Abstract
Neurocognitive (NC) impairment remains prevalent among people living with HIV (PLWH) and may be exacerbated by alcohol and drug use. This cross-sectional study assesses the degree to which alcohol and other drug use, time from HIV diagnosis to treatment, and years living with HIV affect three areas of NC functioning among HIV-seropositive adults. NC functioning in 370 PLWH living in Miami, FL was assessed using the Auditory Verbal Learning Test, the Short Category Test, Booklet Format, and the Color Trails Test 2 (CTT2). Participants reported the number of days using alcohol, marijuana, and cocaine over the previous 3 months, the number of known years living with HIV and length of time from HIV diagnosis to seeking care. Bivariate linear regression and multivariate linear regression were used to test associations between independent and dependent variables. Mean scores on NC measures were significantly lower than published norms; 39% of participants scored ≥1 standard deviation below normative sample means on >2 NC tests. No significant associations were found between alcohol or cocaine use and any NC measure. Years living with HIV was associated with CTT2 in the bivariate analysis (β = 1.031; p = 0.007). In multivariate analysis, each day of marijuana use and years living with HIV were associated with a 0.32 (p = 0.05) point and 1.18 (p = 0.03) points poorer performance score on the CTT2, respectively. Results suggest that both marijuana use and duration of HIV infection may affect cognitive functioning among PLWH in ways that may impair their ability to follow important treatment guidance.
Keywords: HIV, neurocognitive, alcohol, marijuana, Color Trails Test
Introduction
Since the introduction of highly active antiretroviral treatment (ART), the incidence of HIV-associated dementia has declined from 10 to 15% to rates of 2–5% in people living with HIV (PLWH) (1–3). However, milder forms of HIV-associated neurocognitive disorders (HAND) continue to be reported in up to 40% of this population, in part because HIV-positive individuals are living longer with the disease (4–8).
HAND, ranging from mild impairment to profoundly disabling HIV-associated dementia (7), is mostly seen in advanced stages of HIV disease but can occur even in PLWH who have medically asymptomatic HIV infection (9–11). While increased time living with the virus, particularly during the acute phase of HIV infection, is associated with neurocognitive (NC) impairment, HAND has been observed even among PLWH who have consistently maintained low-to-undetectable viral loads on ART (12, 13). Early initiation of ART is known to reduce the risk of developing HAND (14, 15). At the time of this study, treatment guidelines recommended that all HIV-infected adults with a CD4 count of <350 be prescribed ART.
HAND can express clinically as impairment in episodic memory, information processing, attention, and executive functions (16). In patients presenting with even milder forms of HAND, quality of life can be greatly affected, with individuals suffering from difficulties in ability to perform activities of daily living, personal health care management, medication management, and risk behavior reduction (6, 17, 18). Mild or asymptomatic NC impairment is known to be more prevalent than the more debilitating HAND diagnoses (19, 20); however, even when NC impairment is asymptomatic, HAND may still be associated with functional problems such as poorer employment capacity and lower symptom reporting related to lower self-awareness (1, 19, 21).
Abuse of alcohol and other drugs frequently co-occurs with HIV and may further impair NC functioning (22, 23). HIV infection and heavy drug or alcohol use have been observed to have synergistically negative effects on NC functioning and the progression of HAND (24–26). Impaired verbal and auditory working memory, and enhanced cognitive impulsivity have been observed (27, 28). These cognitive functions are associated with the higher-order thinking required to conduct safer sex practices and health management behaviors such as ART adherence (17, 24, 29, 30).
It is important to understand specific domains of impairment associated with exposure to HIV and the use of commonly abused substances such as alcohol, marijuana, and cocaine in order to deliver targeted and effective clinical care, as well as HIV risk reduction and treatment adherence interventions. Using structural equation modeling (SEM), this study sought to identify direct relationships between exposure to HIV and degree of alcohol and drug use upon several major domains of NC functioning including memory, information processing, attention, and executive function. It was expected that longer time living with HIV, longer time from HIV diagnosis to seeking care, and greater use of substances would reduce performance on several NC measures. Outcomes may help identify areas of cognitive functioning affected by substance abuse and HIV in order to improve ways in which health care information is delivered by clinicians and retained by patients.
Materials and Methods
Study design
This study employed a cross-sectional design, utilizing baseline data gathered between 2009 and 2012 as part of a prospective randomized controlled trial for HIV-positive adult alcohol users. Participants (N = 370) were recruited from 13 community-based organizations (CBOs) in densely populated, multicultural, low-income, urban areas of South Florida, primarily Miami-Dade County, with high rates of substance abuse, HIV, violence, and poverty. The CBOs were among the largest in the local area providing substance abuse treatment (inpatient or outpatient) and mental health services to HIV-positive men and women. Recruitment settings were selected from a wide range of non-academic institutions, specialty and primary care, public and private facilities; however, 23.5% of participants were in residential substance abuse treatment at the time of data collection. The inclusion criteria were: being 18 to 60-years-old; being HIV-positive; having consumed any alcohol in the past 3 months; having a history of alcohol abuse or dependence within the past 2 years; facility in English; and currently not showing overt signs of any major psychiatric disorder. Participants in the parent study were randomized to a group level, 8-week intervention to reduce substance abuse, and sex risk among PLWH or to a similarly administered health comparison group providing standard of care content. The research protocol was approved by the Institutional Review Board of Florida International University and all participants provided signed informed consent prior to participating in the study.
Assessment methods consisted of: (1) computer-assisted personal interview (CAPI); (2) audio computer-assisted self-interview for subjective sensitive topics (ACASI); (3) paper and pen as specified for neurological measures; and (4) Timeline Follow-Back (TLFB).
