typically used during a posterolateral surgical approach, and two distinct capsule repair variants using a single suture (b) or six equally-spaced sutures (c). When too few sutures are used, high tensile stresses occur within the suture (white arrow). For parametric analyses of suture position and number (d), posterior capsule incisional repairs with fewer than four sutures were predicted to fail (gray band identifies range of suture failure loads). Caption: Acetabular 1/8 detachment repair ( ); Femoral 1/8 detachment repair (
); Femoral 1/8 detachment repair ( ); Longitudinal incision repairs with two (‡ ), three (
); Longitudinal incision repairs with two (‡ ), three ( ), six (
), six ( ) or nine (
) or nine ( ) sutures.
) sutures.
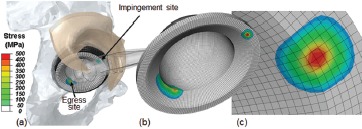
For hard-on-hard (HoH) implants, the extremely localized nature of contact at neck impingement and head egress sites suggests stress magnitudes and stress gradients far more severe than those for comparable impingement events in metal-on-polyethylene implants. A difficulty in quantifying HoH impingement, however, is that whole-implant finite element zonings with enough spatial resolution to accurately capture stresses at impingement/egress sites are logistically prohibitive from a computational resource viewpoint. To overcome this difficulty, a novel multi-stage finite element strategy was devised28: Regional results output from conventionalzoning-resolution FEA of the global THA construct were used as input for high-zoning-resolution FEA at impingement and egress sites. This formulation (Figure 11) allowed ascertaining the extent to which local stress concentrations from impingement/subluxation would challenge the bulk failure strengths of the HoH constituent materials. Independent validation of the computations was possible by comparison with mathematically idealized contact of a sphere (i.e., the femoral head) on a torus (i.e., a circularly radiused acetabular lip), a special case of the well-established family of canonical Hertzian engineering contact analyses for bi-curvilinear elastic surfaces.
Figure 11. (a): FE model of HoH THA impingement, demonstrating stress concentrations at the impingement and egress sites (femoral component rendered translucent, and the posterior half of the capsule transparent, for clarity). Using a submodeling formulation (b,c), highly accurate stress resolutions at these sites are possible, at a minimum of computational cost.
Additionally, a new metric was introduced to enable assessing the relative propensity for debris to be generated from the localized scraping occurring at impingement and egress sites. The basis for this new metric was the premise that liberation of debris from localized scraping might be viewed as analogous to liberation of abrasive/ adhesive wear debris at a bearing surface, in terms of being quantifiable as the product of contact stress times sliding distance times a tribologically-dependent wear factor. Despite being only an analogy and despite the wear factor assignment needing to be somewhat arbitrary, “scraping wear” defined in this manner provided an all-other-factors-equal basis for quantifying the relative tendency of different impingement/subluxation events to generate particulate debris.
After completing the Hertzian validations (average error 8.8%), 154 simulations were parametrically run, investigating cup inclination and cup anteversion, for metal-on-metal (MoM) and ceramic-on-ceramic (CoC) bearings, for two different challenge maneuvers (low seat-to-stand, and stooping)28. The computed stresses demonstrated strong dependence upon cup surgical orientation, with contact stresses at the egress site consistently exceeding those at the impingement site, for all combinations of construct type and component orientation. The tendency to generate scraping debris showed parameter dependencies similar to those for contact stress: Scraping wear was generally higher at the egress site than at the impingement site, and became more severe as cup abduction and/or cup anteversion were increased.
The contact stress magnitudes computed at hard-on-hard THA impingement/egress sites were among the highest yet quantified in the field of orthopaedics. For MoM, the material failure modality of primary concern is that the yield strength of the CoCrMo alloy would be exceeded, leading to localized permanent deformation. For CoC, the concern is liner chipping or even catastrophic fracture. For both bearing types, material failure levels were approached or in some instances even exceeded. While some of the model input parameters necessarily involved approximations and/or assumptions, the computed results nevertheless give clear cause for concern that impingement/subluxation events pose substantial threat to the refined engineering integrity of contemporary hard-on-hard bearings.
Particles generated from scraping at impingement or egress sites would have only a very short migration path to reach the bearing surface to become third bodies. Scraping debris also would constitute direct particulate burden in the peri-articular tissue bed, and/ or in distant organs. And, since the surface-to-volume ratio of metal scrape particles is enormously higher than for bulk implant members, scraping debris is a potent source of metal ions, contributing to genesis of aseptic lymphocytic vasculitis-associated lesions (ALVAL) or to other immunologic reactions46. Despite the nascent nature of the scraping wear concept, the parametric FEA results indicate (1) that some impingement events are far more deleterious than others from a third-body generation standpoint, (2) that the egress site is a substantially greater cause of concern in that regard than the impingement site, and (3) that cup orientations that are most problematic in terms of risk of bulk material failure generally correspond to the cup orientations that are most problematic for scraping wear.
