Abstract
Alleviating myocardial injury associated with ST elevation myocardial infarction is central to improving the global burden of coronary heart disease. The chemokine stromal cell-derived factor 1α (SDF-1α) has dual potential benefit in this regard. Firstly, SDF-1α is up-regulated in experimental and clinical studies of acute myocardial infarction (AMI) and regulates stem cell migration to sites of injury. SDF-1α delivery to the myocardium after AMI is associated with improved stem cell homing, angiogenesis, and left ventricular function in animal models, and improvements in heart failure and quality of life in humans. Secondly, SDF-1α may have a role in remote ischaemic conditioning (RIC), the phenomenon whereby non-lethal ischaemia–reperfusion applied to an organ or tissue remote from the heart protects the myocardium from lethal ischaemia–reperfusion injury (IRI). SDF-1α is increased in the serum of rats subjected to RIC and protects against myocardial IRI in ex vivo studies. Despite these potential pleiotropic effects, a limitation of SDF-1α is its short plasma half-life due to cleavage by dipeptidyl peptidase-4 (DPP-4). However, DPP-4 inhibitors increase the half-life of SDF-1α by preventing its degradation and are also protective against lethal IRI. In summary, SDF-1 potentially delivers a ‘two-pronged’ defence of the myocardium: acutely protecting it from IRI while simultaneously stimulating repair by recruiting stem cells to the site of injury. In this article we examine the evidence for acute and chronic cardioprotective roles of SDF-1α and discuss potential therapeutic manipulations of this mechanism with DPP-4 inhibitors to protect against lethal tissue injury in the clinical setting.
Keywords: SDF, DPP-4, Cardioprotection, AMI, Ischemic conditioning, CXCR4
1. Introduction
Coronary heart disease is the leading cause of death worldwide, accounting for an estimated 7.3 million deaths per year (“Global Atlas on Cardiovascular Disease Prevention and Control”). Untreated, mortality following ST-elevation myocardial infarction (STEMI) may be as high as 15% and strategies to mitigate the deleterious effects of STEMI are therefore paramount (Gibson, 2004). Early reperfusion by primary percutaneous coronary intervention (PPCI) is the most effective strategy for reducing infarct size and improving clinical outcome (Keeley et al., 2003, Gibson, 2004). Other important therapeutic targets include platelet aggregation, subsequent myocardial dysfunction and secondary prevention, including statin therapy. Overall, 30 day mortality following PPCI in the UK is now 6.5% (“BCIS Audit Returns 2012: Adult Interventional Procedures”). Another potential target is the injury inflicted by the therapeutic restoration of blood flow, known as ischaemia–reperfusion injury (IRI), which may account for up to 50% of final infarct size (Braunwald and Kloner, 1985, Piper et al., 1998, Staat et al., 2005, Yellon and Hausenloy, 2007). The chemokine stromal cell-derived factor 1α (SDF-1α) potentially delivers a ‘two-pronged’ defence of the myocardium in this regard: acutely protecting the myocardium from IRI while simultaneously stimulating myocardial repair by recruiting stem cells to the site of injury.
SDF-1α is known to play a central role in stem cell homing, retention, survival, proliferation, cardiomyocyte repair, angiogenesis and ventricular remodelling following myocardial infarction (Kucia, Dawn, et al., 2004, Cheng et al., 2008, Saxena et al., 2008, Tang et al., 2009, Zaruba et al., 2009, Jujo et al., 2010, Takahashi, 2010, Tang et al., 2010, Zaruba and Franz, 2010, Ghadge et al., 2011, Kanki et al., 2011, Dong et al., 2012, Penn et al., 2012). It acts as the unique ligand for its receptor CXCR4 and the SDF-1–CXCR4 axis is up-regulated in both experimental and clinical studies of myocardial infarction (Zaruba & Franz, 2010). SDF-1α–CXCR4 has been utilised to target stem cells to ischaemic tissue, thereby improving left ventricular (LV) dimensions and function (Misao et al., 2006, Sasaki et al., 2007, Saxena et al., 2008, Tang et al., 2010). Importantly, the SDF-1α–CXCR4 signalling axis exerts these effects via a Gα1 dependent mechanism and activation of phosphoinositide 3 kinase (PI3K), mitogen activated protein kinase (MAPK), and Janus kinase (JAK)-signal transducer and activator of transcription (STAT) signalling.
These signalling pathways are the same pathways that it is postulated are responsible for the protection against IRI conferred by all forms of conditioning such as pre, post and remote ischaemic conditioning (Hausenloy and Yellon, 2004, Hausenloy and Yellon, 2007a, Hausenloy and Yellon, 2007b). The latter describes the phenomenon whereby non-lethal ischaemia and reperfusion applied to an organ or tissue remote from the heart protects the myocardium from lethal reperfusion injury (Przyklenk et al., 1993, Whittaker and Przyklenk, 1994, Dickson et al., 2000, Hausenloy and Yellon, 2008a, Hausenloy and Yellon, 2008b). Remote ischaemic conditioning (RIC) can be induced non-invasively by inflating a blood pressure cuff placed on the arm or thigh to above systolic pressure to induce brief ischaemia and then deflating the cuff to allow reperfusion (Kharbanda et al., 2002). When administered pre-hospital this has been shown to reduce myocardial infarct size and improve myocardial salvage in PPCI patients (Botker et al., 2010), and improve outcomes in patients undergoing cardiac surgery or elective PCI (Gunaydin et al., 2000, Hausenloy et al., 2007, Hoole et al., 2009). The mechanism of cardioprotection conferred by RIC is so far unknown, but is thought to be due to a humoral factor that has been shown by biochemical fractionation studies to be a protein between 3.5 kDa and 15 kDa in size (Serejo et al., 2007, Shimizu et al., 2009).
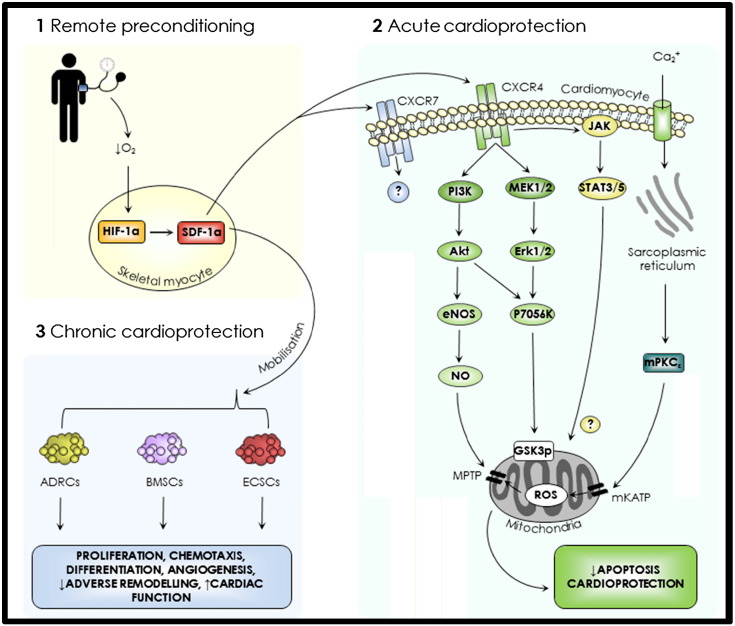
It is therefore proposed that in addition to its chronic effects SDF-1α has a direct role in the protection observed from RIC and a proposed paradigm is described in Fig. 1 (Saxena et al., 2008, Zaruba and Franz, 2010, Huang et al., 2011). Recently published data has demonstrated increased SDF-1α in the serum of rats subjected to RIC. Furthermore, RIC, which was shown to significantly decrease infarct size and improve papillary muscle functional recovery in an ex vivo model, could be blocked by AMD3100, a highly specific inhibitor of CXCR4 (Davidson et al., 2013, De Clercq, 2003). This, together with the identification of SDF-1α as an 8 kDa peptide has made it a prime candidate for a role in RIC (Ceradini et al., 2004). In this review we focus on the evidence for acute and chronic roles of SDF-1α in cardioprotection following STEMI and discuss potential therapeutic manipulations of this mechanism to protect against lethal tissue injury in the clinical setting. In particular we examine the role of inhibitors of the protease dipeptidyl peptidase 4 (DPP-4) to prolong the half-life of SDF-1α to these ends.
Fig. 1.
The SDF-1–CXCR4 signalling axis. Remote preconditioning may enable both acute and chronic cardioprotective pathways (see text for details). SDF-1α, stromal derived factor-1α; HIF-1α, hypoxic inducible factor-1α; ADRCs, adipose tissue derived regenerative cells; BMSCs, bone marrow stem cells (including mesenchymal stem cells, hamatopoietic stem cells and endothelial stem cells); ECSCs, endogenous cardiac stem cells; JAK, Janus kinase; STAT, signal transducer and activator of transcription; PI3K, phosphoinositide 3 kinase; MEK1/2, mitogen-activated protein kinase; Erk, extracellular signal-regulated kinases; eNOS, endothelial nitric oxide synthase; NO, nitric oxide; mPKC, mitochondrial protein kinase C; mKATP, mitochondrial ATP-sensitive potassium channel; ROS, reactive oxygen species; MPTP, mitochondrial permeability transition pore.
2. Stromal derived factor 1α–CXC Chemokine Receptor 4/CXC Chemokine Receptor 7
Chemokines, or chemoattractant cytokines, play a critical role in the regulation and trafficking of leukocytes as well as haematopoietic and other progenitor cells (Ghadge et al., 2011). They are also involved in a variety of other functions, including degranulation, mitogenesis, gene transcription, apoptosis, and angiogenesis (Gerard & Rollins, 2001). There are over 50 human chemokines that are classified according to the position of two N-terminal cysteine residues as CXC, CCC, C or CX3C (Ghadge et al., 2011). In myocardial ischaemia, several chemokines, including CXC and CC subtypes, have been shown to be up-regulated in both experimental and clinical studies of myocardial infarction (Matsumori et al., 1997, Riesenberg et al., 1997, Frangogiannis and Entman, 2005, Takahashi, 2010, Ghadge et al., 2011).
SDF-1 (also known as CXCL12) is a CXC chemokine, so-called because the two amino acids nearest the N-terminus are separated by a single amino acid (Rollins, 1997). It is a small (8 kDa), 89 amino acid peptide that is encoded by a gene originally cloned from murine bone marrow stromal cells, hence its name (Rollins, 1997, Ceradini et al., 2004, Ghadge et al., 2011). It is highly conserved (more than 92% similarity at the protein level) between species and has been shown to confer protection between species (Shirozu et al., 1995, Shimizu et al., 2009). Several isoforms of SDF-1, namely SDF-1α–ζ, arise from alternative splicing and have been identified by PCR (Ghadge et al., 2011). Of interest here and best described is SDF-1α. Note that many studies do not specify which isoform they are investigating and henceforth where this is the case the more general SDF-1 descriptor is adopted. SDF-1α expression has been reported in several organs, tissues and cells, including bone marrow, heart, liver, kidney, thymus, spleen, skeletal muscle, brain and, more recently, platelets (Kucia et al., 2005, Nagasawa, Nakajima, et al., 1996, Ratajczak et al., 2006, Ghadge et al., 2011, Chatterjee and Gawaz, 2013), although it is likely that this also reflects expression in the vascular endothelium of the organ. In the heart, SDF-1α is expressed by stromal cells, endothelial cells and cardiomyocytes (Askari et al., 2003, Segret et al., 2007). It is a chemoattractant for a variety of cell types including T lymphocytes, bone marrow stem cells (BMSCs; including haematopoietic, endothelial and mesenchymal subtypes), adipose-derived regenerative cells (ADRCs) and c-kit+ endogenous cardiac stem cells (eCSCs) (Aiuti et al., 1997, Rollins, 1997, Kondo et al., 2009, Zaruba and Franz, 2010). It also has a role in maintaining hematopoietic stem cell niches in bone marrow (Ghadge et al., 2011). SDF-1α is cleaved from its active form (1–68) to SDF-1α (3–68) by exopeptidases such as dipeptidyl peptidase 4 (DPP-4), matrix metalloproteinase (MMP)-2 and MMP-9 (Segers et al., 2007). It has an estimated half-life in vivo by radiolabelling of 25.8 ± 4.6 min (Misra et al., 2008). However, this represents total SDF-1α only and does not differentiate active and inactive isoforms (Misra et al., 2008). Similarly, commercially available ELISA kits, to our knowledge, measure total and not active SDF-1α only and may therefore offer a skewed view of the role of SDF-1α in myocardial infarction.
SDF-1α exerts its effects by binding the receptors CXCR7 and CXCR4, for which it is the unique ligand (Balabanian et al., 2005, Sierro et al., 2007). CXCR4 is a G protein coupled receptor that, once activated, is thought to initiate a signalling cascade that regulates the functions described (Kucia, Jankowski, et al., 2004, Wong and Korz, 2008, Ghadge et al., 2011). The SDF-1α–CXCR4 axis is also important in embryogenesis, including cell migration and development of neuronal, cardiac, vascular, haematopoietic and craniofacial systems, which is reflected by its expression on a range of progenitor cells, including haematopoietic, endothelial and cardiac stem cells (Rollins, 1997, Zaruba and Franz, 2010, Ghadge et al., 2011). During angiogenesis, expression of CXCR4 on vessel endothelium correlates with areas of high SDF-1α expression (McGrath et al., 1999). In embryonic development transgenic homozygote mice lacking either CXCR4 or SDF-1α have abnormal B-lymphocyte development, as well as abnormal hepatic and cardiac development, including ventricular septal defects, and die in utero (Nagasawa, Hirota, et al., 1996, Tachibana et al., 1998, Zou et al., 1998). Similarly, in humans, CXCR4 mutation causes impaired mobilisation of neutrophils and B-cell lymphopaenia (Hernandez et al., 2003).
