Abstract
Introduction
The indications for vancomycin prophylaxis to prevent Methicillin-resistant Staphylococcus aureus (MRSA) surgical site infections are increasing. The recommended dose of vancomycin has traditionally been 1 gram intravenous. However, the increasing prevalence of obesity in our population coupled with increasing resistance of MRSA to vancomycin has resulted in recent recommendations for weight-based dosing of vancomycin at 15mg/kg. We hypothesize that the standard one gram dose of vancomycin is inadequate to meet the recently recommended dosage of 15mg/kg.
Methods
We performed a retrospective chart review on 216 patients who were screened positive for MRSA prior to undergoing elective total joint or spine surgeries between January 2009 to January 2012. All patients were given 1 gram of vancomycin within an hour prior to surgical incision as prophylaxis. Using the revised dosing protocol of 15mg/kg of body weight for vancomycin, proper dosage was calculated for each patient. These values were then compared to the 1 gram dose given to the patients at time of surgery. Patients were assessed as either underdosed (a calculated weight-based dose >1 gram) or overdosed (a calculated weight-based dose <1 gram). Additionally, we used actual case times and pharmacokinetic equations to determine the vancomycin (VAN) levels at the end of the procedures.
Results
Out of 216 patients who tested positive for MRSA, 149 patients (69%) were determined to be underdosed and 22 patients (10%) patients were determined to be overdosed. The predicted VAN level at the end of procedure was <15 mg/L in 60% of patients with 1 gram dose compared to 12% (p=0.0005) with weight base dose. Six patients developed post-operative MRSA surgical site infections (SSI). Of these six patients; four had strains of MRSA with vancomycin minimum inhibitory concentration of >1.0mg/L. Based on 1g dosing, 5/6 patients with MRSA positive SSIs had wound closure levels of <15 mg/L and all six were <20 mg/L.
Conclusion
In settings such as hospitals, where the risk for resistant bacteria, especially MRSA, is high, it is becoming increasingly important to accurately dose patients who require vancomycin. In order to avoid incorrect dosing of vancomycin health care providers must use weight-based dosing.
Keywords: MRSA, surgical site infections, vancomycin, weight based dosing, total joint surgery, spine surgery
Introduction
As the cost of healthcare continues to escalate at an economically unsustainable rate, the payers of care, including the Federal Government, are focusing on strategies to deflect this cost curve without reducing the quality or accessibility of care. Decreasing the incidence of surgical site infections (SSIs) accomplishes both of these goals. SSIs lead to increasing morbidity and mortality, as well as higher health care costs with longer hospital stays2,9,12,17. To date, the prevalence of SSIs following joint replacement and spine surgery remains at 1-5%. Staphylococcus aureus (SA) is the leading causes of nosocomial infections17, accounting for as many as 48% of all SSIs17. Additionally, an increasing amounts of these infections are caused by resistant bacteria; especially Methicillin-resistant Staphylococcus aureus (MRSA)8. Thus strategies to prevent SSIs caused by both SA and MRSA are gaining importance.
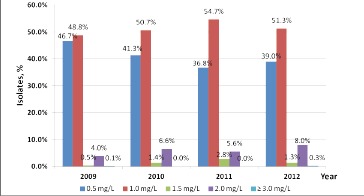
Vancomycin (VAN) remains the standard of treatment and prophylaxis for MRSA5,14,20. The goal of VAN dosing is to achieve a safe and effective minimum inhibitory concentration (MIC)21. With increasing VAN resistance of MRSA, hospitals have witnessed an increasing MIC to successfully treat MRSA infections15. Steinkraus et al.23 reported a 1.5 fold increase in the geometric mean MIC of VAN with nearly 70% of patients having an MIC of 1mg/L, compared to just 10% five years earlier. We have noted a similar trend at our institution. In 2009, 95.5% of our MRSA isolates had a MIC of <1mg/L and only 4.1% were >2mg/L. By 2012 only 90.3% of our MRSA isolates had a MIC of <1mg/L and the percentage of MRSA isolates with a MIC of >2mg/L increased to 8.3%; a 52% increase (Figure 1).
Figure 1. MICs of MRSA isolates at our institution, 2009-third quarter 2012.