Influence of Cup Design on Edge-Loading:
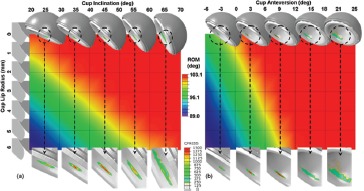
Given the current level of concern with edge loading of HoH bearings, another utility of FEA lies in investigating how changes of implant design might serve to moderate the effect. One important design parameter in that regard is the meridional curvature radius of the liner lip. The larger this radius, the broader the width of the cusp-shaped edge loading rim contact patch, other factors being equal. However, increasing the lip's meridional curvature radius also has the effect of reducing the liner's articular coverage area on the femoral component head, thus reducing construct stability, and therefore making it easier for an edge-loading situation to develop in the first place. A study was undertaken to parametrically explore this interplay for MoM bearings, as a function of surgical positioning of the cup26. Dependent variables of primary interest were kinetic range of motion, resistance to dislocation, contact stress on the cup lip, and propensity for debris generation due to scraping. Seven different cup lip radii were considered, from 0 mm (i.e., a sharp lip edge) up to 6mm, for a range of cup orientations involving univariate combinations of eleven different angles of abduction and ten different angles of anteversion. The data showed that increasing the liner lip radius affected both the kinetic range of motion and the energy necessary to cause dislocation, more strongly than did changes of cup orientation (Figure 12). More than half of the permutations of cup orientation and lip radius that were considered were found to cause peak (von Mises) edge-loading stresses in excess of the yield strength of wrought CoCr alloy. Computed scraping wear tended to exhibit similar dependency upon lip radius and cup inclination as was observed for peak stresses.
Figure 12. Kinetic range of motion (ROM) for 77 permutations of cup lip radius and cup inclination (a) and anteversion (b). In general, ROM was reduced for increased values of cup lip radius and decreased cup inclination and anteversion. The changes in ROM due to lip radius change were most pronounced for the more horizontally oriented cups. Contact pressure contours for the 5-mm lip radius cases are shown in the lower insets.
Significance of Femoral Head Size:
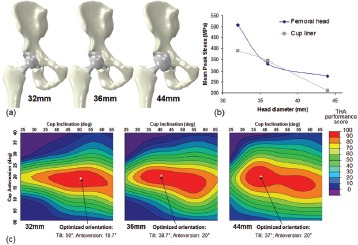
Besides cup design, femoral component geometry is also important to construct performance. By increasing the kinematic range of motion prior to impingement, large femoral heads in principle provide improved stability. However, surgical compromises exist, since concomitantly increasing cup size requires increased acetabular bone stock removal, making accurate cup placement more technically challenging. Historical guidelines for the “safe zone” of implant orientation are based on much smaller head geometries, and consider only implant stability. These concerns have been addressed by multivariate analysis of cup orientation and head size (Figure 13a). Larger head sizes, while providing improved stability and decreased bearing surface stress (Figure 13b), demonstrate similar sensitivity to cup placement as smaller head sizes, especially in terms of requiring high accuracy of anteversion (Figure 13c)
Figure 13. (a) Three head sizes in common usage with HoH THA were studied. Five dislocation maneuvers were considered (predisposing both to anterior and posterior dislocation), for parametric variations in cup orientation straddling the THA “safe zone.” Impingement, instability, and mean peak stresses decreased with larger heads (b). Competing considerations, stability and surface stresses, were combined into a novel metric of THA performance (c), to determine optimal placement as a function of head size.
Fracture of Ceramic Liners:
Another concern regarding HoH impingement/ subluxation is the possibility of liner fracture, in the case of ceramics. This is a very different issue than the now-historical problem with taper-seating/impaction fractures of first generation ceramic femoral heads47, which material and design improvements have reduced to a marginal concern (0.004% prevalence48). Fracture of contemporary ceramic liners, however, occurs on the order of a thousand times more frequently (3.5%49, 1. 12%50, 0.22%51), typically in high-flexion postures or maneuvers plausibly involving impingement. Because of the ongoing particle/ion problems with MoM and the expanding need for joint replacements in younger and more active patients, further improving the performance of ceramic liners is an important goal. This consideration motivated development work to add formal fracture prediction analysis to the existing capabilities of the impingement FEA model29. (To the authors' knowledge, this has been the first application of engineering fracture mechanics to THA acetabular components.) As with earlier aspects of the FEA effort, the rationale was to lay groundwork to understand how specific design factors, surgical factors, and patient factors interact to influence implant performance.
In brittle materials such as ceramics, mechanical stress levels in the near vicinity of a crack tip exhibit what is known as a singularity, effectively an unbounded increase toward infinity. In a given brittle material object subjected to a given load, whether or not cracks will nucleate, whether or not they will propagate once nucleated, and whether or not such propagation will be stable (as in fatigue situations, for example stress fracture of bone) as opposed to unstable/catastrophic, can be quantified in terms of a family of mechanical parameters known as stress intensity factors. Calculating stress intensity factors in FEA requires a specialized meshing structure in the local region of interest. This makes fracture analysis a difficult proposition in complex structures such as orthopaedic constructs, because it requires a priori knowledge of the fracture site, and it requires successive rezonings of the mesh for cracks undergoing propagation.