Measures
“Years living with HIV” was measured by asking the year, they received their first HIV-positive test. This value was subtracted from the year of intake to calculate number of years living with HIV.
Time from HIV diagnosis to seeking medical care was an ordinal item from the Community Programs for Clinical Research on AIDS [CPCRA; (31)] asking “How soon after your positive test for HIV did you first go for medical care for your HIV?” Response options were: (1) within 6 weeks; (2) 6–12 weeks; (3) 3–6 months; (4) 6–12 months; (5) more than a year; and (6) I have not gone for medical care for my HIV. This final response option did not provide an interval time measurement. Two participants selecting option (6) had been diagnosed at least 1 year before interview and were classified as option (5) and one participant had been diagnosed recently so this response was coded as “missing.” It should be noted that this question did not ask when treatment was initiated, only when it was sought.
Alcohol, marijuana, and cocaine use were assessed by TLFB to provide a continuous 3 months history for intensity of drug and alcohol consumption. These variables were measured: total number of “heavy drinking days” (defined as ≥5 drinks), total number of marijuana use days, and total number of cocaine use days. Up to 3 months recall of alcohol and other drug use has proven to yield reliable data (32). TLFB has strong agreement with other measures of substance use and has reliability measures ranging from 0.75 to 0.90 (33).
Three neuropsychological tests were administered to derive scores in various cognitive domains, selected for importance to behaviors associated with maintaining health and reducing risk of transmission among PLWH. For example, information processing and memory are related to comprehending, retaining, and applying instructions such as medical advice and medication dosing (34). The tests selected have been used in previous studies with HIV-positive users of alcohol, marijuana, and cocaine and have well-developed norms, high reliability, and high validity (24, 35–39).
Auditory Verbal Learning Test, University of California Los Angeles/World Health Organization Version
Fifteen words were read to the participant, requesting both immediate and longer-term recall (34, 38). Scores for immediate recall, delayed recall, and percent of words retained can be derived. This study utilized the total of immediate recall scores for trials 1–5, with higher scores indicating greater functioning. This instrument demonstrated high test-retest reliability, with alpha scores ranging from 0.51 to 0.72 (34).
The Color Trails Test 2, Form A (CTT2)
CTT-2 shows two sets of 1–25 numbered circles, and using a pen, the participant must quickly connect the numbers in order, alternating between pink and yellow (34, 40, 41). This analysis used the raw time in seconds the participant required to complete the test, with higher scores indicating poorer functioning. The test is sensitive to a variety of neurological impairments and processes (42) and, because it is entirely numeric, it is considered culturally unbiased (36). Additionally, the instrument has shown strong agreement with other cognitive assessments among PLWH (36, 43) and has displayed good temporal stability with test-retest reliability between 0.85 and 1.00 (41).
The Short Category Test booklet format
This assesses problem-solving ability, requiring the examinee to determine the organizing principle behind a series of visually presented stimuli, based on external feedback from the test administrator (44, 45). This study uses the total raw error score derived, with higher scores indicating poorer functioning. Test-retest coefficients have varied from 0.60 to 0.96 depending upon the severity of impairment in the sample (44).
Analytic strategy
A hypothesized directional model was constructed to assess the degree to which independent relationships exist between HIV exposure, alcohol use, marijuana use, and cocaine use on the outcome of NC functioning as indicated by three different cognitive performance measures.
Means, standard deviations, skewness, and kurtosis levels were calculated for all continuous variables; frequencies were generated for dichotomous and categorical variables. In order to gain an understanding of the overall degree of NC impairment in this sample, one-sample t-tests were used to compare mean raw NC scores with normative scores on each instrument. Raw scores were square root transformed, with confidence intervals representing the difference between the raw, transformed scores, and square roots of the normative scores. Norms for NC measures were obtained from professional manuals provided for the instruments (41, 46, 47). In addition, using chi-square analysis, participants were dichotomized – those who scored >1 standard deviation below the means of normative samples on at least two NC assessments versus those with higher NC performance – and cross tabulated on whether they reported taking prescribed ART. Raw NC scores were used in bivariate and multivariate regression analyses; age, gender, and educational level were entered as covariates to control for their possible impact upon NC scores. Bivariate regression analyses assessed relationships between each independent and dependent variable using SPSS version 21. Maximum Likelihood framework was invoked in MPlus version 5.1 (48) to accommodate non-normality in the multivariate, SEM analysis (49). Approximately 8% of data was missing randomly across variables. With no systematic patterns observed in the missing data, a full information maximum likelihood method was used to accommodate the missing data in the SEM analysis. To assess non-model-based outliers, leverage indices were examined for each participant based on their multivariate profile for the variables included in the model analyses. No observations had a leverage score four times greater than the mean leverage (0.09) in the sample data. After addressing all diagnostic statistics, the final hypothesized model was analyzed using Mplus; strengths of association were reported.
Results
The mean age of participants was 44.79 years; most (63.5%) were men and identified as black (76.2%). Most (55.7%) of participants reported at least high school completion; few (8.2%) were employed. The mean number of years living with HIV was 12.2. While most (58.4%) sought medical care within 6 weeks of receiving a positive HIV test, 24.6% of participants waited over a year before seeking care (Table 1). Although not included in the tested model, the majority (76.7%) reported that they were currently taking ART; 80% reported perfect (100%) treatment adherence; and 45% had an undetectable viral load when last tested. Use of marijuana in this cohort was substantially lower than use of alcohol and cocaine; however, more than half of all participants reported some marijuana use.
Table 1.
