Abstract
Osteochondral lesions of the talus are being recognized as an increasingly common injury. They are most commonly located postero-medially or antero-laterally, while centrally located lesions are uncommon. Large osteochondral lesions have significant biomechanical consequences and often require resurfacing with osteochondral autograft transfer, mosaicplasty, autologous chondrocyte implantation (or similar methods) or osteochondral allograft transplantation. Allograft procedures have become popular due to inherent advantages over other resurfacing techniques. Cartilage viability is one of the most important factors for successful clinical outcomes after transplantation of osteochondral allografts and is related to storage length and intra-operative factors. While there is abundant literature about osteochondral allograft transplantation in the knee, there are few papers about this procedure in the talus. Failure of non-operative management, initial debridement, curettage or microfractures are an indication for resurfacing. Patients should have a functional ankle motion, closed growth plates, absence of cartilage lesions on the tibial side. This paper reviews the published literature about osteochondral allograft transplantation of the talus focusing on indications, pre-operative planning, surgical approaches, postoperative management, results and complications of this procedure.
Introduction
Osteochondral lesions of the talus are being recognized as an increasingly common injury, and may occur in up to 50% of acute ankle sprains and fractures, particularly in association with sports injuries1, 2. They are most commonly located postero-medially (57%) or anterolaterally (43%), while centrally located lesions are uncommon3. There is a consensus regarding non-operative management of an asymptomatic osteochondral lesion of the talus, but in symptomatic patients, non-operative treatment has been reported to be successful in only 45% of the cases. Surgery is often indicated in these cases and multiple techniques have been proposed1. Smaller lesions can be treated with arthroscopic debridement and drilling/microfracture, with satisfactory results in more than 80% of the patients4. On the other hand, large osteochondral lesions have more significant biomechanical consequences and often require resurfacing. The three most common treatments are: osteochondral autograft transfer (OATS)/mosaicplasty from the knee, autologous chondrocyte implantation (ACI), or similar methods such as micronized cartilage matrix (BioCartilage, Arthrex, Naples, Florida) or juvenile cartilage fragments (DeNovo, Zimmer, Warsaw, Indiana)5, and osteochondral allograft transplantation. OATS and ACI have both been reported to have successful results in up to 90% of the patients with large lesions6. Allograft transplantation procedures have become popular due to inherent advantages over other techniques. When compared to OATS/mosaicplasty, allograft has no donor site morbidity, no curvature mismatch, no fibrocartilage growth between plugs, and can be used in cases with irregularly-shaped defects. In addition, there is scant evidence to support the assumption that cartilage from the knee can withstand the tremendous forces sustained by the talar dome. Compared to ACI, and other cartilage scaffolds, allograft is a single stage procedure that also replaces the subchondral bone, and can be applicable to the shoulder of the talus7, therefore may be biomechanically more suitable.
While there is an abundant literature about osteochondral allograft transplantation in the knee, there are few papers about this procedure in the talus8-16. Cartilage viability is one of the most important factors for successful clinical outcomes after transplantation of osteochondral allografts. Donor chondrocytes can be damaged during storage and/or preparation of the graft, but also recipient cartilage can be damaged during surgery. Osteochondral allograft can be stored frozen or fresh, with the latter system having better results in terms of cellular viability, stiffness and matrix content both at the time of implantation than on the long term17-19. Storage length can modify the features of fresh allograft. Williams et al.20 reported that chondrocyte viability and viable cell density remained unchanged after storage up to 14 days and then declined at 28 days, proteoglycan synthesis declined at 14 days and no significant differences were detected in glycosaminoglycan content and in biomechanical properties after storage for 28 days. During surgery, osteochondral grafts require multiple physical manipulations that are prone to cause iatrogenic injury and apoptotic cell death. Harvesting of the donor osteochondral graft from a larger piece of osteochondral tissue may subject the articular cartilage to mechanical damage and subsequent cell death. Motorized coring devices are likely to cause even greater damage and cell death than manual trephines. The subsequent surgical step involves creating an osteochondral defect in the recipient bone with a motorized coring instrument. Drill injuries to articular cartilage can cause extensive apoptosis in the surrounding cartilage that extends outwards from the initial injury site over time. As a result, a rim of relatively acellular matrix surrounds the recipient site. This apoptotic response has been hypothesized to impede effective cartilage integration in cartilage repair procedures. Finally, repetitive impacts necessary to insert donor grafts can cause an extensive cell death21. Currently, fresh osteochondral allograft are used in clinical practice, but the recommendation for storage length vary widely between authors, ranging from 24-48 hours to 42 days8'11-14'16'22.