Determining the extent to which fracture vulnerability of ceramic liners depends upon component malpositioning, and identifying which specific patient challenge maneuvers pose the greatest fracture risks, were two research questions lending themselves to answer by means of a novel meshing approach devised to bypass these traditional difficulties in FEA fracture mechanics29. The essential idea was to automatically zone local FEA meshes for incipient cracks at a large number of provisional locations serially in the liner, allowing the respective stress intensity factors to be compared in order to identify the site(s) most vulnerable to crack nucleation and propagation for any given liner orientation and impingement challenge.
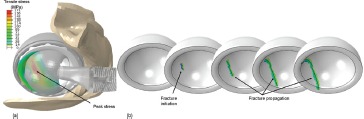
Interestingly, during flexion-dominated impingement challenges such as low-seat-to-stand, the location of greatest fracture vulnerability consistently turned out to be in the posterior region of the cup, at the (edge loaded) head egress site. A second FEA series addressed propagation of cracks presumed to have begun propagating stably from this site, in terms of the effects of cup orientation and various patient motion challenges in tending to cause ongoing stable crack propagation to change from stable to unstable/critical. (Figure 14). The data showed that higher cup inclination and anteversion angles gave rise to higher risk of shift to catastrophic fracture, but only if the impingement kinetics were such that subluxation-associated edge loading occurred. Stooping, bending anteriorly, and squatting - maneuvers clinically linked to liner fracture 49, 52 - were found to pose the worst challenges. The capability to quantify ceramic liner fracture propensity opens inviting new possibilities for systematic evaluation of design factors, surgical factors, and patient factors. This capability nevertheless stops short of constituting actual simulation of a fracture event. Recent advancements with a new computational formulation termed eXtended Finite Element Modeling (XFEM) have enabled that next key step. Working collaboratively with a group of industry-based (Abaqus®) software developers, we now have succeeded in computationally achieving impingement-induced crack propagation in ceramic liners 27. The specific study design involved 36mm CoC implants subjected to squatting and stooping impingement challenges, for 25 variants of cup inclination and anteversion in normal-weight (BMI=25) versus morbidly obese (BMI=50) patients. (The study's clinical motivation was to determine whether optimal cup designs and optimal cup orientations might differ for normal-weight versus obese patients, since obesity has recently been identified as a risk factor for ceramic liner fracture 53) Depending on the specific circumstance considered, usually the liners safely avoided fracture for normal-weight individuals. In the subset of normal-BMI situations for which fracture occurred, the crack usually began posteriorly either at the lip inner edge or at latitude intermediate between the lip and the pole, although there were a handful of cases where the crack started either posteriorly at the lip outer edge or anteriorly at the impingement site. As would be expected intuitively, fracture was much more common in the high-BMI situations. Interestingly, however, the cracks in the high-BMI situations predominantly (87%) initiated posteriorly at intermediate latitudes (Figure 15), rather than at the cup lip. Obviously, much remains to be done to reinforce this new computational advance - experimental validation, in particular. Nevertheless, XFEM represents a very promising new tool for helping further improve the success of THA ceramic bearings.
Figure 14. Linear-elastic fracture mechanics (LEFM) model of ceramic liners. LEFM theory is predicated upon a pre-existing flaw. The existence of microscopic cracks due to manufacturing were presumed to constitute a source of flaws. Specialty quarter-point 2nd order fracture elements were arranged in a rosette pattern (a,b) to allow for fracture propagation calculations. During impingement/subluxation, the crack face (c) opens, with resulting high stress near the crack tip (d). (e) Stress intensity factors (K) for seven impingement-prone motion challenges. Three kinematic sequences developed KI values in excess of alumina's critical stress intensity for fracture propagation (4 MPa m1/2).
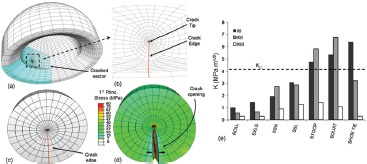
Figure 15. (a) Deep flexion during squatting leads to near-edge loading and development of high stress at an intermediate location between the cup edge and cup pole. These stresses are passed to an XFEM submodel (b), which allowed for both fracture initiation and crack propagation to be modeled, without the requirement for effort-intensive specialized meshes.
THA Instability in Obese Patients
Currently in the US, the prevalence of obesity (BMI > 30) stands at 33.9 percent 54. Besides being linked to earlier and more severe OA of weight-bearing joints and to higher likelihood of requiring THA, there is growing evidence that obesity (especially morbid obesity, BMI > 40) is a significant independent risk factor for dislocation, both in primary55-58 and revision59,60 constructs.