Sociodemographic characteristics of participants at baseline (N = 370).
| Mean (SD) N (%) | Skewness/Kurtosis | |
|---|---|---|
| Age | 44.79 (7.3) | −0.56/−0.13 |
| Gender (male) | 233 (63.5) | −0.56/−0.69 |
| Hispanic ethnicity (yes) | 53 (15) | 1.97/1.90 |
| Race | ||
| Black | 266 (76.2) | −1.24/−0.47 |
| White | 50 (14.3) | 2.05/2.20 |
| Years education | 0.41/−0.17 | |
| <HS diploma | 157 (44.3) | – |
| ≥HS diploma | 198 (55.7) | – |
| Employed (Yes) | 29 (8.2) | 3.07/7.45 |
| Years since HIV diagnosis | 12.15 (7.5) | 0.20/−1.01 |
| Time from HIV diagnosis to treatment | 0.65/−1.41 | |
| Within 6 weeks | 206 (58.4) | – |
| 6–12 weeks | 13 (3.7) | – |
| 3–6 months | 22 (6.2) | – |
| 6 months–1 year | 25 (7.1) | – |
| 1 year+ | 87 (24.6) | – |
| Taking ART (yes) | 273 (76.7) | −1.27/−0.40 |
| Currently in residential substance treatment | 84 (23.5) | 0.66/−1.27 |
| Days using in last 3 months | ||
| Alcohol | 15.77 (23.33) | 1.86/2.83 |
| Marijuana | 9.29 (20.68) | 2.84/7.59 |
| Cocaine | 15.68 (23.89) | 1.89/2.80 |
| Neurocognitive functioning | ||
| CTT2 | 116.31 (49.82)a | 1.71/4.97 |
| SCT | 38.09 (15.58)a | −0.38/−0.86 |
| AVLT | 43.44 (9.63)a | −0.03/−0.22 |
aRaw scores reported. All sample means are significantly different from normative sample at p < 0.01.
Normative scores used for comparison testing represented mean age and education for this sample. Skewness and Kurtosis values for neurocognitive instruments reflect distribution of scores prior to square root transformation.
Single sample t-tests comparing the mean raw NC scores with normative scores on each instrument showed that performance for the study group was significantly poorer (all p < 0.001) than observed in healthy samples as follows: auditory verbal learning test (AVLT), t = −16.25 [95% CI = −0.67, −0.52]; CTT2, t = 6.65 [95% CI = 0.53, 0.97]; and short category test booklet format (SCT), t = 11.52 [95% CI = 0.66, 0.93]. Over a third (39%) of the study population scored at least one standard deviation below the mean for normative samples on at least two NC tests, consistent with clinically significant NC impairment (7). No association was observed between the higher and lower performing groups based upon whether they reported being on prescribed ART (χ2 = 0.021, p = 0.88).
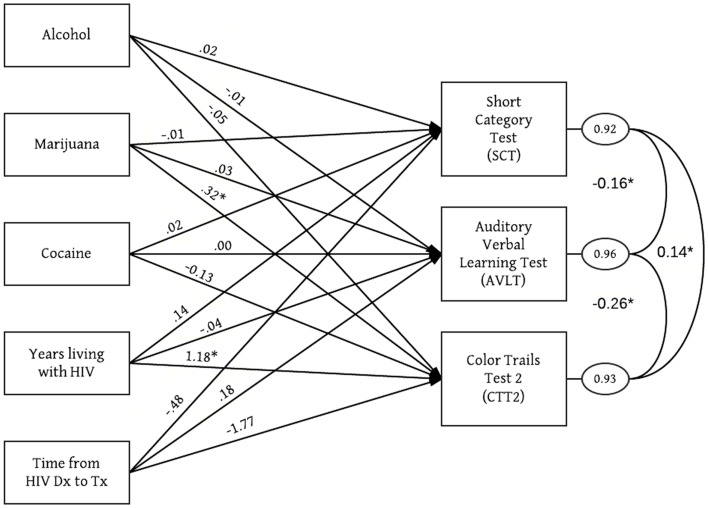
Bivariate analyses (Table 2) identified one significant relationship: years living with HIV was associated with poorer scores on the CTT2 (β = 1.031; 95% CI: 0.285, 1.777; p = 0.007). After examining normality, outliers and patterns of missing data, the final multivariate model was tested. Figure 1 and Table 3 present significant and non-significant unstandardized path coefficients of the final model. This was a just-identified model (χ2 = 0, 0 df; CFI = 1; RMSEA = 0; SRMR = 0). All exogenous variables were assumed to be correlated. Unstandardized path coefficients, standard errors and 95% confidence intervals are listed in Table 3. Residual error terms across three measures of NC functioning were correlated. Focused fit indices did not show evidence of Heywood cases. Standardized residual values were <2. The final model explained 8% of variance in memory, 7% of variance in information processing, and 4% of variance in executive functions. In the final model, two associations approached or achieved statistical significance. On average, for each day of marijuana use, the CTT2 score increased by 0.32 (p = 0.051), indicating poorer performance on this NC measure. Similarly, on average, each year living with HIV increased the CTT2 score by 1.18 (p = 0.03).
Table 2.