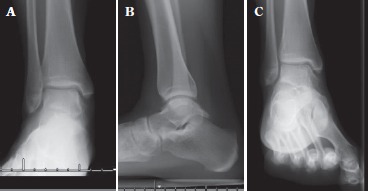
Fig 1. Pre-operative antero-posterior (A), lateral (B) and mortise (C) x-rays of right ankle in a 22-year-old girl. There is no evidence of significant collapse of the talar body and no evidence of arthritis. The arrow shows lucency in the medial dome of the talus. Anterior distal tibial and posterior talar ankle osteophytes can also be observed.
Indications and contraindications
Acute lesions are usually treated by excision particularly if the fragment is small, displaced, or comminuted. Indications for repair are controversial, but if the fragment is large (> 35% talar dome surface area) with a cartilage surface intact, repair may be attempted, particularly with an antero-lateral location2. In chronic lesions, although conservative treatment has fair success rate, most authors consider a three to six month nonoperative management the standard first-line approach for non-displaced lesions. An initial period of immobilization in a cast or controlled ankle motion brace followed by protected weight-bearing, pain medication, physical therapy for range of motion (ROM) and strengthening, and orthotic shoe-wear modification have been utilized12,13,23.
Indications for surgery varied significantly among studies, and in some cases were not reported at all. In general, failure of non-operative management is an indication for surgical intervention. Initial surgical treatment should likely be some form of debridement, curettage or microfracture so there is a stable bleeding base2,3. Failed initial debridement is an indication for resurfacing, with OATS/mosaicplasty or ACI performed first. Generally, osteochondral allograft transplantation is considered as a rescue procedure if they fail, but can be done as a first resurfacing treatment in high-demand patients, or in large lesions that cannot be treated successfully with other resurfacing techniques. Patients should have functional ankle ROM, closed growth plates and absence of cartilage lesions on the tibial side. Contraindications for surgery include degenerative joint disease, reflex sympathetic dystrophy, uncorrectable malalignment, ligamentous instability, active infections and malignancy. Generally, a calcaneal osteotomy or a supramalleolar osteotomy and correction of instability may be of value2,11,12,14,15. Allografts are particularly suitable for large defects with bone loss, where some flexibility is necessary to fill the defect.
Allograft can be either a circular plug, press-fit, or block-shaped to address different locations and different lesion sizes. Plug-shaped allograft are indicated for contained lesions but in some cases larger defects may require the use of multiple plugs in the so-called snowman technique, which consists of placing and fixing the first plug, then drilling a second recipient site next to or partially over the first one. On the other hand, blockshaped allograft are indicated for irregularly shaped lesions, uncontained lesions involving the shoulder of the talus, and lesion that cannot be reached perpendicularly with the plug technique16,24.
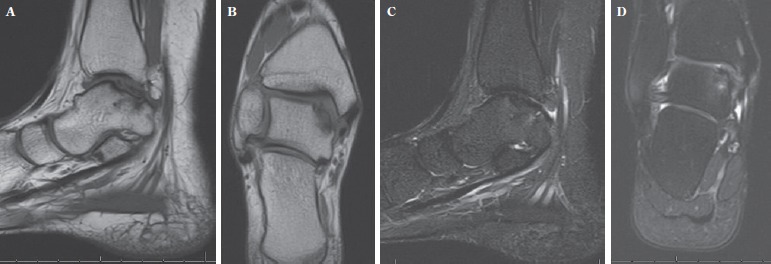
Figure 2. Pre-operative MRI of the same patient of Figure 1 shows a posterior medial talar lesion that measures 8 x 17 mm in its largest dimension (A and B). There is no significant edema on the T2 sequences under the lesion (C and D).
Planning
Pre-operative planning includes X-rays, CT scan, bone scan, and MRI to define the location, size, cartilage surface, and joint condition. Plain X-rays include weight-bearing antero-posterior (AP), lateral, and mortise views (Fig 1). A plantar-flexed mortise view may better visualize postero-medial lesions; conversely, dorsiflexed radiographs are indicated to detect an antero-medial or antero-lateral lesion. A Canale view (15 degree pronation of the foot and the tube angled 75 degrees cephalad) is useful to assess the talar profile25. The sensitivity of routine radiography is 50% to 75%, whereas pickup on bone scan is 99% sensitive2. MRI is indicated if radiographic results are normal and allows a good evaluation of the cartilage surface, underlying stability of the fragment, surrounding bony edema, and other soft tissue injuries2,26,27 (Fig 2). There are controversies on the ability of MRI to accurately assess the dimensions of the lesion, due to overestimation related to bone edema. Some authors prefer to associate a CT scan for a more accurate planning2, 10. Plain radiographs and MRI are sent to the tissue bank in order to obtain a whole talus of a matching size, but the final measurement is up to the surgeon. An allograft differing up to 2 mm from the native talus is considered acceptable28.