More recently it has become apparent that CXCR7 also plays a central role in SDF-1α regulation. For example, Berahovich et al. found increased serum SDF-1α in a murine model of genetic deletion and pharmacological inhibition of CXCR7, which was associated with impaired leukocyte migration (Berahovich et al., 2014). However, the relative contribution and relationship of CXCR4 and CXCR7 is not fully elucidated. Hoffman et al. have demonstrated the rapid spontaneous internalisation of SDF-1α associated with CXCR7, compared to G-protein coupling via CXCR4, and rapid release of SDF-1α degradation products (Hoffmann et al., 2012). They also described a mechanism whereby CXCR7 performs an SDF-1α-scavenging function (Hoffmann et al., 2012). CXCR7 is thought to be up-regulated in inflammation, cancer and autoimmune conditions (Sanchez-Martin et al., 2013). It has been shown to improve migration and paracrine (angiogenesis and mitogenesis) function of mesenchymal stem cells (MSCs) in mice subjected to renal IR, and also reduced apoptosis and improved functional recovery (Liu et al., 2012). Despite this its contribution, if any, to cardioprotection is not yet known (Zaruba & Franz, 2010).
3. Chronic cardioprotection
3.1. Stromal derived factor 1α–CXC Chemokine Receptor 4/CXC Chemokine Receptor 7 after acute myocardial infarction
Much is known about the SDF-1α–CXCR4/CXCR7 axis in the context of myocardial regeneration after acute myocardial infarction (‘chronic cardioprotection’). The mechanism is well described. In hypoxic conditions, the transcription factor HIF-1α is up-regulated and in turn up-regulates SDF-1α, CXCR4 and CXCR7 expression (Pillarisetti and Gupta, 2001, Zaruba and Franz, 2010, Ghadge et al., 2011, Esencay et al., 2013). In this way SDF-1α acts as diffusible ‘homing beacon’ directing cells towards hypoxic tissue. In health, since the bone marrow is physiologically hypoxic, SDF-1α expression results in the retention of bone marrow stem cells (Ceradini et al., 2004, Zaruba and Franz, 2010). In response to acute myocardial infarction (AMI) several clinical studies have demonstrated up-regulation of SDF-1α in infarct and peri-infarct zones, returning to baseline at 7 days (Pillarisetti and Gupta, 2001, Askari and Penn, 2003, Abbott et al., 2004, Hu et al., 2007), and in the serum of patients suffering AMI (Yamani et al., 2005, Leone et al., 2006). SDF-1α–CXCR4 signalling is also reportedly elevated in toxic liver damage, total body irradiation and after chemotherapy (Kucia et al., 2005). In AMI, this transient increase causes the gradient-guided homing of progenitor cells from the bone marrow, as well as adipose and cardiac tissue, to sites of injury and inflammation. Thus, expression of SDF-1 in infarcted myocardium has been associated with recruitment, retention, survival and proliferation of ADRCs, eCSCs and BMSCs (Kucia, Dawn, et al., 2004, Unzek et al., 2007, Kondo et al., 2009, Jujo et al., 2010, Tang et al., 2010, Zaruba and Franz, 2010, Penn et al., 2012). Conversely, decreased recruitment, angiogenesis and blood flow has been demonstrated when CXCR4 is blocked on infused progenitor cells or SDF-1α is blocked in the recipient (Ceradini et al., 2004, Zaruba and Franz, 2010). SDF-1α–CXCR4/CXCR7 exerts these effects by activating intracellular signalling cascades (Zaruba & Franz, 2010). These include the MAPK p42/44 extracellular signal-regulated (Erk1/2), PI3K-Akt, JAK-STAT and protein kinase C (PKC) signalling cascades, as well as inositol-1,4,5-triphosphate (IP3)-induced SR/ER calcium release (Ratajczak et al., 2006, Haider et al., 2008, Gao et al., 2009, Zaruba and Franz, 2010, Ghadge et al., 2011).
Although a number of mechanisms can mobilise stem cells, including cytokines (granulocyte- and granulocyte macrophage-colony stimulating factor (G-CSF, GM-CSF), and stem cell factor (SCF)), interleukins (IL-7, IL-12, IL-3), chemokines (SDF-1α, IL-8), growth factors (VEGF, hepatocyte- and insulin-like growth factor (HGF, IGF)) (Lapidot & Petit, 2002), and chemotherapeutic agents like cyclophosphamide (Ghadge et al., 2011), it is suggested that the SDF-1α–CXCR4 axis is the most potent and central to the process of mobilising progenitor cells (Lapidot and Petit, 2002, Cottler-Fox et al., 2003). For example, G-CSF, a cytokine known to increase the mobilisation of stem cells from bone marrow and widely used therapeutically, does so by disrupting the association of SDF-1 on bone marrow stromal cells, osteoblasts and reticulocytes with CXCR4 on bone marrow stem cells (Petit et al., 2002, Levesque et al., 2003, Zaruba and Franz, 2010). Interestingly, a similar mechanism may explain how AMD3100, a selective CXCR4 antagonist, increases circulating HPCs and endothelial progenitor cells (Liles et al., 2003, Jujo et al., 2010).
3.2. Therapeutic application of stromal derived factor 1α–CXC Chemokine Receptor 4/CXC Chemokine Receptor 7 in chronic cardioprotection
Given the potential therapeutic utility of SDF-1α it has been the subject of several pre-clinical studies that have adopted a range of approaches to manipulate SDF-1α expression. For example, SDF-1α has been delivered by intracardiac injection immediately after AMI in mice and demonstrated activation of Akt within endothelial cells and cardiac myocytes, associated with improved cardiac function up to 28 days after infarction, increased VEGF, increased angiogenesis and reduced infarct size (Sasaki et al., 2007, Saxena et al., 2008). Continued expression of active SDF-1α (peak at 7 days) has been achieved by expression from adenovirus injected in the myocardium after infarction, which resulted in smaller infarct size, improved left ventricular parameters, less fibrosis and a greater density of cardiomyocytes and blood vessels in a rat model of STEMI (Tang et al., 2010). Other reported techniques include intracardiac injection of skeletal myoblasts overexpressing human SDF-1α (Deglurkar et al., 2006, Elmadbouh et al., 2007), MSCs overexpressing SDF-1α (Zhang, Mal, et al., 2007, Zhao et al., 2009), plasmid-based over-expression of SDF-1 (Sundararaman et al., 2011), use of a PEGylated fibrin patch for MSC transplantation (Zhang et al., 2007), and MSCs overexpressing IGF-1 to activate SDF-1α (Haider et al., 2008), all of which have conferred similar beneficial effects. Furthermore, some studies have utilised combinations of techniques. For example, Askari et al. administered cardiac fibroblasts over-expressing SDF-1α to the infarcted myocardium of a rat along with G-CSF and found improved stem cell homing, angiogenesis and cardiac function (Askari et al., 2003). Abbott et al. found that stem cells delivered to the coronary artery following AMI were only retained alongside adenovirus-mediated cardiac expression of SDF-1α, an effect that was abolished by AMD3100 (Abbott et al., 2004). Finally, Misao et al. administered G-CSF or vehicle 3 days after IR in rabbits and found increased serum SDF-1α, reduced infarct size, improved ejection fraction (EF) and improved end-diastolic dimensions in the G-CSF group, suggesting that G-CSF can increase SDF expression independently of its action on the SDF-CXCR4 association in bone marrow (Misao et al., 2006). This was abrogated by AMD3100 (Misao et al., 2006).
Likewise, studies have attempted to manipulate CXCR4 expression. Cheng et al. delivered MSCs overexpressing CXCR4 intravenously to rats 24 h after IR and found improved homing, better preservation of LV dimensions, reduced fibrosis and better LV function, as assessed by echocardiography (Cheng et al., 2008). Similarly, injection of cultured stem cells with CXCR4 specifically up-regulated by cultivation led to better angiogenesis (Shiba et al., 2009). Finally, conditional cardiomyocyte CXCR4 null mice have been used to investigate the requirement for CXCR4 signalling in stem cell-mediated repair following infarction (Dong et al., 2012). In this study, exogenous MSC engraftment was similar regardless of myocardial CXCR4 status, consistent with the hypothesis that, in these animals, the myocardium still produces SDF-1 and the MSCs still express CXCR4 (Dong et al., 2012). The authors noted abrogation of recovery of cardiac function following infarct in CXCR4 deficient mice, which may lend support to an acute role for SDF-1α, although infarct size was not measured acutely in this study (Dong et al., 2012).
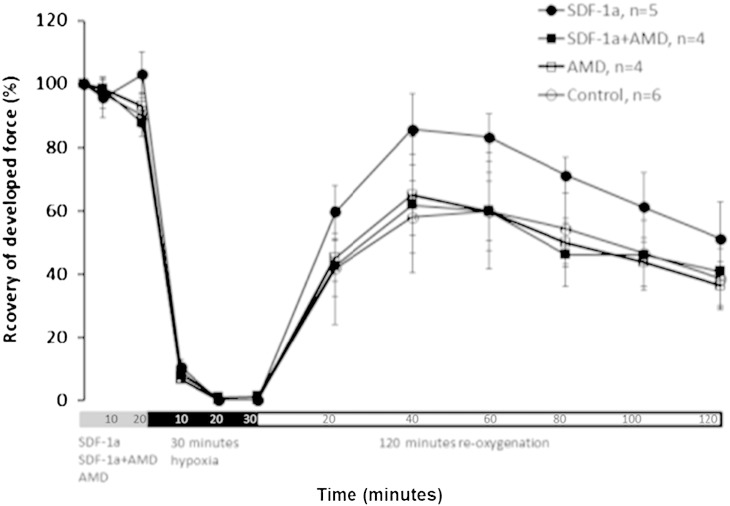
Despite these encouraging results enthusiasm is tempered by a lack of firm evidence for true myocardial regeneration, and it may be that the benefits described above are solely attributable to paracrine actions of SDF-1α (Ghadge et al., 2011). Furthermore, it is important to note that not all experimental data indicates a favourable role of SDF-1α–CXCR4 in AMI. For example, Pyo et al. found a negative inotropic effect of SDF-1 on murine papillary muscles and cardiac myocytes during calcium stimulation, an effect that was exacerbated by adenovirus-mediated over-expression of CXCR4 but attenuated by AMD3100 (Pyo et al., 2006). However, this data is contradicted by data from our laboratory showing that the functional recovery of muscle treated with SDF-1 was significantly improved relative to control muscle (see Fig. 2) (Davidson et al., 2013). Elsewhere, SDF-1α was injected into the peri-infarct zone of pigs that, consistent with previous studies, resulted in increased vessel density (Koch et al., 2006). However, there was no significant improvement in infarct size, myocardial perfusion or left ventricular function compared to controls (Koch et al., 2006). These studies may indicate a requirement for combination therapy to both stimulate stem cell mobilisation from bone marrow niches and homing to sites of injury. In addition, Koch et al. delivered only a single bolus of SDF-1, which could be expected to be rapidly proteolysed (Koch et al., 2006), and administered SDF-1 at 7 days while there is evidence that intravenous MSCs are only effective when given within 4 days (Maekawa et al., 2004).
Fig. 2.
SDF-1α prior to ischaemia improves contractile recovery of rat heart papillary muscle that is isolated and subject to 30 min hypoxia and 2 h reoxygenation. Before hypoxia, the rat papillary muscle was perfused with 1) SDF-1 for 10 min; or 2) AMD3100 for 5 min then AMD3100 plus SDF-1 for 10 min, or 3) AMD3100 alone for 15 min. Control papillary muscle was untreated. Data expressed as mean ± SE. The functional recovery of muscle treated with SDF-1 alone was significantly improved (P < 0.05) (Davidson et al., 2013).
Nevertheless, research into the role of the SDF-1α–CXCR4 axis has begun to be translated to the bedside. For example, Theiss et al. have confirmed in humans that mRNA of SDF-1 and HIF-1α, as well as stem cell factor and vascular cell adhesion molecule, are significantly higher in explanted heart tissue of patients with ischaemic cardiomyopathy versus dilated cardiomyopathy (Theiss et al., 2007). Penn's group have recently completed a Phase I study of plasmid-based endomyocardial SDF-1 delivery to 17 patients with symptomatic ischaemic cardiomyopathy and found improvements in 6-minute walk distance, New York Heart Association classification and quality of life (Penn et al., 2013). Despite these promising results, ejection fraction was non-significantly reduced in all patients and neither inflammation nor scar formation was recorded, which may be relevant with serial myocardial injection.