In addition to increasing resistance, VAN dosing has been affected by the rising trend of obesity10. As an increasing number of patients are above normal healthy weight limits, giving a standard dose based on these guidelines is insufficient to achieve appropriate serum concentrations. Thus an increasing amount of patients are at risk for underdosing of VAN. Weight-based dosing protocols address this issue. Traditionally, the recommended preoperative dosage of VAN has been one gram given intravenously19, however, recent clinical guidelines for antimicrobial prophylaxis recommend that each patient should receive VAN preoperatively according to a 15mg/kg weight-based protocol2,14. The goal of this study is to demonstrate that weight-based VAN dosing is essential to provide adequate serum concentrations to reduce MRSA to eradicate MRSA in patients undergoing joint replacement and spine surgical procedures.
Methods
This study was conducted at a university affiliated, single specialty, orthopedic hospital. We reviewed prospectively collected data on patients with positive MRSA nasal screens undergoing total joint and spine surgical procedures between January 2009 and January 2012. Patients' gender, height, weight, and serum creatinine (Cr) were utilized in the pharmacokinetic analysis. In addition, we noted if a patient within the study developed a SSI during admission. All patients received VAN 1g within 1 hour prior to incision and VAN levels were calculated according to this dose. We calculated the VAN weight-based (WB) dose using the goal of 15mg/ kg of actual body weight (ABW). The actual dose was rounded to the nearest 250mg for each patient. Patients were classified as either underdosed (calculated WB dose >1g dose) or overdosed (calculated WB dose <1g dose). We used pharmacokinetic formulas (Appendix 1) to estimate VAN levels at the time of incision (peak levels). Additionally, we used the actual duration of the operation (op-time) in conjunction with the pharmacokinetic formulas to determine and VAN levels at the time of wound closure. The percent of patients with estimated VAN levels <10 mg/L, 10-15 mg/L, 15-20 mg/L and >20 mg/L at the end of procedure were compared for each dosing regimen. For each patient who developed a MRSA SSI we calculated VAN levels at wound closure and compared these levels to the MICs of the MRSA cultured from that patient. McNemar's test was used for categorical variables and an analysis was performed using SPSS version 20.
Results
There were 216 patients with a positive MRSA nasal culture during our study period. Of the 216 patients, 68% underwent arthroplasty (knee n=75, hip n=66, shoulder n=6), 24% spine fusion, and 8% laminectomy (Table 1). Median surgical time was 124 (29-470) minutes. Mean age was 60 years, mean ABW was 86 kg (68% of patients had an ABW >20% of ideal body weight (IBW)). The mean VAN dose, clearance, and half-life were 12 mg/ kg, 5 L/h and 10 h respectively. Vancomycin 1 g dose was appropriate in 21% of patients, 10% were overdosed, and 69% were underdosed (44% by 250mg, 32% by 500mg, 17% by 750mg, 7% by 1.0g) (Figure 2). There were no adverse effects observed in patients who were overdosed. Eighty-three percent of patients with WB dosing attained a peak level at incision of >20 mg/L, while only 25% of patients with 1g dosing achieved an incisional level of >20 mg/L (Figure 3). Predicted VAN levels at the end of procedure were <10 mg/L in 9% of patients with a 1g dose compared to just 2% of patients with a weight based dose (p= 0.0002). Water-based dosing resulted in end-of-surgery VAN levels of >15mg/L and >20mg/L in 87.5% and 17.1% of patients respectively. In comparison, the standard 1g dose resulted in VAN levels of >15mg/L and >20mg/L in 39.8% and 10.6% of patients respectively. In total, the predicted VAN level at the end of procedure was <15 mg/L in 60% of patients with 1g dose compared to 12% (p=0.0005) with WB dose (Figure 4). Nine patients developed post-operative SSIs, and 6/9 had positive cultures growing MRSA. Of these six patients, four had strains of MRSA with VAN MICs of >1.0mg/L. Based on 1g dosing, 5/6 patients with MRSA positive SSIs had wound closure levels of <15 mg/L and all six were <20 mg/L (Table 2).
Table 1.