Surgical approaches
Most areas of the talar dome can be accessed perpendicularly without the necessity for a malleolar osteotomy. In fact, Muir et al.29 demonstrated that, on average, only 17% of the medial talar dome and 20% of the lateral talar dome could not be accessed without an osteotomy. After an anterolateral osteotomy, they reported an increase of 22% in sagittal exposure, while malleolar osteotomies provided access to the entire medial and lateral talar dome areas with a residual central 15% of the talar dome remaining inaccessible perpendicularly. Critical to all methods of osteotomy is a precise reduction and fixation to avoid fibrous nonunion or malunion. For centrally located lesions an anterior approach to the ankle joint should be performed.
Medial approach
A 10 cm medial incision is carried out from 3 cm above the joint line to the tip of the malleolus and then curved anteriorly toward the tubercle of the navicular. The saphenous vein must be retracted anteriorly. The capsule is incised at the anteromedial corner allowing visualization of the joint and lesion. The tibialis posterior tendon is protected with a small Homan retractor. The medial malleolus is predrilled and a step cut30 or an oblique osteotomy31 is performed, and the fragment is retracted posteriorly. Fluoroscopy can be used if necessary to direct this cut (Fig. 3). The amount of talar dome exposed can be modified by making the osteotomy exit slightly more laterally, but this amount still does not give complete access to the talus. The most posterior capsular attachment can be released to allow retraction of the malleolus on the deltoid ligament. Two Steinmann pins with a Hintermann distractor can be used to improve exposure8,12,28 (Fig. 4).
Fig 3. Fluoroscopic image showing a K-wire used to direct the position of the medial malleolar osteotomy. The amount of talar dome exposed can be modified by making the osteotomy exit slightly more laterally.
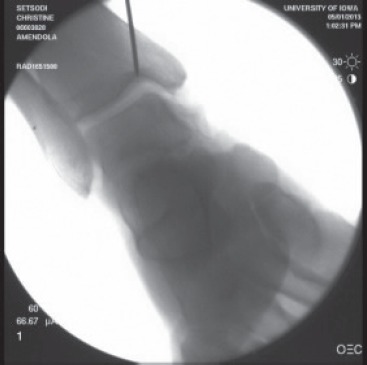
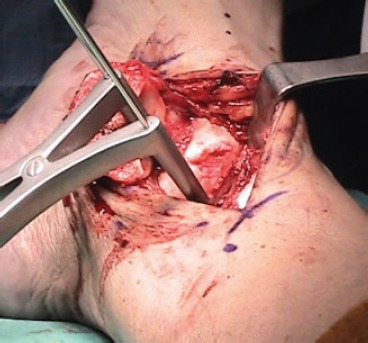
Fig 4. Intraoperative picture of a medial approach to the talus after medial malleolar osteotomy. The malleolar fragment is retracted posteriorly exposing the ankle joint. The most posterior capsular attachment can be released to allow retraction of the malleolus on the deltoid ligament. To increase exposure, temporary joint distraction was applied with two Steinmann pins and a Hintermann distractor.
Lateral approach
A 4 to 6 cm longitudinal incision is made medial to the fibula and centered on the ankle joint line. The branches of the superficial peroneal nerve should be protected. The extensor retinaculum is incised and the extensor digitorum longus is retracted medially. The joint capsule is incised in line with the incision. Slight plantar flexion of the ankle will further facilitate exposure. For large lesions extending posteriorly, an anterior fibular periosteal flap can be created including the origin of the anterior talofibular ligament and, if necessary, the calcaneofibular ligament. The talus can then be drawn forward and rotated downward with the help of a Kirschner wire “joy stick” that is driven through the body of the talus. When a fibular osteotomy is needed, a 6 cm incision is made laterally over the distal fibula, starting from the tip of the lateral malleolus and extending proximally. A microsagittal saw is used to perform a transverse fibular osteotomy 1 cm proximal to the joint line. The distal syndesmotic ligaments are incised, and the distal fibula retracted inferiorly. Two Steinmann pins with a Hintermann distractor can be used to improve exposure28. To improve reduction, some authors suggested placing a semitubular plate and a lag screw and then removing them before the osteotomy16.