4. Acute cardioprotection
4.1. Ischaemic preconditioning
Given the role of SDF-1α–CXCR4/CXCR7 in myocardial repair and the involvement of signalling kinases known to be integral to ischaemic conditioning, namely the Erk1/2, PI3K-Akt, JAK-STAT and PKC signalling cascades (Deglurkar et al., 2006, Hausenloy and Yellon, 2008a), it has been hypothesised that SDF-1α may also be involved in the myocardial protection from IRI conferred by ischaemic conditioning. This is further supported by studies that have successfully used ischaemic preconditioning to up-regulate SDF-1α and improve stem cell engraftment after AMI. For example, Tang et al. applied a preconditioning stimulus directly to the heart in a murine model of AMI and demonstrated up-regulation of CXCR4 on cardiac progenitor cells, increased cardiac progenitor cell migration and recruitment, reduced infarct size and improved functional outcome, all of which were abolished by the addition of AMD3100 (Tang et al., 2009).
The pathophysiology of IRI is not fully understood, but proposed mechanisms include oxidative stress (Zweier, 1988), deranged calcium metabolism (Piper et al., 1998), rapid restoration of physiologic pH (Lemasters et al., 1996), inflammation (Vinten-Johansen, 2004), and deranged metabolism of glucose, insulin and potassium (Opie, 1970). However, manipulation of these mechanisms has largely failed to translate to benefit in clinical trials (Armstrong et al., 2007, Avkiran and Marber, 2002, Bar et al., 2006, Baran et al., 2001, Boden et al., 2000, Faxon et al., 2002, Granger et al., 2003, Hausenloy et al., 2010, Mehta et al., 2005, Mertens et al., 2006, Selker et al., 2012, Zeymer et al., 2001). The finding that the myocardium could be protected from lethal IRI by the application of multiple brief ischaemic episodes was first made by Murry et al., who found a 25% reduction in infarct size in dogs subjected to four 5 minute circumflex occlusions, each separated by 5 min of reperfusion, prior to sustained occlusion of the circumflex artery (Murry et al., 1986). They termed this phenomenon ischaemic preconditioning (IPreC) (Murry et al., 1986). However, despite promising experimental results, its clinical utility is limited by the necessity to intervene before the index ischaemia, which is evidently impossible to predict in STEMI. Further work by Zhao et al. investigated repetitive ischaemia applied in early reperfusion of the left anterior descending (LAD) territory in a canine model, and found a 14% reduction in infarct size (compared to 15% in IPreC in their model), a technique referred to as ischaemic post conditioning (IPostC) (Zhao et al., 2003). Several studies have investigated this approach in a clinical setting, with mixed results (Staat et al., 2005, Thibault et al., 2008, Lonborg et al., 2010, Sorensson et al., 2010, Freixa et al., 2012, Tarantini et al., 2012).
The mechanism of protection conferred by ischaemic conditioning, as it is currently understood, comprises extracellular autacoids acting on cardiomyocyte receptors in response to the conditioning stimulus and triggering protective intracellular signal transduction cascades (Ovize et al., 2010). These are thought to unite at the mitochondria, particularly the mitochondrial permeability transition pore (MPTP), to inhibit apoptosis and preserve cardiomyocyte viability (see Fig. 1) (Shanmuganathan et al., 2005).
Several endogenous extracellular factors are known to mitigate the deleterious effects of IRI and are thought to play a central role in ischaemic conditioning (Simpkin et al., 2007, Smith et al., 2010, Hausenloy and Yellon, 2013). They originate from a variety of sources, including cardiomyocytes, endothelium, smooth muscle cells, nerve endings, inflammatory cells, mast cells and macrophages, in response to the conditioning stimulus (Deussen et al., 1986, Dorge et al., 2002, Schulz et al., 2004, Faigle et al., 2008, Heusch et al., 2008). They are thought to have a number of actions, including reducing the activation of coronary vascular endothelium, reducing the production of pro-inflammatory cytokines and reactive oxygen species (ROS) and reducing adherence of neutrophils to the coronary artery, all of which contribute to ischaemic conditioning (Zhao et al., 2003, Halkos et al., 2004, Ovize et al., 2010). As described, these factors exert these effects on the intracellular effector by recruiting the same protein kinase signalling cascades that are thought to be activated by the SFD-1α–CXCR4/CXCR7 axis (Hausenloy and Yellon, 2004, Hausenloy and Yellon, 2007b, Ovize et al., 2010). The best defined of these are the ‘reperfusion injury salvage kinase’ (RISK) and ‘survivor activating factor enhancement’ (SAFE) pathways (Hausenloy and Yellon, 2007a, Boengler, Hilfiker-Kleiner, Drexler, Heusch and Schulz, 2008, Lacerda et al., 2009, Lecour, 2009).
The RISK pathway was first described by Yellon's group in recognition of the activation of PI3K-Akt pathway and p42/p44 Erk1/2 MAPK by myocardial reperfusion (Yellon and Baxter, 1999, Brar et al., 2000, Baxter et al., 2001, Jonassen et al., 2001, Hausenloy and Yellon, 2004). Pharmacological activation of this pathway has been shown to reduce infarct size by 40–50% at the time of reperfusion (Ovize et al., 2010). Importantly, several of the known protective endogenous factors, including insulin, insulin-like growth factor-1, bradykinin and adenosine, have been shown to protect against IRI by recruiting the RISK pathway (Hausenloy & Yellon, 2004). IPreC has also been shown to protect against lethal IRI by recruiting the RISK pathway (Tong et al., 2000, Fryer et al., 2001, Mocanu et al., 2002); specifically, the Akt1 isoform appears to be essential to IpreC as demonstrated in Akt1-deficient mice that were resistant to protection from IPreC (Kunuthur et al., 2012). Further, it has been shown that the RISK pathway is recruited equally by IPreC, IPostC and RIC, indicating that this signal transduction pathway may represent a common pathway in ischaemic conditioning (Hausenloy et al., 2003, Downey and Cohen, 2005, Hausenloy et al., 2005, Tamareille et al., 2011).
Lecour et al. described an alternative pathway, labelled the SAFE pathway, which activates JAK-STAT signalling (Lecour, 2009). In mice subjected to simulated ischaemia and reperfusion, IPostC reduced infarct size and increased phosphorylated (active) STAT3 (Boengler et al., 2008). In this study, administration of a specific JAK-2 inhibitor (AG-490) reduced phosphorylated STAT3 and abolished the beneficial effect of IPostC (Boengler et al., 2008). Likewise, cardiac-specific STAT3-deficient mice were not protected from IRI by IPostC (Boengler et al., 2008). Further, in a model of pharmacological preconditioning with tumour necrosis factor-α cardioprotection was not affected by inhibition of PI3K-Akt or Erk 1/2 MAPK, but was abolished by inhibition of STAT3 (Lecour et al., 2005). Likewise, the SAFE signalling pathway is also required for RIC (Tamareille et al., 2011). The interplay between RISK and SAFE pathways is not fully defined, however it has been shown that in ex vivo mouse hearts functional protection conferred by IPostC was not only abolished by JAK-STAT inhibition, but also that STAT3 inhibition decreased both functional STAT3 and Akt, suggesting that these signalling cascades are not entirely independent (Goodman et al., 2008). What is known is that both pathways converge on the mitochondria, particularly the MPTP, to affect cardioprotection.
Many of the pathological processes thought to mediate IRI, including oxidative stress, deranged calcium metabolism, and rapid recovery of physiologic pH, exert their effects at the mitochondria, resulting in cardiomyocyte death (Yellon & Hausenloy, 2007). Specifically, the MPTP, a voltage- and calcium-dependent channel in the inner mitochondrial membrane, is implicated (Ovize et al., 2010). This is closed in ischaemia due to acidosis, a high mitochondrial membrane potential, and high concentrations of Mg2+ and ADP (Di Lisa and Bernardi, 2009, Ovize et al., 2010). However, in reperfusion, the MPTP opens in response to binding of cyclophilin D (Cyp-D), which is potentiated by depolarization, increased mitochondrial Ca2+, inorganic phosphate and ROS, and restoration of normal pH (Griffiths and Halestrap, 1995, Di Lisa et al., 2001, Kim et al., 2006, Murata et al., 2001, Matsumoto-Ida et al., 2006, Ovize et al., 2010). Once open, the mitochondrial membrane rapidly dissipates, respiration becomes uncoupled, further elevating ROS formation, which establishes a vicious cycle of further MPTP opening and consequently loss of cell viability (Di Lisa and Bernardi, 2009, Ovize et al., 2010). Importantly, it is known that IPreC and IPostC antagonise MPTP opening and significantly limit infarct size in animal models of IR (Javadov et al., 2003, Hausenloy et al., 2004, Argaud, Gateau-Roesch, Muntean, et al., 2005, Argaud, Gateau-Roesch, Raisky, et al., 2005, Bopassa, Vandroux, Ovize and Ferrera, 2006, Zhao and Vinten-Johansen, 2006). Further, specific chemical inhibitors of MPTP, including NIM811 (Argaud, Gateau-Roesch, Muntean, et al., 2005, Argaud, Gateau-Roesch, Raisky, et al., 2005), cyclosporine A (Griffiths and Halestrap, 1993, Griffiths and Halestrap, 1995, Hausenloy et al., 2004, Argaud, Gateau-Roesch, Muntean, et al., 2005), and N-methyl-4-valine cyclosporine A (Hausenloy et al., 2004), have been shown to reduce MPTP opening, limit apoptosis, improve functional recovery, and limit infarct size. Likewise, transgenic Cyp-D-deficient mice subjected to simulated IR have been shown to have reduced infarct size (Baines et al., 2005, Nakagawa et al., 2005). The improvement in functional recovery conferred by cyclosporine A, which prevents Cyp-D binding (Ovize et al., 2010), has also been demonstrated in human atrial tissue (Shanmuganathan et al., 2005), has been successfully translated to a human pilot study of AMI (Piot et al., 2008), and a phase III trial is currently recruiting (“Cyclosporine & Prognosis in Acute Myocardial Infarction (MI) Patients (CIRCUS)”). Finally, it is notable that several studies have associated activation of the RISK and SAFE pathways with inhibition of MPTP opening, indicating it may represent the final common effector of ischaemic conditioning (Bopassa, Ferrera, Gateau-Roesch, Couture-Lepetit and Ovize, 2006, Davidson et al., 2006, Juhaszova et al., 2004, Smith et al., 2010, Zhao and Vinten-Johansen, 2006).
4.2. Stromal derived factor 1α and ischaemic conditioning
SDF-1α has been shown to be involved in myocardial protection from IRI conferred by ischaemic conditioning in a number of different models (Hu et al., 2007, Huang et al., 2011, Jang et al., 2012). Data from Yellon's group using ex vivo rat papillary muscle indicates that SDF-1α increases recovery of function of muscle subject to simulated IRI and can be blocked by AMD3100 (Fig. 2) (Davidson et al., 2013). Similarly, Huang et al. administered SDF-1 5 min before ischaemia in isolated mouse hearts subject to ischaemia–reperfusion in a model of pharmacological preconditioning (Huang et al., 2011). They found that SDF-1 significantly improved functional recovery, reduced markers of apoptosis and increased activation of STAT3, a central mediator of the SAFE pathway (Huang et al., 2011). These effects were abolished by the addition of AMD3100 (Huang et al., 2011). Interestingly, they did not see any increase in Akt or Erk1/2 phosphorylation, and no attenuation of protection with LY294002, an inhibitor of the Akt pathway (Huang et al., 2011). However, the inhibitors were given prior to ischaemia and not prior to reperfusion and hence it may be that Akt is still integral to mitigating reperfusion injury specifically. Jang et al. used an ex vivo Langendorff model of IR to show that SDF-1 infused from 10 min before reperfusion to 30 min afterwards reduced infarct size significantly more than that seen with IPreC and IPostC (Jang et al., 2012). They also saw an increase in Erk phosphorylation at 5 and 20 min after reperfusion, thereby implicating the RISK pathway in the mechanism (Jang et al., 2012). Hu et al. demonstrated significantly increased SDF-1α released from isolated cardiac myocytes following hypoxia and reoxygenation that resulted in increased phosphorylation of both Erk1/2 and Akt, less lactate dehydrogenase release and less apoptosis (Hu et al., 2007). Pre-treatment with AMD3100 abolished the effect on myocyte survival (Hu et al., 2007). In vivo, they demonstrated pharmacological preconditioning with SDF-1α infused into the left ventricular cavity significantly reduced infarct size, which was abrogated by AMD3100 (Hu et al., 2007). Conversely, Chen et al. used adenovirus-mediated over-expression of CXCR4 administered 7 days prior to IR in rats and found significantly increased scar size, worse fractional shortening, increased inflammatory cell infiltration, increased cardiac myocyte apoptosis and more left ventricular hypertrophy at 24 h (Chen et al., 2010). These effects were attenuated by AMD3100, and it is clear that the window for protection from IRI requires careful evaluation (Chen et al., 2010).