Bivariate regression statistics, 95% confidence intervals, and p-values (N = 370).
| β | 95% CI | P | ||
|---|---|---|---|---|
| Alcohol daysa | AVLTf | − 0.005 | −0.049, 0.039 | 0.82 |
| CTT2g | − 0.137 | −0.386, 0.111 | 0.28 | |
| SCTh | − 0.017 | −0.089, 0.056 | 0.65 | |
| Marijuana daysb | AVLT | 0.009 | −0.044, 0.063 | 0.73 |
| CTT2 | 0.275 | −0.024, 0.573 | 0.07 | |
| SCT | − 0.049 | −0.137, 0.038 | 0.27 | |
| Cocaine daysc | AVLT | 0.011 | −0.032, 0.053 | 0.63 |
| CTT2 | − 0.172 | −0.413, 0.069 | 0.16 | |
| SCT | 0.027 | −0.033, 0.097 | 0.46 | |
| Years of HIV infectiond | AVLT | − 0.075 | −0.208, 0.059 | 0.27 |
| CTT2 | 1.031 | 0.285, 1.777 | 0.007 | |
| SCT | 0.096 | −0.124, 0.316 | 0.39 | |
| Time from HIV diagnosis to seeking medical caree | AVLT | − 0.045 | −0.570, 0.480 | 0.87 |
| CTT2 | 0.384 | −2.509, 3.277 | 0.79 | |
| SCT | − 0.333 | −1.192, 0.526 | 0.447 |
aNumber of days using alcohol in past 3 months.
bNumber of days using marijuana in past 3 months.
cNumber of days using cocaine in past 3 months.
dDuration of known HIV infection in years.
eTime interval from diagnosis of HIV infection to seeking medical care.
fAuditory Verbal Learning Test.
gColor Trails Test 2.
hShort Category Test.
Figure 1.
Significant regression paths among measured variables in the structural equation model among HIV-positive alcohol abusers (N = 370). Model included age, gender and education level as covariates. Regression coefficients (represented as one-way arrows) are unstandardized. *p < 0.05.
Table 3.
Multivariate, unstandardized path coefficients, standard errors, 95% confidence intervals, and p-values (N = 370).
| Coefficient | SE | 95% CI | P | ||
|---|---|---|---|---|---|
| Alcohol daysa | AVLTf | −0.01 | 0.03 | −0.07, 0.51 | 0.79 |
| CTT2g | −0.05 | 0.16 | −0.36, 0.26 | 0.75 | |
| SCTh | 0.02 | 0.04 | −0.06, 0.11 | 0.59 | |
| Marijuana daysb | AVLT | 0.03 | 0.04 | −0.05, 0.11 | 0.45 |
| CTT2 | 0.32** | 0.17 | −0.001, 0.65 | 0.05 | |
| SCT | −0.01 | 0.06 | −0.13, 0.10 | 0.82 | |
| Cocaine daysc | AVLT | 0.00 | 0.03 | −0.05, 0.06 | 0.94 |
| CTT2 | −0.13 | 0.14 | −0.40, 0.15 | 0.37 | |
| SCT | 0.02 | 0.05 | −0.07, 0.11 | 0.65 | |
| Years of HIV infectiond | AVLT | −0.04 | 0.10 | −0.23, 0.15 | 0.66 |
| CTT2 | 1.18* | 0.54 | 0.12, 2.25 | 0.03 | |
| SCT | 0.14 | 0.15 | −0.17, 0.44 | 0.37 | |
| Time from HIV diagnosis to seeking medical caree | AVLT | 0.18 | 0.38 | −0.56, 0.92 | 0.63 |
| CTT2 | −1.77 | 2.33 | −6.34, 2.79 | 0.45 | |
| SCT | −0.48 | 0.61 | −1.68, 0.44 | 0.43 |
Model included age, gender and education level as covariates.
aNumber of days using alcohol in past 3 months.
bNumber of days using marijuana in past 3 months.
cNumber of days using cocaine in past 3 months
dDuration of known HIV infection in years.
eTime interval from diagnosis of HIV infection to seeking medical treatment.
fAuditory Verbal Learning Test.
gColor Trails Test 2.
hShort Category Test.
*Significant at p < 0.05.
**Approaching significance.
Discussion
Neurocognitive impairment was common (39%) in this group; however, other U.S. studies of HIV-seropositive adults using similar NC measures have yielded different results. A U.S. study of adult PLWH found their participants scored better on CTT2 () than participants in this study (50). Another study of HIV-seropositive African-American men also reported significantly better CTT2 scores with mean score of 93.09 for their HIV+ symptomatic group (51). These higher functioning levels could be explained by their samples’ lower mean age and AOD use characteristics.
While not all hypothesized associations were supported in this model, two variables – marijuana use and duration of HIV infection – were associated with poorer performance on one NC measure. Albeit marginal, higher number of days of marijuana use showed some relation with poorer performance on the CTT2, an instrument that measures frontal systems functioning such as attention, executive functions, and information processing (41, 52). Although significant differences in CTT2 scores have been identified between HIV-positive intravenous drug users and non-users (53), previous research on the NC effects of marijuana use has yielded mixed results. Most studies examining marijuana-associated NC functioning found long-term use to impede performance in domains of executive functions, verbal memory, and psychomotor speed (38, 54, 55). While it is believed that chronic illicit drug use can exacerbate NC sequelae of HIV infection (25, 56, 57), little actual exploration of long-term cognitive impact of marijuana use has been conducted among PLWH. One study reported a significant association between marijuana use and poorer NC functioning among PLWH with more advanced disease, with most profound effects in the area of memory performance (58).
Methodological issues associated with NC assessment and effects of marijuana use should be considered when interpreting these and similar study outcomes (54). In this study, a 3-month drug use history was collected with no control for any recent period of drug abstinence; thus, it is possible that NC performance measures may have also captured intoxication effects of marijuana. Additionally, only associations with performance on the CTT2 measure were significant; however, this instrument may measure several domains of functioning, including psychomotor speed attention (41). Without the corroboration of instruments measuring similar NC domains, the exact areas of neuropsychological impact associated with marijuana use cannot be determined with certainty.