Anterior approach
A longitudinal incision is made on the anterior aspect of the leg starting 7.5 cm proximally and extending to about 5 cm distal to the joint. The deep fascia is divided in line with the skin incision. The interval between the anterior tibialis and extensor hallucis longus tendons is developed and the neurovascular bundle is retracted laterally with the long extensor tendons of the toes, and the anterior tibial tendon is retracted medially. The periosteum, capsule, and synovium are incised in line with the skin, and the full width of the ankle joint is exposed anteriorly by subcapsular and subperiosteal dissection32. To increase exposure, temporary joint distraction can be applied with a unipolar external fixator10.
Surgical techniques
Plug-shaped allograft
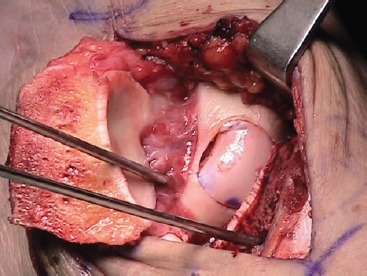
The osteochondral defect is visualized and measured in a medial-lateral direction. Debridement of the chondral surface is carried out with curettes, in order to expose the damaged area and assess its real dimensions. A guide pin is drilled perpendicular to the articular surface and it is used to select the adequate cannulated allograft sizer, that is then replaced with an appropriately sized OATS graduated reamer. The cartilage and the subchondral bone are reamed out at about 7-8 mm deep until a bleeding surface. Care should be taken not to injure the cartilage of the tibial side. The reamer and the guide pin are then removed (Fig. 5). The allograft talus is affixed on the OATS holder and carefully examined as well. The donor talus is marked on the medial (or lateral) side using the sizer. The OATS workstation bushing of corresponding size is placed into the top housing over the graft, and set to the exact angle necessary to match the recipient's contour perpendicularly. Once the correct inclination has been determined, the housing is securely fastened. The graduated donor harvester is drilled through the allograft talus, and the graft is carefully extracted. A graduated OATS dilator is inserted in the reamed portion of the recipient talus to reach 0.5 mm dilatation. The medio-lateral dimension of the recipient site is measured again with a ruler. The allograft core is secured in the specifically designed forceps and trimmed by a saw free hand to achieve the appropriate shape and medio-lateral length. Then the allograft is inserted in the native talus with a gentle impaction (Fig. 6). If necessary, additional fixation can be provided to an unstable graft with chondral darts or pins or screws8,12,14,15.
Fig 5. Intraoperative picture showing the recipient site on the medial side of the talus. Two Steinmann pins with a Hintermann distractor are used to improve exposure. The cartilage and the subchondral bone are reamed out at about 7-8 mm deep until a bleeding surface. Care should was taken not to injure the cartilage of the tibial side.
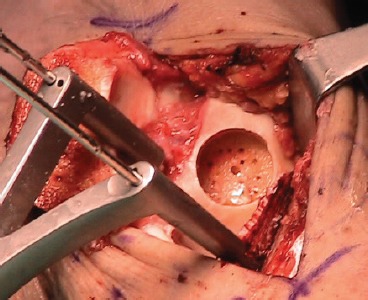
Fig 6. Intraoperative picture showing the allograft inserted in the native talus. If necessary, addiction fixation can be provided to an unstable graft with chondral darts or pins or screws.
Block-shaped allograft
The talar lesion is identified and debrided to stable articular cartilage margins and bleeding cancellous bone. The edges are cut square with a fine saw to allow for correct geometric fitting of the allograft. The dimensions of the lesion are marked onto the corresponding location of the fresh allograft talus. The graft is then cut with an oscillating saw, and it is stabilized with one or two countersunk titanium mini-fragment (2.0 mm) headed cancellous screws11.
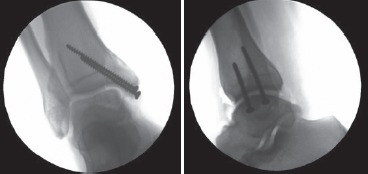
At the end of the surgery malleolar osteotomies are fixed with screws or plates and the syndesmosis should be repaired and fixed with a syndesmotic screw through the plate (if necessary). Intraoperative fluoroscopy should be performed to confirm the placement and fit of the graft and the alignment of the malleolar osteotomy (Fig. 7) and the ankle should be axially loaded and put through ROM to mold the graft10,12,13,15.