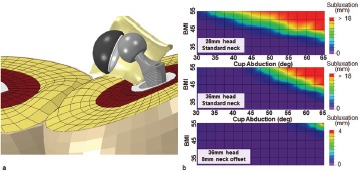
The causality is not well understood. One possible mechanism60 is that thigh-on-thigh soft tissue contact during flexion and adduction induces laterally directed “external” force on the hip, tending to push the head laterally outward from the cup. To study this phenomenon, the THA dislocation FE model was augmented to include thigh-on-thigh soft tissue contact (Figure 16a). The model then was used to assess the extent to which BMI and cup position tended to increase instability due to this mechanism22. Parameters investigated were head size, neck offset, and cup inclination, for eight graded levels of BMI (Figure 16b), four of which were in the morbidly obese range. Physical validation performed using a Tekscan pressure mat showed a 16% average discrepancy of computed versus measured thigh-on-thigh contact force during sit-to-stand maneuvers. Computationally, thigh-on-thigh contact was found to appreciably lower the resistance to dislocation for BMIs of 40 or greater (Figure 17). Dislocation risk increased monotonically above this threshold as a function of cup abduction angle, independent of hardware impingement events. Increased head diameter did not substantially improve joint stability, although high offset necks provided significant benefit.
Figure 16. The obesity dislocation model (a) consisted of THA hardware and the hip capsule, plus mirrored right and left thighs (comprised of skin, adipose tissue, bulk muscle and the femur). Material properties for skin and muscle were assumed linearly elastic, with the adipose tissue treated with a hyperelastic constitutive material model. Material coefficients were obtained from literature. Using anthropometric and cadaveric data, a total of eight graded-levels of obesity were considered (b).
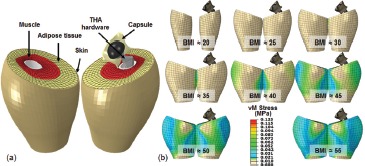
Figure 17. (a) During a sit-to-stand maneuver, thigh-thigh impingement induces a laterally-directed force of sufficient magnitude to cause subluxation and occasionally frank dislocation in the more obese simulations. It was observed that with the 28mm implant (b, top panel), this obesity effect on stability becomes appreciable for BMI values >40. When using a standard offset 36mm implant (b, middle panel), only minor improvement was observed, especially for horizontal cups, and the BMI threshold remained unchanged at 40. However, substantial improvement in stability was observed when using an 8mm high-offset neck (b, bottom panel), with only minor subluxation detected even for the highest BMI simulations.
Impingement Convection of Third Body Debris:
Besides relatively immediate and direct problems in terms of implant damage, impingement is deleterious also because of its indirect linkage with bearing surface wear acceleration, via third-body debris. It is well recognized that the presence of third-body particles within the bearing space can substantially increase wear rates, even by order-of-magnitude61. However, large sub-populations of the third bodies typically found embedded in weightbearing regions of polyethylene bearing surfaces are grossly too large to have migrated through the extremely thin clearance zone between the head and cup surfaces. One possible explanation is that large third body particles might be actively transported into the bearing space by obligate fluid convection during impingement/subluxation events. Since impingement-induced indentation rim damage of UHMWPE cups is a commonplace finding in acetabular components retrieved for revision or at autopsy62, it was inviting to test whether there might be an association between presence/severity of rim indentation damage and the presence/number of embedded third-body particles. An examination was therefore performed of 194 consecutively retrieved cups, which revealed statistically significant association (p<0.01) between presence of impingement rim damage and presence of particles embedded in the bearing, and highly statistically significant correlation (p<0.0001) between severity of impingement rim damage and presence of embedded particles31.
This finding provided encouragement that it might be possible to identify the mechanism. Toward that end, a computational fluid dynamics finite element model was developed of the obligate fluid motions accompanying THA impingement/subluxation32. The computational results were validated by comparison with laser velocimetry of fluid velocity distributions in a directly corresponding physical model. The FE results showed vigorous obligate indraw of peri-articular joint fluid into the bearing region during impingement/subluxation (initially at more than a hundred-fold multiple of the subluxation velocity of the femoral head). The computed fluid pathlines showed that third body particles initially freely suspended just outside the bearing space could reach locations within 11° of the pole of the cup.
As a final step to directly demonstrate causality, new metal-on-polyethylene THA head-liner pairs (n=10) were mounted in the servohydraulic joint motion simulator, with the implants immersed in a synovial fluid analog bath within which CoCrMo particles were suspended30. All THA component pairs were subjected to 7200 cycles of simulated normal level walking. Half of the pairs were also subjected to 20 intermittent impingement/ subluxation events, one per 360 walking cycles. At termination of testing, the number and location of particles embedded on the bearing surface of each of the acetabular liners were then counted using digital image analysis. The specimens that had had the interspersed impingement/subluxation events had 15.7 times as many embedded particles (p=0.017) as the specimens with walking cycles alone. The femoral heads from the walking+impingement group also had dramatically more severe scratching damage than those in the walking-only group. Taken together with the retrievalresults associating impingement rim damage with third body embedment, and with the finite element results documenting vigorous obligate fluid indraw accompanying impingement/subluxation, these motion simulator results convincingly indicate that impingement/subluxation events potently facilitate third body debris gaining access to wear-critical regions of the bearing surface.