Patient Characteristics
| Characteristic | N=216 |
| Mean age (yr) | 60 |
| Male patients | 103 (48%) |
| Mean height (cm) | 169 |
| Mean ideal body weight (kg) | 62 |
| Mean actual body weight (kg) | 86 |
| Mean serum creatinine (mg/dL) | 0.9 |
| Mean estimated creatinine clearance (mL/min)(SD) | 79 (31) |
| Surgical procedure | |
| Arthroplasy | 147 (68) |
| Knee arthroplasty | 75 (51) |
| Hip arthroplasty | 66 (45) |
| Shoulder arthroplasty | 6 (4) |
| Spine fusion | 52 (24) |
| Laminectomy only | 17 (8) |
Figure 2. Percent of patients vs. VAN dosing.
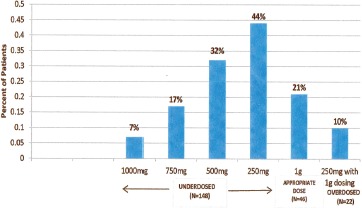
Figure 3. Estimated VAN levels at incision time.
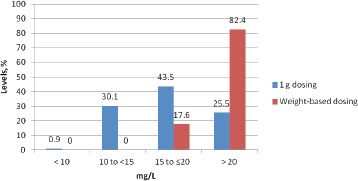
Figure 4. Estimated VAN levels at time of wound closure.
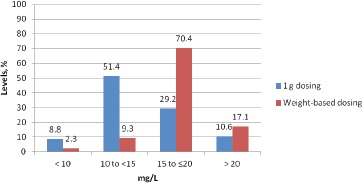
Table 2.
Patients who developed subsequent MRSA SSI post-operatively
| Patient | Age, years | ABW, kg | % above IBW | Procedure | Underdosed with vancomycin dose 1g/mg | Estimated level at wound closure with 1 g dose mg/l | Estimated level at wound closure with weight based dose | MIC of MRSA Isolates mg/L |
|---|---|---|---|---|---|---|---|---|
| 1 | 65 | 96.1 | 213 | TKR | 500 mg | 13.5 | 20.3 | ≤0.5 |
| 2 | 82 | 77.1 | 156 | THR | 250 mg | 16.8 | 21.0 | 1 |
| 3 | 73 | 87.7 | 142 | TKR | 500 mg | 14.2 | 21.3 | 1 |
| 4 | 70 | 92 | 119 | TKR | 500 mg | 13.2 | 18.8 | 1 |
| 5 | 46 | 97.7 | 126 | Spine surgery | 500 mg | 10.1 | 15.2 | ≤0.5 |
| 6 | 70 | 85 | 110 | Spine surgery | 250 mg | 14.1 | 17.7 | 1 |
Discussion
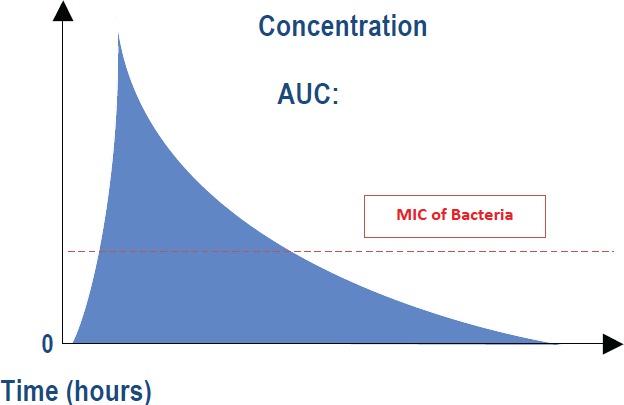
Our analysis determined that due to patient variability in weight and the increasing VAN MIC of MRSA, the standard 1g dose protocol consistently results in either overdosing or underdosing patients. Overdosing can induce severe drug toxicities, while underdosing may not provide sufficient drug concentrations for bactericidal effects. The appropriate dosing goal of VAN is best described by the area under the curve (AUC): MIC ratio (Appendix 2). The AUC:MIC ratio combines the pharmacokinetic (time course of antimicrobial concentrations) and pharmacodynamic (antimicrobial effect of the concentration) factors that determine efficacy. The pharmacokinetic parameters include the peak serum level (or time at incision) and trough level (time at wound closure) from which the AUC is determined. The pharmacodynamic parameter incorporates the amount of time serum drug concentrations remain above the MIC, and therefore have active bactericidal activity. It is imperative that VAN serum concentrations remain above the MIC for the entire surgical procedure (until complete wound closure) to truly have effective prophylactic effects. Taking these factors into account, for isolates with an MIC= 1, the therapeutic level in plasma should be 16-20 mg/L. Gremmel et al.11 echo this recommendation that therapeutic pre-dose levels be set at 15-20 mg/L, which can refer to wound closure level goals for our surgical patients. When used as surgical prophylaxis in a one-time dose, the wound closure levels at the end of surgery must remain at this level to provide coverage throughout the procedure. In addition, the wound closure concentration should always be >10 mg/L to prevent resistance development. Steinkraus et al. highlighted the issues of increasing resistance by reporting a four-fold increase in the percentage of MRSA isolates that required an MIC of 2 mg/L23.