Figure 7. Intraoperative fluoroscopy pictures taken at the end of surgery confirm the placement and fit of the graft and the alignment of the malleolar osteotomy.
Post-operative protocol
Different protocols have been reported after surgery. In general, patients are immobilized in a splint or a short leg cast and non-weightbearing after surgery. Subsequently, a below-knee fracture boot or a splint is applied and active, assisted ROM of the ankle is permitted outside of the boot. Weightbearing as tolerated is started at 6-8 weeks postoperatively, and patients are fully weightbearing by 12 weeks. A physical therapy program focusing on joint mobilizations, strength, and balance is initiated at eight weeks10-15,28. The authors performing both plug- and block-shaped allograft used the same protocol for all the patients14. Imaging to follow healing includes X-rays, but an MRI or CT scan may be utilized to ensure there is bony union of the graft13-15.
Results
There are few papers about osteochondral allograft of the talus, and most of them have low level of evidence and small sample size8-16. Improvement of function, reduction of pain and good patient satisfaction are reported in all these studies. Different outcome scores were used, so a direct comparison between studies is not possible. Additional surgery after the index procedure is common, with ankle arthroscopy, hardware removal, non-union or malunion of malleolar osteotomy, supramalleolar or calcaneal osteotomy been described. Success rates of osteochondral allograft of the talus are reported to range between 73% and 100%. Failures can be treated with a revision allograft transplantation (bipolar or not), ankle fusion or total ankle arthroplasty.
Gross et al.8 retrospectively reviewed nine patients with stage IV lesions of the talus treated with a blockshaped allograft. At mean follow-up of 12 years (range, 4-20), ankle arthrodesis was performed in three cases because of resorption and fragmentation of the graft, rather than arthritic deterioration. The average overall survival of the nine grafts was nine years (range, 3-19). Two grafts appeared to be raised 1 mm above the host articular surface. One graft was 50% resorbed, with no evidence of osteoarthritis, whereas the other exhibited an area of fragmentation that involved less than one- third of the graft. No evidence of gross or microscopic rejection was found.
Raikin9 reviewed 15 cases of fresh block-shaped allograft of the talus. At a mean follow-up of 44 months (range, 26-88), some evidence of collapse or resorption of the graft was observed in 10 patients. Additionally, nine ankles demonstrated some narrowing of the joint space overlying the graft area. Two patients underwent ankle fusion.
Gortz et al.10 followed up prospectively, using a clinical database, 11 patients (12 ankles) treated with a block-shaped allograft. At a mean follow-up of 38 months (range, 24-107), two failures were reported (one underwent arthrodesis and one revision allograft), 90% of the patients were satisfied, 80% reported less pain and 60% reported improved function.
Hahn et al.11 retrospectively reviewed 13 patients with a block-shaped allograft at a mean follow-up of 48 months. All the patients were all satisfied with the results, all but one had osteophytes and other mild arthritic changes were seen in two patients. Five patients had complications related to the allograft and needed additional surgery (four had hardware removal and one had debridement of an impingement spur). All 13 allografts healed, but in two cases it took longer.
Janis et al.12 in their retrospective study on 15 blockshaped allografts of the talus, evaluated at a mean follow- up of 1.6 years (range, 0.9-2.1), reported no graft-related complications, no subsequent surgical procedures and no delamination. Six grafts revealed mild lucency but no resorption, four lesions had a step-off of less than 1 mm and two had step-off of 1 mm or greater. Arthrosis was mild in four patients and severe in two.
Adams et al.13 in a retrospective study reported the results of eight patients treated with a block-shaped allograft. At a mean follow-up of 48 months (range, 25-91), three grafts had partial lucency at the interface with the host bone, but they were asymptomatic. In one case lucency and graft resorption were suspected at X-rays, but CT scan showed only lucency on the anterior graft- host interface. In one case a non-union of the graft was suspected.
Berlet et al.14 retrospectively reported the results of 12 patients at a mean follow-up of 3.3 years (range, 2.0-4.6). A plug-shaped allograft was used in six cases and a blockshaped allograft in the remaining cases. No graft-related complications were noted. Eight patients had MRI at the last follow-up showing graft incorporation in seven cases, radiolucencies were observed in three cases. The authors also reported on a patient that did not meet the inclusion criteria of the study, but showed graft collapse after 2.7 years, which required revision. The authors reported that their sample size was underpowered to determine a statistical difference in SF-12, but not in AOFAS outcomes.