4.3. Remote ischaemic conditioning
Despite promising results for mechanical IPreC and IPostC, the common problem is that both mandate an invasive approach to cardioprotection that may be both impractical and even harmful, as they increase the risk of procedural complications, including access site complications, coronary artery dissection or perforation, arrhythmias and stroke. In response to these concerns RIC has emerged as an exciting potential alternative, whereby brief cycles of ischaemia and reperfusion are applied to an organ or tissue remote from the heart. This was first shown to be protective by Przyklenk et al., 1993, who applied four episodes of 5 minute circumflex occlusion separated by 5 min of reperfusion, before 1 h of sustained LAD occlusion and reperfusion for 4.5 h in a canine model (Przyklenk et al., 1993). They found a 10% reduction in infarct size in the circumflex preconditioned dogs (Przyklenk et al., 1993). This has been developed by others who showed similar cardioprotective effects after applying a preconditioning stimulus to other remote organs and tissues, including the kidneys and skeletal muscle (Mcclanahan et al., 1993, Birnbaum et al., 1997), and using a model of remote ischaemic postconditioning (Andreka et al., 2007). More recently, it has been demonstrated that the non-invasive application of brief cycles of ischaemia and reperfusion to a limb using a tourniquet has the same effect (Oxman et al., 1997, Kharbanda et al., 2002), a finding which has greatly accelerated the rate of clinical trials demonstrating RIC to be protective in a variety of clinical settings (Candilio et al., 2013). These include prior to coronary artery bypass surgery (CABG) (Gunaydin et al., 2000, Cheung et al., 2006, Hausenloy et al., 2007, Venugopal et al., 2009, Thielmann et al., 2010, Hausenloy et al., 2012, Thielmann et al., 2013), elective abdominal aortic aneurysm repair (Ali et al., 2007, Walsh et al., 2009), elective cervical decompression surgery (Hu et al., 2010), elective PCI (Hoole et al., 2009), and in PPCI for STEMI (Kerendi et al., 2005, Schmidt et al., 2007, Botker et al., 2010). For patients suffering STEMI, Botker et al. randomised patients to receive PPCI with or without a pre-hospital RIC protocol. The primary endpoint of improved myocardial salvage index at 30 days, measured by myocardial perfusion imaging, was met (Botker et al., 2010). Importantly, studies have demonstrated similar effects with RIC applied before (Gunaydin et al., 2000, Hausenloy et al., 2007, Rentoukas et al., 2010), during (Botker et al., 2010), and after the index ischaemia (Kerendi et al., 2005, Andreka et al., 2007), thereby improving its clinical utility and potential to mitigate IRI in patients.
It is suggested that the protective effect of RIC may be due to a humoral factor(s), which may be one or a combination of the factors described above, or a novel molecule(s), which are carried by the blood from the transiently ischaemic limb to the remote target organ where they activate endogenous pro-survival signalling pathways. Evidence for this comes from studies wherein the cardioprotective effect of RIC applied to the lower limb is abrogated by occlusion of the femoral vein (Lim et al., 2010). Further, infarct size is significantly reduced when the effluent from an isolated perfused heart that is preconditioned is used to perfuse a second isolated heart prior to index ischaemia (Dickson et al., 1999, Leung et al., 2013). Interestingly, there also appears to be a neural component to RIC whereby severing the femoral and sciatic nerve in an in vivo mouse model of IR abolishes the protection conferred by RIC, although the relationship between neural and humoral components of this phenomenon is debated (Lim et al., 2010).
4.4. Stromal derived factor 1α and remote ischaemic conditioning
For the reasons described, SDF-1α is a prime candidate for a role in RIC. Although only a limited number of studies have investigated the possible role of SDF-1α–CXCR4 in RIC, it so far satisfies the criteria for an endogenous mediator defined by the Working Group of Cellular Biology of the Heart of the European Society of Cardiology (Ovize et al., 2010); namely that RIC can be abrogated by specific receptor blockade or inhibition of the mediator's production and that RIC can be mimicked by exogenous administration of the mediator. Jiang et al. used remote ischaemic postconditioning to improve the retention of improved MSCs in a murine model of myocardial infarction (Jiang et al., 2013). They found increased serum and myocardial SDF-1α, significantly increased MSC retention in the myocardium, and improved cardiac function at 1 month, all of which was abrogated by administration of anti-rat CXCR4 polyclonal antibody as a single intraperitoneal injection after the RIC procedure (Jiang et al., 2013). Kamota et al. conferred RIC using cyclical occlusion of the abdominal aorta in mice prior to IR of the LAD territory (Kamota et al., 2009). They found increased VEGF and SDF-1α acutely (1 and 3 h) and significantly increased CD34+ stem cells in the peripheral blood at 12 and 24 h (Kamota et al., 2009). Both phases of protection independently resulted in improved LV dimensions and function, and less apoptosis (Kamota et al., 2009). Interestingly, blocking recruitment of bone marrow stem cells only abrogated cardioprotection in the late phase in their model, which all suggests that SDF-1α has the ability to potentially repair myocardial damage, by directing the homing of stem cells from the bone marrow to the site of damage, in combination with its potential to directly protect the myocardium from IRI via the pathways described above. Most recently, Davidson et al. demonstrated the involvement of SDF-1α in acute RIC by showing that RIC significantly reduced infarct size, an effect which was blocked by AMD3100 (Davidson et al., 2013). The also confirmed that SDF-1α was elevated in the plasma of rats subjected to hind-limb RIC (Davidson et al., 2013). Improved functional recovery in isolated rat cardiac papillary muscle subjected to simulated IR after RIC was also blocked by AMD3100 and in this model significant functional recovery was also seen with pharmacological preconditioning with SDF-1α (Davidson et al., 2013).
5. Dipeptidyl peptidase-4 inhibitors
Despite these exciting results, an important drawback is the relatively short plasma half-life of SDF-1α, which might limit its therapeutic utility (Valenzuela-Fernandez et al., 2002, Segers et al., 2007). However, bioengineered SDF-1 that is resistant to cleavage by DPP-4 and MMP-2 has been associated with improved stem cell homing, angiogenesis and ejection fraction (Segers et al., 2007). Another approach to extracting the maximum potential from SDF-1α relates to its potential manipulation by a new class of anti-diabetic drugs. DPP-4 inhibitors such as Sitagliptin, Vildagliptin, Alogliptin and Saxagliptin, have been designed to prevent the breakdown of the incretin glucagon-like peptide 1 (GLP-1) by inhibiting the protease DPP-4 thereby increasing insulin and lowering glucose (Ravassa et al., 2012). Active SDF-1α is also cleaved by DPP-4 and thus, similar to GLP-1, DPP-4 inhibition increases the half-life of SDF-1α by preventing its degradation (Crump et al., 1997, Zaruba et al., 2009). DPP-4 is found on many of the same cell types as CXCR4, including B and T lymphocytes, endothelial cells and CD34+ HPCs, as well as being present in plasma (Christopherson et al., 2003, Zaruba and Franz, 2010).
Several studies have attempted to exploit this proteolytic mechanism. Christopherson et al. first showed that DPP-4 inhibition increased stem cell homing to bone marrow (Christopherson et al., 2004), following which Zaruba et al. combined DPP-4 inhibition using Diprotin A with G-CSF-mediated stem cell mobilisation in a murine model of myocardial infarction (Zaruba et al., 2009). They found decreased DPP-4 activity, which was associated with the stabilisation of active SDF-1α (Zaruba et al., 2009). This consequently increased CXCR4+ EPCs homing (an effect that was abrogated by administration of AMD3100), reduced cardiac remodelling and apoptosis, and improved EF and survival (Zaruba et al., 2009). This approach was recently tested in a phase III clinical trial using Sitagliptin (Safety and efficacy of SITAgliptin plus Granulocyte-colony-stimulating factor in patients suffering from Acute Myocardial Infarction, SITAGRAMI). They randomised patients to either G-CSF and Sitagliptin or placebo after PPCI for AMI in a multi-centre, double-blind design. The primary endpoint of improved combined global left and right ventricular ejection fraction as assessed by magnetic resonance imaging was not met, and while there was a trend towards reduced major adverse cardiac events this was not significant (“Safety and efficacy of SITAgliptin plus Granulocyte-colony stimulating factor in patients suffering from Acute Myocardial Infarction — SITAGRAMI trial”). This may be explained by the inclusion of only 21% of patients with left ventricular ejection fraction below 50%, thereby obfuscating any potential benefit of this therapy.
Similarly, two large multicentre clinical trials have recently failed to demonstrate a benefit of DPP-4 inhibitors on cardiovascular outcomes in patients with type 2 diabetes at high risk for cardiovascular events (Scirica et al., 2013, White et al., 2013). For example, SAVOR-TIMI 53 compared Saxagliptin and placebo in 16,492 patients with a history of, or at risk for, cardiovascular events (Scirica et al., 2013). After median follow-up of 2.1 years they found no significant difference in the primary endpoint of cardiovascular death, myocardial infarction or stroke. Similarly, EXAMINE compared Alogliptin with placebo in 5380 patients with type 2 diabetes and recent acute coronary syndrome (ACS) over median follow-up of 18 months and found no significant difference in the primary endpoint of cardiovascular death, nonfatal myocardial infarction or nonfatal stroke (White et al., 2013). That these important studies failed to show any long-term benefit of DPP-4 inhibitors is disappointing, but may have several explanations. Patients in SAVOR-TIMI 53 had not necessarily had a recent ACS or other hypoxic stimulus to SDF-1α production and it may be the case that, as in the preclinical studies described, a combination of SDF-1α up-regulation and DPP-4 inhibition is necessary. In EXAMINE, although a recent ACS was an inclusion criteria, re-admissions with heart failure were not recorded.
It is equally important to note that these studies did not specifically examine any acute role of DPP-4 inhibition, specifically on IRI. Indeed, relatively few groups have done so. Kanki et al. developed a bioengineered form of SDF-1 resistant to MMP-2 and DPP-4 (SSDF-1(S4V)), which they injected into the LV cavity after reperfusion in a rat model of IR (Kanki et al., 2011). This resulted in improved retention in the ischaemic myocardium (Kanki et al., 2011). They also found improved function and capillary density, although this was several weeks after the initial insult making it difficult to separate the relative contribution of acute cardioprotection and possible cardiac regeneration. It has also been demonstrated that the new anti-diabetic agents described above have direct cardioprotective effects by preventing cell death and limiting infarct size in ex vivo models (Hausenloy et al., 2013). Hausenloy et al. pretreated control and diabetic rats with DPP-4 inhibitors Vildagliptin or Sitagliptin and found a significant reduction in infarct size (Hausenloy et al., 2013). Interestingly, they found this to be dependent on elevated glucose and inhibited by Exendin, a GLP-1 receptor antagonist (Hausenloy et al., 2013). However, the contribution, if any, of SDF-1–CXCR4 was not elucidated.
6. Conclusions
Reperfusion injury makes an important contribution to myocardial injury and poor clinical outcomes after a lethal ischaemic insult in animal models, although there remains a paucity of data from clinical studies. Ischaemic conditioning has emerged as a powerful protective phenomenon and has translated to the clinical application of remote ischaemic conditioning, which is a clinically feasible, non-invasive, cost-effective therapeutic intervention that has been shown to mitigate acute myocardial injury in preliminary studies. This has heralded a hunt for the potential mechanism, which is thought to be due to a humoral factor(s) carried from the preconditioned organ or tissue to the heart, where endogenous pro-survival signalling pathways are activated. SDF-1α confers protection against myocardial ischaemia–reperfusion injury via the same signalling pathways implicated in ischaemic conditioning, namely the RISK and SAFE pathways. It is also central to the mobilisation and migration of stem cells and has been used to target them to sites of ischaemic injury. SDF-1α therefore potentially has pleiotropic effects on ischaemic myocardium: directly protecting via intracellular pro-survival signal transduction pathways while activating stem cell mobilisation and gradient-guided homing to augment myocardial recovery. One potential avenue for translating these findings to the bedside is with regard to the potentially significant role of SDF-1α in the mechanism of action of the anti-diabetic DPP-4 inhibitors to protect the myocardium from lethal reperfusion injury. Finding the factor(s) responsible for RIC is both novel and paramount for maximising its potential in cardiac patients. Further work should focus on clarifying the role of SDF-1α–CXCR4 in cardioprotection and whether this can be mimicked using DPP-4 inhibitors acutely.
Acknowledgments
We are grateful to the members of the lab, particularly Jose Vicencio, for discussions and figures.
Ongoing work is supported by the Medical Research Council [MR/K002066/1] and British Heart Foundation [RG/08/015/26411]. In addition ongoing work was undertaken at UCLH/UCL who received a proportion of funding from the Department of Health's NIHR Biomedical Research Centres funding scheme to which DM Yellon is a Senior Investigator.
Footnotes
None of the authors have any actual or potential conflict of interest including any financial, personal or other relationships with individuals or organisations within three years of initiating the work that could inappropriately influence, or be perceived to influence, the study design or data interpretation.