In our study, 69% of the patients receiving perioperative VAN prophylaxis were underdosed by >250mg and more than half of those patients were underdosed by >500mg. This underdosing translates to 30% of patients failing to obtain the recommended 15-20 mg/L levels for a MIC=1 at their peak concentrations. Furthermore, 8.8% had wound closure levels <10 mg/L at the end of their operations, which is the minimum level required to avoid developing resistant strains. Most notably four of the six patients in our study who developed MRSA SSIs each had serum VAN levels which were not high enough to address the actual MICs of the MRSA strains cultured from each of the patients. Obtaining these VAN concentration levels is essential as MRSA isolate MICs continue to increase and the development of resistance becomes more of an issue.
Steinkraus et al highlighted the issues of increasing resistance by reporting a four-fold increase in the percentage of MRSA isolates that required an MIC of 2 mg/L. As defined by Tenover et al., SA isolates with an MIC >1 mg/L are considered heteroresistant and small populations of these isolates may progress to become VAN resistant, and are able to grow in VAN concentration of 8-16 mg/L24. If patients are consistently underdosed in the hospital setting, SA will be more adept at evolving and gaining resistance genes, thereby furthering the bacteria's resistance to VAN25.
Not only has increasing MRSA resistance caused potential underdosing, VAN dosing is a dynamic process, as levels vary according to multiple factors and change over time. Cheymol et al.6, illustrated that obesity increases both the volume of distribution (Vd), clearance of VAN (CL) thus decreasing its half-life. Vance-Bryan et al.26 demonstrated the increase in Vd, meaning that obese patients may require increased dosage to obtain serum concentrations greater than the necessary MIC. Bauer et al.3 explained that because VAN is renally excreted, and obese patients have higher CL rates, the drug will be excreted faster, requiring higher doses to maintain appropriate AUC:MIC ratios. Blouin et al4 also recognized the increased Vd and CL in obese patients compared to those of normal, healthy body weights. Each of these authors recommended dosing VAN based upon patients' total body weight due to the described pharmacokinetic factors.
It is also important to consider that not only are obese patients being underdosed perioperatively, but also that obese patients are at greater risk to undergo surgery. Obesity is a major risk factor for the development of osteoarthritis and patients undergoing lower extremity joint replacement and spine surgery are on average heavier than those not undergoing these surgeries1,22. Because of this, more and more obese patients are undergoing joint replacement surgery and spine surgery, and may be at risk to be underdosed for MRSA prophylaxis perioperatively. This can be seen in our patient population, as 61/75 (81.5%) of patients undergoing knee-replacement surgery had an ABW >20% over IBW, as defined by Equation 1 of Appendix 1. Additionally, patients with positive swabs, such as those included in our study, are at an increased risk of SSIs and will remain so if VAN prophylaxis fails to reach the proper therapeutic levels13,18,27. The purpose of our study was to demonstrate that weight-based VAN dosing is essential to provide VAN serum concentrations high enough to be effective in protecting patients against MRSA. One may use a priori reasoning to correlate this increased dosing to a decrease in the risk of developing MRSA SSIs. However, the study was not designed to address this issue and is statistically underpowered to make any such claims.