An additional finding of this research was that the number of years living with HIV predicted poorer performance on the CTT2 instrument; however, no significant association was found between NC functioning and the length of time from HIV diagnosis to seeking care. Although ART has significantly reduced incidence of severe NC disorders among PLWH, milder HAND remains common even among virally suppressed patients (59). In this sample, it should be noted that participants were heterogeneous, in varying stages of ART treatment and exhibiting different degrees of viral suppression. There are several possible explanations for poorer NC functioning observed among those living longer with HIV, but not necessarily associated with delayed ART initiation. An inflammatory response can occur when immune functioning rebounds after treatment with ART, known as immune reconstitution inflammatory syndrome (IRIS). However, regardless of possible IRIS, greater immunosuppression remains a strong predictor of NC impairment among PLWH (1, 60); thus, early initiation of ART is reported to be one of the most important preventive measures against developing HAND (13, 61) and continuous adherence remains a protective factor against NC impairment (17). While some participants in this study may have initiated treatment early, only about 77% of participants in this group report currently taking ART. It is also possible that the association found between years living with HIV and poorer CTT2 performance may be a function of age-related cognitive decline (62); although the analyses controlled for age.
Certain limitations of this study are important to note. First, the tested model was just identified and future studies may seek to assess models where non-significant paths are trimmed as one avenue for replicating these study findings. Also, reporting bias should be considered in interpreting outcomes. Nearly a quarter of the study population was in abstinence-based, residential addiction treatment at the time of this study, and it is possible that recent substance use was underreported. Residential treatment status was not a covariate in this model primarily because all participants had a history of substance use and frequently utilized substance abuse services – both inpatient and outpatient. While residential treatment status could have been responsible for underreporting of recent substance use, including this variable in the model could have created excessive noise. Moreover, underreporting may have varied by substance; marijuana use is likely to be less stigmatized than cocaine use (63) and possibly less than alcohol use. Measurement issues also should be considered. HIV or drug/alcohol use can affect other cognitive functions not evaluated here, such as prospective memory and cognitive impulsivity (16, 64, 65). The NC instruments used, however, are commonly utilized in similar research and considered to be reliable. Of greater concern is that poor mean NC scores for this group may also suggest poorer recall on self-report measures. In addition, the CTT2 may be non-specific, evaluating several domains of functioning. Finally, the measurement of time from HIV diagnosis to seeking care did not provide information on actual time to ART initiation. For the purpose of this study, the measure was used as a proxy for ART initiation – i.e., one who sought care sooner might also be treated with ART sooner.
Counter to reports that cocaine and alcohol use exacerbate NC impairment among PLWH (24, 26, 66, 67), this particular study did not find such associations. Because abuse of alcohol and illicit substances is inextricably linked to HIV (22), further investigation into interaction effects between long-term drug/alcohol use and HIV is recommended. Further, future research should consider the contributions of substance use to overall NC functioning for the delivery of clinical care and health behavior interventions. Early HIV care can help decrease potential NC impairment and yield better health outcomes for HIV-infected populations; however, people suffering from substance use disorders or cognitive impairment may find it difficult to follow important medical recommendations (17, 24, 29, 68–70). Outcomes from this study provide further evidence of the prevalence of NC impairment that can help to guide and enhance interventions for PLWH who use alcohol and drugs.
Conclusion
Associations found with the CTT2 in particular points to the utility of this instrument for HAND assessment. In order to deliver effective health care to PLWH, especially those who use substances, it is important to understand the areas of functioning most affected by HAND and potentially incorporate into care remediation strategies that will help improve patient engagement. The delivery of health interventions and clinical care should consider taking a harm-reduction approach: working with the patient’s current substance use status to improve HIV treatment adherence and service utilization and delivering interventions and services that are sensitive to cognitive difficulties (71–73). Finally, it may be advised to consider including information about HAND into HIV prevention efforts, particularly to encourage testing and early treatment. It is unclear to date how knowledgeable at risk populations are about NC effects of HIV; however, growing evidence shows that NC impairment is lower among PLWH who are treated early (74).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Funding for this study was provided by NIAAA Grant 1 R01 AA017405. NIAAA had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