References
- Abbott J.D., Huang Y., Liu D., Hickey R., Krause D.S., Giordano F.J. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- Aiuti A., Webb I.J., Bleul C., Springer T., Gutierrez-Ramos J.C. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali Z.A., Callaghan C.J., Lim E., Ali A.A., Nouraei S.A., Akthar A.M., et al. Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: a randomized controlled trial. Circulation. 2007;116:I98–I105. doi: 10.1161/circulationaha.106.679167. [DOI] [PubMed] [Google Scholar]
- Andreka G., Vertesaljai M., Szantho G., Font G., Piroth Z., Fontos G., et al. Remote ischaemic postconditioning protects the heart during acute myocardial infarction in pigs. Heart. 2007;93:749–752. doi: 10.1136/hrt.2006.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argaud L., Gateau-Roesch O., Muntean D., Chalabreysse L., Loufouat J., Robert D., et al. Specific inhibition of the mitochondrial permeability transition prevents lethal reperfusion injury. J Mol Cell Cardiol. 2005;38:367–374. doi: 10.1016/j.yjmcc.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Argaud L., Gateau-Roesch O., Raisky O., Loufouat J., Robert D., Ovize M. Postconditioning inhibits mitochondrial permeability transition. Circulation. 2005;111:194–197. doi: 10.1161/01.CIR.0000151290.04952.3B. [DOI] [PubMed] [Google Scholar]
- Armstrong P.W., Granger C.B., Adams P.X., Hamm C., Holmes D., Jr., O'Neill W.W., et al. Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: a randomized controlled trial. JAMA. 2007;297:43–51. doi: 10.1001/jama.297.1.43. [DOI] [PubMed] [Google Scholar]
- Askari A.T., Penn M.S. Stromal cell-derived factor-1 mediates stem cell homing and tissue regeneration. Discov Med. 2003;3:46–47. [PubMed] [Google Scholar]
- Askari A.T., Unzek S., Popovic Z.B., Goldman C.K., Forudi F., Kiedrowski M., et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- Avkiran M., Marber M.S. Na+/H+ exchange inhibitors for cardioprotective therapy: progress, problems and prospects. J Am Coll Cardiol. 2002;39:747–753. doi: 10.1016/s0735-1097(02)01693-5. [DOI] [PubMed] [Google Scholar]
- Baines C.P., Kaiser R.A., Purcell N.H., Blair N.S., Osinska H., Hambleton M.A., et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Balabanian K., Lagane B., Infantino S., Chow K.Y., Harriague J., Moepps B., et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280:35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- Bar F.W., Tzivoni D., Dirksen M.T., Fernandez-Ortiz A., Heyndrickx G.R., Brachmann J., et al. Results of the first clinical study of adjunctive CAldaret (MCC-135) in patients undergoing primary percutaneous coronary intervention for ST-Elevation Myocardial Infarction: the randomized multicentre CASTEMI study. Eur Heart J. 2006;27:2516–2523. doi: 10.1093/eurheartj/ehl304. [DOI] [PubMed] [Google Scholar]
- Baran K.W., Nguyen M., McKendall G.R., Lambrew C.T., Dykstra G., Palmeri S.T., et al. Double-blind, randomized trial of an anti-CD18 antibody in conjunction with recombinant tissue plasminogen activator for acute myocardial infarction: limitation of myocardial infarction following thrombolysis in acute myocardial infarction (LIMIT AMI) study. Circulation. 2001;104:2778–2783. doi: 10.1161/hc4801.100236. [DOI] [PubMed] [Google Scholar]
- Baxter G.F., Mocanu M.M., Brar B.K., Latchman D.S., Yellon D.M. Cardioprotective effects of transforming growth factor-beta1 during early reoxygenation or reperfusion are mediated by p42/p44 MAPK. J Cardiovasc Pharmacol. 2001;38:930–939. doi: 10.1097/00005344-200112000-00015. [DOI] [PubMed] [Google Scholar]
- BCIS Audit Returns 2012: Adult Interventional Procedures (accessed March, 2014, at http://www.bcis.org.uk/resources/BCIS_Audit_2012_for_web_V2_14-10-20131.pdf).
- Berahovich R.D., Zabel B.A., Lewen S., Walters M.J., Ebsworth K., Wang Y., et al. Endothelial expression of CXCR7 and the regulation of systemic CXCL12 levels. Immunology. 2014;141(1):111–122. doi: 10.1111/imm.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum Y., Hale S.L., Kloner R.A. Ischemic preconditioning at a distance: reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit. Circulation. 1997;96:1641–1646. doi: 10.1161/01.cir.96.5.1641. [DOI] [PubMed] [Google Scholar]
- Boden W.E., van Gilst W.H., Scheldewaert R.G., Starkey I.R., Carlier M.F., Julian D.G., et al. Diltiazem in acute myocardial infarction treated with thrombolytic agents: a randomised placebo-controlled trial. Incomplete Infarction Trial of European Research Collaborators Evaluating Prognosis post-Thrombolysis (INTERCEPT) Lancet. 2000;355:1751–1756. doi: 10.1016/s0140-6736(00)02262-5. [DOI] [PubMed] [Google Scholar]
- Boengler K., Buechert A., Heinen Y., Roeskes C., Hilfiker-Kleiner D., Heusch G., et al. Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Circ Res. 2008;102:131–135. doi: 10.1161/CIRCRESAHA.107.164699. [DOI] [PubMed] [Google Scholar]
- Boengler K., Hilfiker-Kleiner D., Drexler H., Heusch G., Schulz R. The myocardial JAK/STAT pathway: from protection to failure. Pharmacol Ther. 2008;120:172–185. doi: 10.1016/j.pharmthera.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Bopassa J.C., Ferrera R., Gateau-Roesch O., Couture-Lepetit E., Ovize M. PI 3-kinase regulates the mitochondrial transition pore in controlled reperfusion and postconditioning. Cardiovasc Res. 2006;69:178–185. doi: 10.1016/j.cardiores.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Bopassa J.C., Vandroux D., Ovize M., Ferrera R. Controlled reperfusion after hypothermic heart preservation inhibits mitochondrial permeability transition-pore opening and enhances functional recovery. Am J Physiol Heart Circ Physiol. 2006;291:H2265–H2271. doi: 10.1152/ajpheart.00209.2006. [DOI] [PubMed] [Google Scholar]
- Botker H.E., Kharbanda R., Schmidt M.R., Bottcher M., Kaltoft A.K., Terkelsen C.J., et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727–734. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- Brar B.K., Jonassen A.K., Stephanou A., Santilli G., Railson J., Knight R.A., et al. Urocortin protects against ischemic and reperfusion injury via a MAPK-dependent pathway. J Biol Chem. 2000;275:8508–8514. doi: 10.1074/jbc.275.12.8508. [DOI] [PubMed] [Google Scholar]
- Braunwald E., Kloner R.A. Myocardial reperfusion: a double-edged sword? J Clin Invest. 1985;76:1713–1719. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candilio L., Malik A., Hausenloy D.J. Protection of organs other than the heart by remote ischemic conditioning. J Cardiovasc Med (Hagerstown) 2013;14:193–205. doi: 10.2459/JCM.0b013e328359dd7b. [DOI] [PubMed] [Google Scholar]
- Ceradini D.J., Kulkarni A.R., Callaghan M.J., Tepper O.M., Bastidas N., Kleinman M.E., et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- Chatterjee M., Gawaz M. Platelet-derived CXCL12 (SDF-1alpha): basic mechanisms and clinical implications. J Thromb Haemost. 2013;11(11):1954–1967. doi: 10.1111/jth.12404. [DOI] [PubMed] [Google Scholar]
- Chen J., Chemaly E., Liang L., Kho C., Lee A., Park J., et al. Effects of CXCR4 gene transfer on cardiac function after ischemia–reperfusion injury. Am J Pathol. 2010;176:1705–1715. doi: 10.2353/ajpath.2010.090451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z., Ou L., Zhou X., Li F., Jia X., Zhang Y., et al. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008;16:571–579. doi: 10.1038/sj.mt.6300374. [DOI] [PubMed] [Google Scholar]
- Cheung M.M., Kharbanda R.K., Konstantinov I.E., Shimizu M., Frndova H., Li J., et al. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol. 2006;47:2277–2282. doi: 10.1016/j.jacc.2006.01.066. [DOI] [PubMed] [Google Scholar]
- Christopherson K.W., II, Cooper S., Broxmeyer H.E. Cell surface peptidase CD26/DPPIV mediates G-CSF mobilization of mouse progenitor cells. Blood. 2003;101:4680–4686. doi: 10.1182/blood-2002-12-3893. [DOI] [PubMed] [Google Scholar]
- Christopherson K.W., II, Hangoc G., Mantel C.R., Broxmeyer H.E. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- Cottler-Fox M.H., Lapidot T., Petit I., Kollet O., DiPersio J.F., Link D., et al. Stem cell mobilization. Hematology Am Soc Hematol Educ Program. 2003:419–437. doi: 10.1182/asheducation-2003.1.419. [DOI] [PubMed] [Google Scholar]
- Crump M.P., Gong J.H., Loetscher P., Rajarathnam K., Amara A., Arenzana-Seisdedos F., et al. Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. EMBO J. 1997;16:6996–7007. doi: 10.1093/emboj/16.23.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyclosporine and Prognosis in Acute Myocardial Infarction (MI) Patients (CIRCUS) (accessed December, 2013, at http://clinicaltrials.gov/show/NCT01502774).
- Davidson S.M., Hausenloy D., Duchen M.R., Yellon D.M. Signalling via the reperfusion injury signalling kinase (RISK) pathway links closure of the mitochondrial permeability transition pore to cardioprotection. Int J Biochem Cell Biol. 2006;38:414–419. doi: 10.1016/j.biocel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Davidson S.M., Selvaraj P., He D., Boi-Doku C., Yellon R.L., Vicencio J.M., et al. Remote ischaemic preconditioning involves signalling through the SDF-1alpha/CXCR4 signalling axis. Basic Res Cardiol. 2013;108:377. doi: 10.1007/s00395-013-0377-6. [DOI] [PubMed] [Google Scholar]
- De Clercq E. The bicyclam AMD3100 story. Nat Rev Drug Discov. 2003;2:581–587. doi: 10.1038/nrd1134. [DOI] [PubMed] [Google Scholar]
- Deglurkar I., Mal N., Mills W.R., Popovic Z.B., McCarthy P., Blackstone E.H., et al. Mechanical and electrical effects of cell-based gene therapy for ischemic cardiomyopathy are independent. Hum Gene Ther. 2006;17:1144–1151. doi: 10.1089/hum.2006.17.1144. [DOI] [PubMed] [Google Scholar]
- Deussen A., Moser G., Schrader J. Contribution of coronary endothelial cells to cardiac adenosine production. Pflugers Arch. 1986;406:608–614. doi: 10.1007/BF00584028. [DOI] [PubMed] [Google Scholar]
- Di Lisa F., Bernardi P. A CaPful of mechanisms regulating the mitochondrial permeability transition. J Mol Cell Cardiol. 2009;46:775–780. doi: 10.1016/j.yjmcc.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Di Lisa F., Menabo R., Canton M., Barile M., Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem. 2001;276:2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- Dickson E.W., Lorbar M., Porcaro W.A., Fenton R.A., Reinhardt C.P., Gysembergh A., et al. Rabbit heart can be “preconditioned” via transfer of coronary effluent. Am J Physiol. 1999;277:H2451–H2457. doi: 10.1152/ajpheart.1999.277.6.H2451. [DOI] [PubMed] [Google Scholar]
- Dickson E.W., Porcaro W.A., Fenton R.A., Heard S.O., Reindhardt C.P., Renzi F.P., et al. “Preconditioning at a distance” in the isolated rabbit heart. Acad Emerg Med. 2000;7:311–317. doi: 10.1111/j.1553-2712.2000.tb02228.x. [DOI] [PubMed] [Google Scholar]
- Dong F., Harvey J., Finan A., Weber K., Agarwal U., Penn M.S. Myocardial CXCR4 expression is required for mesenchymal stem cell mediated repair following acute myocardial infarction. Circulation. 2012;126:314–324. doi: 10.1161/CIRCULATIONAHA.111.082453. [DOI] [PubMed] [Google Scholar]
- Dorge H., Schulz R., Belosjorow S., Post H., van de Sand A., Konietzka I., et al. Coronary microembolization: the role of TNF-alpha in contractile dysfunction. J Mol Cell Cardiol. 2002;34:51–62. doi: 10.1006/jmcc.2001.1489. [DOI] [PubMed] [Google Scholar]
- Downey J.M., Cohen M.V. We think we see a pattern emerging here. Circulation. 2005;111:120–121. doi: 10.1161/01.CIR.0000153622.49496.10. [DOI] [PubMed] [Google Scholar]
- Elmadbouh I., Haider H., Jiang S., Idris N.M., Lu G., Ashraf M. Ex vivo delivered stromal cell-derived factor-1alpha promotes stem cell homing and induces angiomyogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2007;42:792–803. doi: 10.1016/j.yjmcc.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esencay M., Sarfraz Y., Zagzag D. CXCR7 is induced by hypoxia and mediates glioma cell migration towards SDF-1alpha. BMC Cancer. 2013;13:347. doi: 10.1186/1471-2407-13-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faigle M., Seessle J., Zug S., El Kasmi K.C., Eltzschig H.K. ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS One. 2008;3:e2801. doi: 10.1371/journal.pone.0002801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faxon D.P., Gibbons R.J., Chronos N.A., Gurbel P.A., Sheehan F. The effect of blockade of the CD11/CD18 integrin receptor on infarct size in patients with acute myocardial infarction treated with direct angioplasty: the results of the HALT-MI study. J Am Coll Cardiol. 2002;40:1199–1204. doi: 10.1016/s0735-1097(02)02136-8. [DOI] [PubMed] [Google Scholar]
- Frangogiannis N.G., Entman M.L. Chemokines in myocardial ischemia. Trends Cardiovasc Med. 2005;15:163–169. doi: 10.1016/j.tcm.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Freixa X., Bellera N., Ortiz-Perez J.T., Jimenez M., Pare C., Bosch X., et al. Ischaemic postconditioning revisited: lack of effects on infarct size following primary percutaneous coronary intervention. Eur Heart J. 2012;33:103–112. doi: 10.1093/eurheartj/ehr297. [DOI] [PubMed] [Google Scholar]
- Fryer R.M., Pratt P.F., Hsu A.K., Gross G.J. Differential activation of extracellular signal regulated kinase isoforms in preconditioning and opioid-induced cardioprotection. J Pharmacol Exp Ther. 2001;296:642–649. [PubMed] [Google Scholar]
- Gao H., Priebe W., Glod J., Banerjee D. Activation of signal transducers and activators of transcription 3 and focal adhesion kinase by stromal cell-derived factor 1 is required for migration of human mesenchymal stem cells in response to tumor cell-conditioned medium. Stem Cells. 2009;27:857–865. doi: 10.1002/stem.23. [DOI] [PubMed] [Google Scholar]
- Gerard C., Rollins B.J. Chemokines and disease. Nat Immunol. 2001;2:108–115. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- Ghadge S.K., Muhlstedt S., Ozcelik C., Bader M. SDF-1alpha as a therapeutic stem cell homing factor in myocardial infarction. Pharmacol Ther. 2011;129:97–108. doi: 10.1016/j.pharmthera.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Gibson C.M. NRMI and current treatment patterns for ST-elevation myocardial infarction. Am Heart J. 2004;148:S29–S33. doi: 10.1016/j.ahj.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Global Atlas on Cardiovascular Disease Prevention and Control (accessed November, 2013, at http://whqlibdoc.who.int/publications/2011/9789241564373_eng.pdf).