Due to increasing cases of MRSA, resistant strains, MICs of MRSA are continually increasing. This, paired with a rise in obesity throughout the population and increased surgical procedures within the obese population, has made it more evident that a standard 1g dose of VAN for perioperative prophylaxis may no longer be sufficient. If the trend of underdosing continues, the MIC of VAN required to treat MRSA will continue to increase, forging more resistant strains. As described in our study, a protocol of weight-based dosing exposed the standard 1g dose as insufficient for a majority of patients. A weight based protocol provides necessary perioperative MRSA prophylaxis potentially decreasing the progression toward VAN-resistant strains, and preventing SSIs.
Conflict of Interest
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Ethical Review
We have received IRB approval for our study.
Appendix 128
Equation 1: Estimated creatinine clearance (CrCl) by Cockroft-Gault equation, mL/min
CrCl (male) = [(140 - age) x ideal body weight (IBW)]/(72 x SCr)
CrCl (female) = [(140 - age) x IBW (kg)]/(72 x SCr) x 0.85
Used minimum serum creatinine of 1 mg/dL if age > 70 y
IBW (males), kg = 50 + (2.3 x height in inches over 60 inches)
IBW (females), kg = 45 + (2.3 x height in inches over 60 inches)
Equation 2: Vancomycin clearance (Clearance), L/h Clearance= CrCl x 0.06
Equation 3: Vancomycin volume of distribution (Vd), L
Vd = DW x 0.7 L/kg
DW = total (actual) body weight, kg
Equation 4: Vancomycin Ke, h−1
Ke= Cl/Vd
Equation 5: Vancomycin half-life (T1/2), h
T1/2 = 0.693/Ke
Equation 6: Estimated peak level (Cmax), mg/L
Cmax= Dose/Vd
Dose= vancomycin dose of 1000 mg or vancomycin calculated weight-based dose (15 mg/kg x DW, rounded to the nearest 250 mg)
Equation 7: Estimated level at the end of surgical procedure (Cmin), mg/L
Cmin = Cmax x e-Ke x T
T = opTime
Appendix 2
References
- 1.Altman R, Fries JF, Block DA, et al. Radiographic assessment of progression in osteoarthritis. Arthritis Rheum. 1987;30:1214–1225. doi: 10.1002/art.1780301103. [DOI] [PubMed] [Google Scholar]
- 2.Astagneau P, Rioux C, Golliot F, Brucker G. Morbidity and mortality associated with surgical site infections: results from the 1997-1999 INCISO surveillance. J Hosp Infect. 2001;48:267–274. doi: 10.1053/jhin.2001.1003. [DOI] [PubMed] [Google Scholar]
- 3.Bauer LA, Black DJ, Lill JS. Vancomycin dosing in morbidly obese patients. Eur J Clin Pharmacol. 1998;54:621–5. doi: 10.1007/s002280050524. [DOI] [PubMed] [Google Scholar]
- 4.Blouin RA, Bauer LA, Miller DD, et al. Vancomycin pharmacokinetics in normal and morbidly obese subjects. Antimicrob Agents Chemother. 1982;21:575–80. doi: 10.1128/aac.21.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70:195283. doi: 10.2146/ajhp120568. [DOI] [PubMed] [Google Scholar]
- 6.Cheymol G. Effects of obesity on pharmacokinetics: implications for drug therapy. Clin Pharmacokinet. 2000;39:215–31. doi: 10.2165/00003088-200039030-00004. [DOI] [PubMed] [Google Scholar]
- 7.DeRyke CA, Alexander DP. Optimizing vancomycin dosing trough pharmacodynamic assessment targeting area under the concentration-time curve/minimum inhibitory concentration. Hospital Pharmacy. 2009;44:751–765. [Google Scholar]
- 8.Anderson DJ, Sexton DJ, Kanafani ZA, Auten G, Kaye KS. Severe surgical site infection in community hospitals: epidemiology, key procedures, and the hanging prevalence of methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28:1047–1053. doi: 10.1086/520731. pp. [DOI] [PubMed] [Google Scholar]
- 9.Engemann JJ, Carmeli Y, Cosgrove SE, et al. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infections. Clin Infect Dis. 2003;36:592–598. doi: 10.1086/367653. [DOI] [PubMed] [Google Scholar]
- 10.Flegal KM. Epidemiologic aspects of overweight and obesity in the United States. Physiol Behav. 2005;86:599–602. doi: 10.1016/j.physbeh.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 11.Gemmell C, Edwards D, Fraise A, Gould F, Ridgeway G, Warren R. Guidelines for the prophylaxis and treatment of MRSA infections in the UK. Journal of Antimicrobial Chemotherapy. 2006;57:589–608. doi: 10.1093/jac/dkl017. [DOI] [PubMed] [Google Scholar]