References
- 1.Heaton R, Clifford D, Franklin D, Woods S, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology (2010) 75(23):2087–96 10.1212/WNL.0b013e318200d727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS (2007) 21(14):1915–21 10.1097/QAD.0b013e32828e4e27 [DOI] [PubMed] [Google Scholar]
- 3.Sacktor N, McDermott M, Marder K, Schifitto G, Selnes O, McArthur J, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol (2002) 8(2):136–42 10.1080/13550280290049615 [DOI] [PubMed] [Google Scholar]
- 4.Sacktor N, Skolasky R, Selnes OA, Watters M, Poff P, Shiramizu B, et al. Neuropsychological test profile differences between young and old human immunodeficiency virus-positive individuals. J Neurovirol (2007) 13(3):203–9 10.1080/13550280701258423 [DOI] [PubMed] [Google Scholar]
- 5.Sacktor N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. J Neurovirol (2002) 8(Suppl 2):115–21 10.1080/13550280701258423 [DOI] [PubMed] [Google Scholar]
- 6.McArthur JC. HIV dementia: an evolving disease. J Neuroimmunol (2004) 157(1–2):3–10 10.1016/j.jneuroim.2004.08.042 [DOI] [PubMed] [Google Scholar]
- 7.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology (2007) 69(18):1789–99 10.1212/01.WNL.0000287431.88658.8b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durvasula R, Norman L, Malow R. Current perspectives on the neuropsychology of HIV. In: Pope C, White R, Malow R, editors. HIV/AIDS: Global Frontiers in Prevention/Intervention. New York: Routledge; (2009). p. 177–90 [Google Scholar]
- 9.Heaton R, Grant I, Butters N, White D, Kirkson D, Atkinson J, et al. The HNRC 500-neuropsychology of HIV infection at different disease stages. J Int Neuropsychol Soc (1995) 1(3):231–51 10.1017/S1355617700000230 [DOI] [PubMed] [Google Scholar]
- 10.White D, Heaton R, Monsch A. Neuropsychological studies of asymptomatic Human immunodeficiency virus-type-1 infected individuals. J Int Neuropsychol Soc (1995) 1(3):304–15 10.1017/S1355617700000308 [DOI] [PubMed] [Google Scholar]
- 11.Grant I, Atkinson J, Hesselink J, Kennedy C, Richman D, Spector S, et al. Evidence for early central nervous system involvement in the acquired immunodeficiency syndrome (AIDS) and other human immunodeficiency virus (HIV) infections. Ann Intern Med (1987) 107:828–36 10.7326/0003-4819-107-6-828 [DOI] [PubMed] [Google Scholar]
- 12.Simioni S, Cavassini M, Annoni J-M, Rimbault Abraham A, Bourquin I, Schiffer V, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS (2010) 24(9):1243–50 10.1097/QAD.0b013e3283354a7b [DOI] [PubMed] [Google Scholar]
- 13.Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-comandini U, et al. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients prevalence and risk factors. J Acquir Immune Defic Syndr (2007) 45(2):174–82 10.1097/QAI.0b013e318042e1ee [DOI] [PubMed] [Google Scholar]
- 14.Cross H, Combrinck M, Joska J. HIV-associated neurocognitive disorders: antiretroviral regimen, central nervous system penetration effectiveness, and cognitive outcomes. S Afr Med J (2013) 103(10):758–62 10.7196/SAMJ.6677 [DOI] [PubMed] [Google Scholar]
- 15.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol (2011) 17(1):3–16 10.1007/s13365-010-0006-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev (2009) 19(2):152–68 10.1007/s13365-010-0006-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinkin C, Castellon S, Durvasula R. Medication adherence among HIV+ adults: effects of cognitive dysfunction and regimen complexity. Neurology (2002) 59(12):1944–50 10.1212/01.WNL.0000038347.48137.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malow R, Devieux J, Stein J, Rosenberg R, Lerner B, Attonito J, et al. Neurological function, information-motivation-behavioral skills factors, and risk behaviors among HIV-positive alcohol users. AIDS Behav (2012) 16(8):2297–308 10.1007/s10461-012-0246-6 [DOI] [PubMed] [Google Scholar]
- 19.Blackstone K, Moore DJ, Heaton RK, Franklin DR, Woods SP, Clifford DB, et al. Diagnosing symptomatic HIV-associated neurocognitive disorders: self-report versus performance-based assessment of everyday functioning. J Int Neuropsychol Soc (2012) 18(1):79–88 10.1017/S135561771100141X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan LG, Kandiah N, Chua A. HIV-associated neurocognitive disorders (HAND) in a South Asian population – contextual application of the 2007 criteria. BMJ Open (2012) 2(1):e000662. 10.1136/bmjopen-2011-000662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiao S, Rosen HJ, Nicolas K, Wendelken LA, Alcantar O, Rankin KP, et al. Deficits in self-awareness impact the diagnosis of asymptomatic neurocognitive impairment in HIV. AIDS Res Hum Retroviruses (2013) 29(6):949–56 10.1089/aid.2012.0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volkow ND. Drug Abuse and HIV. Washington, DC: National Institute of Drug Abuse; (2012). Available from: http://www.drugabuse.gov/sites/default/files/rrhiv.pdf [Google Scholar]
- 23.Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-41, HHS Publication No. (SMA) 11-4658. Rockville, MD: (2011). [Google Scholar]
- 24.Rothlind JC, Greenfield TM, Bruce AV, Dieter J, Flenniken DL, Lindgren JA, et al. Heavy alcohol consumption in individuals with HIV infection: effects on neuropsychological performance. J Int Neuropsychol Soc (2005) 11(1):70–83 10.1017/S1355617705050095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anthony I, Arango J, Stephens B, Simmonds P, Bell J. The effects of illicit drugs on the HIV-infected brain. Front Biosci (2008) 13:1294–307 10.2741/2762 [DOI] [PubMed] [Google Scholar]