- Goodman M.D., Koch S.E., Fuller-Bicer G.A., Butler K.L. Regulating RISK: a role for JAK-STAT signaling in postconditioning? Am J Physiol Heart Circ Physiol. 2008;295:H1649–H1656. doi: 10.1152/ajpheart.00692.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger C.B., Mahaffey K.W., Weaver W.D., Theroux P., Hochman J.S., Filloon T.G., et al. Pexelizumab, an anti-C5 complement antibody, as adjunctive therapy to primary percutaneous coronary intervention in acute myocardial infarction: the COMplement inhibition in Myocardial infarction treated with Angioplasty (COMMA) trial. Circulation. 2003;108:1184–1190. doi: 10.1161/01.CIR.0000087447.12918.85. [DOI] [PubMed] [Google Scholar]
- Griffiths E.J., Halestrap A.P. Protection by Cyclosporin A of ischemia/reperfusion-induced damage in isolated rat hearts. J Mol Cell Cardiol. 1993;25:1461–1469. doi: 10.1006/jmcc.1993.1162. [DOI] [PubMed] [Google Scholar]
- Griffiths E.J., Halestrap A.P. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J. 1995;307(Pt 1):93–98. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin B., Cakici I., Soncul H., Kalaycioglu S., Cevik C., Sancak B., et al. Does remote organ ischaemia trigger cardiac preconditioning during coronary artery surgery? Pharmacol Res. 2000;41:493–496. doi: 10.1006/phrs.1999.0611. [DOI] [PubMed] [Google Scholar]
- Haider H., Jiang S., Idris N.M., Ashraf M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ Res. 2008;103:1300–1308. doi: 10.1161/CIRCRESAHA.108.186742. [DOI] [PubMed] [Google Scholar]
- Halkos M.E., Kerendi F., Corvera J.S., Wang N.P., Kin H., Payne C.S., et al. Myocardial protection with postconditioning is not enhanced by ischemic preconditioning. Ann Thorac Surg. 2004;78:961–969. doi: 10.1016/j.athoracsur.2004.03.033. (discussion 969) [DOI] [PubMed] [Google Scholar]
- Hausenloy D.J., Baxter G., Bell R., Botker H.E., Davidson S.M., Downey J., et al. Translating novel strategies for cardioprotection: the Hatter Workshop Recommendations. Basic Res Cardiol. 2010;105:677–686. doi: 10.1007/s00395-010-0121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausenloy D.J., Candilio L., Laing C., Kunst G., Pepper J., Kolvekar S., et al. Effect of remote ischemic preconditioning on clinical outcomes in patients undergoing coronary artery bypass graft surgery (ERICCA): rationale and study design of a multi-centre randomized double-blinded controlled clinical trial. Clin Res Cardiol. 2012;101:339–348. doi: 10.1007/s00392-011-0397-x. [DOI] [PubMed] [Google Scholar]
- Hausenloy D.J., Mocanu M.M., Yellon D.M. Activation of the pro-survival kinases (PI3 kinase-Akt and Erk 1/2) at reperfusion is essential for preconditioning-induced protection. Circulation. 2003;108(17):62-62. (Abstract) [Google Scholar]
- Hausenloy D.J., Mwamure P.K., Venugopal V., Harris J., Barnard M., Grundy E., et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet. 2007;370:575–579. doi: 10.1016/S0140-6736(07)61296-3. [DOI] [PubMed] [Google Scholar]
- Hausenloy D.J., Tsang A., Yellon D.M. The reperfusion injury salvage kinase pathway: a common target for both ischemic preconditioning and postconditioning. Trends Cardiovasc Med. 2005;15:69–75. doi: 10.1016/j.tcm.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Hausenloy D.J., Whittington H.J., Wynne A.M., Begum S.S., Theodorou L., Riksen N., et al. Dipeptidyl peptidase-4 inhibitors and GLP-1 reduce myocardial infarct size in a glucose-dependent manner. Cardiovasc Diabetol. 2013;12:154. doi: 10.1186/1475-2840-12-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausenloy D.J., Yellon D.M. New directions for protecting the heart against ischaemia–reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448–460. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Hausenloy D.J., Yellon D.M. Preconditioning and postconditioning: united at reperfusion. Pharmacol Ther. 2007;116:173–191. doi: 10.1016/j.pharmthera.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Hausenloy D.J., Yellon D.M. Reperfusion injury salvage kinase signalling: taking a RISK for cardioprotection. Heart Fail Rev. 2007;12:217–234. doi: 10.1007/s10741-007-9026-1. [DOI] [PubMed] [Google Scholar]
- Hausenloy D.J., Yellon D.M. Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res. 2008;79:377–386. doi: 10.1093/cvr/cvn114. [DOI] [PubMed] [Google Scholar]
- Hausenloy D.J., Yellon D.M. Time to take myocardial reperfusion injury seriously. N Engl J Med. 2008;359:518–520. doi: 10.1056/NEJMe0803746. [DOI] [PubMed] [Google Scholar]
- Hausenloy D.J., Yellon D.M. Myocardial ischemia–reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausenloy D.J., Yellon D.M., Mani-Babu S., Duchen M.R. Preconditioning protects by inhibiting the mitochondrial permeability transition. Am J Physiol Heart Circ Physiol. 2004;287:H841–H849. doi: 10.1152/ajpheart.00678.2003. [DOI] [PubMed] [Google Scholar]
- Hernandez P.A., Gorlin R.J., Lukens J.N., Taniuchi S., Bohinjec J., Francois F., et al. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet. 2003;34:70–74. doi: 10.1038/ng1149. [DOI] [PubMed] [Google Scholar]
- Heusch G., Boengler K., Schulz R. Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation. 2008;118:1915–1919. doi: 10.1161/CIRCULATIONAHA.108.805242. [DOI] [PubMed] [Google Scholar]
- Hoffmann F., Muller W., Schutz D., Penfold M.E., Wong Y.H., Schulz S., et al. Rapid uptake and degradation of CXCL12 depend on CXCR7 carboxyl-terminal serine/threonine residues. J Biol Chem. 2012;287:28362–28377. doi: 10.1074/jbc.M111.335679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoole S.P., Heck P.M., Sharples L., Khan S.N., Duehmke R., Densem C.G., et al. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) study: a prospective, randomized control trial. Circulation. 2009;119:820–827. doi: 10.1161/CIRCULATIONAHA.108.809723. [DOI] [PubMed] [Google Scholar]
- Hu X., Dai S., Wu W.J., Tan W., Zhu X., Mu J., et al. Stromal cell derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury: role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis. Circulation. 2007;116:654–663. doi: 10.1161/CIRCULATIONAHA.106.672451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Dong H.L., Li Y.Z., Luo Z.J., Sun L., Yang Q.Z., et al. Effects of remote ischemic preconditioning on biochemical markers and neurologic outcomes in patients undergoing elective cervical decompression surgery: a prospective randomized controlled trial. J Neurosurg Anesthesiol. 2010;22:46–52. doi: 10.1097/ANA.0b013e3181c572bd. [DOI] [PubMed] [Google Scholar]
- Huang C., Gu H., Zhang W., Manukyan M.C., Shou W., Wang M. SDF-1/CXCR4 mediates acute protection of cardiac function through myocardial STAT3 signaling following global ischemia/reperfusion injury. Am J Physiol Heart Circ Physiol. 2011;301:H1496–H1505. doi: 10.1152/ajpheart.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Y.H., Kim J.H., Ban C., Ahn K., Cheong J.H., Kim H.H., et al. Stromal cell derived factor-1 (SDF-1) targeting reperfusion reduces myocardial infarction in isolated rat hearts. Cardiovasc Ther. 2012;30:264–272. doi: 10.1111/j.1755-5922.2011.00301.x. [DOI] [PubMed] [Google Scholar]
- Javadov S.A., Clarke S., Das M., Griffiths E.J., Lim K.H., Halestrap A.P. Ischaemic preconditioning inhibits opening of mitochondrial permeability transition pores in the reperfused rat heart. J Physiol. 2003;549:513–524. doi: 10.1113/jphysiol.2003.034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q., Song P., Wang E., Li J., Hu S., Zhang H. Remote ischemic postconditioning enhances cell retention in the myocardium after intravenous administration of bone marrow mesenchymal stromal cells. J Mol Cell Cardiol. 2013;56:1–7. doi: 10.1016/j.yjmcc.2012.12.016. [DOI] [PubMed] [Google Scholar]
- Jonassen A.K., Sack M.N., Mjos O.D., Yellon D.M. Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circ Res. 2001;89:1191–1198. doi: 10.1161/hh2401.101385. [DOI] [PubMed] [Google Scholar]
- Juhaszova M., Zorov D.B., Kim S.H., Pepe S., Fu Q., Fishbein K.W., et al. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jujo K., Hamada H., Iwakura A., Thorne T., Sekiguchi H., Clarke T., et al. CXCR4 blockade augments bone marrow progenitor cell recruitment to the neovasculature and reduces mortality after myocardial infarction. Proc Natl Acad Sci U S A. 2010;107:11008–11013. doi: 10.1073/pnas.0914248107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamota T., Li T.S., Morikage N., Murakami M., Ohshima M., Kubo M., et al. Ischemic pre-conditioning enhances the mobilization and recruitment of bone marrow stem cells to protect against ischemia/reperfusion injury in the late phase. J Am Coll Cardiol. 2009;53:1814–1822. doi: 10.1016/j.jacc.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Kanki S., Segers V.F., Wu W., Kakkar R., Gannon J., Sys S.U., et al. Stromal cell-derived factor-1 retention and cardioprotection for ischemic myocardium. Circ Heart Fail. 2011;4:509–518. doi: 10.1161/CIRCHEARTFAILURE.110.960302. [DOI] [PubMed] [Google Scholar]
- Keeley E.C., Boura J.A., Grines C.L. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- Kerendi F., Kin H., Halkos M.E., Jiang R., Zatta A.J., Zhao Z.Q., et al. Remote postconditioning. Brief renal ischemia and reperfusion applied before coronary artery reperfusion reduces myocardial infarct size via endogenous activation of adenosine receptors. Basic Res Cardiol. 2005;100:404–412. doi: 10.1007/s00395-005-0539-2. [DOI] [PubMed] [Google Scholar]
- Kharbanda R.K., Mortensen U.M., White P.A., Kristiansen S.B., Schmidt M.R., Hoschtitzky J.A., et al. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106:2881–2883. doi: 10.1161/01.cir.0000043806.51912.9b. [DOI] [PubMed] [Google Scholar]
- Kim J.S., Jin Y., Lemasters J.J. Reactive oxygen species, but not Ca2+ overloading, trigger pH- and mitochondrial permeability transition-dependent death of adult rat myocytes after ischemia–reperfusion. Am J Physiol Heart Circ Physiol. 2006;290:H2024–H2034. doi: 10.1152/ajpheart.00683.2005. [DOI] [PubMed] [Google Scholar]
- Koch K.C., Schaefer W.M., Liehn E.A., Rammos C., Mueller D., Schroeder J., et al. Effect of catheter-based transendocardial delivery of stromal cell-derived factor 1alpha on left ventricular function and perfusion in a porcine model of myocardial infarction. Basic Res Cardiol. 2006;101:69–77. doi: 10.1007/s00395-005-0570-3. [DOI] [PubMed] [Google Scholar]
- Kondo K., Shintani S., Shibata R., Murakami H., Murakami R., Imaizumi M., et al. Implantation of adipose-derived regenerative cells enhances ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:61–66. doi: 10.1161/ATVBAHA.108.166496. [DOI] [PubMed] [Google Scholar]
- Kucia M., Dawn B., Hunt G., Guo Y., Wysoczynski M., Majka M., et al. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. Circ Res. 2004;95:1191–1199. doi: 10.1161/01.RES.0000150856.47324.5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucia M., Jankowski K., Reca R., Wysoczynski M., Bandura L., Allendorf D.J., et al. CXCR4–SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35:233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- Kucia M., Reca R., Miekus K., Wanzeck J., Wojakowski W., Janowska-Wieczorek A., et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1–CXCR4 axis. Stem Cells. 2005;23:879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- Kunuthur S.P., Mocanu M.M., Hemmings B.A., Hausenloy D.J., Yellon D.M. The Akt1 isoform is an essential mediator of ischaemic preconditioning. J Cell Mol Med. 2012;16:1739–1749. doi: 10.1111/j.1582-4934.2011.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacerda L., Somers S., Opie L.H., Lecour S. Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res. 2009;84:201–208. doi: 10.1093/cvr/cvp274. [DOI] [PubMed] [Google Scholar]