El-Rashidy et al.15 in their retrospective study reported 4 failures in 38 patients with a plug-shaped allograft at a mean follow-up of 37.7 months (range, 6-22). Three MRI scans showed moderate arthritis while one showed advanced joint space collapse. In five cases at least one sign of graft instability was found, and one case showed graft collapse. Four cases showed slight articular irregularity ad one showed complete discontinuity.
Haene et al.16 reported prospectively the results of 17 block-shaped allografts for treatment of osteochondral lesions of the talus. At a mean follow-up of 4.1 years (range, 2-7), five ankles were considered failures, two had poor results, six good and four excellent.
Complications
Allograft-associated infection is rare but may potentially be fatal. Clostridium contamination risk increases with the length of time between donor death and procure- ment33-34. Safety guidelines established by the American Association of Tissue Banks (AATB) advocate donor screening, extensive serologic, bacterial, and viral testing; procurement and storage requirements; and graft quarantine until negative testing results are ensured35. Deep infection following allograft transplantation should be considered for surgical debridement and graft removal. To improve the safety of allograft use, tissue banks and surgeons must keep tracking information and report any allograft-related infection36.
Failure in the osseous portion of the allograft is most common, where subchondral collapse, delayed union or nonunion may occur. Larger and bulkier grafts are associated with higher risks of these complications. Graft fragmentation and collapse are among the main failure mechanisms usually presenting as new onset of pain, joint effusion, and mechanical symptoms. As a milder complication, allograft subsidence may occur37. Whenever patients remain asymptomatic despite radiological graft subsidence, observation may be indicated. Ideally, postoperative imaging reveals a well-incorporated flush graft with marrow signal consistent with fat, and an articular cartilage signal that is isointense to normal cartilage38.
Conclusions
Fresh osteochondral allograft transplantation can improve function of the patients with talar dome lesions. Risk factors for a poor outcome are large lesions, multiple previous surgeries and bipolar defects. The high rates of secondary arthroscopic debridement and clinical failure suggest that the current indications for this surgery should be carefully evaluated and the patient should be properly selected and educated before surgery.
References
- 1.O'Loughlin PF, Heyworth BE, Kennedy JG. Current concepts in the diagnosis and treatment of osteochondral lesions of the ankle. Am J Sports Med. 2010;38(2):392–404. doi: 10.1177/0363546509336336. Feb doi: 10.1177/0363546509336336. Epub 2009 Jun 26. Review. [DOI] [PubMed] [Google Scholar]
- 2.Amendola A, Panarella L. Osteochondral lesions: medial versus lateral, persistent pain, cartilage restoration options and indications. Foot Ankle Clin. 2009;14(2):215–27. doi: 10.1016/j.fcl.2009.03.004. Jun doi: 10.1016/j.fcl.2009.03.004. Review. [DOI] [PubMed] [Google Scholar]
- 3.Berndt AL, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 1959;41-A:988–1020. [PubMed] [Google Scholar]
- 4.Verhagen RA, Struijs PA, Bossuyt PM, van Dijk CN. Systematic review of treatment strategies for osteochondral defects of the talar dome. Foot Ankle Clin. 2003;8(2):233–42. doi: 10.1016/s1083-7515(02)00064-5. Jun viii-ix. [DOI] [PubMed] [Google Scholar]
- 5.Coetzee JC, Giza E, Schon LC, Berlet GC, Neufeld S, Stone RM, Wilson EL. Treatment of Osteochondral Lesions of the Talus With Particulated Juvenile Cartilage. Foot Ankle Int. 2013 doi: 10.1177/1071100713485739. Apr 10. [DOI] [PubMed] [Google Scholar]
- 6.Giza E. Operative techniques for osteochondral lesions of the talus. Foot Ankle Spec. 2008;1:250–2. doi: 10.1177/1938640008321387.. [DOI] [PubMed] [Google Scholar]