Directions forward:
This summarized body of research hopefully has contributed to reducing an important set of clinical problems. However, it remains a work in progress. Going forward, three broad areas of effort suggest themselves. The first of these is to continue developing and improving the current finite element model paradigm, by specific tangible steps such as validating the ceramic liner fracture analysis, by including passive constraint effects and active loadings of individual muscles at the hip, by refining (and validating) the scraping damage metric, etc., and by ongoing internal technical improvements algorithmically. The second broad direction forward, by contrast largely outside the scope of the present model paradigm, is that there needs to be a much better base of knowledge regarding the numbers and frequencies of impingement/dislocation challenge events in THA patients' habitual activity regimens. In a controlled laboratory setting, replicate captures of kinetic and kinetic data for well-prescribed challenge events are encouragingly reproducible13, but this alone provides an unduly narrow window into the real-world of impingement and dislocation challenges that THA constructs encounter. The third broad direction forward is that the work needs to move beyond its present paradigm of delineating causality relationships for individual parameters. Basis needs to be developed for integrating multiple concurrent risk factors, in order to be able to make reasonable trade-offs between directly competing individual variables, and to have rationale for relative weighting of multiple independent variables.
Acknowledgments
The authors gratefully acknowledge the invaluable contributions of many collaborators in this work. These primarily are colleagues and/or students who have shared in the authorship of the individual studies summarized. Within this group, we particularly wish to recognize Mr. Tom Baer, Dr. Dick Brand, Mr. Ben Ellis, Dr. Devon Goetz, Dr. Anneliese Heiner, Dr. Dick Johnston, Dr. Steve Lu, Dr. Hannah Lundberg, Mr. Mark Nadzadi, Dr. Phil Noble, Dr. Jim Rudert, Dr. Chris Scifert, Mr. Kit Stewart, Mr. Nick Stroud, Dr. Yuki Tochigi, Dr. Jeff Weiss, and Dr. John Yack. Ms. Julie Mock and Ms. Lori Yoder provided wonderful behind-the-scenes administrative support.
Conflict of Interest Statement:
Funding for the work summarized in this manuscript came from NIH/NIAMS (AR046601, AR047653, and AR053553) (TDB), from Merit Awards from the US Department of Veterans Affairs (JJC), and from DePuy Orthopaedics Inc. (TDB/JJC). Dr. Brown is a consultant for Smith and Nephew, Inc., and Dr. Callaghan is a consultant for DePuy, Inc., neither of which relationships played a role in the design or conduct of this research.
References
- 1.Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J.Bone Joint Surg. Am. 2009;91:128–133. doi: 10.2106/JBJS.H.00155. [DOI] [PubMed] [Google Scholar]
- 2.Alberton GM, High WA, Morrey BF. Dislocation after revision total hip arthroplasty: An analysis of risk factors and treatment options. J Bone Joint Surg Am. 2002;84:1788–1792. [PubMed] [Google Scholar]
- 3.Paterno SA, Lachiewicz PF, Kelley SS. The influence of patient-related factors and the position of the acetabular component on the rate of dislocation after total hip replacement. J.Bone Joint Surg.Am. 1997;79:1202–1210. doi: 10.2106/00004623-199708000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez-Sotelo J, Berry DJ. Epidemiology of instability after total hip replacement. Orthop.Clin. North Am. 2001;32:543–52. doi: 10.1016/s0030-5898(05)70225-x. [DOI] [PubMed] [Google Scholar]
- 5.von Knoch M, Berry DJ, Harmsen WS, Morrey BF. Late dislocation after total hip arthroplasty. J.Bone Joint Surg.Am. 2002;84-A:1949–1953. doi: 10.2106/00004623-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Callaghan JJ, Heithoff BE, Goetz DD, Sullivan PM, Pedersen DR, Johnston RC. Prevention of dislocation after hip arthroplasty: Lessons from longterm followup. Clin.Orthop. 2001;393:157–162. doi: 10.1097/00003086-200112000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Heithoff BE, Callaghan JJ, Goetz DD, Sullivan PM, Pedersen DR, Johnston RC. Dislocation after total hip arthroplasty: A single surgeon's experience. Orthop.Clin.North Am. 2001;32:587–591. doi: 10.1016/s0030-5898(05)70229-7. [DOI] [PubMed] [Google Scholar]