- Lapidot T., Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–981. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- Lecour S. Activation of the protective Survivor Activating Factor Enhancement (SAFE) pathway against reperfusion injury: does it go beyond the RISK pathway? J Mol Cell Cardiol. 2009;47:32–40. doi: 10.1016/j.yjmcc.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Lecour S., Suleman N., Deuchar G.A., Somers S., Lacerda L., Huisamen B., et al. Pharmacological preconditioning with tumor necrosis factor-alpha activates signal transducer and activator of transcription-3 at reperfusion without involving classic prosurvival kinases (Akt and extracellular signal-regulated kinase) Circulation. 2005;112:3911–3918. doi: 10.1161/CIRCULATIONAHA.105.581058. [DOI] [PubMed] [Google Scholar]
- Lemasters J.J., Bond J.M., Chacon E., Harper I.S., Kaplan S.H., Ohata H., et al. The pH paradox in ischemia–reperfusion injury to cardiac myocytes. EXS. 1996;76:99–114. doi: 10.1007/978-3-0348-8988-9_7. [DOI] [PubMed] [Google Scholar]
- Leone A.M., Rutella S., Bonanno G., Contemi A.M., de Ritis D.G., Giannico M.B., et al. Endogenous G-CSF and CD34+ cell mobilization after acute myocardial infarction. Int J Cardiol. 2006;111:202–208. doi: 10.1016/j.ijcard.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Leung C.H., Wang L., Nielsen J.M., Tropak M.B., Fu Y.Y., Kato H., et al. Remote cardioprotection by transfer of coronary effluent from ischemic preconditioned rabbit heart preserves mitochondrial integrity and function via adenosine receptor activation. Cardiovasc Drugs Ther. 2013;28(1):7–17. doi: 10.1007/s10557-013-6489-2. [DOI] [PubMed] [Google Scholar]
- Levesque J.P., Hendy J., Takamatsu Y., Simmons P.J., Bendall L.J. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111:187–196. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liles W.C., Broxmeyer H.E., Rodger E., Wood B., Hubel K., Cooper S., et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102:2728–2730. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- Lim S.Y., Yellon D.M., Hausenloy D.J. The neural and humoral pathways in remote limb ischemic preconditioning. Basic Res Cardiol. 2010;105:651–655. doi: 10.1007/s00395-010-0099-y. [DOI] [PubMed] [Google Scholar]
- Liu H., Liu S., Li Y., Wang X., Xue W., Ge G., et al. The role of SDF-1–CXCR4/CXCR7 axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PLoS One. 2012;7:e34608. doi: 10.1371/journal.pone.0034608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonborg J., Kelbaek H., Vejlstrup N., Jorgensen E., Helqvist S., Saunamaki K., et al. Cardioprotective effects of ischemic postconditioning in patients treated with primary percutaneous coronary intervention, evaluated by magnetic resonance. Circ Cardiovasc Interv. 2010;3:34–41. doi: 10.1161/CIRCINTERVENTIONS.109.905521. [DOI] [PubMed] [Google Scholar]
- Maekawa Y., Anzai T., Yoshikawa T., Sugano Y., Mahara K., Kohno T., et al. Effect of granulocyte-macrophage colony-stimulating factor inducer on left ventricular remodeling after acute myocardial infarction. J Am Coll Cardiol. 2004;44:1510–1520. doi: 10.1016/j.jacc.2004.05.083. [DOI] [PubMed] [Google Scholar]
- Matsumori A., Furukawa Y., Hashimoto T., Yoshida A., Ono K., Shioi T., et al. Plasma levels of the monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 are elevated in patients with acute myocardial infarction. J Mol Cell Cardiol. 1997;29:419–423. doi: 10.1006/jmcc.1996.0285. [DOI] [PubMed] [Google Scholar]
- Matsumoto-Ida M., Akao M., Takeda T., Kato M., Kita T. Real-time 2-photon imaging of mitochondrial function in perfused rat hearts subjected to ischemia/reperfusion. Circulation. 2006;114:1497–1503. doi: 10.1161/CIRCULATIONAHA.106.628834. [DOI] [PubMed] [Google Scholar]
- Mcclanahan T.B., Nao B.S., Wolke L.J., Martin B.J., Mertz T.E., Gallagher K.P. Brief renal occlusion and reperfusion reduces myocardial infarct size in rabbits. FASEB J. 1993;7(3):A118-A118. (Abstract) [Google Scholar]
- McGrath K.E., Koniski A.D., Maltby K.M., McGann J.K., Palis J. Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Dev Biol. 1999;213:442–456. doi: 10.1006/dbio.1999.9405. [DOI] [PubMed] [Google Scholar]
- Mehta S.R., Yusuf S., Diaz R., Zhu J., Pais P., Xavier D., et al. Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: the CREATE-ECLA randomized controlled trial. JAMA. 2005;293:437–446. doi: 10.1001/jama.293.4.437. [DOI] [PubMed] [Google Scholar]
- Mertens P., Maes A., Nuyts J., Belmans A., Desmet W., Esplugas E., et al. Recombinant P-selectin glycoprotein ligand-immunoglobulin, a P-selectin antagonist, as an adjunct to thrombolysis in acute myocardial infarction. The P-Selectin Antagonist Limiting Myonecrosis (PSALM) trial. Am Heart J. 2006;152(125):e121–e128. doi: 10.1016/j.ahj.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Misao Y., Takemura G., Arai M., Ohno T., Onogi H., Takahashi T., et al. Importance of recruitment of bone marrow-derived CXCR4+ cells in post-infarct cardiac repair mediated by G-CSF. Cardiovasc Res. 2006;71:455–465. doi: 10.1016/j.cardiores.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Misra P., Lebeche D., Ly H., Schwarzkopf M., Diaz G., Hajjar R.J., et al. Quantitation of CXCR4 expression in myocardial infarction using 99mTc-labeled SDF-1alpha. J Nucl Med. 2008;49:963–969. doi: 10.2967/jnumed.107.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocanu M.M., Bell R.M., Yellon D.M. PI3 kinase and not p42/p44 appears to be implicated in the protection conferred by ischemic preconditioning. J Mol Cell Cardiol. 2002;34:661–668. doi: 10.1006/jmcc.2002.2006. [DOI] [PubMed] [Google Scholar]
- Murata M., Akao M., O'Rourke B., Marban E. Mitochondrial ATP-sensitive potassium channels attenuate matrix Ca2+ overload during simulated ischemia and reperfusion: possible mechanism of cardioprotection. Circ Res. 2001;89:891–898. doi: 10.1161/hh2201.100205. [DOI] [PubMed] [Google Scholar]
- Murry C.E., Jennings R.B., Reimer K.A. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Nagasawa T., Hirota S., Tachibana K., Takakura N., Nishikawa S., Kitamura Y., et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- Nagasawa T., Nakajima T., Tachibana K., Iizasa H., Bleul C.C., Yoshie O., et al. Molecular cloning and characterization of a murine pre-B-cell growth-stimulating factor/stromal cell-derived factor 1 receptor, a murine homolog of the human immunodeficiency virus 1 entry coreceptor fusin. Proc Natl Acad Sci U S A. 1996;93:14726–14729. doi: 10.1073/pnas.93.25.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Shimizu S., Watanabe T., Yamaguchi O., Otsu K., Yamagata H., et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- Opie L. The glucose hypothesis: relation to acute myocardial ischemia. J Mol Cell Cardiol. 1970;1:107–115. [Google Scholar]
- Ovize M., Baxter G.F., Di Lisa F., Ferdinandy P., Garcia-Dorado D., Hausenloy D.J., et al. Postconditioning and protection from reperfusion injury: where do we stand? Position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2010;87:406–423. doi: 10.1093/cvr/cvq129. [DOI] [PubMed] [Google Scholar]
- Oxman T., Arad M., Klein R., Avazov N., Rabinowitz B. Limb ischemia preconditions the heart against reperfusion tachyarrhythmia. Am J Physiol. 1997;273:H1707–H1712. doi: 10.1152/ajpheart.1997.273.4.H1707. [DOI] [PubMed] [Google Scholar]
- Penn M.S., Mendelsohn F.O., Schaer G.L., Sherman W., Farr M., Pastore J., et al. An open-label dose escalation study to evaluate the safety of administration of nonviral stromal cell-derived factor-1 plasmid to treat symptomatic ischemic heart failure. Circ Res. 2013;112:816–825. doi: 10.1161/CIRCRESAHA.111.300440. [DOI] [PubMed] [Google Scholar]
- Penn M.S., Pastore J., Miller T., Aras R. SDF-1 in myocardial repair. Gene Ther. 2012;19:583–587. doi: 10.1038/gt.2012.32. [DOI] [PubMed] [Google Scholar]
- Petit I., Szyper-Kravitz M., Nagler A., Lahav M., Peled A., Habler L., et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- Pillarisetti K., Gupta S.K. Cloning and relative expression analysis of rat stromal cell derived factor-1 (SDF-1)1: SDF-1 alpha mRNA is selectively induced in rat model of myocardial infarction. Inflammation. 2001;25:293–300. doi: 10.1023/a:1012808525370. [DOI] [PubMed] [Google Scholar]
- Piot C., Croisille P., Staat P., Thibault H., Rioufol G., Mewton N., et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- Piper H.M., Garcia-Dorado D., Ovize M. A fresh look at reperfusion injury. Cardiovasc Res. 1998;38:291–300. doi: 10.1016/s0008-6363(98)00033-9. [DOI] [PubMed] [Google Scholar]
- Przyklenk K., Bauer B., Ovize M., Kloner R.A., Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–899. doi: 10.1161/01.cir.87.3.893. [DOI] [PubMed] [Google Scholar]
- Pyo R.T., Sui J., Dhume A., Palomeque J., Blaxall B.C., Diaz G., et al. CXCR4 modulates contractility in adult cardiac myocytes. J Mol Cell Cardiol. 2006;41:834–844. doi: 10.1016/j.yjmcc.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak M.Z., Zuba-Surma E., Kucia M., Reca R., Wojakowski W., Ratajczak J. The pleiotropic effects of the SDF-1–CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia. 2006;20:1915–1924. doi: 10.1038/sj.leu.2404357. [DOI] [PubMed] [Google Scholar]
- Ravassa S., Zudaire A., Diez J. GLP-1 and cardioprotection: from bench to bedside. Cardiovasc Res. 2012;94:316–323. doi: 10.1093/cvr/cvs123. [DOI] [PubMed] [Google Scholar]
- Rentoukas I., Giannopoulos G., Kaoukis A., Kossyvakis C., Raisakis K., Driva M., et al. Cardioprotective role of remote ischemic periconditioning in primary percutaneous coronary intervention: enhancement by opioid action. JACC Cardiovasc Interv. 2010;3:49–55. doi: 10.1016/j.jcin.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Riesenberg K., Levy R., Katz A., Galkop S., Schlaeffer F. Neutrophil superoxide release and interleukin 8 in acute myocardial infarction: distinction between complicated and uncomplicated states. Eur J Clin Invest. 1997;27:398–404. doi: 10.1046/j.1365-2362.1997.1270667.x. [DOI] [PubMed] [Google Scholar]
- Rollins B.J. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- Safety and efficacy of SITAgliptin plus Granulocyte-colony stimulating factor in patients suffering from Acute Myocardial Infarction — SITAGRAMI trial (accessed 3rd March, 2014, at http://my.americanheart.org/idc/groups/ahamah-public/@wcm/@sop/@scon/documents/downloadable/ucm_458267.pdf). [DOI] [PubMed]
- Sanchez-Martin L., Sanchez-Mateos P., Cabanas C. CXCR7 impact on CXCL12 biology and disease. Trends Mol Med. 2013;19:12–22. doi: 10.1016/j.molmed.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Fukazawa R., Ogawa S., Kanno S., Nitta T., Ochi M., et al. Stromal cell-derived factor-1alpha improves infarcted heart function through angiogenesis in mice. Pediatr Int. 2007;49:966–971. doi: 10.1111/j.1442-200X.2007.02491.x. [DOI] [PubMed] [Google Scholar]
- Saxena A., Fish J.E., White M.D., Yu S., Smyth J.W., Shaw R.M., et al. Stromal cell-derived factor-1alpha is cardioprotective after myocardial infarction. Circulation. 2008;117:2224–2231. doi: 10.1161/CIRCULATIONAHA.107.694992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M.R., Smerup M., Konstantinov I.E., Shimizu M., Li J., Cheung M., et al. Intermittent peripheral tissue ischemia during coronary ischemia reduces myocardial infarction through a KATP-dependent mechanism: first demonstration of remote ischemic perconditioning. Am J Physiol Heart Circ Physiol. 2007;292:H1883–H1890. doi: 10.1152/ajpheart.00617.2006. [DOI] [PubMed] [Google Scholar]