- 7.Giannini S, Vannini F. Operative treatment of osteochondral lesions of the talar dome: current concepts review. Foot Ankle Int. 2004;25(3):168–75. doi: 10.1177/107110070402500311. Mar Review. [DOI] [PubMed] [Google Scholar]
- 8.Gross AE, Agnidis Z, Hutchison CR. Osteochondral defects of the talus treated with fresh osteochondral allograft transplantation. Foot Ankle Int. 2001;22(5):385–91. doi: 10.1177/107110070102200505. May. [DOI] [PubMed] [Google Scholar]
- 9.Raikin SM. Fresh osteochondral allografts for large- volume cystic osteochondral defects of the talus. J Bone Joint Surg Am. 2009;91(12):2818–26. doi: 10.2106/JBJS.I.00398. Dec doi: 10.2106/JBJS.I.00398. [DOI] [PubMed] [Google Scholar]
- 10.Görtz S, De Young AJ, Bugbee WD. Fresh osteochondral allografting for osteochondral lesions of the talus. Foot Ankle Int. 2010;31(4):283–90. doi: 10.3113/FAI.2010.0283. Apr doi: 10.3113/FAI.2010.0283. [DOI] [PubMed] [Google Scholar]
- 11.Hahn DB, Aanstoos ME, Wilkins RM. Osteochondral lesions of the talus treated with fresh talar allografts. Foot Ankle Int. 2010;31(4):277–82. doi: 10.3113/FAI.2010.0277. Apr doi: 10.3113/FAI.2010.0277. [DOI] [PubMed] [Google Scholar]
- 12.Janis L, Kaplansky DB, DeCarbo WT. Early clinical experience with a fresh talar transplant inlay allograft for the treatment of osteochondral lesions of the talus. J Am Podiatr Med Assoc. 2010;100(1):25–34. doi: 10.7547/1000025. Jan-Feb. [DOI] [PubMed] [Google Scholar]
- 13.Adams SB Jr, Viens NA, Easley ME, Stinnett SS, Nunley JA 2nd. Midterm results of osteochondral lesions of the talar shoulder treated with fresh osteochondral allograft transplantation. J Bone Joint Surg Am. 2011;93(7):648–54. doi: 10.2106/JBJS.J.00141. Apr 6 doi: 10.2106/JBJS.J.00141. [DOI] [PubMed] [Google Scholar]
- 14.Berlet GC, Hyer CF, Philbin TM, Hartman JF, Wright ML. Does fresh osteochondral allograft transplantation of talar osteochondral defects improve function? Clin Orthop Relat Res. 2011;469(8):2356–66. doi: 10.1007/s11999-011-1813-2. Aug doi: 10.1007/s11999-011-1813-2. Epub 2011 Feb 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Rashidy H, Villacis D, Omar I, Kelikian AS. Fresh osteochondral allograft for the treatment of cartilage defects of the talus: a retrospective review. J Bone Joint Surg Am. 2011;93(17):1634–40. doi: 10.2106/JBJS.J.00900. Sep 7 doi: 10.2106/JBJS.J.00900. [DOI] [PubMed] [Google Scholar]
- 16.Haene R, Qamirani E, Story RA, Pinsker E, Daniels TR. Intermediate outcomes of fresh talar osteochondral allografts for treatment of large osteochondral lesions of the talus. J Bone Joint Surg Am. 2012;94(12):1105–10. doi: 10.2106/JBJS.J.02010. Jun 20 doi: 10.2106/JBJS.J.02010. [DOI] [PubMed] [Google Scholar]
- 17.Enneking WF, Mindell ER. Observations on massive retrieved human allografts. J Bone Joint Surg Am. 1991;73(8):1123–42. Sep. [PubMed] [Google Scholar]
- 18.Enneking WF, Campanacci DA. Retrieved human allografts: a clinicopathological study. J Bone Joint Surg Am. 2001;83-A(7):971–86. Jul. [PubMed] [Google Scholar]
- 19.Pallante AL, Görtz S, Chen AC, Healey RM, Chase DC, Ball ST, Amiel D, Sah RL, Bugbee WD. Treatment of articular cartilage defects in the goat with frozen versus fresh osteochondral allografts: effects on cartilage stiffness, zonal composition, and structure at six months. J Bone Joint Surg Am. 2012;94(21):1984–95. doi: 10.2106/JBJS.K.00439. Nov 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams SK, Amiel D, Ball ST, Allen RT, Wong VW, Chen AC, Sah RL, Bugbee WD. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am. 2003;85-A(11):2111–20. doi: 10.2106/00004623-200311000-00008. Nov doi: 10.2106/JBJS.K.00439. [DOI] [PubMed] [Google Scholar]