- 12.Kirkland K, Briggs J, Trivette S, Wilkinson W, Sexton D. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infection Control and Hospital Epidemiology. 1999;20(11):725–730. doi: 10.1086/501572. Vol. No. November, pp. [DOI] [PubMed] [Google Scholar]
- 13.Kluytmans J, Mouton J, Ijzerman E, Vanden-broucke-Grauls C, Maat J, Wagenvoort J, Verbrugh H. Nasal carriage of S. aureus as a major risk factor for wound infections after cardiac surgery. The Journal of Infectious Diseases. 1995;171:216–219. doi: 10.1093/infdis/171.1.216. [DOI] [PubMed] [Google Scholar]
- 14.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 15.Moise PA, Sakoulas G, Forrest A, Schentag JJ. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Anti-microb Agents Chemother. 2007;51:2582–6. doi: 10.1128/AAC.00939-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Nosocomial Infections Surveillance (NNIS) report, data summary from October 1986-April 1996, issued May 1996: a report from the National Nosocomial Infections Surveillance (NNIS) System. Am J Infect Control. 1996;24:380–388. [PubMed] [Google Scholar]
- 17.Perencevich EN, Sands KE, Cosgrove SE, Guadagnoli E, Meara E, Platt R. Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerg Infect Dis. 2003;9:196–203. doi: 10.3201/eid0902.020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perl T, Cullen J, Wenzel R, Zimmerman B, Pfaller M, Sheppard D, Twombley J, French P, Herwaldt L. Intranasal mupirocin to prevent postoperative S. Aureus infections. N Engl J Med. 2002;24:1871–1877. doi: 10.1056/NEJMoa003069. 346. [DOI] [PubMed] [Google Scholar]
- 19.Ritter M, Barzilauskas C, Faris P, Keating E. Vancomycin prophylaxis and elective total joint arthroplasty. orthopedics. 1989;10:1333–1336. doi: 10.3928/0147-7447-19891001-09. 12. [DOI] [PubMed] [Google Scholar]
- 20.Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Craig W, Billeter M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 21.Rybak MJ, Lomaestro BM, Rotschafer JC, et al. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the Infectious Diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009;49:325–7. doi: 10.1086/600877. [DOI] [PubMed] [Google Scholar]
- 22.Schouten JS, van den Ouweland F, Valkenburg HA. A 12 year follow up study in the general population on prognostic factors of cartilage loss in osteoarthritis of the knee. Ann Rheum Dis. 1992;51:932–7. doi: 10.1136/ard.51.8.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinkraus G, White R, Friedrich L. Vancomycin MIC creep in nonvancomycin-intermediate Staphylococcus aureus (VISA), vancomycinsusceptible clinical methicillin-resistant S.aureus (MRSA) blood isolates from 2001-05. J Antimicrob Chemother. 2007;60:788–94. doi: 10.1093/jac/dkm258. [DOI] [PubMed] [Google Scholar]
- 24.Tenover FC, Biddle JW, Lancaster MV. Increasing resistance to vancomycin and other glycopeptides in Staphylococcus aureus. Emerg Infect Dis. 2001;7:327–32. doi: 10.3201/eid0702.010237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Brien TF. Emergence, spread, and environmental effect of antimicrobial resistance: how use of an antimicrobial anywhere can increase resistance to any antimicrobial anywhere else. Clin Infect Dis. 2002;34(suppl 3):S78–S84. doi: 10.1086/340244. [DOI] [PubMed] [Google Scholar]
- 26.Vance-Bryan K, Guay DRP, Gilliland SS, et al. Effect of obesity on vancomycin pharmacokinetic parameters as determined by using a Bayesian forecasting technique. Antimicrob Agents Chemother. 1993;37:436–40. doi: 10.1128/aac.37.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wertheim H, Melles D, van Leeuwen W, van Belkum A, Verburgh H, Nouwen J. The role of nasal carriage in S. Aureus infections. Lancet Infect Dis. 2005;5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]