- 26.Nath A, Maragos W, Avison M, Schmitt F, Berger J. Acceleration of HIV dementia with methamphetamine and cocaine. J Neurovirol (2001) 7(1):66–71 10.1080/135502801300069737 [DOI] [PubMed] [Google Scholar]
- 27.Martin EM, Pitrak DL, Weddington W, Rains NA, Nunnally G, Nixon H, et al. Cognitive impulsivity and HIV serostatus in substance dependent males. J Int Neuropsychol Soc (2004) 10(7):931–8 10.1017/S1355617704107054 [DOI] [PubMed] [Google Scholar]
- 28.Farinpour R, Martin EM, Seidenberg M, Pitrak DL, Pursell KJ, Mullane KM, et al. Verbal working memory in HIV-seropositive drug users. J Int Neuropsychol Soc (2000) 6(5):548–55 10.1017/S1355617700655042 [DOI] [PubMed] [Google Scholar]
- 29.Bryant KJ. Expanding research on the role of alcohol consumption and related risks in the prevention and treatment of HIV/AIDS. Subst Use Misuse (2006) 41(10–12):1465–507 10.1080/10826080600846250 [DOI] [PubMed] [Google Scholar]
- 30.Meade CS, Conn NA, Skalski LM, Safren SA. Neurocognitive impairment and medication adherence in HIV patients with and without cocaine dependence. J Behav Med (2011) 34(2):128–38 10.1007/s10865-010-9293-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mannheimer S, Matts J, Telzak E, Chesney M. Psychological and socio- medical aspects of AIDS/HIV quality of life in HIV-infected individuals receiving antiretroviral therapy is related to adherence. AIDS Care (2010) 17(1):10–22 10.1080/09540120412331305098 [DOI] [PubMed] [Google Scholar]
- 32.Schroder K, Carey M, Vanable P. Methodological challenges in research on sexual risk behavior: II. Accuracy of self-reports. Ann Behav Med (2003) 26(2):104–23 10.1207/S15324796ABM2602_03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline follow back reports of psychoactive substance use by drug-abusing patients: psychometric properties. J Consult Clin Psychol (2000) 68(1):134–44 10.1037/0022-006X.68.1.134 [DOI] [PubMed] [Google Scholar]
- 34.Lezak M. Neuropsychological Assessment. 4th ed New York: Oxford University Press; (2004). [Google Scholar]
- 35.Durvasula R, Miller E, Myers H, Wyatt G. Predictors of neuropsychological performance in HIV positive women. J Clin Exp Neuropsychol (2001) 23(2):149–64 10.1076/jcen.23.2.149.1211 [DOI] [PubMed] [Google Scholar]
- 36.Maj M, Satz P, Janssen R, Zaudig M, Starace F, Schulte G, et al. WHO neuropsychiatric AIDS study, cross-sectional phase II. Arch Gen Psychiatry (1994) 51:51–61 10.1001/archpsyc.1994.03950010051007 [DOI] [PubMed] [Google Scholar]
- 37.Mason KI, Campbell A, Hawkins P, Madhere S, Johnson K, Takushi-Chinen R. Neuropsychological functioning in HIV-positive African-American women with a history of drug use. J Natl Med Assoc (1998) 90(11):665–74 [PMC free article] [PubMed] [Google Scholar]
- 38.Messinis L, Kyprianidou A, Malefaki S, Papathanasopoulos P. Neuropsychological deficits in long- term frequent cannabis users. Neurology (2006) 66:737–9 10.1212/01.wnl.0000201279.83203.c6 [DOI] [PubMed] [Google Scholar]
- 39.Di Sclafani V, Tolou-Shams M, Price LJ, Fein G. Neuropsychological performance of individuals dependent on crack-cocaine, or crack-cocaine and alcohol, at 6 weeks and 6 months of abstinence. Drug Alcohol Depend (2002) 66(2):161–71 10.1016/S0376-8716(01)00197-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D’Elia L, Satz P. Color Trails 1 and 2. Odessa, FL: Psychological Assessment Resources; (1989). [Google Scholar]
- 41.D’Elia L, Satz P, Uchiyama C, White T. Color Trails Test Professional Manual. Odessa, FL: Psychological Assessment Resources; (1996). [Google Scholar]
- 42.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol (2004) 19(2):203–14 10.1016/S0887-6177(03)00039-8 [DOI] [PubMed] [Google Scholar]
- 43.Maj M, D’Elia L, Satz P, Janssen R, Zaudig M, Uchiyama C, et al. Evaluation of two new neuropsychological tests designed to minimize cultural bias in the assessment of HIV-1 seropositive persons: a WHO study. Arch Clin Neuropsychol (1993) 8(2):123–35 10.1016/0887-6177(93)90030-5 [DOI] [PubMed] [Google Scholar]
- 44.Wetzel L, Boll T. Short Category Test, Booklet Format. Los Angeles: Western Psychological Services; (1987). [Google Scholar]
- 45.Calvin WH. Brain and Intelligence. Chicago: University of Chicago Press; (1947). [Google Scholar]
- 46.Mitrushina M, Boone K, Razan J, D’Elia L. Handbook of Normative Data for Neuropsychological Assessment. 2nd ed New York: Oxford University Press; (2005). [Google Scholar]
- 47.Van der Elst W, van Boxtel MPJ, van Breukelen GJP, Jolles J. Rey’s verbal learning test: normative data for 1855 healthy participants aged 24-81 years and the influence of age, sex, education, and mode of presentation. J Int Neuropsychol Soc (2005) 11(3):290–302 10.1017/S1355617705050344 [DOI] [PubMed] [Google Scholar]
- 48.Muthen B, Muthen L. Mplus Version 5.1. Los Angeles: Muthen & Muthen; (2008). [Google Scholar]
- 49.Yuan K, Bentler P. Three likelihood-based methods for mean and covariance structure analysis with nonnormal missing data. Sociol Methodol (2000) 30(1):165–200 10.1111/0081-1750.00078 [DOI] [Google Scholar]
- 50.Ammassari A, Antinori A, Aloisi M, Trotta M, Murri R, Bartoli L, et al. Depressive symptoms, neurocognitive impairment, and adherence to highly active antiretroviral therapy among HIV-infected persons. Psychosomatics (2004) 45(5):394–402 10.1176/appi.psy.45.5.394 [DOI] [PubMed] [Google Scholar]