- 8.Brown TD, Callaghan JJ. Impingement in total hip replacement: Mechanisms and consequences. Curr Orthop. 2008;22:376–391. doi: 10.1016/j.cuor.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouchard SM, Stewart KJ, Pedersen DR, Callaghan JJ, Brown TD. Design factors influencing performance of constrained acetabular liners: Finite element characterization. J.Biomech. 2006;39:885–893. doi: 10.1016/j.jbiomech.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 10.Bouchard SM. M.S. Thesis (Supervision Brown, TD) University of Iowa: Department of Biomedical Engineering; 2004. A Finite Element Study of Constrained Acetabular Cups. [Google Scholar]
- 11.Callaghan JJ, Brown TD, Pedersen DR, Johnston RC. Choices and compromises in the use of small head sizes in total hip arthroplasty. Clin.Orthop. Relat.Res. 2002;405:144–149. doi: 10.1097/00003086-200212000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Nadzadi ME, Pedersen DR, Callaghan JJ, Brown TD. Effects of acetabular component orientation on dislocation propensity for small-head-size total hip arthroplasty. Clin.Biomech. 2002;17:32–40. doi: 10.1016/s0268-0033(01)00096-1. [DOI] [PubMed] [Google Scholar]
- 13.Nadzadi ME, Pedersen DR, Yack HJ, Callaghan JJ, Brown TD. Kinematics, kinetics, and finite element analysis of commonplace maneuvers at risk for total hip dislocation. J.Biomech. 2003;36:577–591. doi: 10.1016/s0021-9290(02)00232-4. [DOI] [PubMed] [Google Scholar]
- 14.Nadzadi ME. M.S. Thesis (Supervisor: Brown TD) University of Iowa: Department of Biomedical Engineering; 2001. Formulation Advancements for Finite Element Investigation of Dislocation of Total Hip Arthroplasty. [Google Scholar]
- 15.Pedersen DR, Callaghan JJ, Brown TD. Activity-dependence of the “safe zone” for impingement versus dislocation avoidance. Med.Eng.Phys. 2005;27:323–328. doi: 10.1016/j.medengphy.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Scifert CF, Brown TD, Lipman JD. Finite element analysis of a novel design approach to resisting total hip dislocation. Clin.Biomech. 1999;14:697–703. doi: 10.1016/s0268-0033(99)00054-6. [DOI] [PubMed] [Google Scholar]
- 17.Scifert CF, Brown TD, Pedersen DR, Callaghan JJ. A finite element analysis of factors influencing total hip dislocation. Clin.Orthop.Relat.Res. 1998;355:152–162. doi: 10.1097/00003086-199810000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Scifert CF, Brown TD, Pedersen DR, Heiner AD, Callaghan JJ. Development and physical validation of a finite element model of total hip dislocation. Comput Methods Biomech Biomed Engin. 1999;2:139–147. doi: 10.1080/10255849908907983. [DOI] [PubMed] [Google Scholar]
- 19.Scifert CF, Noble PC, Brown TD, Bartz RL, Kadakia N, Sugano N, Johnston RC, Pedersen DR, Callaghan JJ. Experimental and computational simulation of total hip arthroplasty dislocation. Orthop Clin North Am. 2001;32:553–567. doi: 10.1016/s0030-5898(05)70226-1. [DOI] [PubMed] [Google Scholar]
- 20.Scifert CF. Ph.D. Thesis (Supervisor: Brown TD) University of Iowa: Department of Biomedical Engineering; 1999. A Finite Element Investigation into the Biomechanics of Total Artificial Hip Dislocation. [Google Scholar]
- 21.Elkins JM, Stroud NJ, Rudert MJ, Tochigi Y, Pedersen DR, Ellis BJ, Callaghan JJ, Weiss JA, Brown TD. The capsule's contribution to total hip construct stability-a finite element analysis. J Orthop Res. 2011;29:1642–1648. doi: 10.1002/jor.21435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elkins JM, Matej D, Pedersen DR, Singh B, Yack HJ, Callaghan JJ, Brown TD. Morbid obesity may increase dislocation in total hip patients: A biomechanical analysis. Clin Orthop Relat Res. 2013;471:971–980. doi: 10.1007/s11999-012-2512-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart KJ, Edmonds-Wilson RH, Brand RA, Brown TD. Spatial distribution of hip capsule structural and material properties. J.Biomech. 2002;35:1491–1498. doi: 10.1016/s0021-9290(02)00091-x. [DOI] [PubMed] [Google Scholar]
- 24.Stewart KJ, Pedersen DR, Callaghan JJ, Brown TD. Implementing capsule representation in a total hip dislocation finite element model. Iowa Orthop J. 2004;24:1–8. [PMC free article] [PubMed] [Google Scholar]
- 25.Stroud NJ. M.S. Thesis (Supervisor: Brown TD) University of Iowa: Department of Biomedical Engineering; 2010. Advancements of a servohydraulic human hip joint motion simulator for experimental investigation of hip joint impingement/dislocation. [Google Scholar]
- 26.Elkins JM, Kruger KM, Pedersen DR, Callaghan JJ, Brown TD. Edge-loading severity as a function of cup lip radius in metal-on-metal total hips - A finite element analysis. J.Orthop.Res. 2012;30:169–177. doi: 10.1002/jor.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elkins JM, Liu C, Qin X, Du Z, Brown TD. Ceramic total hip bearing fracture modeling in abaqus using co-simulation and extended finite element modeling. Proceedings of the 2011 SIMULIA Customer Conference. 2011:670–679. [Google Scholar]