- 21.Kim HT, Teng MS, Dang AC. Chondrocyte apopto-sis: implications for osteochondral allograft transplantation. Clin Orthop Relat Res. 2008;466(8):1819–25. doi: 10.1007/s11999-008-0304-6. Aug doi: 10.1007/s11999-008-0304-6. Epub 2008 May 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranawat AS, Vidal AF, Chen CT, Zelken JA, Turner AS, Williams RJ 3rd. Material properties of fresh cold-stored allografts for osteochondral defects at 1 year. Clin Orthop Relat Res. 2008;466(8):1826–36. doi: 10.1007/s11999-008-0311-7. doi: 10.1007/s11999-008-0311-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGahan PJ, Pinney SJ. Current concept review: osteochondral lesions of the talus. Foot Ankle Int. 2010;31(1):90–101. doi: 10.3113/FAI.2010.0090. http://dx.doi.org/10.3113/FAI.2009.0090. [DOI] [PubMed] [Google Scholar]
- 24.Demange M, Gomoll AH. The use of osteochondral allografts in the management of cartilage defects. Curr Rev Musculoskelet Med. 2012;5(3):229–35. doi: 10.1007/s12178-012-9132-0. Sep doi: 10.1007/s12178-012-9132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canale ST, Kelly FB Jr. Fractures of the neck of the talus: long-term evaluation of seventy-one cases. J Bone Joint Surg Am. 1978;60(2):143–156. [PubMed] [Google Scholar]
- 26.Schachter AK, Chen AL, Reddy PD, et al. Osteochondral lesions of the talus. J Am Acad Orthop Surg. 2005;13(3):152–8. doi: 10.5435/00124635-200505000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Verhagen RA, Maas M, Dijkgraaf MG, et al. Prospective studyon diagnostic strategies in osteochondral lesions of the talus. Is MRI superior to helical CT? J Bone Joint Surg Br. 2005;87(1):41–6. [PubMed] [Google Scholar]
- 28.Dragoni M, Bonasia DE, Amendola A. Osteochondral talar allograft for large osteochondral defects: technique tip. Foot Ankle Int. 2011;32(9):910–6. doi: 10.3113/FAI.2011.0910. Sep. [DOI] [PubMed] [Google Scholar]
- 29.Muir D, Saltzman CL, Tochigi Y, et al. Talar dome access for osteochondral lesions. Am J Sports Med. 2006;34(9):1457–63. doi: 10.1177/0363546506287296. http://dx.doi.org/10.1177/0363546506287296. [DOI] [PubMed] [Google Scholar]
- 30.Alexander IJ, Watson JT. Step-cut osteotomy of the medial malleolus for exposure of the medial ankle joint space. Foot Ankle. 1991;11(4):242–3. doi: 10.1177/107110079101100413. [DOI] [PubMed] [Google Scholar]
- 31.Spatt JF, Frank NG, Fox IM. Transchondral fractures of the domeof the talus. J Foot Surg. 1986;25:68–72. [PubMed] [Google Scholar]
- 32.Gortz S, Bugbee WD. Fresh osteochondral resurfacing of the ankle. Oper Tech Orthop. 2006;16(4):244–249. [Google Scholar]
- 33.Kainer MA, Linden JV, Whaley DN, Holmes HT, Jarvis WR, Jernigan DB, Archibald LK. Clostridium infections associated with musculoskeletal-tissue allografts. N Engl J Med. 2004;350(25):2564–71. doi: 10.1056/NEJMoa023222. doi: 10.1056/NEJMoa023222. [DOI] [PubMed] [Google Scholar]
- 34.Malinin TI, Buck BE, Temple HT, Martinez OV, Fox WP. Incidence of clostridial contamination in donors' musculoskeletal tissue. J Bone Joint Surg Br. 2003;85(7):1051–4. doi: 10.1302/0301-620x.85b7.14438. doi: 10.1302/0301-620X.85B7.14438. [DOI] [PubMed] [Google Scholar]
- 35.Mroz TE, Joyce MJ, Steinmetz MP, Lieberman IH, Wang JC. Musculoskeletal allograft risks and recalls in the United States. J Am AcadOrthop Surg. 2008;16(10):559–65. doi: 10.5435/00124635-200810000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Hinsenkamp M, Muylle L, Eastlund T, Fehily D, Noel L, Strong DM. Adverse reactions and events related to musculoskeletal allografts: reviewed by the World Health Organisation Project NOTIFY. Int Orthop. 2011 doi: 10.1007/s00264-011-1391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marwin S, Gross A. Harner C, Fu FH, Vince KG, editors. Fresh osteochondral allografts in knee reconstruction. Baltimore: Williams & Wilkins. Knee surgery. 1994:1223–1234. editors. pp. [Google Scholar]
- 38.Williams RJ 3rd, Ranawat AS, Potter HG, Carter T, Warren RF. Fresh stored allografts for the treatment of osteochondral defects of the knee. J Bone Joint Surg Am. 2007;89(4):718–26. doi: 10.2106/JBJS.F.00625. doi: 10.2106/JBJS.F.00625. [DOI] [PubMed] [Google Scholar]