- 51.Richardson MA, Satz PF, Myers HF, Miller EN, Bing EG, Fawzy FI, et al. Effects of depressed mood versus clinical depression on neuropsychological test performance among African American men impacted by HIV/AIDS. J Clin Exp Neuropsychol (1999) 21(6):769–83 10.1076/jcen.21.6.769.860 [DOI] [PubMed] [Google Scholar]
- 52.Horton A., Jr Some suggestions regarding the clinical interpretation of the Trail Making Test. Clin Neuropsychol (1979) 1(1):20–3 [Google Scholar]
- 53.Starace F, Baldassarre C, Biancolilli V, Fea M, Serpelloni G, Bartoli L, et al. Early neuropsychological impairment in HIV-seropositive intravenous drug users: evidence from the Italian Multicentre Neuropsychological HIV Study. Acta Psychiatr Scand (1998) 97(2):132–8 10.1111/j.1600-0447.1998.tb09975.x [DOI] [PubMed] [Google Scholar]
- 54.Gonzalez R, Carey C, Grant I. Nonacute (residual) neuropsychological effects of cannabis use: a qualitative analysis and systematic review. J Clin Pharmacol (2002) 42:48S–57S 10.1002/j.1552-4604.2002.tb06003.x [DOI] [PubMed] [Google Scholar]
- 55.Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology (2006) 59:1337–43 10.1212/01.WNL.0000031422.66442.49 [DOI] [PubMed] [Google Scholar]
- 56.Basso MR, Bornstein RA. Neurobehavioural consequences of substance abuse and HIV infection. J Psychopharmacol (2000) 14(3):228–37 10.1177/026988110001400306 [DOI] [PubMed] [Google Scholar]
- 57.Norman L, Basso M, Kumar A, Malow R. Neuropsychological consequences of HIV and substance abuse: a literature review and implications for treatment and future research. Curr Drug Abuse Rev (2009) 2(2):143–56 10.2174/1874473710902020143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cristiani SA, Pukay-Martin ND, Bornstein RA. Marijuana use and cognitive function in HIV-infected people. J Neuropsychiatry Clin Neurosci (2004) 16(3):330–5 10.1176/appi.neuropsych.16.3.330 [DOI] [PubMed] [Google Scholar]
- 59.McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: mind the gap. Ann Neurol (2010) 67(6):699–714 10.1002/ana.22053 [DOI] [PubMed] [Google Scholar]
- 60.Gupta JD, Satishchandra P, Gopukumar K, Wilkie F, Waldrop-Valverde D, Ellis R, et al. Neuropsychological deficits in human immunodeficiency virus type 1 clade C-seropositive adults from South India. J Neurovirol (2007) 13(3):195–202 10.1080/13550280701258407 [DOI] [PubMed] [Google Scholar]
- 61.Müller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis (2010) 10(4):251–61 10.1016/S1473-3099(10)70026-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mateen FJ, Mills EJ. Aging and HIV-related cognitive loss. J Am Med Assoc (2012) 308(4):349–50 10.1001/jama.2012.8538 [DOI] [PubMed] [Google Scholar]
- 63.Flom PL, Friedman SR, Kottiri BJ, Neaigus A, Curtis R, DesJarlais D, et al. Stigmatized drug use, sexual partner concurrency, and other sex risk network and behavior characteristics of 18-24 year-old youth in a high-risk neighborhood. Sex Transm Dis (2001) 28(10):598–607 10.1097/00007435-200110000-00006 [DOI] [PubMed] [Google Scholar]
- 64.Reger M, Welsh R, Razani J, Martin D, Boone K. A meta-analysis of the neuropsychological sequelae of HIV infection. J Int Neuropsychol Soc (2002) 8(3):410–24 10.1017/S1355617702813212 [DOI] [PubMed] [Google Scholar]
- 65.Schuster RM, Gonzalez R. Substance abuse, hepatitis C, and aging in HIV: common cofactors that contribute to neurobehavioral disturbances. Neurobehav HIV Med (2012) 4:15–34 10.2147/NBHIV.S17408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nath A. Human immunodeficiency virus-associated neurocognitive disorder: pathophysiology in relation to drug addiction. Ann N Y Acad Sci (2010) 1187:122–8 10.1111/j.1749-6632.2009.05277.x [DOI] [PubMed] [Google Scholar]
- 67.Bates ME, Bowden SC, Barry D. Neurocognitive impairment associated with alcohol use disorders: implications for treatment. Exp Clin Psychopharmacol (2002) 10(3):193–212 10.1037//1064-1297.10.3.193 [DOI] [PubMed] [Google Scholar]
- 68.Baum MK, Rafie C, Lai S, Sales S, Page JB, Campa A. Alcohol use accelerates HIV disease progression. AIDS Res Hum Retroviruses (2010) 26(5):511–8 10.1089/aid.2009.0211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Braithwaite SR, Bryant KJ. Influence of alcohol consumption on adherence to and toxicity of antiretroviral therapy and survival. Alcohol Res Health (2010) 33(3):280–7 [PMC free article] [PubMed] [Google Scholar]
- 70.Cook R, Sereika S, Hunt S, Woodward W, Erlen J, Conigliaro J. Problem drinking and medication adherence among persons with HIV infection. J Gen Intern Med (2001) 16(2):83–8 10.1111/j.1525-1497.2001.00122.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Margolin A, Avants S, Warburton L, Hawkins K, Shi J. A randomized clinical trial of a manual-guided risk reduction intervention for HIV-positive injection drug users. Health Psychol (2003) 22(2):223–8 10.1037/0278-6133.22.2.223 [DOI] [PubMed] [Google Scholar]
- 72.Margolin A, Avants S, Warburton L, Hawkins K. Factors affecting cognitive functioning in a sample of human immunodeficiency virus-positive injection drug users. AIDS Patient Care STDS (2002) 16(6):255–67 10.1089/10872910260066697 [DOI] [PubMed] [Google Scholar]
- 73.Miller L. Psychotherapy of the Brain-Injured Patient: Reclaiming the Shattered Self. Chicago: WW Norton & Co; (1993). [Google Scholar]
- 74.Crum-Cianflone NF, Moore DJ, Letendre S, Poehlman Roediger M, Eberly L, Weintrob A, et al. Low prevalence of neurocognitive impairment in early diagnosed and managed HIV-infected persons. Neurology (2013) 80(4):371–9 10.1212/WNL.0b013e31827f0776 [DOI] [PMC free article] [PubMed] [Google Scholar]