- 28.Elkins JM, O'Brien MK, Stroud NJ, Pedersen DR, Callaghan JJ, Brown TD. Hard-on-hard total hip impingement causes extreme contact stress concentrations. Clin.Orthop.Relat.Res. 2011;469:454–463. doi: 10.1007/s11999-010-1632-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elkins JM, Pedersen DR, Callaghan JJ, Brown TD. Fracture propagation propensity of ceramic liners during impingement-subluxation. J.Arthroplasty. 2011;27(4):520–526. doi: 10.1016/j.arth.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heiner AD, Lundberg HJ, Baer TE, Pedersen DR, Callaghan JJ, Brown TD. Effects of episodic subluxation events on third body ingress and embedment in the THA bearing surface. J.Biomech. 2008;41:2090–2096. doi: 10.1016/j.jbiomech.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lundberg HJ, Liu SS, Callaghan JJ, Pedersen DR, O'Rourke MR, Goetz DD, Vittetoe DA, Clohisy JC, Brown TD. Association of third body embedment with rim damage in retrieved acetabular liners. Clin.Orthop.Relat.Res. 2007;465:133–139. doi: 10.1097/BLO.0b013e31815c5a7b. [DOI] [PubMed] [Google Scholar]
- 32.Lundberg HJ, Pedersen DR, Baer TE, Muste M, Callaghan JJ, Brown TD. Effects of implant design parameters on fluid convection, potentiating third-body debris ingress into the bearing surface during THA impingement/subluxation. J.Biomech. 2007;40:1676–1685. doi: 10.1016/j.jbiomech.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Callaghan JJ, Brown TD, Pedersen DR, Johnston RC. Orthopaedic crossfire--larger femoral heads: A triumph of hope over reason! in the affirmative. J.Arthroplasty. 2003;18:82–84. doi: 10.1054/arth.2003.50072. [DOI] [PubMed] [Google Scholar]
- 34.Bremner BR, Goetz DD, Callaghan JJ, Capello WN, Johnston RC. Use of constrained acetabular components for hip instability: An average 10-year follow-up study. J.Arthroplasty. 2003;18:131–137. doi: 10.1016/s0883-5403(03)00295-x. [DOI] [PubMed] [Google Scholar]
- 35.Callaghan JJ, Parvizi J, Novak CC, Bremner B, Shrader W, Lewallen DG, Johnston RC, Goetz DD. A constrained liner cemented into a secure cementless acetabular shell. J Bone Joint Surg Am. 2004;86:2206–2211. doi: 10.2106/00004623-200410000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Callaghan JJ, O'Rourke MR, Goetz DD, Lewallen DG, Johnston RC, Capello WN. Use of a constrained tripolar acetabular liner to treat intraoperative instability and postoperative dislocation after total hip arthroplasty: A review of our experience. Clin.Orthop. 2004;429:117–123. doi: 10.1097/01.blo.0000150276.98701.95. [DOI] [PubMed] [Google Scholar]
- 37.Flint JH, Phisitkul P, Callaghan JJ. Closed reduction of a dislocated constrained total hip arthroplasty using a novel technique with a peg board. Orthopedics. 2010;10:201–203. doi: 10.3928/01477447-20100129-28. [DOI] [PubMed] [Google Scholar]
- 38.Goetz DD, Capello WN, Callaghan JJ, Brown TD, Johnston RC. Salvage of a recurrently dislocating total hip prosthesis with use of a constrained acetabular component. A retrospective analysis of fifty-six cases. J Bone Joint Surg A. 1998;80:502–509. doi: 10.2106/00004623-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Goetz DD, Bremner BR, Callaghan JJ, Capello WN, Johnston RC. Salvage of a recurrently dislocating total hip prosthesis with use of a constrained acetabular ComponentA concise follow-up of a previous report. J Bone Joint Surg Am. 2004;86:2419–2423. doi: 10.2106/00004623-200411000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Goetz DD, Capello WN, Callaghan JJ, Brown TD, Johnston RC. Salvage of total hip instability with a constrained acetabular component. Clin.Orthop. 1998;355:171–181. doi: 10.1097/00003086-199810000-00018. [DOI] [PubMed] [Google Scholar]
- 41.Maxian TA, Brown TD, Pedersen DR, Callaghan JJ. A sliding-distance-coupled finite element formulation for polyethylene wear in total hip arthroplasty. J.Biomech. 1996;29:687–692. doi: 10.1016/0021-9290(95)00125-5. [DOI] [PubMed] [Google Scholar]
- 42.Haft GF, Heiner AD, Dorr LD, Brown TD, Callaghan JJ. A biomechanical analysis of polyethylene liner cementation into a fixed metal acetabular shell. J.Bone Joint Surg.Am. 2003;85-A:1100–1110. doi: 10.2106/00004623-200306000-00019. [DOI] [PubMed] [Google Scholar]
- 43.Gerber C, Schneeberger AG, Beck M, Schlegel U. Mechanical strength of repairs of the rotator cuff. J.Bone Joint Surg.Br. 1994;76:371–380. [PubMed] [Google Scholar]