- Schulz R., Aker S., Belosjorow S., Heusch G. TNFalpha in ischemia/reperfusion injury and heart failure. Basic Res Cardiol. 2004;99:8–11. doi: 10.1007/s00395-003-0431-x. [DOI] [PubMed] [Google Scholar]
- Scirica B.M., Bhatt D.L., Braunwald E., Steg P.G., Davidson J., Hirshberg B., et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- Segers V.F., Tokunou T., Higgins L.J., MacGillivray C., Gannon J., Lee R.T. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116:1683–1692. doi: 10.1161/CIRCULATIONAHA.107.718718. [DOI] [PubMed] [Google Scholar]
- Segret A., Rucker-Martin C., Pavoine C., Flavigny J., Deroubaix E., Chatel M.A., et al. Structural localization and expression of CXCL12 and CXCR4 in rat heart and isolated cardiac myocytes. J Histochem Cytochem. 2007;55:141–150. doi: 10.1369/jhc.6A7050.2006. [DOI] [PubMed] [Google Scholar]
- Selker H.P., Beshansky J.R., Sheehan P.R., Massaro J.M., Griffith J.L., D'Agostino R.B., et al. Out-of-hospital administration of intravenous glucose-insulin-potassium in patients with suspected acute coronary syndromes: the IMMEDIATE randomized controlled trial. JAMA. 2012;307:1925–1933. doi: 10.1001/jama.2012.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serejo F.C., Rodrigues L.F., Jr., da Silva Tavares K.C., de Carvalho A.C., Nascimento J.H. Cardioprotective properties of humoral factors released from rat hearts subject to ischemic preconditioning. J Cardiovasc Pharmacol. 2007;49:214–220. doi: 10.1097/FJC.0b013e3180325ad9. [DOI] [PubMed] [Google Scholar]
- Shanmuganathan S., Hausenloy D.J., Duchen M.R., Yellon D.M. Mitochondrial permeability transition pore as a target for cardioprotection in the human heart. Am J Physiol Heart Circ Physiol. 2005;289:H237–H242. doi: 10.1152/ajpheart.01192.2004. [DOI] [PubMed] [Google Scholar]
- Shiba Y., Takahashi M., Hata T., Murayama H., Morimoto H., Ise H., et al. Bone marrow CXCR4 induction by cultivation enhances therapeutic angiogenesis. Cardiovasc Res. 2009;81:169–177. doi: 10.1093/cvr/cvn247. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Tropak M., Diaz R.J., Suto F., Surendra H., Kuzmin E., et al. Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clin Sci. 2009;117:191–200. doi: 10.1042/CS20080523. [DOI] [PubMed] [Google Scholar]
- Shirozu M., Nakano T., Inazawa J., Tashiro K., Tada H., Shinohara T., et al. Structure and chromosomal localization of the human stromal cell-derived factor 1 (SDF1) gene. Genomics. 1995;28:495–500. doi: 10.1006/geno.1995.1180. [DOI] [PubMed] [Google Scholar]
- Sierro F., Biben C., Martinez-Munoz L., Mellado M., Ransohoff R.M., Li M., et al. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci U S A. 2007;104:14759–14764. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkin J.C., Yellon D.M., Davidson S.M., Lim S.Y., Wynne A.M., Smith C.C. Apelin-13 and apelin-36 exhibit direct cardioprotective activity against ischemia–reperfusion injury. Basic Res Cardiol. 2007;102:518–528. doi: 10.1007/s00395-007-0671-2. [DOI] [PubMed] [Google Scholar]
- Smith C.C., Dixon R.A., Wynne A.M., Theodorou L., Ong S.G., Subrayan S., et al. Leptin-induced cardioprotection involves JAK/STAT signaling that may be linked to the mitochondrial permeability transition pore. Am J Physiol Heart Circ Physiol. 2010;299:H1265–H1270. doi: 10.1152/ajpheart.00092.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensson P., Saleh N., Bouvier F., Bohm F., Settergren M., Caidahl K., et al. Effect of postconditioning on infarct size in patients with ST elevation myocardial infarction. Heart. 2010;96:1710–1715. doi: 10.1136/hrt.2010.199430. [DOI] [PubMed] [Google Scholar]
- Staat P., Rioufol G., Piot C., Cottin Y., Cung T.T., L'Huillier I., et al. Postconditioning the human heart. Circulation. 2005;112:2143–2148. doi: 10.1161/CIRCULATIONAHA.105.558122. [DOI] [PubMed] [Google Scholar]
- Sundararaman S., Miller T.J., Pastore J.M., Kiedrowski M., Aras R., Penn M.S. Plasmid-based transient human stromal cell-derived factor-1 gene transfer improves cardiac function in chronic heart failure. Gene Ther. 2011;18:867–873. doi: 10.1038/gt.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana K., Hirota S., Iizasa H., Yoshida H., Kawabata K., Kataoka Y., et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- Takahashi M. Role of the SDF-1/CXCR4 system in myocardial infarction. Circ J. 2010;74:418–423. doi: 10.1253/circj.cj-09-1021. [DOI] [PubMed] [Google Scholar]
- Tamareille S., Mateus V., Ghaboura N., Jeanneteau J., Croue A., Henrion D., et al. RISK and SAFE signaling pathway interactions in remote limb ischemic perconditioning in combination with local ischemic postconditioning. Basic Res Cardiol. 2011;106:1329–1339. doi: 10.1007/s00395-011-0210-z. [DOI] [PubMed] [Google Scholar]
- Tang J., Wang J., Song H., Huang Y., Yang J., Kong X., et al. Adenovirus-mediated stromal cell-derived factor-1 alpha gene transfer improves cardiac structure and function after experimental myocardial infarction through angiogenic and antifibrotic actions. Mol Biol Rep. 2010;37:1957–1969. doi: 10.1007/s11033-009-9642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y.L., Zhu W., Cheng M., Chen L., Zhang J., Sun T., et al. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;104:1209–1216. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini G., Favaretto E., Marra M.P., Frigo A.C., Napodano M., Cacciavillani L., et al. Postconditioning during coronary angioplasty in acute myocardial infarction: the POST-AMI trial. Int J Cardiol. 2012;162:33–38. doi: 10.1016/j.ijcard.2012.03.136. [DOI] [PubMed] [Google Scholar]
- Theiss H.D., David R., Engelmann M.G., Barth A., Schotten K., Naebauer M., et al. Circulation of CD34+ progenitor cell populations in patients with idiopathic dilated and ischaemic cardiomyopathy (DCM and ICM) Eur Heart J. 2007;28:1258–1264. doi: 10.1093/eurheartj/ehm011. [DOI] [PubMed] [Google Scholar]
- Thibault H., Piot C., Staat P., Bontemps L., Sportouch C., Rioufol G., et al. Long-term benefit of postconditioning. Circulation. 2008;117:1037–1044. doi: 10.1161/CIRCULATIONAHA.107.729780. [DOI] [PubMed] [Google Scholar]
- Thielmann M., Kottenberg E., Boengler K., Raffelsieper C., Neuhaeuser M., Peters J., et al. Remote ischemic preconditioning reduces myocardial injury after coronary artery bypass surgery with crystalloid cardioplegic arrest. Basic Res Cardiol. 2010;105:657–664. doi: 10.1007/s00395-010-0104-5. [DOI] [PubMed] [Google Scholar]
- Thielmann M., Kottenberg E., Kleinbongard P., Wendt D., Gedik N., Pasa S., et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet. 2013;382:597–604. doi: 10.1016/S0140-6736(13)61450-6. [DOI] [PubMed] [Google Scholar]
- Tong H., Chen W., Steenbergen C., Murphy E. Ischemic preconditioning activates phosphatidylinositol-3-kinase upstream of protein kinase C. Circ Res. 2000;87:309–315. doi: 10.1161/01.res.87.4.309. [DOI] [PubMed] [Google Scholar]
- Unzek S., Zhang M., Mal N., Mills W.R., Laurita K.R., Penn M.S. SDF-1 recruits cardiac stem cell-like cells that depolarize in vivo. Cell Transplant. 2007;16:879–886. doi: 10.3727/096368907783338271. [DOI] [PubMed] [Google Scholar]
- Valenzuela-Fernandez A., Planchenault T., Baleux F., Staropoli I., Le-Barillec K., Leduc D., et al. Leukocyte elastase negatively regulates stromal cell-derived factor-1 (SDF-1)/CXCR4 binding and functions by amino-terminal processing of SDF-1 and CXCR4. J Biol Chem. 2002;277:15677–15689. doi: 10.1074/jbc.M111388200. [DOI] [PubMed] [Google Scholar]
- Venugopal V., Hausenloy D.J., Ludman A., Di Salvo C., Kolvekar S., Yap J., et al. Remote ischaemic preconditioning reduces myocardial injury in patients undergoing cardiac surgery with cold-blood cardioplegia: a randomised controlled trial. Heart. 2009;95:1567–1571. doi: 10.1136/hrt.2008.155770. [DOI] [PubMed] [Google Scholar]
- Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res. 2004;61:481–497. doi: 10.1016/j.cardiores.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Walsh S.R., Boyle J.R., Tang T.Y., Sadat U., Cooper D.G., Lapsley M., et al. Remote ischemic preconditioning for renal and cardiac protection during endovascular aneurysm repair: a randomized controlled trial. J Endovasc Ther. 2009;16:680–689. doi: 10.1583/09-2817.1. [DOI] [PubMed] [Google Scholar]
- White W.B., Cannon C.P., Heller S.R., Nissen S.E., Bergenstal R.M., Bakris G.L., et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- Whittaker P., Przyklenk K. Reduction of infarct size in vivo with ischemic preconditioning: mathematical evidence for protection via non-ischemic tissue. Basic Res Cardiol. 1994;89:6–15. doi: 10.1007/BF00788673. [DOI] [PubMed] [Google Scholar]
- Wong D., Korz W. Translating an antagonist of chemokine receptor CXCR4: from bench to bedside. Clin Cancer Res. 2008;14:7975–7980. doi: 10.1158/1078-0432.CCR-07-4846. [DOI] [PubMed] [Google Scholar]
- Yamani M.H., Ratliff N.B., Cook D.J., Tuzcu E.M., Yu Y., Hobbs R., et al. Peritransplant ischemic injury is associated with up-regulation of stromal cell-derived factor-1. J Am Coll Cardiol. 2005;46:1029–1035. doi: 10.1016/j.jacc.2005.04.059. [DOI] [PubMed] [Google Scholar]
- Yellon D.M., Baxter G.F. Reperfusion injury revisited: is there a role for growth factor signaling in limiting lethal reperfusion injury? Trends Cardiovasc Med. 1999;9:245–249. doi: 10.1016/s1050-1738(00)00029-3. [DOI] [PubMed] [Google Scholar]
- Yellon D.M., Hausenloy D.J. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- Zaruba M.M., Franz W.M. Role of the SDF-1–CXCR4 axis in stem cell-based therapies for ischemic cardiomyopathy. Expert Opin Biol Ther. 2010;10:321–335. doi: 10.1517/14712590903460286. [DOI] [PubMed] [Google Scholar]
- Zaruba M.M., Theiss H.D., Vallaster M., Mehl U., Brunner S., David R., et al. Synergy between CD26/DPP-IV inhibition and G-CSF improves cardiac function after acute myocardial infarction. Cell Stem Cell. 2009;4:313–323. doi: 10.1016/j.stem.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Zeymer U., Suryapranata H., Monassier J.P., Opolski G., Davies J., Rasmanis G., et al. The Na+/H+ exchange inhibitor eniporide as an adjunct to early reperfusion therapy for acute myocardial infarction. Results of the evaluation of the safety and cardioprotective effects of eniporide in acute myocardial infarction (ESCAMI) trial. J Am Coll Cardiol. 2001;38:1644–1650. doi: 10.1016/s0735-1097(01)01608-4. [DOI] [PubMed] [Google Scholar]
- Zhang M., Mal N., Kiedrowski M., Chacko M., Askari A.T., Popovic Z.B., et al. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007;21:3197–3207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- Zhang G., Nakamura Y., Wang X., Hu Q., Suggs L.J., Zhang J. Controlled release of stromal cell-derived factor-1 alpha in situ increases c-kit+ cell homing to the infarcted heart. Tissue Eng. 2007;13:2063–2071. doi: 10.1089/ten.2006.0013. [DOI] [PubMed] [Google Scholar]
- Zhao Z.Q., Corvera J.S., Halkos M.E., Kerendi F., Wang N.P., Guyton R.A., et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- Zhao Z.Q., Vinten-Johansen J. Postconditioning: reduction of reperfusion-induced injury. Cardiovasc Res. 2006;70:200–211. doi: 10.1016/j.cardiores.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Zhao T., Zhang D., Millard R.W., Ashraf M., Wang Y. Stem cell homing and angiomyogenesis in transplanted hearts are enhanced by combined intramyocardial SDF-1alpha delivery and endogenous cytokine signaling. Am J Physiol Heart Circ Physiol. 2009;296:H976–H986. doi: 10.1152/ajpheart.01134.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y.R., Kottmann A.H., Kuroda M., Taniuchi I., Littman D.R. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- Zweier J.L. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. J Biol Chem. 1988;263:1353–1357. [PubMed] [Google Scholar]