- 44.Stähelin T, Drittenbass L, Hersche O, Miehlke W, Munzinger U. Failure of capsular enhanced short external rotator repair after total hip replacement. Clin.Orthop.Relat.Res. 2004;420:199–204. doi: 10.1097/00003086-200403000-00028. [DOI] [PubMed] [Google Scholar]
- 45.Kao JT, Woolson ST. Piriformis tendon repair failure after total hip replacement. Orthop.Rev. 1992;21:171–174. [PubMed] [Google Scholar]
- 46.Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Koster G, Lohmann CH. Metal-on-metal bearings and hypersensitivity in patients with ar tificial hip joints. A clinical and histomorphological study. J.Bone Joint Surg.Am. 2005;87:28–36. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]
- 47.Knahr K, Böhler M, Frank P, Plenk H, Salzer M. Survival analysis of an uncemented ceramic acetabular component in total hip replacement. Arch. Orthop.Trauma Surg. 1987;106:297–300. doi: 10.1007/BF00454337. [DOI] [PubMed] [Google Scholar]
- 48.Willmann G. Ceramic femoral head retrieval data. Clin.Orthop.Relat.Res. 2000;379:22–28. doi: 10.1097/00003086-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 49.Ha YC, Kim SY, Kim HJ, Yoo JJ, Koo KH. Ceramic liner fracture after cementless alumina-on-alumina total hip arthroplasty. Clin.Orthop.Relat.Res. 2007;458:106–110. doi: 10.1097/BLO.0b013e3180303e87. [DOI] [PubMed] [Google Scholar]
- 50.Park YS, Hwang SK, Choy WS, Kim YS, Moon YW, Lim SJ. Ceramic failure after total hip arthroplasty with an alumina-on-alumina bearing. J.Bone Joint Surg.Am. 2006;88:780–787. doi: 10.2106/JBJS.E.00618. [DOI] [PubMed] [Google Scholar]
- 51.Toni A, Traina F, Stea S, Sudanese A, Visentin M, Bordini B, Squarzoni S. Early diagnosis of ceramic liner fracture. guidelines based on a twelve-year clinical experience. J.Bone Joint Surg.Am. 2006;4(88 Suppl):55–63. doi: 10.2106/JBJS.F.00587. [DOI] [PubMed] [Google Scholar]
- 52.Min BW, Song KS, Kang CH, Bae KC, Won YY, Lee KY. Delayed fracture of a ceramic insert with modern ceramic total hip replacement. J.Arthroplasty. 2007;22:136–139. doi: 10.1016/j.arth.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 53.Poggie RA, Turgeon TR, Coutts RD. Failure analysis of a ceramic bearing acetabular component. J.Bone Joint Surg.Am. 2007;89:367–375. doi: 10.2106/JBJS.F.00148. [DOI] [PubMed] [Google Scholar]
- 54.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 55.Andrew JG, Palan J, Kurup HV, Gibson P, Murray DW, Beard DJ. Obesity in total hip replacement. J.Bone Joint Surg.Br. 2008;90:424–429. doi: 10.1302/0301-620X.90B4.20522. [DOI] [PubMed] [Google Scholar]
- 56.Grant JA, Viens N, Bolognesi MP, Olson SA, Cook CE. Two-year outcomes in primary THA in obese male veterans administration medical center patients. Rheumatol.Int. 2008;28:1105–1109. doi: 10.1007/s00296-008-0575-y. [DOI] [PubMed] [Google Scholar]
- 57.Lubbeke A, Stern R, Garavaglia G, Zurcher L, Hoffmeyer P. Differences in outcomes of obese women and men undergoing primary total hip arthroplasty. Arthritis Rheum. 2007;57:327–334. doi: 10.1002/art.22542. [DOI] [PubMed] [Google Scholar]
- 58.Sadr Azodi O, Adami J, Lindstrom D, Eriksson KO, Wladis A, Bellocco R. High body mass index is associated with increased risk of implant dislocation following primary total hip replacement: 2,106 patients followed for up to 8 years. Acta Orthop. 2008;79:141–147. doi: 10.1080/17453670710014897. [DOI] [PubMed] [Google Scholar]
- 59.Lubbeke A, Moons KG, Garavaglia G, Hoffmeyer P. Outcomes of obese and nonobese patients undergoing revision total hip arthroplasty. Arthritis Rheum. 2008;59:738–745. doi: 10.1002/art.23562. [DOI] [PubMed] [Google Scholar]
- 60.Kim Y, Morshed S, Joseph T, Bozic K, Ries MD. Clinical impact of obesity on stability following revision total hip arthroplasty. Clin.Orthop.Relat.Res. 2006;453:142–146. doi: 10.1097/01.blo.0000238874.09390.a1. [DOI] [PubMed] [Google Scholar]
- 61.Minakawa H, Stone MH, Wroblewski BM, Lancaster JG, Ingham E, Fisher J. Quantification of third-body damage and its effect on UHMWPE wear with different types of femoral head. J.Bone Joint Surg.Br. 1998;80:894–899. doi: 10.1302/0301-620x.80b5.8675. [DOI] [PubMed] [Google Scholar]
- 62.Shon WY, Baldini T, Peterson MG, Wright TM, Salvati EA. Impingement in total hip arthroplasty a study of retrieved acetabular components. J.Arthroplasty. 2005;20:427–435. doi: 10.1016/j.arth.2004.09.058. [DOI] [PubMed] [Google Scholar]
- 63.Amstutz HC, Lodwig RM, Schurman DJ, Hodgson AG. Range of motion studies for total hip replacements. A comparative study with a new experimental apparatus. Clin.Orthop.Relat.Res. 1975:124–130. doi: 10.1097/00003086-197509000-00016. [DOI] [PubMed] [Google Scholar]


